Abstract
Objective
To evaluate the efficacy and safety of a novel, continuous intravenous infusion of ketorolac, a powerful nonopioid analgesic, for postoperative pain control.
Patients and Methods
A prospective, double-blind, randomized, placebo-controlled trial of a continuous infusion of ketorolac tromethamine in 1 L of normal saline vs placebo was performed in 135 patients aged 18 to 75 years after laparoscopic donor nephrectomy or percutaneous nephrolithotomy completed from October 7, 2008, through July 21, 2010. Primary study end points were the 24-hour differences in visual analog pain scores and total narcotic consumption, whereas secondary end points were differences in urine output, serum creatinine level, and hemoglobin level.
Results
The study was stopped after randomization of 135 patients (68 in the ketorolac group and 67 in the placebo group) when interim analysis indicated that the difference in mean pain scores between the 2 groups (difference, 0.6) was smaller than the 1-point threshold set forth in the power calculations. No statistically significant change was noted in hemoglobin levels from preoperative to postoperative values (P=.13) or in postoperative serum creatinine levels (P=.13).
Conclusion
Although continuous infusion of ketorolac produced only a modest decrease in the use of narcotics, it appears to offer a safe therapeutic option for nonnarcotic pain control.
Trial Registration
clinicaltrials.gov Identifiers: NCT00765128 and NCT00765232
Abbreviations and Acronyms: LDN, laparoscopic donor nephrectomy; NSAID, nonsteroidal anti-inflammatory drug; PNL, percutaneous nephrolithotomy; VAS, visual analog scale
Inadequate postsurgical pain control may lead to delayed hospital discharge, unanticipated readmissions, delayed convalescence, and increased health care costs.1 Risks associated with pain management include opiate overdose, medication adverse effects, and required administration by nursing staff. Nonnarcotic pain medications may decrease patient morbidity, expedite discharge, and help contain cost.2 All these factors are especially important for elective urologic operations, such as laparoscopic donor nephrectomy or percutaneous nephrolithotomy, which are often performed on healthy individuals who desire an uncomplicated recovery and a short convalescence.
The cornerstone of postoperative pain management remains opioid-based narcotics, which have the adverse effects of nausea, vomiting, pruritus, confusion, and respiratory depression.3 In contrast, nonsteroidal anti-inflammatory drugs (NSAIDs), such as ketorolac tromethamine, are not sedating, are not addictive, and do not affect bowel function but nonetheless offer considerable pain relief. In addition, after major surgery, many patients are initially unable to take oral medications. Ketorolac is one of the few nonopioid medications available for intravenous and intramuscular use.4
Patients and physicians may be apprehensive about using ketorolac because of the risk of bleeding, diathesis, or renal impairment. Previous studies have established the safety of bolus administration of ketorolac and have found that the use of ketorolac decreases length of stay, reduces narcotic requirements, and results in faster return to bowel function.5-7 In addition, selected reports highlight the use of continuous infusions of ketorolac with good efficacy in patients with cancer pain.8-10
We sought to evaluate the efficacy and safety of a novel continuous infusion of ketorolac for pain control in patients undergoing laparoscopic donor nephrectomy (LDN) or percutaneous nephrolithotomy (PNL). This study is a prospective, double-blind, randomized, placebo-controlled trial of a continuous intravenous infusion of ketorolac in the postoperative setting. We chose continuous infusion over traditional bolus-dosing regimens to try to decrease the peaks and troughs associated with bolus dosing and thus to provide a better steady state of pain control. We hope these results will help guide clinicians toward a new and safer pain management modality.
Methods
Patients
After approval from the Mayo Clinic Institutional Review Board, we conducted a prospective, double-blind, randomized, placebo-controlled trial of ketorolac vs placebo for postoperative pain control in LDN and PNL patients treated at the Mayo Clinic Hospital in Phoenix, Arizona, from October 7, 2008, through July 21, 2010. Men and women aged 18 to 75 years who were undergoing LDN or PNL were candidates for participation. Exclusion criteria included pregnancy, inability to provide informed consent, NSAID allergy, asthma, long-term opioid use, intraoperative blood loss greater than 300 mL, peptic ulcer disease, bleeding diathesis, creatinine level greater than 2.0 mg/dL (to convert to μmol/L, multiply by 88.4), and probenecid use. Both LDN and PNL were performed using standard approaches previously described by Lallas et al11 and Miller et al,12 respectively.
After providing written informed consent, patients were assigned to receive either ketorolac or placebo at a random 1:1 ratio. The project statistician (J.G.H.) created the randomized treatment allocation schedule using a computerized random number generator. A separate randomized treatment allocation schedule was used for each type of procedure, and the allocation schedule was stored at the Mayo Clinic Hospital pharmacy. Patients, anesthesiologists, operating room staff, postanesthesia care unit staff, urology nurses, urology attending physicians and residents, and research coordinators were all masked to the treatment assignments until the last patient completed the study.
