Abstract
The evolution of radiotherapy has been ontogenetically linked to medical imaging. Over the years, major technological innovations have resulted in substantial improvements in radiotherapy planning, delivery, and verification. The increasing use of computed tomography imaging for target volume delineation coupled with availability of computer-controlled treatment planning and delivery systems have progressively led to conformation of radiation dose to the target tissues while sparing surrounding normal tissues. Recent advances in imaging technology coupled with improved treatment delivery allow near-simultaneous soft-tissue localization of tumor and repositioning of patient. The integration of various imaging modalities within the treatment room for guiding radiation delivery has vastly improved the management of geometric uncertainties in contemporary radiotherapy practice ushering in the paradigm of image-guided radiation therapy (IGRT). Image-guidance should be considered a necessary and natural corollary to high-precision radiotherapy that was long overdue. Image-guided radiation therapy not only provides accurate information on patient and tumor position on a quantitative scale, it also gives an opportunity to verify consistency of planned and actual treatment geometry including adaptation to daily variations resulting in improved dose delivery. The two main concerns with IGRT are resource-intensive nature of delivery and increasing dose from additional imaging. However, increasing the precision and accuracy of radiation delivery through IGRT is likely to reduce toxicity with potential for dose escalation and improved tumor control resulting in favourable therapeutic index. The radiation oncology community needs to leverage this technology to generate high-quality evidence to support widespread adoption of IGRT in contemporary radiotherapy practice.
Keywords: Conformal radiotherapy, high-precision, image-guidance, verification
Introduction
Radiation therapy has been an image-guided intervention and the evolution of radiotherapy is ontogenetically linked to medical imaging.[1] The radiation oncology community has a strong tradition of using imaging for diagnosis, management, and prognosis in cancer.[2] Imaging has been used for cancer detection, staging, target volume delineation, treatment planning, delivery verification, and response assessment. In the past, treatment planning was largely based on two-dimensional (2D) fluoroscopic imaging designed to cover target volumes without major emphasis on normal tissue shielding. Over the years, major technological innovations have resulted in substantial improvements in planning, delivery and verification. The increasing use of computed tomography (CT) imaging for target volume delineation coupled with availability of computer-controlled treatment planning and delivery systems[3] have progressively led to conformation of radiation dose to the target tissues while sparing surrounding normal structures i.e., three-dimensional conformal radiotherapy (3D-CRT). Daily treatment set-up is reproduced by in-room laser-alignment to either skin marks or fixation aids such as thermoplastic devices or vacuum bags. This is verified periodically by 2D portal imaging assuring reproducibility within certain limits. Such portal images can indicate the location of the isocenter reasonably well relative to bony landmarks. However, the tumor being treated is often a mobile soft tissue mass and patient repositioning based on bony anatomy alone is subject to error. This is particularly more relevant with refined conformal techniques such as intensity-modulated radiation therapy (IMRT) defined as ‘an advanced form of conformal radiotherapy that uses non-uniform radiation beam intensities that have been determined using various computer-based optimization techniques to achieve the desired dose-distribution’ that offer the ability to sculpt radiation dose even more closely.[4] Recent advances in imaging technology coupled with improved treatment delivery allow near-simultaneous soft-tissue localization of tumor and repositioning of the patient.[5] The integration of various imaging modalities within the treatment room for guiding radiation delivery has vastly improved the management of geometric uncertainties in contemporary radiotherapy practice ushering in the paradigm of image-guided radiation therapy (IGRT).[6,7]
Definition of IGRT
There is no standardized or consensus definition of IGRT. It has been rather malleable and author dependent, meaning different things to different people. Some define it very broadly i.e., use of imaging for detection and diagnosis; delineation of target volumes and organs-at-risk (OARs); determining biological attributes; dose distribution design; dose delivery verification and assurance; and deciphering treatment response.[1] This is more aptly termed image-based radiation therapy rather than IGRT. A more focussed and accepted definition of IGRT is ‘use of frequent imaging within the radiation treatment room, with decisions based on imaging to improve precision of radiation therapy delivery i.e., process of in-room imaging guiding radiation delivery’.[2,5] Imaging includes but may not be limited to planar imaging, cine-imaging, volumetric imaging, marker localization, marker tracking, surface-matching, and surface-tracking. The treatment delivery methods in IGRT could include 3D-CRT, IMRT, and stereotactic radiosurgery/radiotherapy (SRS/SRT).
