Abstract
T helper 17 (Th17) cells play critical roles in the pathogenesis of inflammatory and autoimmune diseases, as well as in host protection against pathogens. The contribution of Th17 cells to human tumor immunity, however, remains largely unknown. Since their identification in 2005, Th17 cells have been extensively studied in mouse tumor models and human cancer patients. Although accumulating data suggest the importance of Th17 cells to tumor immunity, conclusions regarding the functional role of Th17 cells remain controversial. In this review, we summarize current knowledge regarding the regulation and functional role of Th17 cells in human cancers. In particular, we emphasize several recently identified characteristics of Th17 cells, including plasticity, their relationship with regulatory T cells, and Th17 cell heterogeneity in the tumor microenvironment. Improved understanding of these issues is critical to elucidating the role of Th17 cells in antitumor immunity and for the design of novel therapeutic approaches specifically targeting Th17 cells.
CME Accreditation Statement: This activity (“ASIP 2013 AJP CME Program in Pathogenesis”) has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint sponsorship of the American Society for Clinical Pathology (ASCP) and the American Society for Investigative Pathology (ASIP). ASCP is accredited by the ACCME to provide continuing medical education for physicians.
The ASCP designates this journal-based CME activity (“ASIP 2013 AJP CME Program in Pathogenesis”) for a maximum of 48 AMA PRA Category 1 Credit(s)™. Physicians should only claim credit commensurate with the extent of their participation in the activity.
CME Disclosures: The authors of this article and the planning committee members and staff have no relevant financial relationships with commercial interests to disclose.
The identification in 2005 of T helper 17 (Th17) cells as a third subset of T helper cells changed the classical Th1/Th2 paradigm of T helper cell differentiation.1,2 Compared with other T-cell lineages, Th17 cells are characterized by their production of IL-17, expression of unique transcription factors, and the performance of specific biological functions.3,4 Th17 cell differentiation and regulation have been extensively studied during the past 6 years. Differentiation of mouse Th17 cells is dependent on the specific cytokine combination of TGF-β and IL-6.5–7 Furthermore, IL-6 induces IL-21 production, which synergizes with TGF-β and IL-23 to promote the differentiation of Th17 cells in mice.8,9 IL-1 is required and important for the early differentiation of murine Th17 cells.10 IL-1 is a critical inducer for human Th17 cell differentiation, and the combination of IL-1, IL-6, and IL-23 is the optimal cytokine milieu for human Th17 generation.11 Molecular programming of transcription regulation is a determinant for Th17 development, in addition to cytokine regulation. At least six transcription factors are critical and required for Th17 cell development: signal transducer and activator of transcription 3 (Stat3), retinoid-related orphan receptor γt (ROR-γt), nuclear receptor ROR-α, IFN regulatory factor 4 (IRF-4), B-cell-activating transcription factor (B-ATF), and hypoxia-inducible factor 1 α (HIF1-α).12–15
Th17 cells are important in host defense against microbial infections, including bacteria, mycobacteria, viruses, and parasites.16,17 They also appear to be key mediators in the pathogenesis of a broad array of inflammatory and autoimmune diseases, including rheumatoid arthritis, psoriasis, and inflammatory bowel disease.17 Despite significant efforts by many research groups in this important area, the functional role of Th17 cells in tumor immunity remains unclear. Here, we review recently published articles that characterize Th17 cells in different types of human cancer. Specifically, we focus on the mechanisms for Th17 cell accumulation in tumor microenvironments, phenotypic features, regulation, and plasticity of tumor-infiltrating Th17 cells. We also discuss the potential role of Th17 cells in tumor immunity.
Prevalence of Th17 Cells in Tumor Microenvironments
Accumulating evidence suggests a close association of chronic infection and inflammation with tumorigenesis. Local inflammation in the tumor microenvironment recruits several different types of immune cells, including αβ T cells, γδ T cells, and natural killer (NK) T cells, all of which can play critical roles in tumor immunity.18,19 Given that Th17 cells have been identified as important players in the immunopathogenesis of inflammation, the presence of Th17 cells in a tumor microenvironment is expected. In fact, recent studies from our group and others have demonstrated that the development of Th17 cells in tumor-infiltrating lymphocytes is a general feature of cancers. Th17 cells have been found in many different types of human tumors, including lymphoma,20 myeloma,21,22 breast cancer,23,24 colon cancer,24–26 gastric cancer,27,28 hepatocellular cancer,25,29 melanoma,24,25,30 ovarian cancer,25,31–34 pancreatic cancer,25 and prostate cancer.35,36
Phenotypic Features of Tumor-Infiltrating Th17 Cells
Cytokines
In addition to IL-17, tumor-infiltrating Th17 cells also secrete other cytokines. Our group recently generated human Th17 clones from bulk tumor-infiltrating lymphocyte lines derived from melanoma, breast, and colon cancers. These tumor-derived Th17 clones secreted large amounts of IL-8 and TNF-α, small amounts of IL-6, but no IL-2, IL-4, IL-12, or IL-23,24 consistent with previous reports characterizing Th17 cells from other tissue sites.11,37 In addition, these tumor-infiltrating Th17 cells also secreted moderate amounts of IL-10 and TGF-β1,24 suggesting that Th17 cells may perform regulatory functions in tumor microenvironments.38,39 However, studies from another group have shown that human ovarian cancer-derived Th17 cells express high levels of IL-2, GM-CSF, and IFN-γ, but negligible levels of IL-10.25 These varied cytokine profiles of tumor-infiltrating Th17 cells further suggest the heterogeneity (IL-17+IFN-γ+ and IL-17+IL-10+) and polyfunction (effector or regulatory) of Th17 cells in tumor microenvironments.40,41 The differences may also reflect the fact that the various Th17 cells were obtained from patients with different types and/or different stages of cancer.
Chemokine Receptors
Th17 cells mediate inflammatory responses through selective migration and accumulative retention at specific sites. Recent studies have shown that the inflammatory microenvironment promotes production of CCL20, which preferentially recruits CC-chemokine receptor type 6 (CCR6)-expressing Th17 cells in human rheumatoid arthritis, psoriasis, and other chronic inflammatory diseases.42,43 In addition to universal expression of CCR6, Th17 cells can express Th1-associated (CCR2, CXCR3, CCR5, and CXCR6), Th2-associated (CCR4), and nonlymphoid tissue trafficking receptors (CCR4, CCR5, CCR6, CXCR3, and CXCR6), as well as homeostatic chemokine receptors (CD62L, CCR6, CCR7, CXCR4, and CXCR5) that are implicated in T-cell migration to and within lymphoid tissues.42 Our group analyzed chemokine receptor expression on tumor-infiltrating Th17 cells derived from melanoma, breast, and colon cancers. All of the Th17 clones expressed CCR2, CCR4, CCR5, CCR6, CCR7, and CXCR3, similar to the expression pattern in other types of T cells.24 These data suggest that tumor-infiltrating Th17 cells express homeostatic chemokine receptors as well as trafficking receptors, and also share major chemokine receptors with other T-cell lineages. Others, however, have reported that tumor-infiltrating Th17 cells express high levels of CXCR4 and CCR6, but not CCR2, CCR5, or CCR7.25 The difference between these studies may be due to different origins of Th17 cells from patients with different types of cancers.
