Abstract
Microbial biofilms are encased in a protein, DNA and polysaccharide matrix that protects the community, promotes interactions with the environment, and helps cells to adhere together. The protein component of these matrices is often a remarkably stable, β-sheet-rich polymer called amyloid. Amyloids form ordered, self-templating fibers that are highly aggregative, making them a valuable biofilm component. Some eukaryotic proteins inappropriately adopt the amyloid fold and these misfolded protein aggregates disrupt normal cellular proteostasis, which can cause significant cytotoxicity. Indeed, until recently amyloids were considered solely the result of protein misfolding. However, research over the past decade has revealed how various organisms have capitalized on the amyloid fold by developing sophisticated biogenesis pathways that coordinate gene expression, protein folding, and secretion so that amyloid-related toxicities are minimized. How microbes manipulate amyloids, by augmenting their advantageous properties and by reducing their undesirable properties, will be the subject of this review.
Keywords: amyloid, biofilm, toxin, fiber, extracellular matrix
Introduction
A biofilm is a community of microbes bound together by an extracellular matrix that includes proteins, polysaccharides, and DNA. Proteins in the extracellular matrix can take on many structures, but one of the most commonly found is amyloid. β-sheet amyloid fibers have long been the hallmark of human neurodegenerative diseases, but amyloids are increasingly appreciated as a functionally diverse protein fold. Amyloids are remarkably stable protein polymers that form β-sheet rich fibers with a diameter of 5–10 nm. The amyloid fold is unique in that it is adopted by a variety of proteins with varying primary sequences.
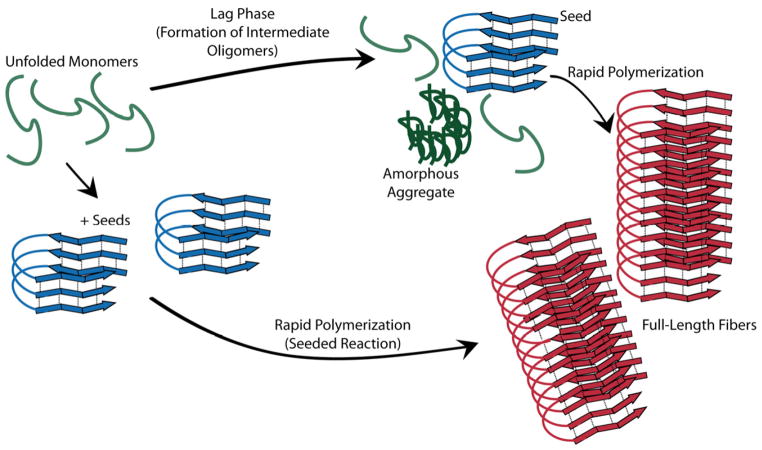
The first step in amyloid fiber formation is aggregation of monomers into intermediate oligomers, or seeds. Once seeds form, they nucleate rapid fiber elongation, with the final amyloid structure being essentially a stack of β-sheet rich monomers, aligned so that each β-strand is perpendicular to the fiber axis (Fig. 1). Dense hydrogen bonding between adjacent β-sheets then provides fiber stability (Harper and Lansbury, 1997, Nelson et al., 2005, O’Nuallain et al., 2004, Scheibel et al., 2001, Serpell et al., 1997, Sunde et al., 1997, Wetzel, 2006). Amyloidogenic proteins can polymerize in the absence of an energy source, so it is not surprising that amyloid fibers are a common component of the microbial extracellular matrix, where energy sources can be scarce and environmental conditions can wreak havoc on lesser protein folds (Chapman et al., 2002, Dueholm et al., 2010, Jordal et al., 2009, Larsen et al., 2007, Romero et al., 2010, Schwartz et al., 2012).
Figure 1.
General schematic for how amyloids form. Unfolded monomers form intermediate oligomers, some of which are toxic, during the lag phase of amyloid assembly. Once the on-pathway oligomers, or seeds, have formed rapid fiber assembly ensues. The lag phase can be bypassed by addition of pre-formed seeds to monomers.
The necessary structural features of an amyloid fiber, β-sheet formation and hydrogen bonding between β-sheets, are partly conferred by the polypeptide backbone and do not require complex amino acids (Chamberlain et al., 2000, Dobson, 2004, Gordon and Meredith, 2003, Kheterpal et al., 2000, Makin et al., 2005). These properties are proposed to lend themselves to the universality of the amyloid fold, and, indeed, there is a hypothesis that the amyloid fold, with its tendency towards elementary amino acid composition and resistance to harsh environmental conditions, was a fundamental structure in the early history of life (Carny and Gazit, 2005). Most proteins can adopt the amyloid fold, although the majority of ‘non-native’ amyloids require exposure to harsh denaturing conditions to destabilize their native structure before they can re-organize into an amyloid (Chiti et al., 1999, Chiti et al., 2000, Ramirez-Alvarado et al., 2000). Conversely, some proteins readily form amyloids. These polypeptides often contain specific regions that promote the amyloid fold (Esteras-Chopo et al., 2005, Pastor et al., 2007, Tenidis et al., 2000). Transfer of amyloidogenic stretches of amino acids to normally non-amyloidogenic proteins can force amyloid formation (Ivanova et al., 2004, Ventura et al., 2004).
Similarly, the amyloid fold confers common biophysical characteristics to proteins that might be dissimilar at the primary sequence level. For instance, a defining characteristic of an amyloid, regardless of primary amino acid sequence, is the ability to bind certain dyes such as Congo red and thioflavin T (Elghetany and Saleem, 1988). Additionally, antibodies have been designed that recognize general epitopes of amyloid fibers or amyloid oligomers (Hrncic et al., 2000, Kayed et al., 2003, Kayed et al., 2007, Larsen et al., 2007, O’Nuallain and Wetzel, 2002), and small molecules have been identified that affect fiber formation of multiple amyloids (Horvath et al., 2012).
Amyloids have been thoroughly studied because of their historical association with neurodegenerative conditions such as Alzheimer’s disease. The Alzheimer’s amyloid β (Aβ) peptide was one of the first described disease-associated amyloids and is among the most thoroughly studied (Hardy and Selkoe, 2002). Amyloid formation is also the hallmark of Parkinson’s disease (Breydo et al., 2012), Huntington’s disease (Zuccato et al., 2010), and type II diabetes (Westermark et al., 2011). Because amyloid fibers were identified in connection with various diseases, it was assumed that amyloid fibrils themselves were toxic (Caughey and Lansbury, 2003). Although new data suggest that mature amyloid fibers are relatively inert, non-cytotoxic, and maybe even protective (Caughey and Lansbury, 2003). Instead, amyloid-related toxicity is likely caused by small oligomers formed as an intermediate step in fiber polymerization (Bucciantini et al., 2002, Caughey and Lansbury, 2003, Glabe and Kayed, 2006, Kayed et al., 2003, Lambert et al., 1998). Indeed, the oligomeric species of various disease causing amyloids have been shown to be toxic (Engel, 2009, Haass and Selkoe, 2007, Volles and Lansbury, 2003).
Although many amyloids in multicellular organisms are traditionally associated with protein misfolding and disease, the last decade has ushered in the emergence of the functional amyloid – where amyloid formation clearly benefits cellular physiology. Molecular studies of functional amyloids have revealed elegant systems designed to enhance and fine-tune amyloid formation spatially and temporally within biological systems and to limit amyloid-associated toxicity. While disease-causing amyloids were the first to be described, the rapid discovery of functional amyloids in a variety of organisms, used for a variety of purposes, is forcing us to reconsider which amyloids are the exceptions and which are the rule. This review will detail microbial systems dedicated to functional amyloid formation, focusing on the versatility of the amyloid fold and the functions for which microbes utilize amyloids.
Amyloids as Biofilm Matrix Material
Curli
The first bacterial functional amyloid to be described was curli, extracellular proteinaceous fibers produced by enterics such as Escherichia coli and Salmonella enterica (Chapman et al., 2002, Collinson et al., 1991, Olsen et al., 1989, Romling et al., 1998). Curli expression is controlled by a variety of environmental signals, with maximum induction typically occurring under low salt, low nutrient conditions (Olsen et al., 1993, Provence and Curtiss, 1992, Romling et al., 1998). Intriguingly, although CsgA is able to polymerize under a wide variety of environmental conditions, salt concentration affects polymerization kinetics (Dueholm et al., 2011), implying that environmental signals can alter both curli expression and curli aggregation. Curli fibers are important for various biofilm-related phenotypes such as attachment to abiotic surfaces and development of biofilm architecture (Castonguay et al., 2006, Pawar et al., 2005, Vidal et al., 1998). In E. coli and S. enterica, curli and the extracellular polysaccharide cellulose are co-expressed, as CsgD also activates transcription of a diguanylate cyclase, adrA, which functions to initiate cellulose synthesis (Romling et al., 2000, Zogaj et al., 2001). Curli and cellulose can act synergistically, and some phenotypes such as resistance to desiccation and attachment to epithelial cells are dependent on the presence of both (Gualdi et al., 2008, Saldana et al., 2009, White et al., 2006). Other biofilm-related phenotypes are more dependent on one matrix component or the other. For example, cellulose confers greater resistance to bleach and curli are more important for adherence to spinach leaves (Macarisin et al., 2012, White et al., 2006).
TasA
The co-appearance of amyloids and polysaccharides in biofilm matrices is commonly observed. The Gram-positive soil organism, Bacillus subtilis, expresses a master biofilm regulator, SinR, that controls expression of matrix components (Kearns et al., 2005). SinR negatively regulates the epsA-O operon, whose gene products produce an extracellular polysaccharide (EPS) that is necessary for robust biofilm formation either on a solid surface or at the air/liquid interface of a broth culture (Kearns et al., 2005). Chu et al. showed that SinR also represses expression of a three-gene operon tapA-sipW-tasA (formerly yqxM-sipW-tasA) (Chu et al., 2006), and TasA was shown to be a major protein component of B. subtilis biofilms (Branda et al., 2006). Purified TasA readily forms amyloid fibers (Romero et al., 2010), revealing a system in which an amyloidogenic protein and an extracellular polysaccharide are members of the same regulon. EPS and TasA, like cellulose and qcurli in E. coli and S. enterica, act cooperatively to a llow biofilm formation (Romero et al., 2010).
The B. subtilis biofilm system also provides an answer to the interesting question of how a cell can escape a self-produced amyloid cage. The first gene in the tapA-sipW-tasA operon, TapA, promotes TasA fiber assembly and anchors the amyloid to the cell surface (Branda et al., 2006, Romero et al., 2011). Deletion of tapA leads to secretion of smaller amounts of TasA that do not appear to be surface attached (Romero et al., 2011). Interestingly, D-amino acids, which have been described to signal biofilm disassembly (Kolodkin-Gal et al., 2010), act through TapA in order to disassociate B. subtilis cells from the amyloid fiber (Romero et al., 2011). This mechanism circumvents the need to break down an amyloid fiber, a process that would likely be difficult due to the resistance of amyloid fibers to proteases.
Phenol Soluble Modulins
Recently amyloidogenic extracellular fibers composed of small peptides called phenol soluble modulins (PSMs) were identified as biofilm components in Staphylococcus aureus (Schwartz et al., 2012). S. aureus is a Gram-positive coccus that can colonize the body as a commensal organism in the nasal pharynx and can also cause a variety of illnesses, ranging from minor skin infections to bacteremia and sepsis, many of which involve formation of in-host biofilms (Otto, 2008). The ability of PSMs to form amyloid is particularly novel because soluble PSMs have a variety of reported functions. PSMs, either isolated from S. aureus or S. epidermidis, have been reported to recruit, activate, and lyse human neutrophils and to kill competing bacteria (Cogen et al., 2010, Cogen et al., 2010, Marchand et al., 2011, Wang et al., 2007). Soluble PSMs are also able to effectively act as a biofilm dissociation factor (Periasamy et al., 2012, Vuong et al., 2000), but upon amyloid fibrillization, PSMs lose that ability (Schwartz et al., 2012). Fibrous PSMs are, however, required for resistance of S. aureus biofilms to various dispersion agents such as Dispersin B, DNAse I, Protease K, and to mechanical stress (Schwartz et al., 2012), demonstrating functional roles in both the monomeric and fibrous states (Fig. 2). How fiber formation contributes to the non-biofilm roles attributed to PSMs, as well as how each of the seven PSMs, whose genes are encoded on three separate operons throughout the S. aureus genome (Janzon et al., 1989, Mehlin et al., 1999, Wang et al., 2007), contributes to fiber formation is unclear. Intriguingly, the recently described B. subtilis amyloid TasA may also perform roles as a toxin and as a biofilm stability factor, as prior to its described amyloid properties, TasA was shown to display antimicrobial activity (Stover and Driks, 1999).
Figure 2.
Structure-function dynamics of Staphylococcal phenol-soluble modulins (PSMs). Soluble PSMs are able to mediate S. aureus biofilm disassembly, to recruit and activate neutrophils, and to lyse neutrophils and niche bacteria such as group A Streptococcus. Once polymerized, PSMs lose the ability to disrupt S. aureus biofilms. Fibrous PSMs stabilize biofilms and confer resistance to proteinase K, dispersin B, and DNase I.