Intervention
The study infusions were prepared by the hospital pharmacist. The study drug consisted of 90 mg of ketorolac in 1 L of 0.9% normal saline infused at 40 mL/h. The placebo was 0.9% normal saline infused at the same rate. Infusions were started in the postanesthesia care unit within 30 minutes of completion of the surgical procedure and were continued when the patient was transferred to the medical-surgical inpatient ward. No additional ketorolac administration was allowed. Nursing staff could increase the infusion to 120 mL/h for 15 minutes each hour for pain levels greater than 5. Per our clinical protocol, all patients were allowed liberal access to supplemental opioids for refractory pain (Figure 1).
FIGURE 1.
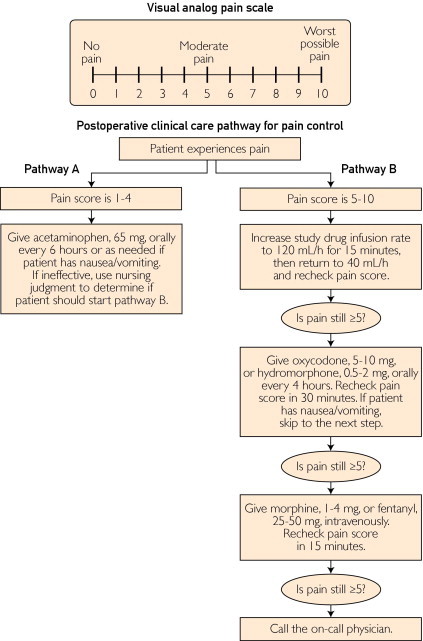
Visual analog scale (top) and pain control algorithm (bottom).
Pain was assessed and recorded preoperatively, again at initiation of the postoperative infusion, and then every 4 hours thereafter using a validated visual analog scale (VAS) administered by nursing staff (Figure 1). The VAS was selected because it has been found to be methodologically sound, conceptually simple, and easily administered; it also requires no reading or writing skills.13,14 Each patient's participation in the study was complete 24 hours after initiation of the ketorolac or placebo infusion.
Serum electrolyte levels, creatinine level, and a complete blood cell count were measured in the postanesthesia care unit and on postoperative day 1. Time to flatus, oral intake of fluids, and ambulation were also documented by the nursing staff. In addition to the study infusion, intravenous fluids were administered, for a total fluid allotment of at least 125 mL/h. The amount of supplemental opioid analgesics consumed was documented and converted into morphine equivalents.
Study End Points and Statistical Analyses
The primary outcome measures were the differences in VAS scores and total amounts of supplemental morphine equivalents used at 24 hours. Secondary outcomes were changes in serum creatinine level, urine output, and hemoglobin level between cohorts. Two primary outcome measures were accounted for using the Hochberg method. Statistical significance and adjusted means were calculated using a general linear model with terms for treatment and procedure.
Sample size was set at 160 patients with an interim analysis after accrual of 128 (80.0%). A stopping rule after interim analysis was established using the α-spending method with an O'Brien-Fleming boundary function. At the interim analysis, results were considered statistically significant (global P<.05) if both primary outcome measures had nominal P<.02 or if either primary outcome measure had nominal P<.01.
The sample size for the interim analysis was determined on the basis of the power to detect the effect size as reported by Schlachta et al15 for ketorolac treatment in laparoscopic colon surgery (Δ = 1.9 points; SD = 1.9 points). The sample size for the final analysis was determined on the basis of the power to detect a difference of 1.0 point, which was considered the minimum clinically important difference. The primary comparison included both types of surgical procedure. Each surgical procedure was also evaluated separately using the gatekeeper method. That is, the ketorolac effect in the LDN subgroup would be evaluated for statistical significance only if the effect in the combined analysis was statistically significant, and the ketorolac effect in the PNL subgroup would be evaluated for statistical significance only if the effect in the LDN subgroup was statistically significant. This sequence was selected because we expected to enroll more patients in the LDN subgroup than in the PNL subgroup, and we expected the LDN subgroup to report more pain.
The sample had 80% power (global α=.05) for the final analysis and 60% power for the interim analysis if ketorolac reduced pain in the target population by at least 1 point. Secondary variables measured on a continuous scale were assessed using a general linear model. Dichotomous measures were assessed using the Fisher exact test.