Other Markers
Studies from our group demonstrated that tumor-derived Th17 clones uniformly express the memory T-cell phenotype CCR7+CD62Ldim/− and CD45RA−CD45RO+.24,32 Moreover, tumor-derived Th17 cells had minimal or no expression of the cytotoxicity-associated markers CD56, granzyme A and B, or Fas ligand, nor of the inhibitory molecule PD-1.24,25 Notably, tumor-infiltrating Th17 cells express some CTLA-4, CD25, and FOXP3, implying that tumor-infiltrating Th17 cells may have developmental plasticity and overlap phenotypically with T-regulatory cells (Tregs).24 Indeed, IL-17+FOXP3+CD4+ populations have recently been observed in human colon and esophageal cancers.44,45 Furthermore, tumor-derived Th17 clones can significantly alter their phenotypes and can differentiate into FOXP3+ Tregs with potent suppressive function after in vitro repetitive T-cell receptor stimulation.46
Potential Mechanisms Responsible for the Accumulation of Th17 Cells in Tumor Microenvironments
Tumor Microenvironment Factors Mediate Recruitment and Expansion of Th17 Cells
Recent studies suggest several potential mechanisms responsible for the accumulation of Th17 cells in tumor sites. One suggested mechanism is that the tumor microenvironment preferentially recruits Th17 cells. Our group recently showed that tumor cells, as well as tumor-derived fibroblasts, secrete MCP-1 (the ligand for CCR2 or CCR4; alias CCL2) and RANTES (the ligand for CCR1, CCR3, or CCR5; alias CCL5), both of which strongly attract Th17 cell migration.24 These studies suggest that tumor microenvironments may use migratory mechanisms to selectively recruit Th17 cells from the periphery into tumor sites. Furthermore, human primary tumor-infiltrating Th17 cells isolated from melanoma, colon, hepatocellular, ovarian, pancreatic, and renal cell carcinomas express high levels of CXCR4 and CCR6, several CD49 integrins, and the C-type lectin receptor CD161 (alias KLRB1).25,47 In addition, high levels of CXCL12 (the ligand for CXCR4) and CCL20 (the ligand for CCR6) have been found in human tumor microenvironments, which could facilitate Th17 cell trafficking and migration into the tumor sites (Figure 1).47
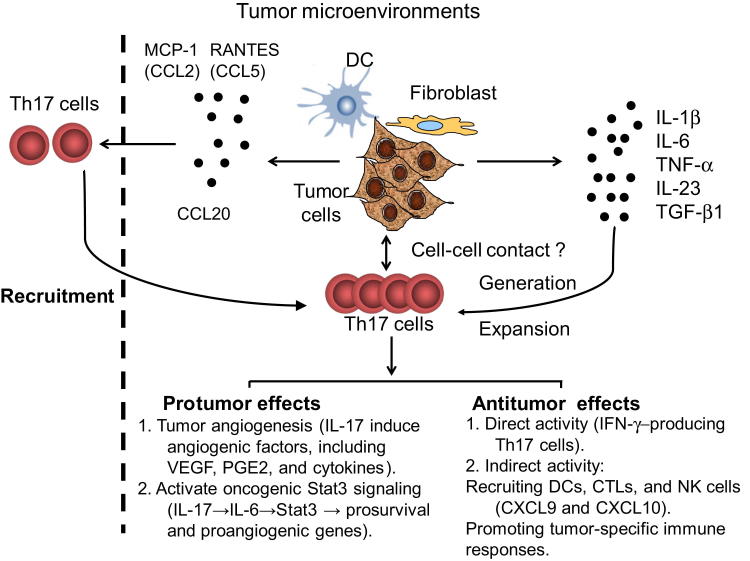
Figure 1.
Recruitment, induction, expansion, and roles of Th17 cells in tumor microenvironments. Tumor microenvironmental chemokines including RANTES (CCL5), MCP-1 (CCL2), and CCL20 secreted by tumor cells and tumor-derived fibroblasts or dendritic cells mediate the recruitment of Th17 cells. Tumor cells, tumor-derived fibroblasts, and dendritic cells produce a proinflammatory cytokine milieu (IL-1β, IL-6, TNF-α, IL-23, and TGF-β1) as well as provide cell-cell contact engagements that facilitate the generation and expansion of Th17 cells. IL-17 secreted by Th17 cells may play protumor roles by promoting tumor angiogenesis through the induction of angiogenic factors, including VEGF, PGE2, and cytokines, and by activating oncogenic stat3 signaling (IL-17→IL-6→Stat3→prosurvival and proangiogenic genes). Th17 cells may also play antitumor roles. Th17 cells can directly eradicate tumor cells through production of IFN-γ, and Th17 cells can also indirectly enhance antitumor immunity through the recruitment of dendritic cells (DC), natural killer (NK) cells, and CD8+ cytotoxic T lymphocytes (CTLs) into the tumor sites and promote specific antitumor immune responses.
Aside from the chemokine-mediated recruitment of Th17 cells into tumor sites, tumor microenvironment factors (tumor cells, as well as tumor-derived stromal cells such as fibroblasts and antigen-presenting cells) may also contribute to Th17 cell differentiation and expansion.24,32 Tumor cells and tumor environment stromal cells produce the proinflammatory cytokines IL-1β, IL-6, IL-23, and TGF-β, which can form an optimal proinflammatory cytokine milieu suitable for human Th17 cell differentiation and expansion.24,25,32 Blockade of IL-1β, but not IL-6 or TGFβ, decreases Th17 induction by human ovarian cancer-associated myeloid antigen-presenting cells, suggesting that IL-1β is crucial for Th17 cell generation in this tumor microenvironment.25,32 In addition to cytokines, other tumor microenvironment factors may also be critical for regulating Th17 cell differentiation and generation. One potential factor is retinoic acid, which enforces the generation of Tregs and inhibits the differentiation of Th17 cells.48 Aryl hydrocarbon receptor (AhR) ligand is another potential factor; AhR regulates Stat1 activation and participates in the development of Th17 cells.49,50 In addition, hypoxia-derived metabolites in the tumor microenvironment, such as adenosine, may influence Th17 cell differentiation.51 Adenosine acts via A(2B) adenosine receptor [A(2B)AR] in dendritic cells to promote the development of Th17 cells.52 Notably, recent studies from our group suggest that tumor cells and tumor-derived fibroblasts play a great role in the expansion rather than the differentiation of human Th17 cells.24 Tumor cells and tumor environment stromal cells not only provide soluble cytokines, but also unknown cell-cell contact signaling for the expansion of human Th17 cells, with the latter being more critical for Th17 cell regulation in the tumor microenvironment.24
Inflammatory Tumor Microenvironments Promote Th17 Cell Attraction and Generation
Chronic infection and inflammation are clearly important environmental factors for tumorigenesis. Infection-induced inflammation is triggered by interactions between pathogens and Toll-like receptors (TLRs) or other innate receptors expressed on immune and other cells.53 Recent studies have shown that activation of dendritic cells, monocytes, and peripheral blood mononuclear cells by TLR and nucleotide-binding oligomerization domain-containing protein (Nod) signaling can potentiate human Th17 cell differentiation and induction.54,55 TLR2 also plays an important role in regulating Th17 differentiation. Effects of TLR2 in CD4+ T lymphocytes can promote Th17-cell immune responses and regulate autoimmune disease pathogenesis.56 In addition, TLR2 stimulation can convert human naïve and effector Tregs into a Th17-like phenotype with reduced suppressive function.57 Recent findings from our group suggest that innate signaling may also influence the prevalence of Th17 cells in tumor microenvironments. In one study, TLR and Nod2 signaling increased MCP-1 and RANTES expression on tumor cells and tumor-derived fibroblasts, resulting in increased migration and trafficking of Th17 cells.24 In addition, TLR and Nod2 signaling accelerated the expansion of Th17 cells by promoting the secretion of inflammatory cytokines, including IL-1β, IL-6, IL-23, and TGF-β1, as well as by providing cell-contact engagement of tumor cells and tumor-derived fibroblasts.24 These results suggest that signaling mediated by local chronic inflammation and infections at tumor sites can directly influence tumor cells and tumor-derived stromal cells, which may also contribute to the accumulation of Th17 cells in tumor microenvironments.