Amyloids in Environmental Biofilms
To determine if amyloids are a common component of naturally occurring biofilms, Daniel Otzen, Per H. Nielsen and colleagues have utilized the amyloid-specific dye, thioflavin-T, and conformational-specific antibodies to probe environmental biofilm samples. They found that among biofilms from seven different habitats, 5–40% of DAPI-positive bacteria were associated with amyloid adhesions. Among these bacteria were representatives from Proteobacteria, Bacteriodetes, Chloroflexi, and Actinobacteria (Larsen et al., 2007). In a follow up study, a Pseudomonas fluorescens strain was shown to produce an amyloid in the extracellular matrix of mixed biofilms (Dueholm et al., 2010). The genes necessary for formation of this amyloid were traced to the fapA-F operon, which is conserved in many Pseudomonas species, including the pathogenic P. aeruginosa. Expression of the fapA-F operon in E. coli resulted in flocculation and biofilm formation (Dueholm et al., 2010). Similar large-scale screening with conformational-specific antibodies demonstrated amyloid production in various members of the Actinobacteria and Firmicutes phyla (Jordal et al., 2009), further implying that amyloid formation is likely a widespread phenomenon in biofilms.
Amyloids as Shared Biofilm Resources
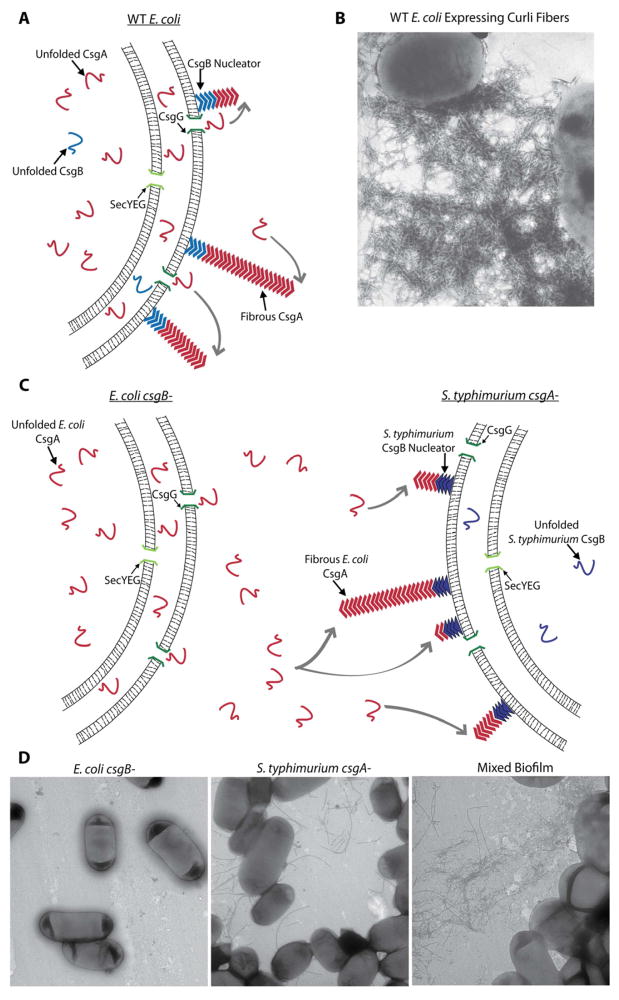
The abundance of amyloids in natural biofilms could potentially lead to interactions between different types of amyloidogenic proteins. Under curli-inducing conditions, large amounts of monomeric CsgA – the major component of curli fibers – are secreted into the extracellular milieu. Once outside the cell, CsgA encounters the outer-membrane anchored minor fiber component CsgB (Hammar et al., 1996, Loferer et al., 1997). CsgB is able to form amyloid seeds on the bacterial surface that nucleate CsgA fiber formation (Hammer et al., 2007, Hammer et al., 2012) (Fig. 3 A, B). If other bacteria use similar secretion/polymerization amyloid assembly processes, seeds from one species could nucleate monomers from another species. Indeed, CsgA derived from various enteric bacteria, including E. coli, S. typhimurium LT2, and Citrobacter koseri, as well as from the distantly related Proteobacteria Shewanella oneidensis, can cross-seed in vitro (Zhou et al., 2012). Moreover, mixed biofilms of an E. coli csgB mutant (able to secrete soluble CsgA) and a S. typhimurium LT2 csgA mutant (able to assemble cell-associated CsgB nucleators) formed heterogeneous amyloid fibers (Fig. 3 C, D). Fiber formation between these two species increased surface attachment of the entire mixed-species community, demonstrating functional, inter-species amyloid cross-seeding (Zhou et al., 2012).
Figure 3.
Secretion/polymerization curli fiber assembly. (A) WT E. coli secretes soluble CsgA proteins into the extracellular milieu. Once outside the cell, CsgA encounters surface-attached CsgB seeds that nucleate CsgA amyloid formation. (B) Transmission electron microscopy (TEM) image of curliated E. coli. (C) Inter-bacterial complementation between a CsgA expressing E. coli csgB mutant and a CsgB expressing S. typhimurium csgA mutant. E. coli secretes monomeric CsgA that can interact with CsgB nucleators on the surface of S. typhimurium. (D) TEM images of an E. coli csgB mutant, a S. typhimurium csgA mutant (producing flagella), and a mixed culture biofilm where curli fibers can be seen.
Amyloids with Surface Active Properties
Clearly, amyloids can serve as strong, relatively inert, scaffolding material in the extracellular matrix, but amyloids can also be dynamic participants in a variety of cellular processes.
Chaplin amyloids help Streptomyces coelicolor form aerial hyphae. S. coelicolor is a filamentous, soil-dwelling bacterium whose life cycle is similar to filamentous fungi. The vegetative form of S. coelicolor, the feeding submerged mycelium, secretes enzymes onto food substrates from which it derives nutrients. Once starvation signals are sensed, aerial hyphae emerge from the submerged mycelium and ascend towards the surface. Chains of spores develop in these hyphae, and once the soil surface has been breached, the spores are released to colonize elsewhere (Flardh and Buttner, 2009). Vegetative S. coelicolor cell surfaces are hydrophilic, so to break the soil/air interface, the cells must first develop a hydrophobic coat. To this end, S. coelicolor secretes monomeric chaplin proteins (encoded by chpA-H) (Claessen et al., 2003, Elliot et al., 2003). These hydrophobic proteins have been shown to form β-sheet rich amyloid fibers on contact either with air (Claessen et al., 2003) or with hydrophobic Teflon (de Jong et al., 2009). Hyphae protrusion from the soil is dependent on formation of a sheath layer of these hydrophobic chaplin amyloids along with a class of proteins called rodlins (Claessen et al., 2004). Although S. coelicolor encodes eight chaplin proteins, a strain producing only chpC, chpE, and chpH is able to produce aerial hyphae. In this minimal strain, ChpH is the major chaplin subunit (Capstick et al., 2011, Di Berardo et al., 2008). To investigate whether the amyloid fold of the chaplins was important in their ability to allow formation of aerial hyphae, Capstick et. al. identified and mutagenized two amyloid domains within the ChpH sequence. Mutagenesis of these domains, while not affecting protein stability or localization, prevented amyloidogenesis of ChpH and abolished aerial hyphae formation, indicating that amyloid formation is essential for proper chaplin function (Capstick et al., 2011). In addition to the layered chaplin coat, S. coelicolor can also produce chaplin fibers that appear as fimbriae-like appendages instead of the smooth coating associated with aerial hyphae. In this form, the chaplin fibers help attach to hydrophobic surfaces (de Jong et al., 2009), much like curli fibers of E. coli help the bacteria attach to polystyrene (Pawar et al., 2005). As with E. coli and B. subtilis, S. coelicolor amyloid fiber formation occurs concurrently with production of an extracellular polysaccharide, in this case cellulose. Interestingly, the cellulose fibers produced by S. coelicolor appear to help attach the chaplin fibers to the cell surface (de Jong et al., 2009).
Recent work has begun to reveal how the chaplins are anchored to the cell surface. The chaplins can be divided into long chaplins (chpA-C) and short chaplins (chpD-H), based on the protein product either being roughly 225 or 63 amino acids in length (Claessen et al., 2003, Elliot et al., 2003). The short chaplins are proposed to coat aerial hyphae and act as the amyloidogenic surfactants, but the role of the long chaplins is more ambiguous. Interestingly, the long chaplins all contain sortase signals, and indeed sortase activity is necessary for proper development of aerial hyphae and for cell wall anchoring of ChpC (Claessen et al., 2003, Duong et al., 2012, Elliot et al., 2003). However, a mutant of all three long chaplin genes was less defective at short chaplin surface assembly than the sortase mutant, indicating an additional, undescribed role for sortases in development of aerial hyphae (Duong et al., 2012).
The S. coelicolor chaplins are similar to an extracellular fiber produced by filamentous fungi called class I hydrophobins (Wosten and de Vocht, 2000, Wosten and Willey, 2000). Indeed, hydrophobins were one of the first functional amyloids to be described (Butko et al., 2001, de Vocht et al., 2000, Mackay et al., 2001, Wosten and de Vocht, 2000). Like chaplins, hydrophobins are secreted as soluble, monomeric proteins that polymerize into a layer of amyloid fibers at hydrophobic/hydrophilic interfaces such as those found at the air/soil boundary (Morris et al., 2011, Wosten et al., 1993, Wosten et al., 1994). The growing hyphae can then emerge from the liquid environment into the hydrophilic face of the amyloid coat, facilitating extension into the air (Beever and Dempsey, 1978, Wosten et al., 1993, Wosten and de Vocht, 2000). Structural analysis of a soluble hydrophobin, EAS, reveals a model for how the amyloid fold can be effectively tailored to the formation of an amphipathic coat (Kwan et al., 2006). Six of the eight charged amino acids in the EAS sequence are localized to one face of a central, hydrophobic core, while the opposite surface is composed chiefly of hydrophobic amino acids. Kwan et. al proposed a model where the central β-barrel core of each monomer aligns to form a cross-β structure, and the fiber is then divided into a charged face and a hydrophobic face. Lateral alignments of multiple fibers would then produce a biphasic surface layer into which the aerial hyphae could protrude (Kwan et al., 2006). Further work with EAS has identified a particular stretch of residues that is important for amyloid formation. Mutation of residues within this stretch yielded a protein with an increased lag phase, and purified peptides derived from a wild-type version of the predicted amyloid domains readily formed fibers in vitro (Macindoe et al., 2012). Furthermore, transfer of this amyloid-forming domain into a class II hydrophobin, which normally will not form amyloid, conferred fiber formation and thioflavin T binding (Macindoe et al., 2012). These examples speak once again to the pliability of the amyloid fold, as a single polypeptide can be engineered to both form an amyloid fiber and to have distinct properties, such as amphipathicity, in the amyloid fold.
Interplay between Microbial Amyloids and the Host
Various interactions between microbial amyloids and host systems have been reported. Curli mediate binding to and internalization by host cells (Gophna et al., 2001, Johansson et al., 2001, Kikuchi et al., 2005), with these attributes being at least partly imbued by curli’s fibronectin-binding capacity (Gophna et al., 2002, Olsen et al., 1989). Curli have also been shown to interact with major histocompatibility complex I (MHC-I), but whether or not the amyloid properties of curli fibers affect these interactions is unclear (Olsen et al., 1998).
In contrast, the amyloid properties of curli might impact human amyloidosis. Amyloidosis is a condition in which aberrantly folded amyloids deposit on tissues or organs, disrupting proper function and causing inflammation (Pepys, 2001). AA amyloidosis results from the abnormal deposition of a protein fragment from the typically soluble serum amyloid A (SAA) protein. These aggregates can be seeded by small SAA fragments, and indeed by other amyloid-like fibrils, thus exacerbating the symptoms of amyloidosis (Ganowiak et al., 1994, Kisilevsky et al., 1999, Johan et al., 1998, Niewold et al., 1987). Lundmark et. al. showed that exogenously added amyloid-proteins including Sup35 from Saccharomyces cerevisiae, silk from Bombyx mori, and either purified curli or curliated E. coli increased the occurrence of SAA aggregates in mice (Lundmark et al., 2005). Further work exploring whether there are direct interactions between SAA and microbial amyloids will help to clarify the role of cross-seeding in amyloidosis.
There is strong evidence that the cross-β structure of curli fibers interferes with host blood clotting mechanisms (Gebbink et al., 2005). Fibrin, a major component of blood clots, displays a cross β-sheet structure and other amyloid-like characteristics (Kranenburg et al., 2002). Fibrin activates the mammalian tissue-type plasminogen activator (tPA) by promoting contact between tPA and its substrate, plasminogen. tPA can then cleave plasminogen into the active serine protease plasmin, which degrades fibrin and results in blood clot dissolution. (Nieuwenhuizen, 2001). Kranenburg et. al. showed that the cross-β structure specifically allows for tPA activation (Kranenburg et al., 2002). Indeed, various other amyloid-like proteins, such as Aβ and IAPP (the amyloid associated with β-cell toxicity in type II diabetes) have been shown to effectively activate tPA and trigger the degradation either of themselves or of fibrin (Gebbink, 2011, Kranenburg et al., 2002). Curli, either purified or on the surface of E. coli or S. enterica, is both able to sequester tPA and plasminogen from plasma, and both of these proteins are functional when bound to curliated bacteria (Sjobring et al., 1994).
