Abstract
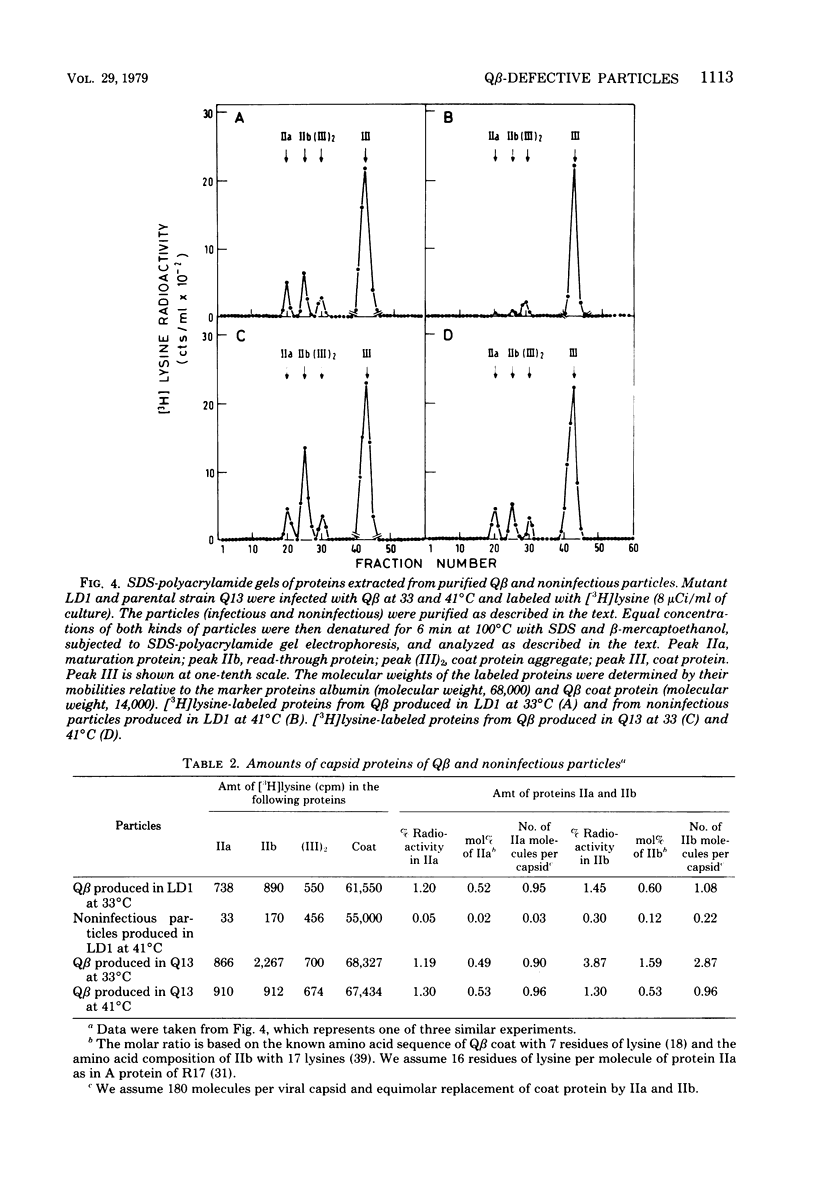
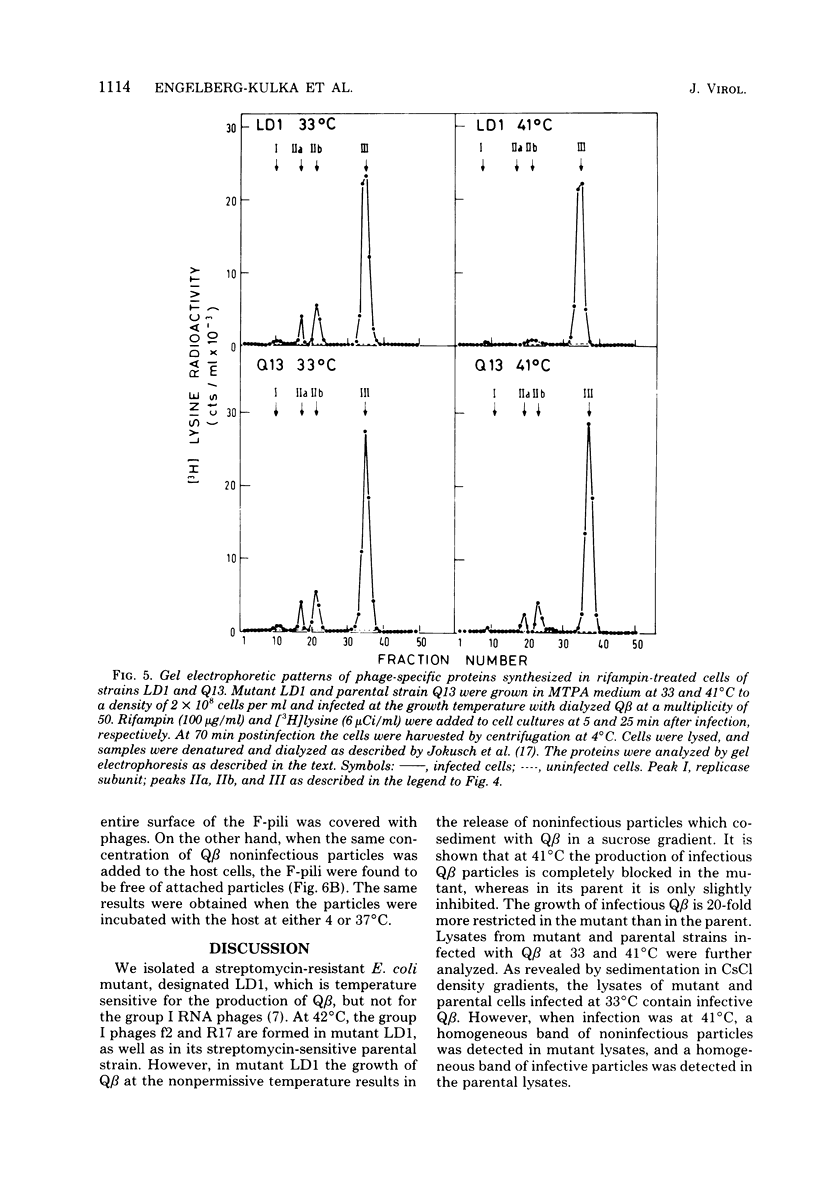
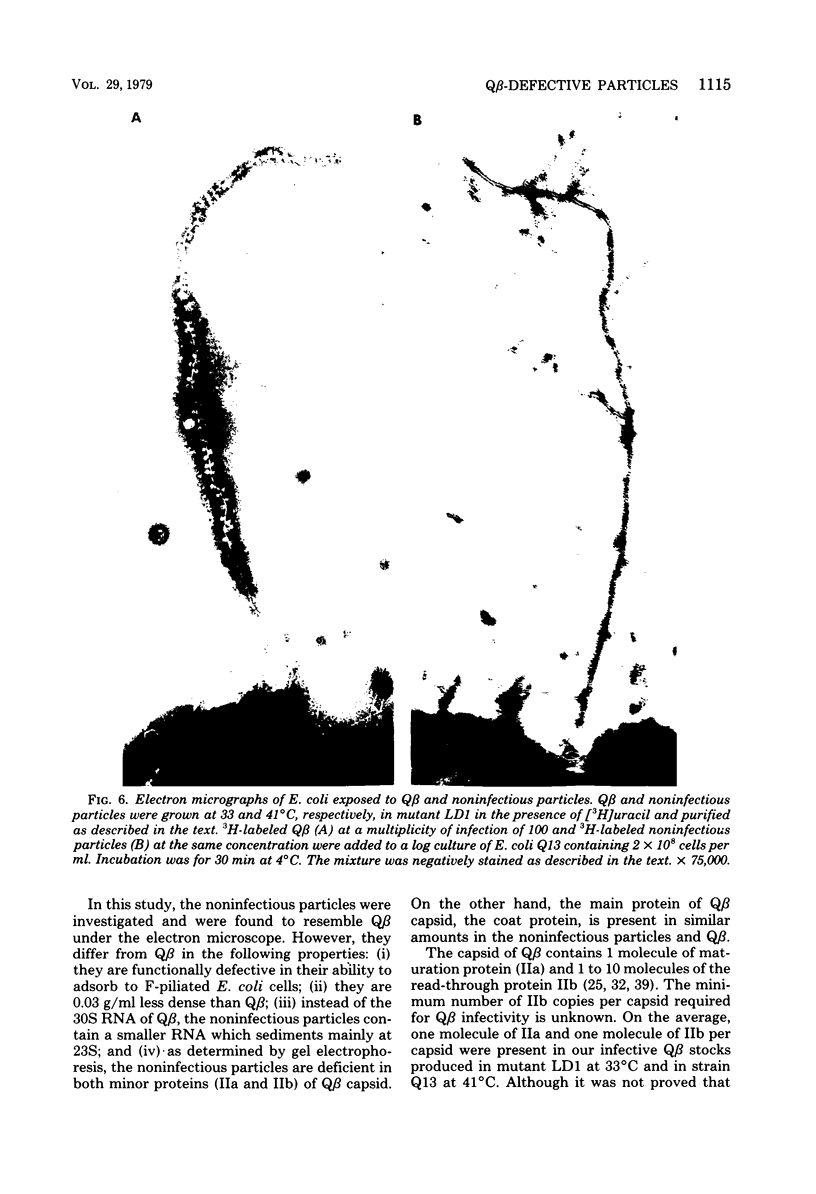
This paper describes Q beta noninfectious particles produced at 41 degrees C in a streptomycin-resistant Escherichia coli mutant which is temperature sensitive for suppression of a nonsense codon. The noninfectious particles resembled Q beta under the electron microscope and contained coat protein molecules in an amount similar to the amount in Q beta. However, they did not adsorb to F-piliated bacteria, and they were deficient in both minor capsid proteins of Q beta, maturation (IIa) and read-through (IIb). Proteins IIa and IIb were not produced in Qbeta-infected mutant cells at 41 degrees C. In addition, instead of the 30S RNA of Q beta, a shorter RNA, which sedimented mainly at 23 S, was found in the defective particles. The results are discussed in relation to the roles of proteins IIa and IIb of Q beta.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chan T. S., Webster R. E., Zinder N. D. Suppression of UGA codon by a tryptophan tRNA. J Mol Biol. 1971 Feb 28;56(1):101–116. doi: 10.1016/0022-2836(71)90087-8. [DOI] [PubMed] [Google Scholar]
- Engelberg H., Soudry E. Ribonucleic acid bacteriophage release: requirement for host-controlled protein synthesis. J Virol. 1971 Sep;8(3):257–264. doi: 10.1128/jvi.8.3.257-264.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feix G., Slor H., Weissmann C. Replication of viral RNA. 13. The early product of phage RNA synthesis in vitro. Proc Natl Acad Sci U S A. 1967 May;57(5):1401–1408. doi: 10.1073/pnas.57.5.