Results
We prospectively identified a total of 218 patients who were scheduled to undergo either LDN or PNL at our hospital. Twenty-seven patients were not eligible because of exclusion criteria, 51 declined consent, and 4 did not order the study in time, leaving a total of 135 patients who were randomized to ketorolac (n=68) or placebo (n=67) (Figure 2). Seven patients who consented did not complete the study because the planned procedure was not performed (6 patients) or because ketorolac was given during the procedure itself (1 patient), and these patients were not included in the final analysis. Thus, a total of 128 patients received the allocated intervention: 65 patients in the ketorolac group (57 LDN and 8 PNL) and 63 in the placebo group (54 LDN and 9 PNL). Because the study was performed in the hospital during the 24-hour postoperative period, all 128 patients completed the study. After interim analysis revealed that the difference in mean pain scores between the ketorolac and placebo groups (difference, 0.6) was smaller than the 1 point set forth in the power calculations, the study was stopped.
FIGURE 2.
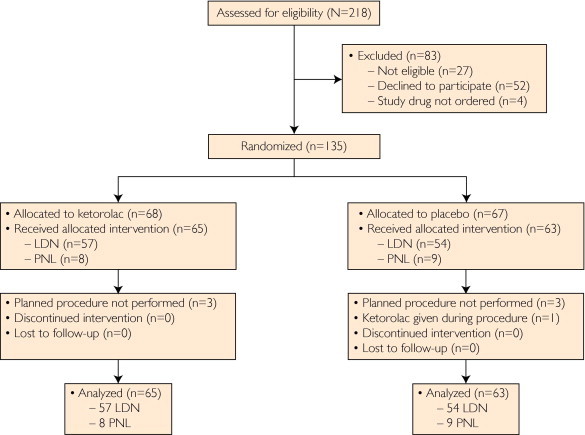
Flowchart of randomized controlled trial of ketorolac vs placebo for pain after renal surgery. LDN = laparoscopic donor nephrectomy; PNL = percutaneous nephrolithotomy.
Baseline demographic information is summarized in Table 1. The mean age of the patients was 52 years (range, 20-76 years). Three of the 8 patients in the PNL ketorolac group were actively using narcotics at the time of randomization but did not meet exclusion criteria because the narcotic use occurred in the context of nephrolithiasis pain and lasted less than 5 days. More patients in the PNL group (n=17) were taking medications for diabetes mellitus or hypertension, and the overall body mass index (weight in kilograms divided by the square of height in meters) was higher in the PNL patients. For the 17 PNL patients, no differences were found in body mass index or medication use between the ketorolac and placebo groups.
TABLE 1.
| Characteristic | LDN group (n=111) |
PNL group (n=17) |
||
|---|---|---|---|---|
| Ketorolac (n=57) | Placebo (n=54) | Ketorolac (n=8) | Placebo (n=9) | |
| Mean age (y) | 43 | 43 | 59 | 62 |
| Women | 23 (40.4) | 33 (61.1) | 3 (37.5) | 4 (44.4) |
| Current narcotic use | 0 | 0 | 3 (37.5) | 0 |
| Current tobacco use | 7 (12.3) | 8 (14.8) | 2 (25.0) | 0 |
| Current use of medications for | ||||
| Diabetes mellitus | 0 | 0 | 1 (12.5) | 1 (11.1) |
| Hypertension | 3 (5.3) | 2 (3.7) | 4 (50.0) | 4 (44.4) |
| Thyroid | 7 (12.3) | 4 (7.4) | 0 | 2 (22.2) |
| Mean weight (kg) | 77 | 78 | 92 | 87 |
| Mean BMI | 25.9 | 26.5 | 33.1 | 30.5 |
| Mean preoperative baseline pain score (scale of 0-10) | 0.04 | 0.09 | 1.6 | 0.28 |
BMI = body mass index; LDN = laparoscopic donor nephrectomy; PNL = percutaneous nephrolithotomy.
Data are presented as No. (percentage) unless otherwise indicated.
During the 24-hour postoperative period, patients receiving ketorolac infusion had a larger numerical but not statistically significant decrease in mean pain score compared with that in patients receiving placebo (1.1 vs. 0.6 points, respectively; P=.10) (Table 2 and Figure 3). At 20 postoperative hours, the difference was statistically significant in the LDN group (P=.02) (Table 4). During the study period, the mean morphine equivalents consumed was 38 mg in the ketorolac group and 41 mg in the placebo group, a difference of 3 mg (P=.79) (Table 2).
TABLE 2.