Curliated E. coli and Salmonella spp. are also able to interfere with the host contact system, which is an enzymatic cascade triggered when blood contacts surface material (Gebbink et al., 2005). As part of the contact cascade, the activated form of plasma zymogen Factor XII (FXII) can cleave another plasma protein, high-molecular weight kininogen (HK). Cleavage of HK results in the release of the peptide hormone bradykinin (BK), which leads to an inflammatory response. Curliated E. coli or S. typhimurium can bind and sequester various contact phase proteins such as FXII, HK, and fibrinogen, resulting in release of the pro-inflammatory signals BK and fibrinopeptides (BenNasr et al., 1996, Herwald et al., 1998, Persson et al., 2000, Persson et al., 2003). Intriguingly, although the in vivo activators of FXII are not completely understood, Maas et al. demonstrated that various unfolded proteins, including pre-fibrillar Aβ, are able to induce FXII autoactivation (Maas et al., 2008), leading to the question of the folding state of CsgA upon binding to FXII. Concurrent with an increase in inflammation, curliated bacteria reduced clotting in isolated plasma as well as in an in vivo mouse model, likely due to curli-mediated sequestration of blood clotting elements such as fibrinogen (Herwald et al., 1998, Persson et al., 2000, Persson et al., 2003). Finally, Bian et. al. demonstrated that serum from patients diagnosed with E. coli bacteremia contained anti-CsgA antibodies, while no such antibodies were present in sera from healthy patients (Bian et al., 2000). Collectively, these results allude to the interesting hypothesis that bacteria could use curli, and potentially other amyloids, as a mimetic of host amyloids in order to trigger specific pathways.
The importance of amyloid fibers in microbial pathogenesis is further cemented by reports of interactions between amyloid fibers and the immune system. Human macrophages were shown to produce increased levels of pro-inflammatory cytokines, including interleukin-8 (IL-8), when exposed to curliated E. coli compared to non-curliated E. coli (Bian et al., 2000). Likewise, mice injected with E. coli showed curli-dependent increases in expression of nitric oxide synthase, increases in nitric oxide levels, and decreased blood pressure (Bian et al., 2001). In studies with S. typhimurium, curli-mediated induction of nitric oxide and IL-8 in HEK293 cells was shown to be dependent on the host toll-like receptor 2 (TLR2) (Tukel et al., 2005, Tukel et al., 2009). Different bacterial structures are recognized by varying TLRs, either alone or in combination. For example, TLR2 can also bind lipoprotein, lipoteichoic acid, and peptidoglycan (Aliprantis et al., 1999, Schwandner et al., 1999). TLR4 recognizes LPS (Poltorak et al., 1998), and TLR5 recognizes flagellin (Hayashi et al., 2001). Once bound, a signaling cascade is triggered that results in an immune reponse. TLR5 is specific for flagellin, and cannot be activated by fibrous flagella (Smith et al., 2003). In contrasts, only CsgA curli polymers fully stimulate TLR2 induction of HEK293 cells, while monomeric CsgA has little activity (Tukel et al., 2009). Interestingly, another amyloid, Aβ fibers, is able to induce IL-8 production in human macrophage-like (THP-1) cells in a TLR2-dependent-manner (Tukel et al., 2009). The same pattern was observed with Nos2 mRNA production in microglia cells (Tukel et al., 2009). TLR2 has independently been shown to recognize Aβ fibers as well as SAA, indicating that it may function partly as a general amyloid receptor (Chen et al., 2010, Cheng et al., 2008, He et al., 2009, Jana et al., 2008, Udan et al., 2008). However, curli fiber recognition additionally requires TLR1 signaling, while Aβ recognition also involves TLR4 and TLR6, indicating that the interactions between amyloids and the immune system are likely complex (Liu et al., 2012, Tukel et al., 2010, Udan et al., 2008).
Similarly, S. aureus PSMs are recognized by the neutrophil-expressed formyl peptide receptor 2 (FPR2) (Kretschmer et al., 2010, Rautenberg et al., 2011). FPRs trigger neutrophil chemotaxis in response to peptides of bacterial origin (Fu et al., 2006), both formylated and nonformylated (Kretschmer et al., 2012). As with TLR2, FPR2 has also been demonstrated to recognize Aβ and SAA (Liang et al., 2000, Tiffany et al., 2001), although whether amyloid formation of these agonists is necessary for stimulation is unknown.
Amyloids have also been reported to interact with host-derived antimicrobial peptides (AMPs). LL-37 is a human AMP important for resistance to bacterial infection of the urinary tract (Chromek et al., 2006), and curli was shown to be expressed on 59% of E. coli urinary tract infection (UTI) isolates (Kai-Larsen et al., 2010). Expression of curli increased the ability of an E. coli isolate to survive LL-37 exposure in broth culture. Additionally, LL-37 was shown to bind to curli fibers and monomers by surface plasmon resonance and was demonstrated to inhibit in vitro polymerization of CsgA (Kai-Larsen et al., 2010), implying a dynamic interplay between LL-37 and CsgA within the host. Staphylococcus epidermidis PSMs have also been demonstrated to directly interact with LL-37 (Cogen et al., 2010). Interestingly, LL-37 itself has been shown to form fibrous structures that induce CR birefringence when exposed to liposomes (Sood et al., 2008).
The Weaponization of the Amyloid Fold
A common characteristic of amyloids is passage through a toxic oligomer stage en route to mature fibrils. The structurally dynamic oligomeric species can disrupt lipid membranes (Demuro et al., 2005, Kayed et al., 2004, Kourie et al., 2002, Lashuel and Lansbury, 2006, Valincius et al., 2008). Microbes have managed to harness the inherent toxicity of amyloid oligomers to kill surrounding cells. The small hydrophobic microcin E492 (MccE492) protein produced by Klebsiella pneumoniae exemplifies this idea. Like many bacteriocins, MccE492 exerts toxicity by forming pores in lipid membranes (de Lorenzo and Pugsley, 1985, Destoumieux-Garzon et al., 2003, Lagos et al., 1993). This mode of action would seem to confer toxicity to a broad range of target microbes, however MccE492 only has demonstrable antimicrobial activity against a small range of enteric bacteria (de Lorenzo et al., 1984, Destoumieux-Garzon et al., 2003). The explanation for this phenomenon lies in the fact that MccE492 is post-translationally modified with a salmochelin-like molecule (Nolan et al., 2007, Thomas et al., 2004). Salmochelin is a siderophore produced by many enteric bacteria that is recognized and imported by the FepA/Fiu/Cir outer membrane complex (Hantke et al., 2003, Mercado et al., 2008). The toxicity of MccE492 relies on the target bacteria expressing a functional copy of this receptor complex, in order for MccE492 to be transported across the outer membrane and have access to the susceptible inner membrane, (Patzer et al., 2003, Pugsley et al., 1986, Strahsburger et al., 2005) where it can disrupt membrane stability (Destoumieux-Garzon et al., 2006, Lagos et al., 2009). This targeted toxicity is made all the more interesting by the fact that MccE492 readily forms amyloid fibers in vitro and in vivo (Arranz et al., 2012, Bieler et al., 2005). Fiber formation by MccE492 completely ablates toxicity to susceptible target cells, likely due to the concurrent loss of the toxic oligomer species that would permeate membranes (Bieler et al., 2005). Further studies with MccE492 revealed that the amyloid form could be destabilized under particular conditions, including high pH or low ionic strength, and destabilization correlated both with release of oligomeric species and with an increase in toxicity. Moreover, changing conditions back to those that favor fiber formation could reverse the presence of oligomers and once again increase the number of fibers (Shahnawaz and Soto, 2012). These results indicate that amyloid formation by MccE492 is a dynamic process and imply the potential for environmental triggering of toxic oligomer release from amyloid reservoirs (Shahnawaz and Soto, 2012).
Changes in environmental pH also affect the aggregation of the listeriolysin O (LLO) protein of Listeria monocytogenes. Among other functions, LLO forms pores in the phagolysosome, allowing L. monocytogenes to escape into the cytoplasm during infection (Hamon et al., 2012). Bavdek et al. showed that under alkaline pH and high temperatures, LLO readily aggregated into fibrous structures that bind to the amyloid dyes thioflavin T and Congo red. As with MccE492, LLO did not demonstrate pore-forming capabilities when present in the fibrous form (Bavdek et al., 2012).
Plant pathogens have also been reported to employ the pore-forming capabilities of amyloidogenic proteins (Oh et al., 2007). Harpins are heat-stable, type III-secreted proteins that plant pathogens inject into host cells and that induce a hypersensitive response (Kim et al., 2003, Wei et al., 1992). Although the mode of harpin toxicity is not completely understood, various harpins have been shown to interact with synthetic lipid membranes (Lee et al., 2001) and to depolarize plant cell membranes (Ahmad et al., 2001, Pike et al., 1998). Oh et al. demonstrated that the harpin HpaG produced by Xanthomonas axonopodis pv. Glycines 8ra readily converts from a soluble α-helix monomer to β-sheet rich amyloid fibers (Oh et al., 2007). Mutants unable to convert to the amyloid fiber were unable to elicit a hypersensitive response in host cells, indicating that the ability to form amyloids is likely necessary for HpaG function. The authors went on to investigate the amyloidogenicity of other harpins, XopA from Xanthomonas campestris pv. Vescatoria, HrpN from E. amylovora, and HrpZ from P. syringae pv. Syringae. Unmodified HrpN and HrpZ readily formed amyloids, while XopA did not. XopA may be the most informative of these, as a WT version of this protein is unable to cause a hypersensitive response. A mutant version of XopA that does elicit such a response, however, was able to form amyloid fibers (Oh et al., 2007).
The utilization of amyloids is not limited to cellular life, as influenza A virus PB1-F2 protein is one of three reported viral amyloids (Chevalier et al., 2010, Freire et al., 2008, Wetzler et al., 2007). PB1-F2 is necessary for WT virulence in a mouse model, induces apoptosis in macrophages and monocytes, and has been shown to form pores in planar lipid membranes (Krumbholz et al., 2011). When exposed to non-polar solvents or membrane-mimicking agents, PB1-F2 was further demonstrated to form amyloids (Chevalier et al., 2010). Again, the ability of PB1-F2 to puncture membranes was lost upon fiber formation, indicating that the toxic activity is limited to pre-fibrillar aggregates (Chevalier et al., 2010).
Recent work with antimicrobial peptides (AMPs) derived from higher organisms further implicates membrane disruption as a common characteristic of amyloidogenesis (Kagan et al., 2012). In addition to the potential amyloidogenesis of LL-37, human antimicrobial peptide Protegrin-1 forms thioflavin T-binding fibers with similar morphology to Aβ after contact with a hydrophilic surface (Jang et al., 2011). Interestingly, atomic force microscopy revealed that Protegrin-1 formed small oligomeric species instead of full-length fibers upon contact with lipid bilayers (Jang et al., 2011). Various AMPs isolated from frog skin have also shown the ability to form amyloid fibers (Auvynet et al., 2008, Gossler-Schofberger et al., 2009, Gossler-Schofberger et al., 2012). Moreover, recent reports have demonstrated that the amyloid component of Alzheimer’s Disease plaques, Aβ, displays antimicrobial activity (Harris et al., 2012, Papareddy et al., 2012, Soscia et al., 2010), calling into question the native function of Aβ and potentially blurring the line between what is seen as a functional vs. a disease-associated amyloid. Altogether these results support the hypothesis that during polymerization, an amyloidogenic protein passes through a toxic oligomeric stage, at which time it is able to disrupt lipid membranes (Kagan et al., 2012). Evidence is mounting that biological systems utilize the generic pore-forming capabilities of the amyloid fold to generate toxins.
How Microbes Avoid Self-induced Amyloid Toxicity
Because of the common features that the amyloid fold confers on divergent proteins, the usefulness of microbial amyloids as a model for amyloidogenesis during human disease is apparent. As the ultimate goal from this model would be development of therapeutics to negate the harmful effects of amyloid-associated diseases, the most pertinent research question becomes “How do microbes prevent amyloid-associated toxicity?” It turns out there are a variety of answers to this question as microbes utilize various chaperones, nucleators, trafficking systems, and the protein sequence of the amyloid itself to promote innocuous amyloid formation.
Since small oligomers are widely considered to be the toxic species in amyloid formation, one mechanism for avoiding toxicity has been hypothesized to be rapid passage through the oligomeric stage. In agreement with this hypothesis, E. coli uses a nucleator protein, CsgB, to seed rapid polymerization of the major curli component, CsgA, on the cell surface. CsgB itself can form amyloid fibers in vitro and the addition of CsgB seeds allows CsgA to bypass the characteristic lag phase associated with amyloid fiber formation (Hammer et al., 2007, Hammer et al., 2012). Specific amino acids in CsgA mediate interaction between these two fiber subunits, and mutation of these residues results in a CsgA protein that is secreted from the cell in a soluble state (Wang and Chapman, 2008, Wang et al., 2008). The main functions of CsgB in vivo then seem to be templating extracellular CsgA amyloid formation and, along with the surface-exposed CsgF protein, anchoring CsgA fibers to the cell (Hammer et al., 2007, Nenninger et al., 2009), outlining a model where CsgA monomers are kept unfolded and soluble until they are transported across the outer-membrane where they encounter CsgB seeds. CsgA then rapidly folds into an amyloid fiber, potentially bypassing the toxic oligomeric stage.