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiers W., Contreras R., Duerinck F., Haegeman G., Iserentant D., Merregaert J., Min Jou W., Molemans F., Raeymaekers A., Van den Berghe A. Complete nucleotide sequence of bacteriophage MS2 RNA: primary and secondary structure of the replicase gene. Nature. 1976 Apr 8;260(5551):500–507. doi: 10.1038/260500a0. [DOI] [PubMed] [Google Scholar]
- GESTELAND R. F., BOEDTKER H. SOME PHYSICAL PROPERTIES OF BACTERIOPHAGE R17 AND ITS RIBONUCLEIC ACID. J Mol Biol. 1964 Apr;8:496–507. doi: 10.1016/s0022-2836(64)80007-3. [DOI] [PubMed] [Google Scholar]
- Gartner T. K., Orias E. Effects of mutations to streptomycin resistance on the rate of translation of mutant genetic information. J Bacteriol. 1966 Mar;91(3):1021–1028. doi: 10.1128/jb.91.3.1021-1028.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garwes D., Sillero A., Ochoa S. Virus-specific proteins in Escherichia coli infected with phage Qb. Biochim Biophys Acta. 1969 Jul 22;186(1):166–172. doi: 10.1016/0005-2787(69)90499-7. [DOI] [PubMed] [Google Scholar]
- Gorini L., Beckwith J. R. Suppression. Annu Rev Microbiol. 1966;20:401–422. doi: 10.1146/annurev.mi.20.100166.002153. [DOI] [PubMed] [Google Scholar]
- Hofstetter H., Monstein H. J., Weissmann C. The readthrough protein A1 is essential for the formation of viable Q beta particles. Biochim Biophys Acta. 1974 Dec 6;374(2):238–251. doi: 10.1016/0005-2787(74)90366-9. [DOI] [PubMed] [Google Scholar]
- Horiuchi K., Webster R. E., Matsuhashi S. Gene products of bacteriophage Q beta. Virology. 1971 Aug;45(2):429–439. doi: 10.1016/0042-6822(71)90343-6. [DOI] [PubMed] [Google Scholar]
- Hung P. P. Use of DEAE-cellulose for isolation of ribonucleic acids from bacteriophages Q beta and MS. Biochim Biophys Acta. 1969 Jul 22;186(1):220–222. doi: 10.1016/0005-2787(69)90506-1. [DOI] [PubMed] [Google Scholar]