Overall Outcomes of 128 Patients Who Underwent Laparoscopic Donor Nephrectomy or Percutaneous Nephrolithotomya,b
| Variable | Ketorolac (n=65) | Placebo (n=63) | Δ (95% CI) | P value |
|---|---|---|---|---|
| Postoperative pain (scale of 0-10) | ||||
| 0 Hours | 2.6 | 2.7 | −0.1 (−1.1 to 1.0) | .91 |
| 4 Hours | 2.5 | 3.0 | −0.5 (−1.2 to 0.3) | .22 |
| 8 Hours | 2.3 | 2.5 | −0.2 (−1.0 to 0.5) | .58 |
| 12 Hours | 2.0 | 2.2 | −0.2 (−0.8 to 0.5) | .58 |
| 16 Hours | 1.6 | 2.1 | −0.6 (−1.2 to 0.1) | .11 |
| 20 Hours | 1.6 | 2.3 | −0.6 (−1.3 to 0.0) | .06 |
| 24 Hours | 1.5 | 2.1 | −0.6 (−1.3 to 0.1) | .10 |
| Morphine equivalents (mg) | ||||
| 24 Hours | 38 | 41 | −3 (−20 to 15) | .79 |
| Discharge | 51 | 52 | −1 (−24 to 22) | .94 |
| Duration of study drug infusion (h) | 23.3 | 22.7 | 0.5 (−0.4 to 1.4) | .25 |
| Blood transfusion, No. (%) | 0 | 0 | 0.00 (−0.06 to 0.06) | >.99 |
| Urine output (mL) | 3200 | 4500 | −1300 (−2000 to −500) | .001 |
| Time to oral fluids (h) | 3.3 | 3.7 | −0.5 (−1.5 to 0.5) | .32 |
| Time to general diet (h) | 14 | 16 | −2.0 (−5.2 to 1.1) | .20 |
| Time to flatus (h) | 17 | 19 | −1.7 (−6.6 to 3.3) | .50 |
| Time to ambulation (h) | 9.7 | 12 | −2.0 (−4.2 to 0.3) | .09 |
| Postoperative weight gain (kg) | 85 | 86 | −1.1 (−7.0 to 4.9) | .72 |
CI = confidence interval.
Data are presented as mean unless otherwise indicated.
FIGURE 3.
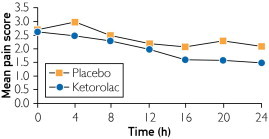
Mean pain score in first 24 hours.
TABLE 4.
Mean Laboratory Values on Postoperative Day 1 for 128 Patients Who Underwent Laparoscopic Donor Nephrectomy or Percutaneous Nephrolithotomy
| Value | Ketorolac (n=65) | Placebo (n=63) | Δ (95% CI) | P value |
|---|---|---|---|---|
| Overall group (N=128) | ||||
| Hemoglobin (g/L) | 11.5 | 12.1 | −0.6 (−1.1 to −0.1) | .01 |
| WBC count (×109/L) | 8.7 | 9.8 | −1.1 (−2.1 to −0.1) | .03 |
| Sodium (mmol/L) | 137.0 | 136.8 | 0.2 (−0.7 to 1.1) | .65 |
| Potassium (mmol/L) | 4.12 | 4.10 | 0.03 (−0.17 to 0.23) | .79 |
| Chloride (mmol/L) | 102.0 | 99.7 | 2.3 (−0.8 to 5.4) | .14 |
| Carbon dioxide (mmol/L) | 26.8 | 26.2 | 0.6 (−0.2 to 1.4) | .12 |
| BUN ratio | 13.4 | 12.2 | 1.2 (−0.2 to 2.5) | .09 |
| Creatinine (mg/dL) | 1.19 | 1.12 | 0.08 (−0.02 to 0.17) | .13 |
| Laparoscopic donor nephrectomy subgroup (n=111) | ||||
| Hemoglobin (g/L) | 11.6 | 12.1 | −0.5 (−1.0 to 0.0) | .04 |
| WBC count (×109/L) | 9.2 | 10.5 | −1.3 (−2.3 to −0.3) | .01 |
| Sodium (mmol/L) | 136.8 | 136.3 | 0.5 (−0.3 to 1.3) | .24 |
| Potassium (mmol/L) | 4.03 | 3.97 | 0.06 (−0.16 to 0.28) | .59 |
| Chloride (mmol/L) | 103.2 | 102.0 | 1.2 (0.3 to 2.2) | .01 |
| Carbon dioxide (mmol/L) | 27.4 | 26.8 | 0.6 (−0.3 to 1.4) | .19 |
| BUN ratio | 11.7 | 11.0 | 0.7 (−0.6 to 2.1) | .29 |
| Creatinine (mg/dL) | 1.38 | 1.30 | 0.08 (−0.03 to 0.19) | .15 |
| Percutaneous nephrolithotomy subgroup (n=17) | ||||
| Hemoglobin (g/L) | 11.2 | 12.2 | −1.0 (−2.3 to 0.3) | .13 |
| WBC count (×109/L) | 8.6 | 8.6 | 0.0 (−3.5 to 3.5) | >.99 |
| Sodium (mmol/L) | 136.2 | 138.0 | −1.8 (−5.6 to 2.1) | .35 |
| Potassium (mmol/L) | 4.12 | 4.31 | −0.19 (−0.62 to 0.25) | .38 |
| Chloride (mmol/L) | 104.1 | 95 | 9.5 (−15.7 to 34.7) | .44 |
| Carbon dioxide (mmol/L) | 26.5 | 25.4 | 1.1 (−1.4 to 3.5) | .37 |
| BUN ratio | 16.4 | 12.4 | 4.0 (−1.3 to 9.3) | .13 |
| Creatinine (mg/dL) | 0.99 | 0.94 | 0.04 (−0.14 to 0.23) | .63 |
BUN = blood urea nitrogen; CI = confidence interval; WBC = white blood cell.