Perhaps the most direct mechanism for avoiding amyloid toxicity is through manipulation of the amyloid protein’s primary amino acid sequence. The chief component of curli fibers, CsgA, is able to polymerize into amyloid fibers in a wide variety of environmental conditions (Dueholm et al., 2011). Similarly, the S. coelicolor chaplins are able to polymerize in a wide variety of pHs (Sawyer et al., 2011). These results contrast with non-native amyloids that need to be exposed to specific, destabilizing conditions in order to aggregate (Chiti et al., 1999, Chiti et al., 2000, Marcon et al., 2005). Investigations into the primary sequence of CsgA have revealed that particular residues play crucial roles in the polymerization of this amyloid-by-design. The amyloidogenic domain of CsgA is composed of 5 imperfect repeats (R1-R5) that share 30% amino acid identity to each other (Collinson et al., 1999, Wang and Chapman, 2008). The two terminal repeating subunits, R1 and R5, readily form amyloid fibers in vitro, while R2 and R4 do not form fibers and R3 forms short, fibrous aggregates (Wang et al., 2007). Wang et. al. demonstrated that R2, R3, and R4 contain ‘gatekeeper’ residues that inhibit the aggregative propensity of these peptides and therefore the entire CsgA protein (Wang et al., 2010). A CsgA mutant in which the gatekeeper residues were substituted with amino acids that bolster amyloid formation, CsgA*, polymerized more rapidly than WT CsgA in vitro and formed mislocalized fibers without the need for CsgB in vivo. Overexpression of CsgA* was significantly more toxic to the cell than overexpression of WT CsgA (Wang et al., 2010). These data indicate that the CsgA protein sequence has evolved specific traits to allow for manageable amyloid formation by the cell. Additionally, both curli fiber components, CsgA and CsgB, indeed the majority of secreted proteins, are composed of a disproportionate amount of inexpensive amino acids (Smith and Chapman, 2010), indicating that the CsgA protein is designed for both safe amyloid formation and for cost-effectiveness.
A third mechanism to control amyloid formation is the use of cellular chaperones. In curli biogenesis, the role of CsgB as an outer-membrane nucleator implies that CsgA must be maintained in a soluble, unfolded state as it is trafficked through the cytoplasm and periplasm. Consistent with this hypothesis, various E. coli chaperones, including cytoplasmic DnaK and Hsp33 and periplasmic Spy, were demonstrated to inhibit CsgA aggregation (Evans et al., 2011). Once in the periplasm, CsgA encounters a specificity factor for curli secretion, CsgE. In addition to trafficking unfolded CsgA to the outer-membrane pore CsgG during secretion, CsgE has been demonstrated to inhibit CsgA polymerization in vitro (Nenninger et al., 2011). These data are consistent with a model of CsgA maintaining an unfolded, unstructured state on its voyage through the cytoplasm and periplasm, and it appears that various cellular chaperones are able to inhibit inappropriate CsgA aggregation before secretion.
Chaperones also play a major role in amyloid formation of the fungal prions, a fascinating family of intracellular amyloids that have diverse roles in gene regulation and heterokaryon formation. The central principle of fungal prions is that a soluble cytoplasmic protein has a particular function that is altered when the protein takes on an amyloid fold. Once in the amyloid fold, the fiber serves as a sink for soluble monomers of the same protein, effectively diminishing the intracellular concentration, and therefore functional capacity, of the soluble protein. Moreover, the amyloidogenic form of a prion protein acts as an epigenetic element, i.e. it can be passed on to progeny cells or introduced as a functional unit into a prion-negative cell. The prion field has been extensively described in recent reviews (Tuite et al., 2011, Wickner et al., 2007), and will only be briefly mentioned here in regards to amyloid-chaperone interactions. As intracellular amyloids, yeast prions serve as fantastic examples of how amyloid formation can occur safely in the crowded cytoplasmic environment. A complex of chaperones, including those from the Hsp104, Hsp70, and Hsp40 families are involved in ameliorating amyloid related toxicities in human cells as well as yeast (Douglas et al., 2009). The yeast prion [RNQ+] has no known function except to influence the conformation state of other prion proteins such as Sup35 (Stein and True, 2011). However, overexpression of Rnq1 in a [RNQ+] strain is toxic to the cell. This toxicity can be tempered by overexpression of the Hsp40 chaperone Sis1 (Douglas et al., 2008). Sis1, along with Hsp104, is necessary for the propagation of the [RNQ+] state (Sondheimer and Lindquist, 2000, Sondheimer et al., 2001). Douglas et al. demonstrated that [RNQ+] toxicity was not due to sequestration of Sis1, but was instead caused by accumulation of SDS-soluble Rnq1 aggregates. Overexpression of Sis1 leads to a depletion of these aggregates and an increase in SDS-insoluble [RNQ+] amyloids, indicating that an Rnq1 toxic oligomer was likely responsible for the cell death and that Sis1 promotes formation of innocuous [RNQ+] amyloids (Douglas et al., 2008). Altogether these results indicate that microbes have evolved intricate systems in order to avoid amyloid-derived toxicity.
Concluding Remarks
The diversity and ubiquitous nature of the amyloid fold is now easily appreciated. Amyloids can be utilized as structural material, toxins, surface-active fibers, genetic material, adhesins, host mimetics, and likely much more. While amyloid represents a conserved, versatile, and important protein fold, the use of amyloids requires dedicated systems in order to limit toxic amyloid formation in the wrong place and/or at the wrong time. A detailed understanding of these systems will no doubt yield insights into both protein misfolding diseases and microbial physiology.
Acknowledgments
We thank Yizhou Zhou for figure images, and members of the Chapman and Boles labs for discussions. This work was supported by NIH grants RO1 A1073847-01 and T32 GM007544-32, and the University of Michigan Rackham Merit Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
William H. DePas, Email: whdepas@umich.edu.
Matthew R. Chapman, Email: chapmanm@umich.edu.
References
- Ahmad M, Majerczak DR, Pike S, Hoyos ME, Novacky A, Coplin DL. Biological activity of harpin produced by Pantoea stewartii subsp. stewartii. Mol Plant Microbe Interact. 2001;14:1223–1234. doi: 10.1094/MPMI.2001.14.10.1223. [DOI] [PubMed] [Google Scholar]
- Aliprantis AO, Yang RB, Mark MR, Suggett S, Devaux B, Radolf JD, Klimpel GR, Godowski P, et al. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science. 1999;285:736–739. doi: 10.1126/science.285.5428.736. [DOI] [PubMed] [Google Scholar]
- Arranz R, Mercado G, Martin-Benito J, Giraldo R, Monasterio O, Lagos R, Valpuesta JM. Structural characterization of microcin E492 amyloid formation: Identification of the precursors. J Struct Biol. 2012;178:54–60. doi: 10.1016/j.jsb.2012.02.015. [DOI] [PubMed] [Google Scholar]
- Auvynet C, El Amri C, Lacombe C, Bruston F, Bourdais J, Nicolas P, Rosenstein Y. Structural requirements for antimicrobial versus chemoattractant activities for dermaseptin S9. FEBS J. 2008;275:4134–4151. doi: 10.1111/j.1742-4658.2008.06554.x. [DOI] [PubMed] [Google Scholar]
- Bavdek A, Kostanjsek R, Antonini V, Lakey JH, Dalla Serra M, Gilbert RJ, Anderluh G. pH dependence of listeriolysin O aggregation and pore-forming ability. FEBS J. 2012;279:126–141. doi: 10.1111/j.1742-4658.2011.08405.x. [DOI] [PubMed] [Google Scholar]
- Beever RE, Dempsey GP. Function of rodlets on the surface of fungal spores. Nature. 1978;272:608–610. doi: 10.1038/272608a0. [DOI] [PubMed] [Google Scholar]
- BenNasr A, Olsen A, Sjobring U, MullerEsterl W, Bjorck L. Assembly of human contact phase proteins and release of bradykinin at the surface of curli-expressing Escherichia coli. Molecular Microbiology. 1996;20:927–935. doi: 10.1111/j.1365-2958.1996.tb02534.x. [DOI] [PubMed] [Google Scholar]
- Bian Z, Brauner A, Li Y, Normark S. Expression of and cytokine activation by Escherichia coli curli fibers in human sepsis. J Infect Dis. 2000;181:602–612. doi: 10.1086/315233. [DOI] [PubMed] [Google Scholar]
- Bian Z, Yan ZQ, Hansson GK, Thoren P, Normark S. Activation of inducible nitric oxide synthase/nitric oxide by curli fibers leads to a fall in blood pressure during systemic Escherichia coli infection in mice. J Infect Dis. 2001;183:612–619. doi: 10.1086/318528. [DOI] [PubMed] [Google Scholar]
- Bieler S, Estrada L, Lagos R, Baeza M, Castilla J, Soto C. Amyloid formation modulates the biological activity of a bacterial protein. Journal of Biological Chemistry. 2005;280:26880–26885. doi: 10.1074/jbc.M502031200. [DOI] [PubMed] [Google Scholar]
- Branda SS, Chu F, Kearns DB, Losick R, Kolter R. A major protein component of the Bacillus subtilis biofilm matrix. Mol Microbiol. 2006;59:1229–1238. doi: 10.1111/j.1365-2958.2005.05020.x. [DOI] [PubMed] [Google Scholar]
- Breydo L, Wu JW, Uversky VN. Alpha-synuclein misfolding and Parkinson’s disease. Biochim Biophys Acta. 2012;1822:261–285. doi: 10.1016/j.bbadis.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Bucciantini M, Giannoni E, Chiti F, Baroni F, Formigli L, Zurdo J, Taddei N, Ramponi G, et al. Inherent toxicity of aggregates implies a common mechanism for protein misfolding diseases. Nature. 2002;416:507–511. doi: 10.1038/416507a. [DOI] [PubMed] [Google Scholar]
- Butko P, Buford JP, Goodwin JS, Stroud PA, McCormick CL, Cannon GC. Spectroscopic evidence for amyloid-like interfacial self-assembly of hydrophobin Sc3. Biochemical and Biophysical Research Communications. 2001;280:212–215. doi: 10.1006/bbrc.2000.4098. [DOI] [PubMed] [Google Scholar]
- Capstick DS, Jomaa A, Hanke C, Ortega J, Elliot MA. Dual amyloid domains promote differential functioning of the chaplin proteins during Streptomyces aerial morphogenesis. Proc Natl Acad Sci U S A. 2011;108:9821–9826. doi: 10.1073/pnas.1018715108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carny O, Gazit E. A model for the role of short self-assembled peptides in the very early stages of the origin of life. Faseb Journal. 2005;19:1051–1055. doi: 10.1096/fj.04-3256hyp. [DOI] [PubMed] [Google Scholar]
- Castonguay MH, van der Schaaf S, Koester W, Krooneman J, van der Meer W, Harmsen H, Landini P. Biofilm formation by Escherichia coli is stimulated by synergistic interactions and co-adhesion mechanisms with adherence-proficient bacteria. Res Microbiol. 2006;157:471–478. doi: 10.1016/j.resmic.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Caughey B, Lansbury PT. Protofibrils, pores, fibrils, and neurodegeneration: separating the responsible protein aggregates from the innocent bystanders. Annu Rev Neurosci. 2003;26:267–298. doi: 10.1146/annurev.neuro.26.010302.081142. [DOI] [PubMed] [Google Scholar]