- Jockusch H., Ball L. A., Kaesberg P. Synthesis of polypeptides directed by the RNA of phage Q beta. Virology. 1970 Oct;42(2):401–414. doi: 10.1016/0042-6822(70)90283-7. [DOI] [PubMed] [Google Scholar]
- Konigsberg W., Maita T., Katze J., Weber K. Amino-acid sequence of the "Qbeta" coat protein. Nature. 1970 Jul 18;227(5255):271–273. doi: 10.1038/227271a0. [DOI] [PubMed] [Google Scholar]
- Lancini G. C., Sartori G. Rifamycins LXI: in vivo inhibition of RNA synthesis of rifamycins. Experientia. 1968 Nov 15;24(11):1105–1106. doi: 10.1007/BF02147783. [DOI] [PubMed] [Google Scholar]
- Miyake T., Haruna I., Shiba T., Ito Y. H., Yamane K. Grouping of RNA phages based on the template specificity of their RNA replicases. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2022–2024. doi: 10.1073/pnas.68.9.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore C. H., Farron F., Bohnert D., Weissmann C. Possible origin of a minor virus specific protein (A1) in Q-beta particles. Nat New Biol. 1971 Sep 15;234(50):204–206. doi: 10.1038/newbio234204a0. [DOI] [PubMed] [Google Scholar]
- Overby L. R., Barlow G. H., Doi R. H., Jacob M., Spiegelman S. Comparison of two serologically distinct ribonucleic acid bacteriophages. I. Properties of the viral particles. J Bacteriol. 1966 Jan;91(1):442–448. doi: 10.1128/jb.91.1.442-448.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabussay D., Zillig W. A rifampicin resistent rna-polymerase from E. coli altered in the beta-subunit. FEBS Lett. 1969 Oct 21;5(2):104–106. doi: 10.1016/0014-5793(69)80305-4. [DOI] [PubMed] [Google Scholar]