Role of Th17 Cells in Antitumor Immunity
Although Th17 cells are prevalent within tumor microenvironments, their functional role in tumor immunity is controversial. Most studies investigating the relationship between Th17 cells and cancer have used mouse models, and results have been contradictory. Information regarding the role of human Th17 cells in cancer patients is limited (Table 1). A fundamental understanding of Th17 cells in antitumor immunity is critical to the development of novel cancer immunotherapeutic strategies (Figure 1).
Table 1.
Model Systems Used to Determine the Effects of Th17 or IL-17 in Tumor Immunity
| Tumor type |
Experimental model |
Key results |
References |
|---|---|---|---|
| Pro-tumor effects of IL-17 or Th17 cells | |||
| Human cervical tumor cell lines | Subdermally injected into nude mice | Cervical cell lines transfected with a cDNA encoding IL-17 exhibited a significant increase of tumor size when transplanted into nude mice. | 58 |
| Human multiple myeloma (MM) | Subcutaneously injected into SCID mice | Th17 cells and IL-17 were elevated in human myeloma. IL-17 promoted myeloma cell growth both in vitro and in vivo via IL-17 receptors and Th1 response inhibition. | 62 |
| B16 melanoma and MB49 bladder carcinoma cell lines | Subcutaneously injected into IL-17−/− mice | Tumor growth was reduced in IL-17−/− mice. IL-17 can promote tumor growth, partly via an IL-6-Stat3 pathway. | 60 |
| Human non-small cell lung cancer (NSCLC) | Subcutaneously injected into SCID mice | Transfection of IL-17 increased NSCLC growth in vivo (SCID mice) via promoting CXCR2-dependent angiogenesis. | 59 |
| Mouse premalignant gastroduodenal lesions | Knockout mice (Smad4 deficiency in T cells) | Loss of Smad4 in T cells led to a chronic increase of gut Th17 cell activity that was associated with the development of premalignant lesions of the gastroduodenal region. | 27 |
| Mouse ovarian cancer cell line (ID8) | Intraperitoneally injected into mice | TNF-α maintained TNFR1-dependent IL-17 production by CD4+ cells leading to myeloid cell recruitment into the tumor microenvironment and enhanced tumor growth in mice. | 33 |
| Acute myeloid leukemia (AML) | Human | The frequencies of Th17 cells and the levels of related cytokines (IL-17, IL-6, and TGF-β1) were increased in patients with AML. | 61 |
| Hepatocellular carcinoma | Human | Th17 cells were significantly increased in tumors of HCC patients and were associated with poor survival. | 29 |
| Human colorectal cancer | Human | Patients with high expression of the Th17 cluster genes had a poor prognosis. | 26 |
| Anti-tumor effects of IL-17 or Th17 cells | |||
|---|---|---|---|
| Mouse plasmocytoma and mastocytoma cell lines | Subcutaneously injected into immunocompetent mice | Transfection of IL-17 inhibited the hematopoietic tumor growth in immunocompetent mice but not in nude mice, and T cells were involved in antitumor activity. | 64 |
| Murine colon cancer cell line (MC38) | Subcutaneously or intravenously injected into IL-17−/− mice | Tumor growth and lung metastasis were enhanced in IL-17−/− mice, which were associated with the decreased IFN-γ+ natural killer cells and tumor specific IFN-γ+ T cells in the tumor draining lymph nodes and tumors. | 66 |
| B16-F10 murine melanoma cells | Intravenously injected into IL-17−/− mice | IL-17A-deficient mice were more susceptible to developing lung melanoma. Adoptive T-cell therapy with tumor-specific Th17 cells prevented tumor development. Th17 cells elicited a protective inflammation that promotes the activation of tumor-specific CD8+ T cells. | 65 |
| B16 melanoma | Pmel-1/Thy1.1 TCR-transgenic mice | Adoptively transferred IL-17-secreting CD8+ T cells resulted in regression of large established B16 melanoma. | 63 |
| B16 melanoma | Subcutaneously injected into TRP-1 TCR transgenic mice | Tumor-specific Th17-polarized cells eradicated established, advanced B16 melanoma in a mouse model and this therapeutic effect was critically dependent on IFN-γ production. | 30 |
| Murine pancreatic cancer cell line | Subcutaneously injected into immunocompetent mice | The addition of IL-6 to the tumor microenvironment skewed the balance toward Th17 cells and improved mouse survival in a murine pancreatic cancer model. | 69 |
| Ovarian cancer | Human | Th17 cells induced Th1-type chemokines and recruited effector cells into the tumor microenvironment. | 25 |
| Lung cancer | Human | Human tumor antigen MAGE-A3-specific Th17 cells converted into IFN-γ−secreting cells as they differentiated into effector T cells in vivo. | 68 |
| Lung cancer | Human | The accumulation of Th17 cells in human malignant pleural effusion predicted improved patient survival. | 67 |
Protumor Effects
Protumor activity mediated by IL-17 and Th17 has been observed both in mouse tumor models and in human cancer patients. The potential mechanisms responsible for the protumor activity of IL-17 or Th17 cells mainly involve angiogenesis and cytokine induction in the tumor microenvironment resulting in the promotion of tumor growth. IL-17 was shown to promote tumorigenicity of human cervical tumors in nude mice, which was associated with increased levels of IL-6 and IL-8 and with recruitment of macrophages at the tumor site.58 Furthermore, a study in a mouse model of colon adenocarcinoma demonstrated that the protumor effect of IL-17 was related to its capacity to induce tumor angiogenesis through the induction of a wide range of angiogenic factors, including VEGF, PGE2, keratinocyte-derived chemokine, and nitric oxide from fibroblasts and tumor cells.70 The same research group further showed that IL-17 increased net angiogenic activity and the growth of human non-small cell lung cancer by promoting CXCR2-dependent angiogenesis in vivo.59 In support of this notion, a recent study showed that accumulation of intratumoral IL-17-producing cells enhances human hepatocellular carcinoma progression by fostering angiogenesis.29 In addition to its involvement in angiogenesis, IL-17 can induce IL-6 production, which in turn activates the oncogenic signal Stat3, resulting in up-regulated prosurvival and proangiogenic genes.60
More recently, several clinical correlation studies in human cancer patients have demonstrated a protumor role of IL-17 or Th17 cells in different types of tumors. Hahn et al27 reported that a chronic increase in Th17 cell activity in the gut was associated with development of premalignant lesions of the gastroduodenal region. Tosolini et al26 analyzed 125 frozen colorectal tumor specimens and reported poor prognosis in patients with high Th17 cluster expression, whereas patients with high Th1 cluster expression had prolonged disease-free survival. A study by Wu et al61 suggested a role for Th17 cells in the pathogenesis of acute myeloid leukemia. They observed that Th17 cell frequencies were significantly increased in peripheral blood samples from untreated patients with acute myeloid leukemia, compared with those from healthy volunteers. Moreover, increased IL-17 concentrations accompanied the increased Th17 cell frequencies in these patients. In multiple myeloma patients, elevated IL-17 produced by Th17 cells promoted myeloma cell growth and inhibited effector immune functions.62 Furthermore, in a study of ovarian cancer, chronic production of TNF-α in the tumor microenvironment increased myeloid cell recruitment in an IL-17-dependent manner that contributed to tumor-promoting actions.33
Antitumor Effects
Despite studies demonstrating a role for Th17 cells in promoting tumor growth, mounting evidence suggests that Th17 cells may also have potent antitumor immune effects. Murine tumor models have shown that Th17 cells can directly eradicate tumor cells. Muranski et al30 demonstrated that tumor-specific Th17-polarized cells reduced established, advanced B16 melanoma in a mouse model, and that this therapeutic effect was critically dependent on IFN-γ production. Furthermore, Hinrichs et al63 reported that adoptively transferred IL-17-secreting CD8+ T cells also enhanced antitumor immunity, resulting in regression of large established B16 melanoma. Transferred IL-17-producing CD8+ T cells converted into IFN-γ-producing effector T cells and mediated antitumor effects. These studies also suggest the plasticity of IL-17-producing T cells in the tumor microenvironment.