No statistically significant differences were found between the treatment and placebo groups in time to oral intake of fluids, flatus, or change from preoperative to postoperative weight either overall (Table 2) or by LDN or PNL subgroup (Table 3). A statistically significant improvement was noted in time to ambulation in the ketorolac LDN group (11 vs 13.5 hours; P=.04) (Table 3).
TABLE 3.
Outcomes by Type of Surgery for 128 Patients Who Underwent Laparoscopic Donor Nephrectomy or Percutaneous Nephrolithotomya,b
| Variable | Ketorolac (n=65) | Placebo (n=63) | Δ (95% CI) | P value |
|---|---|---|---|---|
| Laparoscopic donor nephrectomy (n=111) | ||||
| Pain score (scale of 0-10) | ||||
| 0 Hours | 3.4 | 3.6 | −0.2 (−1.4 to 0.9) | .68 |
| 4 Hours | 3.7 | 4.3 | −0.5 (−1.3 to 0.2) | .18 |
| 8 Hours | 3.5 | 3.7 | −0.2 (−1.0 to 0.7) | .69 |
| 12 Hours | 2.6 | 2.9 | −0.3 (−1.0 to 0.4) | .41 |
| 16 Hours | 2.5 | 3.2 | −0.7 (−1.4 to 0.1) | .08 |
| 20 Hours | 2.4 | 3.2 | −0.9 (−1.6 to −0.1) | .02 |
| 24 Hours | 2.4 | 3.1 | −0.7 (−1.5 to 0.1) | .08 |
| Morphine equivalents (mg) | ||||
| 24 Hours | 65 | 69 | −4 (−24 to 17) | .73 |
| Discharge | 85 | 88 | −3 (−29 to 23) | .83 |
| Duration of study drug infusion (h) | 23.0 | 22.2 | 0.8 (−0.2 to 1.8) | .12 |
| Transfusion, No. (%) | 0 (0) | 0 (0) | 0.00 (−0.07 to 0.06) | >.99 |
| Urine output (mL) | 4600 | 5800 | −1300 (−2100 to −400) | .003 |
| Time to oral fluids (h) | 3.9 | 4.6 | −0.8 (−1.8 to 0.3) | .18 |
| Time to general diet (h) | 16.2 | 17.7 | −1.5 (−5.0 to 1.9) | .38 |
| Time to flatus (h) | 21 | 22 | −1.4 (−7.3 to 4.5) | .64 |
| Time to ambulation (h) | 11.0 | 13.5 | −2.5 (−4.9 to −0.1) | .04 |
| Postoperative weight (kg) | 81 | 81 | 0 (−6 to 6) | .95 |
| Percutaneous nephrolithotomy (n=17) | ||||
| Pain score (scale of 0-10) | ||||
| 0 Hours | 2.5 | 1.3 | 1.2 (−1.6 to 3.9) | .37 |
| 4 Hours | 1.5 | 1.4 | 0.1 (−2.2 to 2.3) | .96 |
| 8 Hours | 0.88 | 1.3 | −0.5 (−1.7 to 0.8) | .44 |
| 12 Hours | 1.6 | 1.1 | 0.5 (−1.6 to 2.6) | .61 |
| 16 Hours | 1.0 | 0.8 | 0.2 (−1.1 to 1.5) | .72 |
| 20 Hours | 1.5 | 0.8 | 0.7 (−1.2 to 2.6) | .44 |
| 24 Hours | 1.0 | 0.9 | 0.1 (−1.3 to 1.5) | .87 |
| Morphine equivalents (mg) | ||||
| 24 Hours | 15 | 10 | 5 (−21 to 31) | .67 |
| Discharge | 24 | 11 | 13 (−18 to 43) | .39 |
| Duration of study drug infusion (h) | 22.7 | 24.0 | −1.2 (−3.2 to 0.7) | .20 |
| Transfusion, % | 0 | 0 | 0 (−0.34 to 0.37) | >.99 |
| Urine output (mL) | 2000 | 3100 | −1200 (−2700 to 400) | .12 |
| Time to oral fluids (h) | 3.5 | 2.2 | 1.3 (−0.2 to 2.8) | .08 |
| Time to oral general diet (h) | 10.3 | 15.2 | −4.9 (−12.9 to 3.2) | .22 |
| Time to flatus (h) | 13.6 | 16.3 | −2.7 (−11.6 to 6.2) | .53 |
| Time to ambulation (h) | 10.0 | 8.6 | 1.4 (−6.0 to 8.8) | .69 |
| Postoperative weight (kg) | 80 | 94 | −14 (−49 to 21) | .37 |
CI = confidence interval.