- Radloff R. J., Kaesberg P. Electrophoretic and other properties of bacteriophage Q : the effect of a variable number of read-through proteins. J Virol. 1973 Jan;11(1):116–128. doi: 10.1128/jvi.11.1.116-128.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. W., Steitz J. E. The reconstitution of infective bacteriophage R17. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1416–1421. doi: 10.1073/pnas.58.4.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCOTT D. W. SEROLOGICAL CROSS REACTIONS AMONG THE RNA-CONTAINING COLIPHAGES. Virology. 1965 May;26:85–88. doi: 10.1016/0042-6822(65)90028-0. [DOI] [PubMed] [Google Scholar]
- SINHA N. K., FUJIMURA R. K., KAESBERG P. RIBONUCLEASE DIGESTION OF R17 VIRAL RNA. J Mol Biol. 1965 Jan;11:84–89. doi: 10.1016/s0022-2836(65)80173-5. [DOI] [PubMed] [Google Scholar]
- Sakurai T., Miyake T., Shiba T., Watanabe I. Isolation of a possible fourth group of RNA phage. Jpn J Microbiol. 1968 Dec;12(4):544–546. doi: 10.1111/j.1348-0421.1968.tb00429.x. [DOI] [PubMed] [Google Scholar]
- Steitz J. A. Identification of the A protein as a structural component of bacteriophage R17. J Mol Biol. 1968 May 14;33(3):923–936. doi: 10.1016/0022-2836(68)90328-8. [DOI] [PubMed] [Google Scholar]
- Steitz J. A. Isolation of the A protein from bacteriphage R17. J Mol Biol. 1968 May 14;33(3):937–945. doi: 10.1016/0022-2836(68)90329-x. [DOI] [PubMed] [Google Scholar]
- Strauss E. G., Kaesberg P. Acrylamide gel electrophoresis of bacteriophage Q beta: electrophoresis of the intact virions and of the viral proteins. Virology. 1970 Oct;42(2):437–452. doi: 10.1016/0042-6822(70)90287-4. [DOI] [PubMed] [Google Scholar]