In addition to direct eradication of tumor cells, Th17 cells appear to have indirect antitumor effects by recruiting other tumor-specific immune cells and/or by promoting their antitumor immune responses. Benchetrit et al64 showed that transfection of IL-17 in immunocompetent mice but not in nude mice inhibited the hematopoietic tumor growth as a result of increased tumor-specific cytolytic T cells. Furthermore, an elegant study by Martin-Orozco et al65 provides strong evidence that Th17 plays an indirect antitumor role by promoting tumor-specific CD8+ T-cell activation. They found that adoptive T-cell therapy with tumor-specific Th17 cells significantly recruited dendritic cells into the tumor site and draining lymph nodes, and also triggered a strong antitumor CD8+ T-cell response. Kryczek et al66 showed that enhanced tumor growth and lung metastases in IL-17-deficient mice were associated with the decreased IFN-γ+ natural killer cells and tumor-specific IFN-γ+ T cells in tumor-draining lymph nodes and tumors. These three studies support the notion that the effects of IL-17 on tumor development are influenced by the existence of an adaptive immune system. In the presence of lymphocytes, IL-17 promotes tumor rejection; in their absence, IL-17 favors tumor growth and angiogenesis.
Most data supporting an antitumor role for Th17 cells are derived from murine models, and the role of human Th17 cells in antitumor immunity is incompletely defined. Kryczek et al25 investigated the functional role of Th17 cells and the associated mechanisms, as well as their clinical significance, in 201 ovarian cancer patients. They showed that Th17 cells can contribute to protective tumor immunity in humans by inducing the Th1-type chemokines CXCL9 and CXCL10, resulting in the recruitment of effector cells to the tumor microenvironment. Another group reported that the accumulation of Th17 cells in human malignant pleural effusions predicted improved patient survival.67 In addition, in lung cancer patients, human Th17 cells specific for the tumor antigen MAGE-A3 converted into IFN-γ secreting cells during differentiation into effector T cells and mediated antitumor effects in vivo.68 Taken together, these data suggest the potential protective function of human Th17 cells in antitumor immunity.
Plasticity and Balance of Th17 Cells and Tregs in Tumor Microenvironments
Although different types of T-cell lineages have distinct gene expression and regulation signatures, each subset retains substantial developmental plasticity. Increasing evidence suggests that Th17 cells and Tregs have greater developmental plasticity than Th1 and Th2 subsets.71,72 Several studies have shown that human CD4+ Tregs can differentiate to IL-17-producing cells (IL-17+FOXP3+), and that Th17 cells can also express both FOXP3 and ROR-γt (ROR-γt+FOXP3+).73,74 Furthermore, the differentiation of human Th17 cells preferentially occurs from naïve FOXP3+ Tregs rather than from conventional naïve CD4+ T cells.75 In addition to differentiation into Th17 cells, human memory Tregs themselves can secrete IL-17 ex vivo and constitutively express ROR-γt. These IL-17-secreting memory Tregs share certain phenotypic and functional features with conventional Th17 cells.76
Recent studies suggest that epigenetic instability of cytokine and transcription factor gene loci may have a role in controlling the plasticity of Th17 cells.77 During the conversion of Tregs into Th17 cells, transcription factors such as Stat3 and Stat4 modulate the molecular process. Stat3 down-regulates FOXP3 expression, and Stat3, ROR-γ, and ROR-α are required for IL-17 expression in Tregs.78 More recently, the transcription factor HIF1-α was also shown to direct Th17 cell and Treg development.15 Lack of HIF1-α diminished Th17 development, but it enhanced Treg differentiation and protected mice from autoimmune neuroinflammation. The mechanism involved is thought to involve an HIF1-α-dependent glycolytic pathway that orchestrates a metabolic checkpoint for the differentiation of TH17 cells and Tregs.15
Th17+FOXP3+ T-cell populations have been observed in tumor environments. Kryczek et al44 reported that high levels of IL-17+FOXP3+CD4+ T cells selectively accumulated in the colitic microenvironment in association with colon carcinoma. IL-17+FOXP3+CD4+ T cells functionally suppressed T-cell activation and stimulated inflammatory cytokine production in colitic tissues.44 Furthermore, another study showed that CD4+IL-17+FOXP3+ T cells exist in human esophageal cancer,45 and our group recently showed that tumor-infiltrating Th17 cells obtained from melanoma, breast, ovarian, and colon cancers also express FOXP3.24 Taken together, these studies provide clear evidence of the commitment and plasticity of Th17 cells and Tregs in various types of tumors. However, whether the IL-17+FOXP3+ T-cell population is derived from Tregs or Th17 cells is still unclear. In addition, whether Th17 cells can reciprocally convert into Tregs has not been fully described. In a study to address these important issues, our group demonstrated that Th17 clones generated from human tumor-infiltrating T lymphocytes can differentiate into FOXP3+ Tregs with potent suppressive function after in vitro repetitive T-cell receptor stimulation.46 This Th17-to-Treg conversion involved the epigenetic modification of FOXP3 and reprogramming of gene expression profiles, including those of lineage-specific transcription factors and cytokine genes. These studies provide critical evidence that human tumor-infiltrating Th17 cells can differentiate into Tregs, and further demonstrate the potential for reciprocal plasticity between Th17 cells and Tregs.46 In addition to potential antigenic stimulation, tumor-infiltrating myeloid-derived suppressor cells may also contribute to the Th17-to-Treg conversion, as well as to the plasticity of human Th17 cells and Tregs in tumor microenvironments.79 The mechanism of Th17 cell plasticity regulated by myeloid-derived suppressor cells is dependent on myeloid-derived suppressor cell-derived TGF-β and retinoic acid (Figure 2).
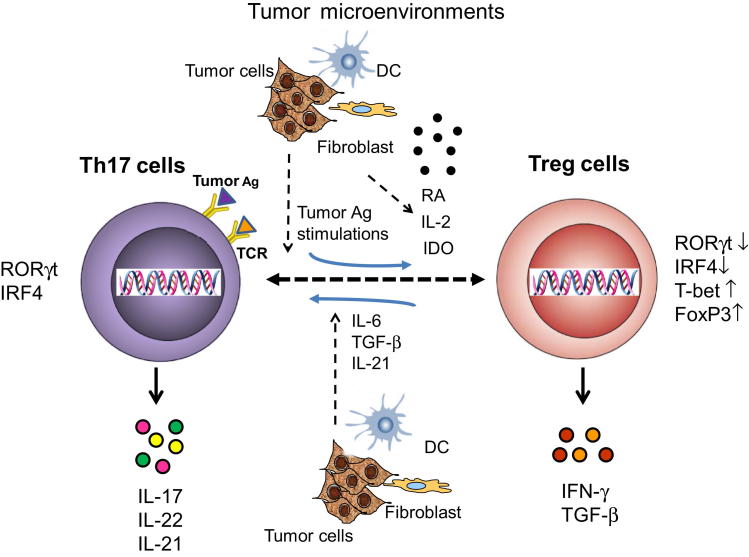
Figure 2.