Data are presented as mean unless otherwise indicated.
Postoperative urine output was lower in the ketorolac group both overall (Table 2) and in the LDN subgroup (Table 3). Hemoglobin level and white blood cell count were lower in the ketorolac group (Table 4). However, no statistically significant difference was found in serum creatinine level between the groups (P=.13 overall, P=.15 in the LDN subgroup, and P=.63 in the PNL subgroup), and both groups had adequate mean urinary output (142 mL/h in the ketorolac group and 175 mL/h in the placebo group). In addition, no statistically significant differences were found in the change from preoperative to postoperative hemoglobin level between groups (1.33 g/dL in the ketorolac group vs 0.94 g/dL in the placebo group [to convert to g/L, multiply by 10]; P=.13; data not shown), and no patient required a blood transfusion.
Discussion
A continuous infusion of ketorolac offered a safe therapeutic option for pain management after LDN or PNL. At the interim analysis, the mean pain score on the VAS for the ketorolac group was 0.6 point lower than that of the placebo group. Because this difference was smaller than the minimum criterion set forth in the power calculations in the study protocol, the study was suspended on the basis of the predetermined stopping criteria. However, we still believe that our report of these findings is an important contribution to the medical literature because it describes a novel administration of ketorolac and highlights the safety of the short-term use of this medication in patients after selected renal surgery.
We hypothesized that the use of a continuous ketorolac infusion would provide patients with a consistent steady state of medication delivery by eliminating the peaks and troughs associated with bolus dosing. This approach has been found to be effective with patient-controlled analgesia because frequent smaller doses provide smoother biodistribution and better matching of analgesic dose and patient requirement.16
Urologic studies describing the use of ketorolac for postoperative pain control are limited, and none of these studies have been double-blind, randomized, placebo-controlled trials.5-7,17-21 This is unfortunate because there can be considerable postoperative narcotic use in certain urologic populations. Lingeman et al22 reported a mean postoperative morphine use of 33.2 mg (range, 7.5-76 mg) during a mean hospital stay of 3.2 days for patients undergoing PNL. In addition, the only clinical studies that describe a continuous ketorolac infusion are in reference to cancer pain or were not randomized.4,8-10 To our knowledge, our findings are the first from a randomized, double-blind, placebo-controlled study to evaluate a continuous ketorolac infusion for postoperative pain control in patients who have undergone LDN or PNL.
Acute renal failure is a well-described complication of NSAID use. The NSAID-mediated inhibition of cyclooxygenases inhibits vasodilatory prostanoid production, thus reducing the diameter of the afferent arteriole and contributing to a decrease in the glomerular filtration rate.23 Patients with underlying volume depletion, which is common in the postoperative setting, are at risk for this phenomenon.23 We believe that adequate intravenous fluid hydration of patients receiving ketorolac is renoprotective. The use of adequate intravenous fluids to prevent intravascular dehydration may mitigate the potential detrimental effects of ketorolac on glomerular filtration rate by increasing hydrostatic pressure and volume, which may explain why no difference was observed in serum creatinine level between the ketorolac and placebo groups. A decrease was noted in the 24-hour urine output in the ketorolac group vs the placebo group (P=.001). However, mean urine output was still excellent in both cohorts, with a mean of 142 and 175 mL/h, respectively. Furthermore, no difference was found in serum creatinine level between groups, which supports the notion that a continuous infusion of ketorolac can be used safely in the period after selected renal surgery. This finding is consistent with those in other reports in the contemporary medical literature. Freedland et al6 investigated 198 patients who underwent open LDN with or without ketorolac analgesia and found no difference at 3-month postsurgical follow-up in creatinine clearance (70% vs 73%; P=.92) between those treated with or without ketorolac. In addition, a review of nearly 20,000 patients treated with ketorolac found that use for less than 5 days did not adversely affect renal function and resulted in no risk of renal failure.24
The use of NSAIDs may cause prostaglandin inhibition and associated platelet dysfunction that increases the risk of bleeding. The mean hemoglobin level was lower in the ketorolac group vs the placebo group on postoperative day 1 (11.5 vs 12.1 g/L; P=.01). However, no patient required a blood transfusion or returned to the operating room for bleeding, and no difference was observed in the change in the preoperative vs the postoperative hemoglobin level (1.33 vs 0.94 g/L; P=.13).