- Strigini P., Gorini L. Ribosomal mutations affecting efficiency of amber suppression. J Mol Biol. 1970 Feb 14;47(3):517–530. doi: 10.1016/0022-2836(70)90319-0. [DOI] [PubMed] [Google Scholar]
- Umezawa H., Mizuno S., Yamazaki H., Nitta K. Inhibition of DNA-dependent RNA synthesis by rifamycins. J Antibiot (Tokyo) 1968 Mar;21(3):234–236. doi: 10.7164/antibiotics.21.234. [DOI] [PubMed] [Google Scholar]
- Vasquez C., Granboulan N., Franklin R. M. Structure of the ribonucleic acid bacteriophage R17. J Bacteriol. 1966 Dec;92(6):1779–1786. doi: 10.1128/jb.92.6.1779-1786.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viñuela E., Algranati I. D., Ochoa S. Synthesis of virus-specific proteins in Escherichia coli infected with the RNA bacteriophage MS2. Eur J Biochem. 1967 Mar;1(1):3–11. doi: 10.1007/978-3-662-25813-2_2. [DOI] [PubMed] [Google Scholar]
- Vollenweider H. J., Koller T. Physical mapping of Qbeta replicase binding sites on Qbeta RNA. J Mol Biol. 1976 Mar 5;101(3):367–377. doi: 10.1016/0022-2836(76)90153-4. [DOI] [PubMed] [Google Scholar]
- Weiner A. M., Weber K. Natural read-through at the UGA termination signal of Q-beta coat protein cistron. Nat New Biol. 1971 Sep 15;234(50):206–209. doi: 10.1038/newbio234206a0. [DOI] [PubMed] [Google Scholar]
- Wittmann H. G. Structure, function and evolution of ribosomes. Eur J Biochem. 1976 Jan 2;61(1):1–13. doi: 10.1111/j.1432-1033.1976.tb09992.x. [DOI] [PubMed] [Google Scholar]
- Yates J. L., Gette W. R., Furth M. E., Nomura M. Effects of ribosomal mutations on the read-through of a chain termination signal: studies on the synthesis of bacteriophage lambda O gene protein in vitro. Proc Natl Acad Sci U S A. 1977 Feb;74(2):689–693. doi: 10.1073/pnas.74.2.689. [DOI] [PMC free article] [PubMed] [Google Scholar]