The plasticity and balance of Th17 and Tregs in tumor microenvironments. The balance and commitment between Th17 and Tregs may be controlled by the tumor microenvironment. In the presence of tumor microenvironmental cytokines IL-6, TGF-β, and IL-21, CD4+FOXP3+ Tregs can differentiate into IL-17-producing Th17 cells (IL-17+/FOXP3+). Th17 cells also have the capacity to reciprocally differentiate into IFN-γ+FOXP3+ Tregs by repetitive tumor antigen (Ag) stimulations. In addition, tumor microenvironmental factors such as IL-2, retinoic acid (RA), or indoleamine 2,3-dioxygenase (IDO) may facilitate this Th17-to-Treg conversion.
Given the significantly different roles and functions of Th17 cells and Tregs in human immunity, appropriate interactions and balance between them are critical for both physiological and pathological conditions. The potential for interconversion and balance between Th17 cells and Tregs has been strongly supported by recent studies in human tumor immunology. These studies indicate a dynamic interaction between Tregs and Th17 cells in tumor microenvironments, one in which Th17 cell and Treg cell numbers are reciprocally associated with tumor progression.28,31,44,47,66 In addition to reciprocal conversion of Tregs and Th17 cells, several additional tumor microenvironmental factors may contribute to the balance between these two subsets of cells. One major factor is the cytokine milieu of tumor microenvironment. Kryczek et al31 showed that IL-2 reduces Th17 cell differentiation in the tumor microenvironment, accompanied by an enhanced Treg compartment both in vitro and in vivo. Tregs can also promote Th17 development in vitro and in vivo through regulation of IL-2.80 Furthermore, Tregs have been shown to inhibit Th17 cell-mediated inflammation in an IL-10-dependent manner.81,82 In addition to cytokine regulation, several other possible regulatory mechanisms have been proposed in nontumor-associated studies. Retinoic acid, a key regulator of TGF-β-dependent immune responses, can inhibit the IL-6-driven induction of proinflammatory Th17 cells by promoting anti-inflammatory Treg differentiation.48 Sharma et al83 showed that indoleamine 2,3-dioxygenase (IDO) functions as a molecular switch in tumor-draining lymph nodes, actively maintaining Tregs in their normal suppressive phenotype but allowing inflammation-induced conversion of Tregs to a polyfunctional T-helper phenotype similar to proinflammatory Th17 cells when blocked. Notably, retinoic acid and IDO are also critical suppressive mediators in tumor suppressive microenvironments. Whether these molecules are also involved in controlling the balance of Th17 cells and Tregs in the tumor microenvironment remains to be determined (Figure 2). A better understanding of the mechanisms and regulations induced by tumor microenvironments will, we trust, facilitate the development of novel strategies to manipulate Th17 cell and Treg commitment and functions for cancer immunotherapy.
Conclusion and Future Perspectives
The recent discovery of Th17 cells not only changes the classical Th1/Th2 paradigm of T helper cell differentiation, but also markedly alters conventional thinking regarding the role of T helper cells in antitumor immunity. Improved understanding of the nature and function of Th17 cells in tumor immunity should lead to opportunities for the development of novel therapeutic approaches for cancer patients. Current knowledge obtained from murine tumor models indicates that Th17 cells can exert both pro- and antitumor effects that depend on the tumor model used and the sources of IL-17 and Th17 cells, as well as on the specific cytokine and inflammatory conditions within the tumor microenvironment (Table 1). The functional contribution of human Th17 cells to tumor immunity remains controversial, and the data are derived mainly from limited clinical correlation studies of Th17 cells in cancer patients and disease progression. Mechanistic studies using knockout technology are urgently needed to firmly establish the role of Th17 cells in either promoting or inhibiting tumor growth and development. Furthermore, extensive investigation of Th17 cells from cancer patients with different types of tumors and different stages is critical for advancing knowledge regarding the role of Th17 cells in the immunopathogenesis of human cancers.
There are many additional challenging issues related to this specific research area. First, Th17 cells retain substantial developmental plasticity within the tumor microenvironment, and they can convert into other subsets of T cells, including Th1 cells and Tregs. Future studies will need to focus on defining the molecular mechanisms and identifying the tumor microenvironment factors responsible for T-cell lineage commitment, plasticity, and reciprocity of Th1 cells, Th17 cells, and Tregs. Such information would be critical to development of strategies using Th17 and/or Th1 cells as potential therapeutic effector cells in cancer patients. Second, increasing evidence suggests a dynamic interaction and reciprocal conversion between Tregs and Th17 cells in tumor microenvironments, and the numbers of Th17 cells and Tregs appear to be reciprocally associated with tumor progression.28,31,44,47,66 Identification of the molecular mechanisms that regulate Th17 cell and Treg interconversion and balance in the tumor microenvironment could also lead to novel target therapeutic strategies for cancer and other human diseases. Another important issue is the potential heterogeneity of Th17 cell subsets in the tumor microenvironment. Different types of Th17 cells, including IL-17+IFN-γ+ and IL-17+IL-10+ Th17 cells, have been documented in autoimmune and inflammatory diseases.38–41 Clearly, these two types of Th17 cells have been observed in tumor-infiltrating lymphocytes; however, whether the generation of these two types of Th17 cells is due to variations in cellular circumstances, disease stage, or type of tumor is still unknown. Furthermore, the precursor origin or origins of these two types of Th17 cells, as well as their specific roles in antitumor immunity, inflammation, or regulatory effects, are as yet undefined. Characterization of the molecular signatures and functional roles of Th17 cells, as well as identification of the mechanisms underlying Th17 cell heterogeneity in individual tumors or during tumor development, is also urgently required for the development of effective and specific antitumor immunotherapies.
Acknowledgments
We acknowledge the contributions of the many researchers whose work could not be cited in this review because of space limitations.
Footnotes
Supported in part by American Cancer Society grant RSG-10-160-01-LIB (G.P.), the Melanoma Research Alliance (G.P.), and NIH grants AI097852 and AI094478 (G.P.).