- Chamberlain AK, MacPhee CE, Zurdo J, Morozova-Roche LA, Hill HA, Dobson CM, Davis JJ. Ultrastructural organization of amyloid fibrils by atomic force microscopy. Biophys J. 2000;79:3282–3293. doi: 10.1016/S0006-3495(00)76560-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman MR, Robinson LS, Pinkner JS, Roth R, Heuser J, Hammar M, Normark S, Hultgren SJ. Role of Escherichia coli curli operons in directing amyloid fiber formation. Science. 2002;295:851–855. doi: 10.1126/science.1067484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ES, Song Z, Willett MH, Heine S, Yung RC, Liu MC, Groshong SD, Zhang Y, et al. Serum amyloid A regulates granulomatous inflammation in sarcoidosis through Toll-like receptor-2. Am J Respir Crit Care Med. 2010;181:360–373. doi: 10.1164/rccm.200905-0696OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng N, He R, Tian J, Ye PP, Ye RD. Cutting edge: TLR2 is a functional receptor for acute-phase serum amyloid A. Journal of Immunology. 2008;181:22–26. doi: 10.4049/jimmunol.181.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier C, Al Bazzal A, Vidic J, Fevrier V, Bourdieu C, Bouguyon E, Le Goffic R, Vautherot JF, et al. PB1-F2 influenza A virus protein adopts a beta-sheet conformation and forms amyloid fibers in membrane environments. The Journal of biological chemistry. 2010;285:13233–13243. doi: 10.1074/jbc.M109.067710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiti F, Taddei N, Bucciantini M, White P, Ramponi G, Dobson CM. Mutational analysis of the propensity for amyloid formation by a globular protein. EMBO J. 2000;19:1441–1449. doi: 10.1093/emboj/19.7.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiti F, Webster P, Taddei N, Clark A, Stefani M, Ramponi G, Dobson CM. Designing conditions for in vitro formation of amyloid protofilaments and fibrils. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:3590–3594. doi: 10.1073/pnas.96.7.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chromek M, Slamova Z, Bergman P, Kovacs L, Podracka L, Ehren I, Hokfelt T, Gudmundsson GH, et al. The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection. Nat Med. 2006;12:636–641. doi: 10.1681/01.asn.0000926856.92699.53. [DOI] [PubMed] [Google Scholar]
- Chu F, Kearns DB, Branda SS, Kolter R, Losick R. Targets of the master regulator of biofilm formation in Bacillus subtilis. Molecular Microbiology. 2006;59:1216–1228. doi: 10.1111/j.1365-2958.2005.05019.x. [DOI] [PubMed] [Google Scholar]
- Claessen D, Rink R, de Jong W, Siebring J, de Vreugd P, Boersma FGH, Dijkhuizen L, Wosten HAB. A novel class of secreted hydrophobic proteins is involved in aerial hyphae formation in Streptomyces coelicolor by forming amyloid-like fibrils. Genes & Development. 2003;17:1714–1726. doi: 10.1101/gad.264303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claessen D, Stokroos I, Deelstra HJ, Penninga NA, Bormann C, Salas JA, Dijkhuizen L, Wosten HA. The formation of the rodlet layer of streptomycetes is the result of the interplay between rodlins and chaplins. Mol Microbiol. 2004;53:433–443. doi: 10.1111/j.1365-2958.2004.04143.x. [DOI] [PubMed] [Google Scholar]
- Cogen AL, Yamasaki K, Muto J, Sanchez KM, Alexander LC, Tanios J, Lai YP, Kim JE, et al. Staphylococcus epidermidis Antimicrobial delta-Toxin (Phenol-Soluble Modulin-gamma) Cooperates with Host Antimicrobial Peptides to Kill Group A Streptococcus. Plos One. 2010:5. doi: 10.1371/journal.pone.0008557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogen AL, Yamasaki K, Sanchez KM, Dorschner RA, Lai YP, MacLeod DT, Torpey JW, Otto M, et al. Selective Antimicrobial Action Is Provided by Phenol-Soluble Modulins Derived from Staphylococcus epidermidis, a Normal Resident of the Skin. Journal of Investigative Dermatology. 2010;130:192–200. doi: 10.1038/jid.2009.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinson SK, Emody L, Muller KH, Trust TJ, Kay WW. Purification and characterization of thin, aggregative fimbriae from Salmonella enteritidis. J Bacteriol. 1991;173:4773–4781. doi: 10.1128/jb.173.15.4773-4781.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinson SK, Parker JM, Hodges RS, Kay WW. Structural predictions of AgfA, the insoluble fimbrial subunit of Salmonella thin aggregative fimbriae. J Mol Biol. 1999;290:741–756. doi: 10.1006/jmbi.1999.2882. [DOI] [PubMed] [Google Scholar]
- de Jong W, Wosten HAB, Dijkhuizen L, Claessen D. Attachment of Streptomyces coelicolor is mediated by amyloidal fimbriae that are anchored to the cell surface via cellulose. Molecular Microbiology. 2009;73:1128–1140. doi: 10.1111/j.1365-2958.2009.06838.x. [DOI] [PubMed] [Google Scholar]
- de Lorenzo V, Martinez JL, Asensio C. Microcin-mediated interactions between Klebsiella pneumoniae and Escherichia coli strains. J Gen Microbiol. 1984;130:391–400. doi: 10.1099/00221287-130-2-391. [DOI] [PubMed] [Google Scholar]
- de Lorenzo V, Pugsley AP. Microcin E492, a low-molecular-weight peptide antibiotic which causes depolarization of the Escherichia coli cytoplasmic membrane. Antimicrob Agents Chemother. 1985;27:666–669. doi: 10.1128/aac.27.4.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vocht ML, Reviakine I, Wosten HA, Brisson A, Wessels JG, Robillard GT. Structural and functional role of the disulfide bridges in the hydrophobin SC3. J Biol Chem. 2000;275:28428–28432. doi: 10.1074/jbc.M000691200. [DOI] [PubMed] [Google Scholar]
- Demuro A, Mina E, Kayed R, Milton SC, Parker I, Glabe CG. Calcium dysregulation and membrane disruption as a ubiquitous neurotoxic mechanism of soluble amyloid oligomers. Journal of Biological Chemistry. 2005;280:17294–17300. doi: 10.1074/jbc.M500997200. [DOI] [PubMed] [Google Scholar]
- Destoumieux-Garzon D, Peduzzi J, Thomas X, Djediat C, Rebuffat S. Parasitism of iron-siderophore receptors of Escherichia coli by the siderophore-peptide microcin E492m and its unmodified counterpart. Biometals. 2006;19:181–191. doi: 10.1007/s10534-005-4452-9. [DOI] [PubMed] [Google Scholar]
- Destoumieux-Garzon D, Thomas X, Santamaria M, Goulard C, Barthelemy M, Boscher B, Bessin Y, Molle G, et al. Microcin E492 antibacterial activity: evidence for a TonB-dependent inner membrane permeabilization on Escherichia coli. Molecular Microbiology. 2003;49:1031–1041. doi: 10.1046/j.1365-2958.2003.03610.x. [DOI] [PubMed] [Google Scholar]
- Di Berardo C, Capstick DS, Bibb MJ, Findlay KC, Buttner MJ, Elliot MA. Function and redundancy of the chaplin cell surface proteins in aerial hypha formation, rodlet assembly, and viability in Streptomyces coelicolor. J Bacteriol. 2008;190:5879–5889. doi: 10.1128/JB.00685-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson CM. Principles of protein folding, misfolding and aggregation. Semin Cell Dev Biol. 2004;15:3–16. doi: 10.1016/j.semcdb.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Douglas PM, Summers DW, Cyr DM. Molecular chaperones antagonize proteotoxicity by differentially modulating protein aggregation pathways. Prion. 2009;3:51–58. doi: 10.4161/pri.3.2.8587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas PM, Treusch S, Ren HY, Halfmann R, Duennwald ML, Lindquist S, Cyr DM. Chaperone-dependent amyloid assembly protects cells from prion toxicity. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:7206–7211. doi: 10.1073/pnas.0802593105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dueholm MS, Nielsen SB, Hein KL, Nissen P, Chapman M, Christiansen G, Nielsen PH, Otzen DE. Fibrillation of the Major Curli Subunit CsgA under a Wide Range of Conditions Implies a Robust Design of Aggregation. Biochemistry. 2011;50:8281–8290. doi: 10.1021/bi200967c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dueholm MS, Petersen SV, Sonderkaer M, Larsen P, Christiansen G, Hein KL, Enghild JJ, Nielsen JL, et al. Functional amyloid in Pseudomonas. Molecular Microbiology. 2010 doi: 10.1111/j.1365-2958.2010.07269.x. [DOI] [PubMed] [Google Scholar]
- Duong A, Capstick DS, Di Berardo C, Findlay KC, Hesketh A, Hong HJ, Elliot MA. Aerial development in Streptomyces coelicolor requires sortase activity. Mol Microbiol. 2012;83:992–1005. doi: 10.1111/j.1365-2958.2012.07983.x. [DOI] [PubMed] [Google Scholar]
- Elghetany MT, Saleem A. Methods for staining amyloid in tissues: a review. Stain Technol. 1988;63:201–212. doi: 10.3109/10520298809107185. [DOI] [PubMed] [Google Scholar]
- Elliot MA, Karoonuthaisiri N, Huang JQ, Bibb MJ, Cohen SN, Kao CM, Buttner MJ. The chaplins: a family of hydrophobic cell-surface proteins involved in aerial mycelium formation in Streptomyces coelicolor. Genes & Development. 2003;17:1727–1740. doi: 10.1101/gad.264403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel MF. Membrane permeabilization by Islet Amyloid Polypeptide. Chem Phys Lipids. 2009;160:1–10. doi: 10.1016/j.chemphyslip.2009.03.008. [DOI] [PubMed] [Google Scholar]
- Esteras-Chopo A, Serrano L, Lopez de la Paz M. The amyloid stretch hypothesis: recruiting proteins toward the dark side. Proc Natl Acad Sci U S A. 2005;102:16672–16677. doi: 10.1073/pnas.0505905102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans ML, Schmidt JC, Ilbert M, Doyle SM, Quan S, Bardwell JC, Jakob U, Wickner S, et al. E. coli chaperones DnaK, Hsp33 and Spy inhibit bacterial functional amyloid assembly. Prion. 2011:5. doi: 10.4161/pri.5.4.18555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flardh K, Buttner MJ. Streptomyces morphogenetics: dissecting differentiation in a filamentous bacterium. Nat Rev Microbiol. 2009;7:36–49. doi: 10.1038/nrmicro1968. [DOI] [PubMed] [Google Scholar]
- Freire E, Oddo C, Frappier L, de Prat-Gay G. Kinetically driven refolding of the hyperstable EBNA1 origin DNA-binding dimeric beta-barrel domain into amyloid-like spherical oligomers. Proteins. 2008;70:450–461. doi: 10.1002/prot.21580. [DOI] [PubMed] [Google Scholar]
- Fu H, Karlsson J, Bylund J, Movitz C, Karlsson A, Dahlgren C. Ligand recognition and activation of formyl peptide receptors in neutrophils. J Leukoc Biol. 2006;79:247–256. doi: 10.1189/jlb.0905498. [DOI] [PubMed] [Google Scholar]
- Ganowiak K, Hultman P, Engstrom U, Gustavsson A, Westermark P. Fibrils from synthetic amyloid-related peptides enhance development of experimental AA-amyloidosis in mice. Biochem Biophys Res Commun. 1994;199:306–312. doi: 10.1006/bbrc.1994.1229. [DOI] [PubMed] [Google Scholar]
- Gebbink MF. Tissue-type plasminogen activator-mediated plasminogen activation and contact activation, implications in and beyond haemostasis. J Thromb Haemost. 2011;9(Suppl 1):174–181. doi: 10.1111/j.1538-7836.2011.04278.x. [DOI] [PubMed] [Google Scholar]
- Gebbink MF, Claessen D, Bouma B, Dijkhuizen L, Wosten HA. Amyloids--a functional coat for microorganisms. Nat Rev Microbiol. 2005;3:333–341. doi: 10.1038/nrmicro1127. [DOI] [PubMed] [Google Scholar]
- Glabe CG, Kayed R. Common structure and toxic function of amyloid oligomers implies a common mechanism of pathogenesis. Neurology. 2006;66:S74–78. doi: 10.1212/01.wnl.0000192103.24796.42. [DOI] [PubMed] [Google Scholar]
- Gophna U, Barlev M, Seijffers R, Oelschlager TA, Hacker J, Ron EZ. Curli fibers mediate internalization of Escherichia coli by eukaryotic cells. Infect Immun. 2001;69:2659–2665. doi: 10.1128/IAI.69.4.2659-2665.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gophna U, Oelschlaeger TA, Hacker J, Ron EZ. Role of fibronectin in curli-mediated internalization. FEMS Microbiol Lett. 2002;212:55–58. doi: 10.1111/j.1574-6968.2002.tb11244.x. [DOI] [PubMed] [Google Scholar]
- Gordon DJ, Meredith SC. Probing the role of backbone hydrogen bonding in beta-amyloid fibrils with inhibitor peptides containing ester bonds at alternate positions. Biochemistry. 2003;42:475–485. doi: 10.1021/bi0259857. [DOI] [PubMed] [Google Scholar]
- Gossler-Schofberger R, Hesser G, Muik M, Wechselberger C, Jilek A. An orphan dermaseptin from frog skin reversibly assembles to amyloid-like aggregates in a pH-dependent fashion. FEBS J. 2009;276:5849–5859. doi: 10.1111/j.1742-4658.2009.07266.x. [DOI] [PubMed] [Google Scholar]
- Gossler-Schofberger R, Hesser G, Reif MM, Friedmann J, Duscher B, Toca-Herrera JL, Oostenbrink C, Jilek A. A stereochemical switch in the aDrs model system, a candidate for a functional amyloid. Arch Biochem Biophys. 2012;522:100–106. doi: 10.1016/j.abb.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gualdi L, Tagliabue L, Bertagnoli S, Ierano T, De Castro C, Landini P. Cellulose modulates biofilm formation by counteracting curli-mediated colonization of solid surfaces in Escherichia coli. Microbiology. 2008;154:2017–2024. doi: 10.1099/mic.0.2008/018093-0. [DOI] [PubMed] [Google Scholar]
- Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- Hammar M, Bian Z, Normark S. Nucleator-dependent intercellular assembly of adhesive curli organelles in Escherichia coli. Proc Natl Acad Sci U S A. 1996;93:6562–6566. doi: 10.1073/pnas.93.13.6562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer ND, McGuffie BA, Zhou Y, Badtke MP, Reinke AA, Brannstrom K, Gestwicki JE, Olofsson A, et al. The C-Terminal Repeating Units of CsgB Direct Bacterial Functional Amyloid Nucleation. J Mol Biol. 2012 doi: 10.1016/j.jmb.2012.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer ND, Schmidt JC, Chapman MR. The curli nucleator protein, CsgB, contains an amyloidogenic domain that directs CsgA polymerization. Proc Natl Acad Sci U S A. 2007;104:12494–12499. doi: 10.1073/pnas.0703310104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamon MA, Ribet D, Stavru F, Cossart P. Listeriolysin O: the Swiss army knife of Listeria. Trends Microbiol. 2012;20:360–368. doi: 10.1016/j.tim.2012.04.006. [DOI] [PubMed] [Google Scholar]
- Hantke K, Nicholson G, Rabsch W, Winkelmann G. Salmochelins, siderophores of Salmonella enterica and uropathogenic Escherichia coli strains, are recognized by the outer membrane receptor IroN. Proc Natl Acad Sci U S A. 2003;100:3677–3682. doi: 10.1073/pnas.0737682100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Harper JD, Lansbury PT., Jr Models of amyloid seeding in Alzheimer’s disease and scrapie: mechanistic truths and physiological consequences of the time-dependent solubility of amyloid proteins. Annu Rev Biochem. 1997;66:385–407. doi: 10.1146/annurev.biochem.66.1.385. [DOI] [PubMed] [Google Scholar]
- Harris F, Dennison SR, Phoenix DA. Aberrant action of amyloidogenic host defense peptides: a new paradigm to investigate neurodegenerative disorders? FASEB J. 2012;26:1776–1781. doi: 10.1096/fj.11-199208. [DOI] [PubMed] [Google Scholar]
- Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, Eng JK, Akira S, et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410:1099–1103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- He RL, Zhou J, Hanson CZ, Chen J, Cheng N, Ye RD. Serum amyloid A induces G-CSF expression and neutrophilia via Toll-like receptor 2. Blood. 2009;113:429–437. doi: 10.1182/blood-2008-03-139923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herwald H, Morgelin M, Olsen A, Rhen M, Dahlback B, Muller-Esterl W, Bjorck L. Activation of the contact-phase system on bacterial surfaces--a clue to serious complications in infectious diseases. Nat Med. 1998;4:298–302. doi: 10.1038/nm0398-298. [DOI] [PubMed] [Google Scholar]
- Horvath I, Weise CF, Andersson EK, Chorell E, Sellstedt M, Bengtsson C, Olofsson A, Hultgren SJ, et al. Mechanisms of protein oligomerization: inhibitor of functional amyloids templates alpha-synuclein fibrillation. J Am Chem Soc. 2012;134:3439–3444. doi: 10.1021/ja209829m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrncic R, Wall J, Wolfenbarger DA, Murphy CL, Schell M, Weiss DT, Solomon A. Antibody-mediated resolution of light chain-associated amyloid deposits. Am J Pathol. 2000;157:1239–1246. doi: 10.1016/S0002-9440(10)64639-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova MI, Sawaya MR, Gingery M, Attinger A, Eisenberg D. An amyloid-forming segment of beta2-microglobulin suggests a molecular model for the fibril. Proc Natl Acad Sci U S A. 2004;101:10584–10589. doi: 10.1073/pnas.0403756101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jana M, Palencia CA, Pahan K. Fibrillar amyloid-beta peptides activate microglia via TLR2: implications for Alzheimer’s disease. J Immunol. 2008;181:7254–7262. doi: 10.4049/jimmunol.181.10.7254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang H, Arce FT, Mustata M, Ramachandran S, Capone R, Nussinov R, Lal R. Antimicrobial Protegrin-1 Forms Amyloid-Like Fibrils with Rapid Kinetics Suggesting a Functional Link. Biophysical Journal. 2011;100:1775–1783. doi: 10.1016/j.bpj.2011.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janzon L, Lofdahl S, Arvidson S. Identification and Nucleotide-Sequence of the Delta-Lysin Gene, Hld, Adjacent to the Accessory Gene Regulator (Agr) of Staphylococcus-Aureus. Molecular & General Genetics. 1989;219:480–485. doi: 10.1007/BF00259623. [DOI] [PubMed] [Google Scholar]
- Johan K, Westermark G, Engstrom U, Gustavsson A, Hultman P, Westermark P. Acceleration of amyloid protein A amyloidosis by amyloid-like synthetic fibrils. Proc Natl Acad Sci U S A. 1998;95:2558–2563. doi: 10.1073/pnas.95.5.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson C, Nilsson T, Olsen A, Wick MJ. The influence of curli, a MHC-I-binding bacterial surface structure, on macrophage-T cell interactions. FEMS Immunol Med Microbiol. 2001;30:21–29. doi: 10.1111/j.1574-695X.2001.tb01545.x. [DOI] [PubMed] [Google Scholar]
- Jordal PB, Dueholm MS, Larsen P, Petersen SV, Enghild JJ, Christiansen G, Hojrup P, Nielsen PH, et al. Widespread abundance of functional bacterial amyloid in mycolata and other gram-positive bacteria. Appl Environ Microbiol. 2009;75:4101–4110. doi: 10.1128/AEM.02107-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan BL, Jang H, Capone R, Teran Arce F, Ramachandran S, Lal R, Nussinov R. Antimicrobial properties of amyloid peptides. Mol Pharm. 2012;9:708–717. doi: 10.1021/mp200419b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kai-Larsen Y, Luthje P, Chromek M, Peters V, Wang XD, Holm A, Kadas L, Hedlund KO, et al. Uropathogenic Escherichia coli Modulates Immune Responses and Its Curli Fimbriae Interact with the Antimicrobial Peptide LL-37. Plos Pathogens. 2010:6. doi: 10.1371/journal.ppat.1001010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayed R, Head E, Sarsoza F, Saing T, Cotman CW, Necula M, Margol L, Wu J, et al. Fibril specific, conformation dependent antibodies recognize a generic epitope common to amyloid fibrils and fibrillar oligomers that is absent in prefibrillar oligomers. Mol Neurodegener. 2007;2:18. doi: 10.1186/1750-1326-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, Glabe CG. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300:486–489. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- Kayed R, Sokolov Y, Edmonds B, McIntire TM, Milton SC, Hall JE, Glabe CG. Permeabilization of lipid bilayers is a common conformation-dependent activity of soluble amyloid oligomers in protein misfolding diseases. Journal of Biological Chemistry. 2004;279:46363–46366. doi: 10.1074/jbc.C400260200. [DOI] [PubMed] [Google Scholar]
- Kearns DB, Chu F, Branda SS, Kolter R, Losick R. A master regulator for biofilm formation by Bacillus subtilis. Mol Microbiol. 2005;55:739–749. doi: 10.1111/j.1365-2958.2004.04440.x. [DOI] [PubMed] [Google Scholar]
- Kheterpal I, Zhou S, Cook KD, Wetzel R. Abeta amyloid fibrils possess a core structure highly resistant to hydrogen exchange. Proc Natl Acad Sci U S A. 2000;97:13597–13601. doi: 10.1073/pnas.250288897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi T, Mizunoe Y, Takade A, Naito S, Yoshida S. Curli fibers are required for development of biofilm architecture in Escherichia coli K-12 and enhance bacterial adherence to human uroepithelial cells. Microbiol Immunol. 2005;49:875–884. doi: 10.1111/j.1348-0421.2005.tb03678.x. [DOI] [PubMed] [Google Scholar]
- Kim JG, Park BK, Yoo CH, Jeon E, Oh J, Hwang I. Characterization of the Xanthomonas axonopodis pv. glycines Hrp pathogenicity island. J Bacteriol. 2003;185:3155–3166. doi: 10.1128/JB.185.10.3155-3166.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisilevsky R, Lemieux L, Boudreau L, Yang DS, Fraser P. New clothes for amyloid enhancing factor (AEF): silk as AEF. Amyloid. 1999;6:98–106. doi: 10.3109/13506129909007309. [DOI] [PubMed] [Google Scholar]
- Kolodkin-Gal I, Romero D, Cao S, Clardy J, Kolter R, Losick R. D-amino acids trigger biofilm disassembly. Science. 2010;328:627–629. doi: 10.1126/science.1188628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourie JI, Culverson AL, Farrelly PV, Henry CL, Laohachai KN. Heterogeneous amyloid-formed ion channels as a common cytotoxic mechanism - Implications for therapeutic strategies against amyloidosis. Cell Biochemistry and Biophysics. 2002;36:191–207. doi: 10.1385/CBB:36:2-3:191. [DOI] [PubMed] [Google Scholar]
- Kranenburg O, Bouma B, Kroon-Batenburg LMJ, Reijerkerk A, Wu YP, Voest EE, Gebbink MFBG. Tissue-type plasminogen activator is a multiligand cross-beta structure receptor. Current Biology. 2002;12:1833–1839. doi: 10.1016/s0960-9822(02)01224-1. [DOI] [PubMed] [Google Scholar]
- Kretschmer D, Gleske AK, Rautenberg M, Wang R, Koberle M, Bohn E, Schoneberg T, Rabiet MJ, et al. Human formyl peptide receptor 2 senses highly pathogenic Staphylococcus aureus. Cell Host Microbe. 2010;7:463–473. doi: 10.1016/j.chom.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretschmer D, Nikola N, Durr M, Otto M, Peschel A. The Virulence Regulator Agr Controls the Staphylococcal Capacity to Activate Human Neutrophils via the Formyl Peptide Receptor 2. Journal of Innate Immunity. 2012;4:201–212. doi: 10.1159/000332142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumbholz A, Philipps A, Oehring H, Schwarzer K, Eitner A, Wutzler P, Zell R. Current knowledge on PB1-F2 of influenza A viruses. Med Microbiol Immunol. 2011;200:69–75. doi: 10.1007/s00430-010-0176-8. [DOI] [PubMed] [Google Scholar]
- Kwan AHY, Winefield RD, Sunde M, Matthews JM, Haverkamp RG, Templeton MD, Mackay JP. Structural basis for rodlet assembly in fungal hydrophobins. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:3621–3626. doi: 10.1073/pnas.0505704103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagos R, Tello M, Mercado G, Garcia V, Monasterio O. Antibacterial and antitumorigenic properties of microcin E492, a pore-forming bacteriocin. Curr Pharm Biotechnol. 2009;10:74–85. doi: 10.2174/138920109787048643. [DOI] [PubMed] [Google Scholar]
- Lagos R, Wilkens M, Vergara C, Cecchi X, Monasterio O. Microcin E492 forms ion channels in phospholipid bilayer membrane. FEBS Lett. 1993;321:145–148. doi: 10.1016/0014-5793(93)80096-d. [DOI] [PubMed] [Google Scholar]
- Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I, et al. Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc Natl Acad Sci U S A. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen P, Nielsen JL, Dueholm MS, Wetzel R, Otzen D, Nielsen PH. Amyloid adhesins are abundant in natural biofilms. Environ Microbiol. 2007;9:3077–3090. doi: 10.1111/j.1462-2920.2007.01418.x. [DOI] [PubMed] [Google Scholar]
- Lashuel HA, Lansbury PT. Are amyloid diseases caused by protein aggregates that mimic bacterial pore-forming toxins? Quarterly Reviews of Biophysics. 2006;39:167–201. doi: 10.1017/S0033583506004422. [DOI] [PubMed] [Google Scholar]
- Lee J, Klusener B, Tsiamis G, Stevens C, Neyt C, Tampakaki AP, Panopoulos NJ, Noller J, et al. HrpZ(Psph) from the plant pathogen Pseudomonas syringae pv. phaseolicola binds to lipid bilayers and forms an ion-conducting pore in vitro. Proc Natl Acad Sci U S A. 2001;98:289–294. doi: 10.1073/pnas.011265298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang TS, Wang JM, Murphy PM, Gao JL. Serum amyloid A is a chemotactic agonist at FPR2, a low-affinity N-formylpeptide receptor on mouse neutrophils. Biochemical and Biophysical Research Communications. 2000;270:331–335. doi: 10.1006/bbrc.2000.2416. [DOI] [PubMed] [Google Scholar]
- Liu S, Liu Y, Hao W, Wolf L, Kiliaan AJ, Penke B, Rube CE, Walter J, et al. TLR2 is a primary receptor for Alzheimer’s amyloid beta peptide to trigger neuroinflammatory activation. J Immunol. 2012;188:1098–1107. doi: 10.4049/jimmunol.1101121. [DOI] [PubMed] [Google Scholar]
- Loferer H, Hammar M, Normark S. Availability of the fibre subunit CsgA and the nucleator protein CsgB during assembly of fibronectin-binding curli is limited by the intracellular concentration of the novel lipoprotein CsgG. Mol Microbiol. 1997;26:11–23. doi: 10.1046/j.1365-2958.1997.5231883.x. [DOI] [PubMed] [Google Scholar]