Because our study is the first to describe the use of a continuous ketorolac infusion for postoperative pain control, the power calculations were defined using a study by Schlachta et al,15 which evaluated pain control after laparoscopic colon surgery. However, the assumption that postoperative pain for renal and colon surgery would be similar, and thus a comparable effect of ketorolac on postoperative pain would be observed, may not have been accurate. In addition, in the colon study, ketorolac was provided in a bolus-dosing regimen and not as a continuous infusion as in our study. These differences in surgical technique, study design, method of drug administration, and patient populations may also account for the erroneous hypothesized pain differences used for the power calculations. Perhaps the lack of difference found in pain scores and morphine equivalents used is indicative of both the subjective nature of patient-perceived pain and the overall adequacy of postoperative pain control by the clinical pathway at our institution. In addition, perhaps our ketorolac infusion dose was too low, and thus a higher dose or infusion rate may be more effective and equally safe. Finally, perhaps our sample size, especially in the PNL group, was too small to allow us to identify a clinically meaningful difference between treatment and placebo groups.
Conclusion
Ketorolac appears to offer a safe therapeutic option for patients in the acute postoperative period after LDN or PNL, with no differences seen in serum creatinine level or changes in hemoglobin level between groups. Although the decreased use of narcotics was only modest, to our knowledge, this study is the first randomized, double-blind, placebo-controlled trial to highlight the novel approach of providing a continuous steady state of ketorolac. As such, it represents the highest level of scientific evidence, which we hope will help guide clinicians in the use of this medication in the pain management armamentarium.
Footnotes
Grant Support: This project was funded by a CR5 Mayo Clinic grant.
Potential Competing Interests: Dr Castle is a speaker for Intuitive Surgical Inc. Dr Humphreys has a consulting agreement with Boston Scientific Corp, Lumenis Inc, and Medafor Inc. Mr Hentz has received grant support for research activity from Allergan Inc, Anodyne Therapy LLC, Astellas Pharma US Inc, Dynatherm Medical Inc, Genentech Inc, InKline Pharmaceuticals Inc, Medtronic Inc, and Millennium Pharmaceuticals Inc.
References
- 1.Palvin D.J., Chen C., Penaloza D.A., Polissar N.L., Buckley F.P. Pain as a factor complicating recovery and discharge after ambulatory surgery. Anesth Analg. 2002;95(3):627–634. doi: 10.1097/00000539-200209000-00025. [DOI] [PubMed] [Google Scholar]
- 2.Knight M.K., DiMarco D.S., Myers R.P. Subjective and objective comparison of critical care pathways for open donor nephrectomy. J Urol. 2002;167(6):2368–2371. [PubMed] [Google Scholar]
- 3.Carr D.B., Jacox A.K., Chapman C.R. Acute Pain Management: Operative or Medical Procedures and Trauma: Clinic Practice Guideline. Agency for Health Care Policy and Research, Public Health Service, US Dept of Health and Human Services; Rockville, MD: 1992. [Google Scholar]
- 4.Ready L.B., Brown C.R., Stahlgren L.H. Evaluation of intravenous ketorolac administered by bolus or infusion for treatment of postoperative pain: a double-blind, placebo-controlled, multicenter study. Anesthesiology. 1994;80(6):1277–1286. doi: 10.1097/00000542-199406000-00015. [DOI] [PubMed] [Google Scholar]
- 5.Breda A., Bui M., Liao J., Schulam P.G. Association of bowel rest and ketorolac analgesia with short hospital stay after laparoscopic donor nephrectomy. Urology. 2007;69(5):828–831. doi: 10.1016/j.urology.2007.01.083. [DOI] [PubMed] [Google Scholar]
- 6.Freedland S.J., Blanco-Yarosh M., Sun S.J. Effect of ketorolac on renal function after donor nephrectomy. Urology. 2002;59(6):826–830. doi: 10.1016/s0090-4295(02)01514-5. [DOI] [PubMed] [Google Scholar]
- 7.Freedland S.J., Blanco-Yarosh M., Sun J.C. Ketorolac-based analgesia improves outcomes for living kidney donors. Transplantation. 2002;73(5):741–745. doi: 10.1097/00007890-200203150-00014. [DOI] [PubMed] [Google Scholar]