References
- 1.Harrington L.E., Hatton R.D., Mangan P.R., Turner H., Murphy T.L., Murphy K.M., Weaver C.T. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 2.Park H., Li Z., Yang X.O., Chang S.H., Nurieva R., Wang Y.H., Wang Y., Hood L., Zhu Z., Tian Q., Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat Rev Immunol. 2008;8:337–348. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- 4.Bettelli E., Korn T., Kuchroo V.K. Th17: the third member of the effector T cell trilogy. Curr Opin Immunol. 2007;19:652–657. doi: 10.1016/j.coi.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Veldhoen M., Hocking R.J., Atkins C.J., Locksley R.M., Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Bettelli E., Carrier Y., Gao W., Korn T., Strom T.B., Oukka M., Weiner H.L., Kuchroo V.K. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 7.Mangan P.R., Harrington L.E., O’Quinn D.B., Helms W.S., Bullard D.C., Elson C.O., Hatton R.D., Wahl S.M., Schoeb T.R., Weaver C.T. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 8.Korn T., Bettelli E., Gao W., Awasthi A., Jäger A., Strom T.B., Oukka M., Kuchroo V.K. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou L., Ivanov I.I., Spolski R., Min R., Shenderov K., Egawa T., Levy D.E., Leonard W.J., Littman D.R. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 10.Chung Y., Chang S.H., Martinez G.J., Yang X.O., Nurieva R., Kang H.S., Ma L., Watowich S.S., Jetten A.M., Tian Q., Dong C. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30:576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson N.J., Boniface K., Chan J.R., McKenzie B.S., Blumenschein W.M., Mattson J.D., Basham B., Smith K., Chen T., Morel F., Lecron J.C., Kastelein R.A., Cua D.J., McClanahan T.K., Bowman E.P., de Waal Malefyt R. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 12.Ivanov I.I., McKenzie B.S., Zhou L., Tadokoro C.E., Lepelley A., Lafaille J.J., Cua D.J., Littman D.R. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 13.Chen Q., Yang W., Gupta S., Biswas P., Smith P., Bhagat G., Pernis A.B. IRF-4-binding protein inhibits interleukin-17 and interleukin-21 production by controlling the activity of IRF-4 transcription factor. Immunity. 2008;29:899–911. doi: 10.1016/j.immuni.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dang E.V., Barbi J., Yang H.Y., Jinasena D., Yu H., Zheng Y., Bordman Z., Fu J., Kim Y., Yen H.R., Luo W., Zeller K., Shimoda L., Topalian S.L., Semenza G.L., Dang C.V., Pardoll D.M., Pan F. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell. 2011;146:772–784. doi: 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi L.Z., Wang R., Huang G., Vogel P., Neale G., Green D.R., Chi H. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J Exp Med. 2011;208:1367–1376. doi: 10.1084/jem.20110278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dandekar S., George M.D., Baumler A.J. Th17 cells, HIV and the gut mucosal barrier. Curr Opin HIV AIDS. 2010;5:173–178. doi: 10.1097/COH.0b013e328335eda3. [DOI] [PubMed] [Google Scholar]
- 17.Tesmer L.A., Lundy S.K., Sarkar S., Fox D.A. Th17 cells in human disease. Immunol Rev. 2008;223:87–113. doi: 10.1111/j.1600-065X.2008.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koebel C.M., Vermi W., Swann J.B., Zerafa N., Rodig S.J., Old L.J., Smyth M.J., Schreiber R.D. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450:903–907. doi: 10.1038/nature06309. [DOI] [PubMed] [Google Scholar]
- 19.Dunn G.P., Old L.J., Schreiber R.D. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 20.Galand C., Donnou S., Crozet L., Brunet S., Touitou V., Ouakrim H., Fridman W.H., Sautès-Fridman C., Fisson S. Th17 cells are involved in the local control of tumor progression in primary intraocular lymphoma. PLoS One. 2011;6:e24622. doi: 10.1371/journal.pone.0024622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dhodapkar K.M., Barbuto S., Matthews P., Kukreja A., Mazumder A., Vesole D., Jagannath S., Dhodapkar M.V. Dendritic cells mediate the induction of polyfunctional human IL17-producing cells (Th17-1 cells) enriched in the bone marrow of patients with myeloma. Blood. 2008;112:2878–2885. doi: 10.1182/blood-2008-03-143222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noonan K., Marchionni L., Anderson J., Pardoll D., Roodman G.D., Borrello I. A novel role of IL-17-producing lymphocytes in mediating lytic bone disease in multiple myeloma. Blood. 2010;116:3554–3563. doi: 10.1182/blood-2010-05-283895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu X., Mulcahy L.A., Mohammed R.A., Lee A.H., Franks H.A., Kilpatrick L., Yilmazer A., Paish E.C., Ellis I.O., Patel P.M., Jackson A.M. IL-17 expression by breast-cancer-associated macrophages: IL-17 promotes invasiveness of breast cancer cell lines. Breast Cancer Res. 2008;10:R95. doi: 10.1186/bcr2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Su X., Ye J., Hsueh E.C., Zhang Y., Hoft D.F., Peng G. Tumor microenvironments direct the recruitment and expansion of human Th17 cells. J Immunol. 2010;184:1630–1641. doi: 10.4049/jimmunol.0902813. [DOI] [PubMed] [Google Scholar]
- 25.Kryczek I., Banerjee M., Cheng P., Vatan L., Szeliga W., Wei S., Huang E., Finlayson E., Simeone D., Welling T.H., Chang A., Coukos G., Liu R., Zou W. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009;114:1141–1149. doi: 10.1182/blood-2009-03-208249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tosolini M., Kirilovsky A., Mlecnik B., Fredriksen T., Mauger S., Bindea G., Berger A., Bruneval P., Fridman W.H., Pagès F., Galon J. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res. 2011;71:1263–1271. doi: 10.1158/0008-5472.CAN-10-2907. [Erratum appeared in Cancer Res 2011, 71:4732] [DOI] [PubMed] [Google Scholar]
- 27.Hahn J.N., Falck V.G., Jirik F.R. Smad4 deficiency in T cells leads to the Th17-associated development of premalignant gastroduodenal lesions in mice. J Clin Invest. 2011;121:4030–4042. doi: 10.1172/JCI45114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maruyama T., Kono K., Mizukami Y., Kawaguchi Y., Mimura K., Watanabe M., Izawa S., Fujii H. Distribution of Th17 cells and FoxP3(+) regulatory T cells in tumor-infiltrating lymphocytes, tumor-draining lymph nodes and peripheral blood lymphocytes in patients with gastric cancer. Cancer Sci. 2010;101:1947–1954. doi: 10.1111/j.1349-7006.2010.01624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang J.P., Yan J., Xu J., Pang X.H., Chen M.S., Li L., Wu C., Li S.P., Zheng L. Increased intratumoral IL-17-producing cells correlate with poor survival in hepatocellular carcinoma patients. J Hepatol. 2009;50:980–989. doi: 10.1016/j.jhep.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 30.Muranski P., Boni A., Antony P.A., Cassard L., Irvine K.R., Kaiser A., Paulos C.M., Palmer D.C., Touloukian C.E., Ptak K., Gattinoni L., Wrzesinski C., Hinrichs C.S., Kerstann K.W., Feigenbaum L., Chan C.C., Restifo N.P. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112:362–373. doi: 10.1182/blood-2007-11-120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kryczek I., Wei S., Zou L., Altuwaijri S., Szeliga W., Kolls J., Chang A., Zou W. Cutting edge: th17 and regulatory T cell dynamics and the regulation by IL-2 in the tumor microenvironment. J Immunol. 2007;178:6730–6733. doi: 10.4049/jimmunol.178.11.6730. [DOI] [PubMed] [Google Scholar]