- Lundmark K, Westermark GT, Olsen A, Westermark P. Protein fibrils in nature can enhance amyloid protein A amyloidosis in mice: Cross-seeding as a disease mechanism. Proc Natl Acad Sci U S A. 2005;102:6098–6102. doi: 10.1073/pnas.0501814102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas C, Govers-Riemslag JW, Bouma B, Schiks B, Hazenberg BP, Lokhorst HM, Hammarstrom P, ten Cate H, et al. Misfolded proteins activate factor XII in humans, leading to kallikrein formation without initiating coagulation. J Clin Invest. 2008;118:3208–3218. doi: 10.1172/JCI35424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macarisin D, Patel J, Bauchan G, Giron JA, Sharma VK. Role of curli and cellulose expression in adherence of Escherichia coli O157:H7 to spinach leaves. Foodborne Pathog Dis. 2012;9:160–167. doi: 10.1089/fpd.2011.1020. [DOI] [PubMed] [Google Scholar]
- Macindoe I, Kwan AH, Ren Q, Morris VK, Yang W, Mackay JP, Sunde M. Self-assembly of functional, amphipathic amyloid monolayers by the fungal hydrophobin EAS. Proc Natl Acad Sci U S A. 2012;109:E804–811. doi: 10.1073/pnas.1114052109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay JP, Matthews JM, Winefield RD, Mackay LG, Haverkamp RG, Templeton MD. The hydrophobin EAS is largely unstructured in solution and functions by forming amyloid-like structures. Structure. 2001;9:83–91. doi: 10.1016/s0969-2126(00)00559-1. [DOI] [PubMed] [Google Scholar]
- Makin OS, Atkins E, Sikorski P, Johansson J, Serpell LC. Molecular basis for amyloid fibril formation and stability. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:315–320. doi: 10.1073/pnas.0406847102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand A, Verdon J, Lacombe C, Crapart S, Hechard Y, Berjeaud JM. Anti-Legionella activity of staphylococcal hemolytic peptides. Peptides. 2011;32:845–851. doi: 10.1016/j.peptides.2011.01.025. [DOI] [PubMed] [Google Scholar]
- Marcon G, Plakoutsi G, Canale C, Relini A, Taddei N, Dobson CM, Ramponi G, Chiti F. Amyloid formation from HypF-N under conditions in which the protein is initially in its native state. Journal of Molecular Biology. 2005;347:323–335. doi: 10.1016/j.jmb.2005.01.034. [DOI] [PubMed] [Google Scholar]
- Mehlin C, Headley CM, Klebanoff S. An inflammatory polypeptide complex from Staphylococcus epidermidis: Isolation and characterization. Journal of Experimental Medicine. 1999;189:907–917. doi: 10.1084/jem.189.6.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercado G, Tello M, Marin M, Monasterio O, Lagos R. The production in vivo of microcin E492 with antibacterial activity depends on salmochelin and EntF. J Bacteriol. 2008;190:5464–5471. doi: 10.1128/JB.00351-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris VK, Ren Q, Macindoe I, Kwan AH, Byrne N, Sunde M. Recruitment of Class I Hydrophobins to the Air: Water Interface Initiates a Multi-step Process of Functional Amyloid Formation. Journal of Biological Chemistry. 2011:286. doi: 10.1074/jbc.M110.214197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R, Sawaya MR, Balbirnie M, Madsen AO, Riekel C, Grothe R, Eisenberg D. Structure of the cross-beta spine of amyloid-like fibrils. Nature. 2005;435:773–778. doi: 10.1038/nature03680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nenninger AA, Robinson LS, Hammer ND, Epstein EA, Badtke MP, Hultgren SJ, Chapman MR. CsgE is a curli secretion specificity factor that prevents amyloid fibre aggregation. Mol Microbiol. 2011;81:486–499. doi: 10.1111/j.1365-2958.2011.07706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nenninger AA, Robinson LS, Hultgren SJ. Localized and efficient curli nucleation requires the chaperone-like amyloid assembly protein CsgF. Proc Natl Acad Sci U S A. 2009;106:900–905. doi: 10.1073/pnas.0812143106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuizen W. Fibrin-mediated plasminogen activation. Ann N Y Acad Sci. 2001;936:237–246. doi: 10.1111/j.1749-6632.2001.tb03512.x. [DOI] [PubMed] [Google Scholar]
- Niewold TA, Hol PR, van Andel AC, Lutz ET, Gruys E. Enhancement of amyloid induction by amyloid fibril fragments in hamster. Lab Invest. 1987;56:544–549. [PubMed] [Google Scholar]
- Nolan EM, Fischbach MA, Koglin A, Walsh CT. Biosynthetic tailoring of microcin e492m: Post-translational modification affords an antibacterial siderophore-peptide conjugate. Journal of the American Chemical Society. 2007;129:14336–14347. doi: 10.1021/ja074650f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Nuallain B, Wetzel R. Conformational Abs recognizing a generic amyloid fibril epitope. Proc Natl Acad Sci U S A. 2002;99:1485–1490. doi: 10.1073/pnas.022662599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Nuallain B, Williams AD, Westermark P, Wetzel R. Seeding specificity in amyloid growth induced by heterologous fibrils. J Biol Chem. 2004;279:17490–17499. doi: 10.1074/jbc.M311300200. [DOI] [PubMed] [Google Scholar]
- Oh J, Kim JG, Jeon E, Yoo CH, Moon JS, Rhee S, Hwang I. Amyloidogenesis of type III-dependent harpins from plant pathogenic bacteria. Journal of Biological Chemistry. 2007;282:13601–13609. doi: 10.1074/jbc.M602576200. [DOI] [PubMed] [Google Scholar]
- Olsen A, Arnqvist A, Hammar M, Sukupolvi S, Normark S. The Rpos Sigma Factor Relieves H-Ns-Mediated Transcriptional Repression of Csga, the Subunit Gene of Fibronectin-Binding Curli in Escherichia-Coli. Molecular Microbiology. 1993;7:523–536. doi: 10.1111/j.1365-2958.1993.tb01143.x. [DOI] [PubMed] [Google Scholar]
- Olsen A, Jonsson A, Normark S. Fibronectin binding mediated by a novel class of surface organelles on Escherichia coli. Nature. 1989;338:652–655. doi: 10.1038/338652a0. [DOI] [PubMed] [Google Scholar]
- Olsen A, Wick MJ, Morgelin M, Bjorck L. Curli, fibrous surface proteins of Escherichia coli, interact with major histocompatibility complex class I molecules. Infect Immun. 1998;66:944–949. doi: 10.1128/iai.66.3.944-949.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto M. Staphylococcal biofilms. Curr Top Microbiol Immunol. 2008;322:207–228. doi: 10.1007/978-3-540-75418-3_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papareddy P, Morgelin M, Walse B, Schmidtchen A, Malmsten M. Antimicrobial activity of peptides derived from human ss-amyloid precursor protein. J Pept Sci. 2012;18:183–191. doi: 10.1002/psc.1439. [DOI] [PubMed] [Google Scholar]
- Pastor MT, Esteras-Chopo A, Serrano L. Hacking the code of amyloid formation: the amyloid stretch hypothesis. Prion. 2007;1:9–14. doi: 10.4161/pri.1.1.4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patzer SI, Baquero MR, Bravo D, Moreno F, Hantke K. The colicin G, H and X determinants encode microcins M and H47, which might utilize the catecholate siderophore receptors FepA, Cir, Fiu and IroN. Microbiology. 2003;149:2557–2570. doi: 10.1099/mic.0.26396-0. [DOI] [PubMed] [Google Scholar]
- Pawar DM, Rossman ML, Chen J. Role of curli fimbriae in mediating the cells of enterohaemorrhagic Escherichia coli to attach to abiotic surfaces. J Appl Microbiol. 2005;99:418–425. doi: 10.1111/j.1365-2672.2005.02499.x. [DOI] [PubMed] [Google Scholar]
- Pepys MB. Pathogenesis, diagnosis and treatment of systemic amyloidosis. Philos Trans R Soc Lond B Biol Sci. 2001;356:203–210. doi: 10.1098/rstb.2000.0766. discussion 210–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Periasamy S, Joo HS, Duong AC, Bach TH, Tan VY, Chatterjee SS, Cheung GY, Otto M. How Staphylococcus aureus biofilms develop their characteristic structure. Proc Natl Acad Sci U S A. 2012;109:1281–1286. doi: 10.1073/pnas.1115006109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson K, Morgelin M, Lindbom L, Alm P, Bjorck L, Herwald H. Severe lung lesions caused by Salmonella are prevented by inhibition of the contact system. J Exp Med. 2000;192:1415–1424. doi: 10.1084/jem.192.10.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson K, Russell W, Morgelin M, Herwald H. The conversion of fibrinogen to fibrin at the surface of curliated Escherichia coli bacteria leads to the generation of proinflammatory fibrinopeptides. J Biol Chem. 2003;278:31884–31890. doi: 10.1074/jbc.M302522200. [DOI] [PubMed] [Google Scholar]
- Pike SM, Adam AL, Pu XA, Hoyos ME, Laby R, Beer SV, Novacky A. Effects of Erwinia amylovora harpin on tobacco leaf cell membranes are related to leaf necrosis and electrolyte leakage and distinct from perturbations caused by inoculated E-amylovora. Physiological and Molecular Plant Pathology. 1998;53:39–60. [Google Scholar]
- Poltorak A, He XL, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: Mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- Provence DL, Curtiss R. Role of Crl in Avian Pathogenic Escherichia-Coli - a Knockout Mutation of Crl Does Not Affect Hemagglutination Activity, Fibronectin Binding, or Curli Production. Infection and Immunity. 1992;60:4460–4467. doi: 10.1128/iai.60.11.4460-4467.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugsley AP, Moreno F, de Lorenzo V. Microcin-E492-insensitive mutants of Escherichia coli K12. J Gen Microbiol. 1986;132:3253–3259. doi: 10.1099/00221287-132-12-3253. [DOI] [PubMed] [Google Scholar]
- Ramirez-Alvarado M, Merkel JS, Regan L. A systematic exploration of the influence of the protein stability on amyloid fibril formation in vitro. Proc Natl Acad Sci U S A. 2000;97:8979–8984. doi: 10.1073/pnas.150091797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautenberg M, Joo HS, Otto M, Peschel A. Neutrophil responses to staphylococcal pathogens and commensals via the formyl peptide receptor 2 relates to phenol-soluble modulin release and virulence. FASEB J. 2011;25:1254–1263. doi: 10.1096/fj.10-175208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero D, Aguilar C, Losick R, Kolter R. Amyloid fibers provide structural integrity to Bacillus subtilis biofilms. Proc Natl Acad Sci U S A. 2010;107:2230–2234. doi: 10.1073/pnas.0910560107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero D, Vlamakis H, Losick R, Kolter R. An accessory protein required for anchoring and assembly of amyloid fibres in B. subtilis biofilms. Mol Microbiol. 2011;80:1155–1168. doi: 10.1111/j.1365-2958.2011.07653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romling U, Bian Z, Hammar M, Sierralta WD, Normark S. Curli fibers are highly conserved between Salmonella typhimurium and Escherichia coli with respect to operon structure and regulation. Journal of Bacteriology. 1998;180:722–731. doi: 10.1128/jb.180.3.722-731.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romling U, Rohde M, Olsen A, Normark S, Reinkoster J. AgfD, the checkpoint of multicellular and aggregative behaviour in Salmonella typhimurium regulates at least two independent pathways. Mol Microbiol. 2000;36:10–23. doi: 10.1046/j.1365-2958.2000.01822.x. [DOI] [PubMed] [Google Scholar]
- Romling U, Sierralta WD, Eriksson K, Normark S. Multicellular and aggregative behaviour of Salmonella typhimurium strains is controlled by mutations in the agfD promoter. Molecular Microbiology. 1998;28:249–264. doi: 10.1046/j.1365-2958.1998.00791.x. [DOI] [PubMed] [Google Scholar]
- Saldana Z, Xicohtencatl-Cortes J, Avelino F, Phillips AD, Kaper JB, Puente JL, Giron JA. Synergistic role of curli and cellulose in cell adherence and biofilm formation of attaching and effacing Escherichia coli and identification of Fis as a negative regulator of curli. Environ Microbiol. 2009;11:992–1006. doi: 10.1111/j.1462-2920.2008.01824.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer EB, Claessen D, Haas M, Hurgobin B, Gras SL. The assembly of individual chaplin peptides from Streptomyces coelicolor into functional amyloid fibrils. Plos One. 2011;6:e18839. doi: 10.1371/journal.pone.0018839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibel T, Kowal AS, Bloom JD, Lindquist SL. Bidirectional amyloid fiber growth for a yeast prion determinant. Curr Biol. 2001;11:366–369. doi: 10.1016/s0960-9822(01)00099-9. [DOI] [PubMed] [Google Scholar]