- 8.Middleton R.K., Lyle J.A., Berger D.L. Ketorolac continuous infusion: a case report and review of the literature. J Pain Symptom Manage. 1996;12(3):190–194. doi: 10.1016/0885-3924(96)00129-7. [DOI] [PubMed] [Google Scholar]
- 9.Gordon R.L. Prolonged central intravenous ketorolac continuous infusion in a cancer patient with intractable bone pain. Ann Pharmacother. 1998;32(2):193–196. doi: 10.1345/aph.17205. [DOI] [PubMed] [Google Scholar]
- 10.Myers K.G., Trotman I.F. Use of ketorolac by continuous subcutaneous infusion for the control of cancer related pain. Postgrad Med J. 1994;70(823):359–362. doi: 10.1136/pgmj.70.823.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lallas C.D., Castle E.P., Andrews P.E., Schlinkert R.T. The development of a laparoscopic donor nephrectomy program in a de novo renal transplant program: evolution of technique and results in over 200 cases. JSLS. 2006;10(2):135–140. [PMC free article] [PubMed] [Google Scholar]
- 12.Miller L.N., Matlaga B.R., Handa S.E., Munch L.C., Lingeman J.E. The presence of horseshoe kidney does not affect the outcome of percutaneous nephrolithotomy. J Endourol. 2008;22(6):1219–1225. doi: 10.1089/end.2008.0051. [DOI] [PubMed] [Google Scholar]
- 13.Coll A.M., Ameen J.R., Mead D. Postoperative pain assessment tools in day surgery: literature review. J Adv Nurs. 2004;46(2):124–133. doi: 10.1111/j.1365-2648.2003.02972.x. [DOI] [PubMed] [Google Scholar]
- 14.Gallagher E.J., Bijur P.E., Latimer C., Silver W. Reliability and validity of a visual analog scale for acute abdominal pain in the ED. Am J Emerg Med. 2002;20(4):287–290. doi: 10.1053/ajem.2002.33778. [DOI] [PubMed] [Google Scholar]
- 15.Schlachta C.M., Burpee S.E., Fernandez C., Chan B., Mamazza J., Poulin E.C. Optimizing recovery after laparoscopic colon surgery (ORAL-CS): effect of intravenous ketorolac on length of hospital stay. Surg Endosc. 2007;21(12):2212–2219. doi: 10.1007/s00464-007-9335-4. [DOI] [PubMed] [Google Scholar]
- 16.Barkin R.L., Schwer W.A., Barkin S.J. Pharmacotherapeutic management of acute and chronic pain. In: Dimock K., Byrnes A., editors. Textbook of Family Medicine. 7th ed. Elsevier Saunders; Philadelphia, PA: 2007. pp. 305–316. [Google Scholar]
- 17.Sandhu D.P., Iacovou J.W., Fletcher M.S., Kaisary A.V., Philip N.H., Arkell D.G. A comparison of intramuscular ketorolac and pethidine in the alleviation of renal colic. Br J Urol. 1994;74(6):690–693. doi: 10.1111/j.1464-410x.1994.tb07107.x. [DOI] [PubMed] [Google Scholar]
- 18.Diblasio C.J., Snyder M.E., Kattan M.W., Russo P. Ketorolac: safe and effective analgesia for the management of renal cortical tumors with partial nephrectomy. J Urol. 2004;171(3):1062–1065. doi: 10.1097/01.ju.0000109961.69936.8e. [DOI] [PubMed] [Google Scholar]
- 19.Chow G.K., Frabrizio M.D., Steer T. Prospective double-blind study of effect of ketorolac administration after laparoscopic urologic surgery. J Endourol. 2001;15(2):171–174. doi: 10.1089/089277901750134502. [DOI] [PubMed] [Google Scholar]
- 20.Kobashi K.C., Chamberlin D.A., Rajpoot D., Shanberg A.M. Retroperitoneal laparoscopic nephrectomy in children. J Urol. 1998;160(3, pt 2):1142–1144. doi: 10.1097/00005392-199809020-00048. [DOI] [PubMed] [Google Scholar]
- 21.Perry K.T., Freedland S.J., Hu J.C. Quality of life, pain and return to normal activities following laparoscopic donor nephrectomy versus open mini-incision donor nephrectomy. J Urol. 2003;169(6):2018–2021. doi: 10.1097/01.ju.0000067975.59772.b6. [DOI] [PubMed] [Google Scholar]
- 22.Lingeman J.E., Coury T.A., Newman D.M. Comparison of results and morbidity of percutaneous nephrostolithotomy and extracorporeal shock wave lithotripsy. J Urol. 1987;138(3):485–490. doi: 10.1016/s0022-5347(17)43236-8. [DOI] [PubMed] [Google Scholar]
- 23.Brenner B.M. Renal complications of NSAIDs. In: Brenner B.M., Levine S.A., editors. Brenner and Rector's The Kidney. 8th ed. Elsevier Saunders; Philadelphia, PA: 2007. [Google Scholar]
- 24.Feldman H.I., Kinman J.L., Berlin J.A. Parenteral ketorolac: the risk of acute renal failure. Ann Intern Med. 1997;126(3):193–199. doi: 10.7326/0003-4819-126-3-199702010-00003. [DOI] [PubMed] [Google Scholar]