- 32.Miyahara Y., Odunsi K., Chen W., Peng G., Matsuzaki J., Wang R.F. Generation and regulation of human CD4+ IL-17-producing T cells in ovarian cancer. Proc Natl Acad Sci USA. 2008;105:15505–15510. doi: 10.1073/pnas.0710686105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Charles K.A., Kulbe H., Soper R., Escorcio-Correia M., Lawrence T., Schultheis A., Chakravarty P., Thompson R.G., Kollias G., Smyth J.F., Balkwill F.R., Hagemann T. The tumor-promoting actions of TNF-alpha involve TNFR1 and IL-17 in ovarian cancer in mice and humans. J Clin Invest. 2009;119:3011–3023. doi: 10.1172/JCI39065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leveque L., Deknuydt F., Bioley G., Old L.J., Matsuzaki J., Odunsi K., Ayyoub M., Valmori D. Interleukin 2-mediated conversion of ovarian cancer-associated CD4+ regulatory T cells into proinflammatory interleukin 17-producing helper T cells. J Immunother. 2009;32:101–108. doi: 10.1097/CJI.0b013e318195b59e. [DOI] [PubMed] [Google Scholar]
- 35.Sfanos K.S., Bruno T.C., Maris C.H., Xu L., Thoburn C.J., DeMarzo A.M., Meeker A.K., Isaacs W.B., Drake C.G. Phenotypic analysis of prostate-infiltrating lymphocytes reveals TH17 and Treg skewing. Clin Cancer Res. 2008;14:3254–3261. doi: 10.1158/1078-0432.CCR-07-5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Derhovanessian E., Adams V., Hähnel K., Groeger A., Pandha H., Ward S., Pawelec G. Pretreatment frequency of circulating IL-17+ CD4+ T-cells, but not Tregs, correlates with clinical response to whole-cell vaccination in prostate cancer patients. Int J Cancer. 2009;125:1372–1379. doi: 10.1002/ijc.24497. [DOI] [PubMed] [Google Scholar]
- 37.Annunziato F., Cosmi L., Santarlasci V., Maggi L., Liotta F., Mazzinghi B., Parente E., Fili L., Ferri S., Frosali F., Giudici F., Romagnani P., Parronchi P., Tonelli F., Maggi E., Romagnani S. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204:1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McGeachy M.J., Bak-Jensen K.S., Chen Y., Tato C.M., Blumenschein W., McClanahan T., Cua D.J. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol. 2007;8:1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 39.McGeachy M.J., Cua D.J. Th17 cell differentiation: the long and winding road. Immunity. 2008;28:445–453. doi: 10.1016/j.immuni.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 40.Peters A., Lee Y., Kuchroo V.K. The many faces of Th17 cells. Curr Opin Immunol. 2011;23:702–706. doi: 10.1016/j.coi.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghoreschi K., Laurence A., Yang X.P., Hirahara K., O’Shea J.J. T helper 17 cell heterogeneity and pathogenicity in autoimmune disease. Trends Immunol. 2011;32:395–401. doi: 10.1016/j.it.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lim H.W., Lee J., Hillsamer P., Kim C.H. Human Th17 cells share major trafficking receptors with both polarized effector T cells and FOXP3+ regulatory T cells. J Immunol. 2008;180:122–129. doi: 10.4049/jimmunol.180.1.122. [DOI] [PubMed] [Google Scholar]
- 43.Hirota K., Yoshitomi H., Hashimoto M., Maeda S., Teradaira S., Sugimoto N., Yamaguchi T., Nomura T., Ito H., Nakamura T., Sakaguchi N., Sakaguchi S. Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J Exp Med. 2007;204:2803–2812. doi: 10.1084/jem.20071397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kryczek I., Wu K., Zhao E., Wei S., Vatan L., Szeliga W., Huang E., Greenson J., Chang A., Rolinski J., Radwan P., Fang J., Wang G., Zou W. IL-17+ regulatory T cells in the microenvironments of chronic inflammation and cancer. J Immunol. 2011;186:4388–4395. doi: 10.4049/jimmunol.1003251. [DOI] [PubMed] [Google Scholar]
- 45.Huang C., Fu Z.X. Localization of IL-17+Foxp3+ T cells in esophageal cancer. Immunol Invest. 2011;40:400–412. doi: 10.3109/08820139.2011.555489. [DOI] [PubMed] [Google Scholar]
- 46.Ye J., Su X., Hsueh E.C., Zhang Y., Koenig J.M., Hoft D.F., Peng G. Human tumor-infiltrating Th17 cells have the capacity to differentiate into IFN-gamma(+) and FOXP3(+) T cells with potent suppressive function. Eur J Immunol. 2011;41:936–951. doi: 10.1002/eji.201040682. [DOI] [PubMed] [Google Scholar]
- 47.Zou W., Restifo N.P. T(H)17 cells in tumour immunity and immunotherapy. Nat Rev Immunol. 2010;10:248–256. doi: 10.1038/nri2742. [Erratum appeared in Nat Rev Immunol 2011, 11:565] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mucida D., Park Y., Kim G., Turovskaya O., Scott I., Kronenberg M., Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 49.Kimura A., Naka T., Nohara K., Fujii-Kuriyama Y., Kishimoto T. Aryl hydrocarbon receptor regulates Stat1 activation and participates in the development of Th17 cells. Proc Natl Acad Sci USA. 2008;105:9721–9726. doi: 10.1073/pnas.0804231105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Veldhoen M., Hirota K., Westendorf A.M., Buer J., Dumoutier L., Renauld J.C., Stockinger B. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- 51.Ohta A., Gorelik E., Prasad S.J., Ronchese F., Lukashev D., Wong M.K., Huang X., Caldwell S., Liu K., Smith P., Chen J.F., Jackson E.K., Apasov S., Abrams S., Sitkovsky M. A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci USA. 2006;103:13132–13137. doi: 10.1073/pnas.0605251103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilson J.M., Kurtz C.C., Black S.G., Ross W.G., Alam M.S., Linden J., Ernst P.B. The A2B adenosine receptor promotes Th17 differentiation via stimulation of dendritic cell IL-6. J Immunol. 2011;186:6746–6752. doi: 10.4049/jimmunol.1100117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 54.van Beelen A.J., Zelinkova Z., Taanman-Kueter E.W., Muller F.J., Hommes D.W., Zaat S.A., Kapsenberg M.L., de Jong E.C. Stimulation of the intracellular bacterial sensor NOD2 programs dendritic cells to promote interleukin-17 production in human memory T cells. Immunity. 2007;27:660–669. doi: 10.1016/j.immuni.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 55.Evans H.G., Suddason T., Jackson I., Taams L.S., Lord G.M. Optimal induction of T helper 17 cells in humans requires T cell receptor ligation in the context of Toll-like receptor-activated monocytes. Proc Natl Acad Sci USA. 2007;104:17034–17039. doi: 10.1073/pnas.0708426104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reynolds J.M., Pappu B.P., Peng J., Martinez G.J., Zhang Y., Chung Y., Ma L., Yang X.O., Nurieva R.I., Tian Q., Dong C. Toll-like receptor 2 signaling in CD4(+) T lymphocytes promotes T helper 17 responses and regulates the pathogenesis of autoimmune disease. Immunity. 2010;32:692–702. doi: 10.1016/j.immuni.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nyirenda M.H., Sanvito L., Darlington P.J., O’Brien K., Zhang G.X., Constantinescu C.S., Bar-Or A., Gran B. TLR2 stimulation drives human naive and effector regulatory T cells into a Th17-like phenotype with reduced suppressive function. J Immunol. 2011;187:2278–2290. doi: 10.4049/jimmunol.1003715. [DOI] [PubMed] [Google Scholar]
- 58.Tartour E., Fossiez F., Joyeux I., Galinha A., Gey A., Claret E., Sastre-Garau X., Couturier J., Mosseri V., Vives V., Banchereau J., Fridman W.H., Wijdenes J., Lebecque S., Sautès-Fridman C. Interleukin 17, a T-cell-derived cytokine, promotes tumorigenicity of human cervical tumors in nude mice. Cancer Res. 1999;59:3698–3704. [PubMed] [Google Scholar]
- 59.Numasaki M., Watanabe M., Suzuki T., Takahashi H., Nakamura A., McAllister F., Hishinuma T., Goto J., Lotze M.T., Kolls J.K., Sasaki H. IL-17 enhances the net angiogenic activity and in vivo growth of human non-small cell lung cancer in SCID mice through promoting CXCR-2-dependent angiogenesis. J Immunol. 2005;175:6177–6189. doi: 10.4049/jimmunol.175.9.6177. [DOI] [PubMed] [Google Scholar]