- Schwandner R, Dziarski R, Wesche H, Rothe M, Kirschning CJ. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. Journal of Biological Chemistry. 1999;274:17406–17409. doi: 10.1074/jbc.274.25.17406. [DOI] [PubMed] [Google Scholar]
- Schwartz K, Syed AK, Stephenson RE, Rickard AH, Boles BR. Functional Amyloids Composed of Phenol Soluble Modulins Stabilize Staphylococcus aureus Biofilms. PLoS Pathog. 2012;8:e1002744. doi: 10.1371/journal.ppat.1002744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serpell LC, Sunde M, Blake CC. The molecular basis of amyloidosis. Cell Mol Life Sci. 1997;53:871–887. doi: 10.1007/s000180050107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahnawaz M, Soto C. Microcin amyloid fibrils A are reservoir of toxic oligomeric species. J Biol Chem. 2012;287:11665–11676. doi: 10.1074/jbc.M111.282533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjobring U, Pohl G, Olsen A. Plasminogen, absorbed by Escherichia coli expressing curli or by Salmonella enteritidis expressing thin aggregative fimbriae, can be activated by simultaneously captured tissue-type plasminogen activator (t-PA) Mol Microbiol. 1994;14:443–452. doi: 10.1111/j.1365-2958.1994.tb02179.x. [DOI] [PubMed] [Google Scholar]
- Smith DR, Chapman MR. Economical evolution: microbes reduce the synthetic cost of extracellular proteins. MBio. 2010:1. doi: 10.1128/mBio.00131-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KD, Andersen-Nissen E, Hayashi F, Strobe K, Bergman MA, Barrett SLR, Cookson BT, Aderem A. Toll-like receptor 5 recognizes a conserved site on flagellin required for protofilament formation and bacterial motility. Nature Immunology. 2003;4:1247–1253. doi: 10.1038/ni1011. [DOI] [PubMed] [Google Scholar]
- Sondheimer N, Lindquist S. Rnq1: an epigenetic modifier of protein function in yeast. Mol Cell. 2000;5:163–172. doi: 10.1016/s1097-2765(00)80412-8. [DOI] [PubMed] [Google Scholar]
- Sondheimer N, Lopez N, Craig EA, Lindquist S. The role of Sis1 in the maintenance of the [RNQ+] prion. EMBO J. 2001;20:2435–2442. doi: 10.1093/emboj/20.10.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sood R, Domanov Y, Pietiainen M, Kontinen VP, Kinnunen PKJ. Binding of LL-37 to model biomembranes: Insight into target vs host cell recognition. Biochimica Et Biophysica Acta-Biomembranes. 2008;1778:983–996. doi: 10.1016/j.bbamem.2007.11.016. [DOI] [PubMed] [Google Scholar]
- Soscia SJ, Kirby JE, Washicosky KJ, Tucker SM, Ingelsson M, Hyman B, Burton MA, Goldstein LE, et al. The Alzheimer’s disease-associated amyloid beta-protein is an antimicrobial peptide. Plos One. 2010;5:e9505. doi: 10.1371/journal.pone.0009505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein KC, True HL. The [RNQ(+)] prion A model of both functional and pathological amyloid. Prion. 2011;5:291–298. doi: 10.4161/pri.5.4.18213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stover AG, Driks A. Secretion, localization, and antibacterial activity of TasA, a Bacillus subtilis spore-associated protein. J Bacteriol. 1999;181:1664–1672. doi: 10.1128/jb.181.5.1664-1672.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strahsburger E, Baeza M, Monasterio O, Lagos R. Cooperative uptake of microcin E492 by receptors FepA, Fiu, and Cir and inhibition by the siderophore enterochelin and its dimeric and trimeric hydrolysis products. Antimicrob Agents Chemother. 2005;49:3083–3086. doi: 10.1128/AAC.49.7.3083-3086.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunde M, Serpell LC, Bartlam M, Fraser PE, Pepys MB, Blake CC. Common core structure of amyloid fibrils by synchrotron X-ray diffraction. J Mol Biol. 1997;273:729–739. doi: 10.1006/jmbi.1997.1348. [DOI] [PubMed] [Google Scholar]
- Tenidis K, Waldner M, Bernhagen J, Fischle W, Bergmann M, Weber M, Merkle ML, Voelter W, et al. Identification of a penta- and hexapeptide of islet amyloid polypeptide (IAPP) with amyloidogenic and cytotoxic properties. J Mol Biol. 2000;295:1055–1071. doi: 10.1006/jmbi.1999.3422. [DOI] [PubMed] [Google Scholar]
- Thomas X, Destoumieux-Garzon D, Peduzzi J, Afonso C, Blond A, Birlirakis N, Goulard C, Dubost L, et al. Siderophore peptide, a new type of post-translationally modified antibacterial peptide with potent activity. J Biol Chem. 2004;279:28233–28242. doi: 10.1074/jbc.M400228200. [DOI] [PubMed] [Google Scholar]
- Tiffany HL, Lavigne MC, Cui YH, Wang JM, Leto TL, Gao JL, Murphy PM. Amyloid-beta induces chemotaxis and oxidant stress by acting at formylpeptide receptor 2, a G protein-coupled receptor expressed in phagocytes and brain. J Biol Chem. 2001;276:23645–23652. doi: 10.1074/jbc.M101031200. [DOI] [PubMed] [Google Scholar]
- Tuite MF, Marchante R, Kushnirov V. Fungal prions: structure, function and propagation. Top Curr Chem. 2011;305:257–298. doi: 10.1007/128_2011_172. [DOI] [PubMed] [Google Scholar]
- Tukel C, Nishimori JH, Wilson RP, Winter MG, Keestra AM, van Putten JPM, Baumler AJ. Toll-like receptors 1 and 2 cooperatively mediate immune responses to curli, a common amyloid from enterobacterial biofilms. Cellular Microbiology. 2010;12:1495–1505. doi: 10.1111/j.1462-5822.2010.01485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukel C, Raffatellu M, Humphries AD, Wilson RP, Andrews-Polymenis HL, Gull T, Figueiredo JF, Wong MH, et al. CsgA is a pathogen-associated molecular pattern of Salmonella enterica serotype Typhimurium that is recognized by Toll-like receptor 2. Mol Microbiol. 2005;58:289–304. doi: 10.1111/j.1365-2958.2005.04825.x. [DOI] [PubMed] [Google Scholar]
- Tukel C, Wilson RP, Nishimori JH, Pezeshki M, Chromy BA, Baumler AJ. Responses to Amyloids of Microbial and Host Origin Are Mediated through Toll-like Receptor 2. Cell Host & Microbe. 2009;6:45–53. doi: 10.1016/j.chom.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udan ML, Ajit D, Crouse NR, Nichols MR. Toll-like receptors 2 and 4 mediate Abeta(1-42) activation of the innate immune response in a human monocytic cell line. J Neurochem. 2008;104:524–533. doi: 10.1111/j.1471-4159.2007.05001.x. [DOI] [PubMed] [Google Scholar]
- Valincius G, Heinrich F, Budvytyte R, Vanderah DJ, McGillivray DJ, Sokolov Y, Hall JE, Losche M. Soluble amyloid beta-oligomers affect dielectric membrane properties by bilayer insertion and domain formation: implications for cell toxicity. Biophys J. 2008;95:4845–4861. doi: 10.1529/biophysj.108.130997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura S, Zurdo J, Narayanan S, Parreno M, Mangues R, Reif B, Chiti F, Giannoni E, et al. Short amino acid stretches can mediate amyloid formation in globular proteins: the Src homology 3 (SH3) case. Proc Natl Acad Sci U S A. 2004;101:7258–7263. doi: 10.1073/pnas.0308249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal O, Longin R, Prigent-Combaret C, Dorel C, Hooreman M, Lejeune P. Isolation of an Escherichia coli K-12 mutant strain able to form biofilms on inert surfaces: involvement of a new ompR allele that increases curli expression. J Bacteriol. 1998;180:2442–2449. doi: 10.1128/jb.180.9.2442-2449.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volles MJ, Lansbury PT. Zeroing in on the pathogenic form of alpha-synuclein and its mechanism of neurotoxicity in Parkinson’s disease. Biochemistry. 2003;42:7871–7878. doi: 10.1021/bi030086j. [DOI] [PubMed] [Google Scholar]
- Vuong C, Saenz HL, Gotz F, Otto M. Impact of the agr quorum-sensing system on adherence to polystyrene in Staphylococcus aureus. J Infect Dis. 2000;182:1688–1693. doi: 10.1086/317606. [DOI] [PubMed] [Google Scholar]
- Wang R, Braughton KR, Kretschmer D, Bach THL, Queck SY, Li M, Kennedy AD, Dorward DW, et al. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nature Medicine. 2007;13:1510–1514. doi: 10.1038/nm1656. [DOI] [PubMed] [Google Scholar]
- Wang X, Chapman MR. Curli provide the template for understanding controlled amyloid propagation. Prion. 2008;2:57–60. doi: 10.4161/pri.2.2.6746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Chapman MR. Sequence determinants of bacterial amyloid formation. J Mol Biol. 2008;380:570–580. doi: 10.1016/j.jmb.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Hammer ND, Chapman MR. The molecular basis of functional bacterial amyloid polymerization and nucleation. J Biol Chem. 2008;283:21530–21539. doi: 10.1074/jbc.M800466200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Smith DR, Jones JW, Chapman MR. In vitro polymerization of a functional Escherichia coli amyloid protein. J Biol Chem. 2007;282:3713–3719. doi: 10.1074/jbc.M609228200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhou Y, Ren JJ, Hammer ND, Chapman MR. Gatekeeper residues in the major curlin subunit modulate bacterial amyloid fiber biogenesis. Proc Natl Acad Sci U S A. 2010;107:163–168. doi: 10.1073/pnas.0908714107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei ZM, Laby RJ, Zumoff CH, Bauer DW, He SY, Collmer A, Beer SV. Harpin, elicitor of the hypersensitive response produced by the plant pathogen Erwinia amylovora. Science. 1992;257:85–88. doi: 10.1126/science.1621099. [DOI] [PubMed] [Google Scholar]
- Westermark P, Andersson A, Westermark GT. Islet amyloid polypeptide, islet amyloid, and diabetes mellitus. Physiol Rev. 2011;91:795–826. doi: 10.1152/physrev.00042.2009. [DOI] [PubMed] [Google Scholar]
- Wetzel R. Kinetics and thermodynamics of amyloid fibril assembly. Acc Chem Res. 2006;39:671–679. doi: 10.1021/ar050069h. [DOI] [PubMed] [Google Scholar]
- Wetzler DE, Castano EM, de Prat-Gay G. A quasi-spontaneous amyloid route in a DNA binding gene regulatory domain: The papillomavirus HPV16 E2 protein. Protein Sci. 2007;16:744–754. doi: 10.1110/ps.062594007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AP, Gibson DL, Kim W, Kay WW, Surette MG. Thin aggregative fimbriae and cellulose enhance long-term survival and persistence of Salmonella. J Bacteriol. 2006;188:3219–3227. doi: 10.1128/JB.188.9.3219-3227.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner RB, Edskes HK, Shewmaker F, Nakayashiki T. Prions of fungi: inherited structures and biological roles. Nat Rev Microbiol. 2007;5:611–618. doi: 10.1038/nrmicro1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wosten H, De Vries O, Wessels J. Interfacial Self-Assembly of a Fungal Hydrophobin into a Hydrophobic Rodlet Layer. Plant Cell. 1993;5:1567–1574. doi: 10.1105/tpc.5.11.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wosten HA, Willey JM. Surface-active proteins enable microbial aerial hyphae to grow into the air. Microbiology. 2000;146(Pt 4):767–773. doi: 10.1099/00221287-146-4-767. [DOI] [PubMed] [Google Scholar]
- Wosten HAB, de Vocht ML. Hydrophobins, the fungal coat unravelled. Biochimica Et Biophysica Acta-Reviews on Biomembranes. 2000;1469:79–86. doi: 10.1016/s0304-4157(00)00002-2. [DOI] [PubMed] [Google Scholar]
- Wosten HAB, Schuren FHJ, Wessels JGH. Interfacial Self-Assembly of a Hydrophobin into an Amphipathic Protein Membrane Mediates Fungal Attachment to Hydrophobic Surfaces. Embo Journal. 1994;13:5848–5854. doi: 10.1002/j.1460-2075.1994.tb06929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Smith D, Leong BJ, Brannstrom K, Almqvist F, Chapman MR. Promiscuous cross-seeding between bacterial amyloids promotes interspecies biofilms. J Biol Chem. 2012 doi: 10.1074/jbc.M112.383737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zogaj X, Nimtz M, Rohde M, Bokranz W, Romling U. The multicellular morphotypes of Salmonella typhimurium and Escherichia coli produce cellulose as the second component of the extracellular matrix. Mol Microbiol. 2001;39:1452–1463. doi: 10.1046/j.1365-2958.2001.02337.x. [DOI] [PubMed] [Google Scholar]
- Zuccato C, Valenza M, Cattaneo E. Molecular mechanisms and potential therapeutical targets in Huntington’s disease. Physiol Rev. 2010;90:905–981. doi: 10.1152/physrev.00041.2009. [DOI] [PubMed] [Google Scholar]