- 60.Wang L., Yi T., Kortylewski M., Pardoll D.M., Zeng D., Yu H. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J Exp Med. 2009;206:1457–1464. doi: 10.1084/jem.20090207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu C., Wang S., Wang F., Chen Q., Peng S., Zhang Y., Qian J., Jin J., Xu H. Increased frequencies of T helper type 17 cells in the peripheral blood of patients with acute myeloid leukaemia. Clin Exp Immunol. 2009;158:199–204. doi: 10.1111/j.1365-2249.2009.04011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Prabhala R.H., Pelluru D., Fulciniti M., Prabhala H.K., Nanjappa P., Song W., Pai C., Amin S., Tai Y.T., Richardson P.G., Ghobrial I.M., Treon S.P., Daley J.F., Anderson K.C., Kutok J.L., Munshi N.C. Elevated IL-17 produced by TH17 cells promotes myeloma cell growth and inhibits immune function in multiple myeloma. Blood. 2010;115:5385–5392. doi: 10.1182/blood-2009-10-246660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hinrichs C.S., Kaiser A., Paulos C.M., Cassard L., Sanchez-Perez L., Heemskerk B., Wrzesinski C., Borman Z.A., Muranski P., Restifo N.P. Type 17 CD8+ T cells display enhanced antitumor immunity. Blood. 2009;114:596–599. doi: 10.1182/blood-2009-02-203935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Benchetrit F., Ciree A., Vives V., Warnier G., Gey A., Sautès-Fridman C., Fossiez F., Haicheur N., Fridman W.H., Tartour E. Interleukin-17 inhibits tumor cell growth by means of a T-cell-dependent mechanism. Blood. 2002;99:2114–2121. doi: 10.1182/blood.v99.6.2114. [DOI] [PubMed] [Google Scholar]
- 65.Martin-Orozco N., Muranski P., Chung Y., Yang X.O., Yamazaki T., Lu S., Hwu P., Restifo N.P., Overwijk W.W., Dong C. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity. 2009;31:787–798. doi: 10.1016/j.immuni.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kryczek I., Wei S., Szeliga W., Vatan L., Zou W. Endogenous IL-17 contributes to reduced tumor growth and metastasis. Blood. 2009;114:357–359. doi: 10.1182/blood-2008-09-177360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ye Z.J., Zhou Q., Gu Y.Y., Qin S.M., Ma W.L., Xin J.B., Tao X.N., Shi H.Z. Generation and differentiation of IL-17-producing CD4+ T cells in malignant pleural effusion. J Immunol. 2010;185:6348–6354. doi: 10.4049/jimmunol.1001728. [DOI] [PubMed] [Google Scholar]
- 68.Hamaï A., Pignon P., Raimbaud I., Duperrier-Amouriaux K., Senellart H., Hiret S., Douillard J.Y., Bennouna J., Ayyoub M., Valmori D. Human T(H)17 immune cells specific for the tumor antigen MAGE-A3 convert to IFN-gamma-secreting cells as they differentiate into effector T cells in vivo. Cancer Res. 2012;72:1059–1063. doi: 10.1158/0008-5472.CAN-11-3432. [DOI] [PubMed] [Google Scholar]
- 69.Gnerlich J.L., Mitchem J.B., Weir J.S., Sankpal N.V., Kashiwagi H., Belt B.A., Porembka M.R., Herndon J.M., Eberlein T.J., Goedegebuure P., Linehan D.C. Induction of Th17 cells in the tumor microenvironment improves survival in a murine model of pancreatic cancer. J Immunol. 2010;185:4063–4071. doi: 10.4049/jimmunol.0902609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Numasaki M., Fukushi J., Ono M., Narula S.K., Zavodny P.J., Kudo T., Robbins P.D., Tahara H., Lotze M.T. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003;101:2620–2627. doi: 10.1182/blood-2002-05-1461. [DOI] [PubMed] [Google Scholar]
- 71.Zhou L., Chong M.M., Littman D.R. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30:646–655. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 72.Lee Y.K., Mukasa R., Hatton R.D., Weaver C.T. Developmental plasticity of Th17 and Treg cells. Curr Opin Immunol. 2009;21:274–280. doi: 10.1016/j.coi.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 73.Koenen H.J., Smeets R.L., Vink P.M., van Rijssen E., Boots A.M., Joosten I. Human CD25highFoxp3pos regulatory T cells differentiate into IL-17-producing cells. Blood. 2008;112:2340–2352. doi: 10.1182/blood-2008-01-133967. [DOI] [PubMed] [Google Scholar]
- 74.Voo K.S., Wang Y.H., Santori F.R., Boggiano C., Arima K., Bover L., Hanabuchi S., Khalili J., Marinova E., Zheng B., Littman D.R., Liu Y.J. Identification of IL-17-producing FOXP3+ regulatory T cells in humans. Proc Natl Acad Sci USA. 2009;106:4793–4798. doi: 10.1073/pnas.0900408106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Valmori D., Raffin C., Raimbaud I., Ayyoub M. Human RORgammat+ TH17 cells preferentially differentiate from naive FOXP3+Treg in the presence of lineage-specific polarizing factors. Proc Natl Acad Sci USA. 2010;107:19402–19407. doi: 10.1073/pnas.1008247107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ayyoub M., Deknuydt F., Raimbaud I., Dousset C., Leveque L., Bioley G., Valmori D. Human memory FOXP3+ Tregs secrete IL-17 ex vivo and constitutively express the T(H)17 lineage-specific transcription factor RORgamma t. Proc Natl Acad Sci USA. 2009;106:8635–8640. doi: 10.1073/pnas.0900621106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mukasa R., Balasubramani A., Lee Y.K., Whitley S.K., Weaver B.T., Shibata Y., Crawford G.E., Hatton R.D., Weaver C.T. Epigenetic instability of cytokine and transcription factor gene loci underlies plasticity of the T helper 17 cell lineage. Immunity. 2010;32:616–627. doi: 10.1016/j.immuni.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chaudhry A., Rudra D., Treuting P., Samstein R.M., Liang Y., Kas A., Rudensky A.Y. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science. 2009;326:986–991. doi: 10.1126/science.1172702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hoechst B., Gamrekelashvili J., Manns M.P., Greten T.F., Korangy F. Plasticity of human Th17 cells and iTregs is orchestrated by different subsets of myeloid cells. Blood. 2011;117:6532–6541. doi: 10.1182/blood-2010-11-317321. [DOI] [PubMed] [Google Scholar]
- 80.Chen Y., Haines C.J., Gutcher I., Hochweller K., Blumenschein W.M., McClanahan T., Hämmerling G., Li M.O., Cua D.J., McGeachy M.J. Foxp3(+) regulatory T cells promote T helper 17 cell development in vivo through regulation of interleukin-2. Immunity. 2011;34:409–421. doi: 10.1016/j.immuni.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 81.Huber S., Gagliani N., Esplugues E., O’Connor W., Jr., Huber F.J., Chaudhry A., Kamanaka M., Kobayashi Y., Booth C.J., Rudensky A.Y., Roncarolo M.G., Battaglia M., Flavell R.A. Th17 cells express interleukin-10 receptor and are controlled by Foxp3- and Foxp3+ regulatory CD4+ T cells in an interleukin-10-dependent manner. Immunity. 2011;34:554–565. doi: 10.1016/j.immuni.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chaudhry A., Samstein R.M., Treuting P., Liang Y., Pils M.C., Heinrich J.M., Jack R.S., Wunderlich F.T., Brüning J.C., Müller W., Rudensky A.Y. Interleukin-10 signaling in regulatory T cells is required for suppression of Th17 cell-mediated inflammation. Immunity. 2011;34:566–578. doi: 10.1016/j.immuni.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sharma M.D., Hou D.Y., Liu Y., Koni P.A., Metz R., Chandler P., Mellor A.L., He Y., Munn D.H. Indoleamine 2,3-dioxygenase controls conversion of Foxp3+ Tregs to TH17-like cells in tumor-draining lymph nodes. Blood. 2009;113:6102–6111. doi: 10.1182/blood-2008-12-195354. [DOI] [PMC free article] [PubMed] [Google Scholar]