IGRT Hypothesis and Rationale
‘Increasing the precision and accuracy of radiation delivery will reduce toxicity with potential for dose escalation and improved tumor control’ is the basic hypothesis of IGRT.[8] With the close conformity of high-dose envelope to target volumes, the accuracy of daily treatment delivery becomes even more crucial. Intensity-modulated radiation therapy is associated with a steep decline in dose outside the target, calling for stringent requirements for control of geometric uncertainties (such as set-up errors and organ motion) and a need for enhanced target delineation at planning and target localization before treatment delivery. High-precision techniques are relatively intolerant to set-up errors and mandate image-guidance for precise delivery. Therefore, it is essential that the daily patient position and anatomy at every fraction be as similar if not identical to that at treatment planning. There are several sources of uncertainty and error in radiotherapy planning and execution.[9] These include but may not be limited to uncertainty in target volume delineation, unknown extent of microscopic tumor, organ positional variation within the patient, and set-up errors. Image-guided radiation therapy aims at reducing geometrical uncertainties by evaluating the patient geometry at treatment and either altering the patient position or adapting the treatment plan with respect to anatomical changes that occur during a course of radiotherapy.
The ability to image the patient in the treatment room immediately prior to irradiation presents many possibilities to generate a more accurate picture of the tumor's extent and coordinates in 3D space.[10] Verification imaging - obtained before, during, or after treatment - records a patient's position at the time of radiotherapy. It creates a record for quality assurance and educates staff on treatment practices and geometric uncertainties. With verification imaging, interventions to reduce errors might be implemented sooner than usual, leading to an overall improvement in quality of treatment. Imaging at the time of treatment might also increase awareness of the range of organ motion, set-up errors, and changes in tumor size and shape that can take place in clinical practice. This information can provide motivation to keep patients immobile during treatment, reduce organ movement, and optimise irradiated volumes. Conformation of the dose around the tumor achieves greater healthy tissue sparing, which facilitates safe implementation of short-course or hypo-fractionated regimens, with potential for better resource utilization, cost savings, and enhanced patient and care-giver convenience.[11,12] However, the requirement for precision increases further with such hypo-fractionated schedules. Imaging at the time of treatment is needed to ensure that radiotherapy is delivered as intended. Volumetric and temporal imaging during radiotherapy provides an opportunity to adapt to changes in the tumor or healthy tissues that arise during a course of treatment, which previously were not apparent. Such interventions can lead to further clinical gains. Apart from reducing toxicity and/or improving tumor control, IGRT could potentially improve patient selection, treatment delivery, and outcome assessments.[8]
IGRT: How, when, what, and where?
Process map and workflow
Image-guided radiation therapy involves a series of inter-connected steps involving treatment planning, delivery and verifications during the course of treatment [Figure 1] requiring a multi-disciplinary team. The step unique to IGRT is the in-room imaging before treatment. The three essential steps for IGRT are i) acquire an image (to provide positional information on targets, surrogates, or avoidance structures); ii) obtain target registration error (via image registration); and iii) perform an intervention (implement a correction strategy). Typically, an IGRT system includes software with the capacity to accomplish an automated fusion of the acquired images with the expected image appearance. The software then calculates the vector displacement in 3D space of the actual target location from the expected location. In some cases, rotational errors are also calculated in addition to translational displacements. The x, y, and z axis displacements (and sometimes rotational errors) are then corrected by moving the couch on which the patient is immobilized.
Figure 1.
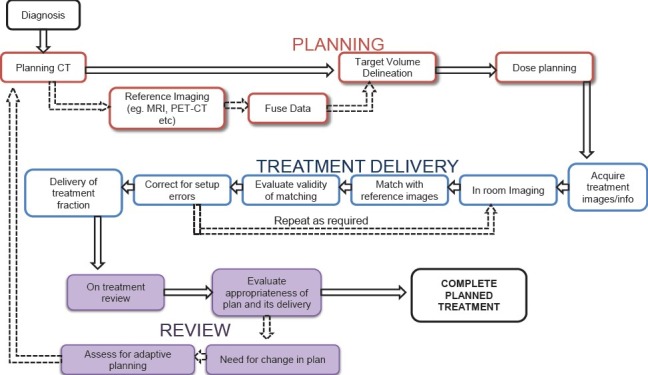
Process map and workflow of IGRT showing a series of inter-connected steps of treatment planning, delivery, and verification with a feedback loop
Clinical implementation and correction strategies
Image-guided radiation therapy is a complex and challenging modality mandating substantial increase in physician, physicist, and therapist's time and commitment for proper implementation in the clinic. The following questions need to be answered prior to implementing IGRT in clinical practice:
What is the optimal imaging modality: Ultrasound, video, planar, or volumetric?
What has to be imaged: part of target, full target, or surrogate?
Which type of X-ray imaging should be used: Kilovoltage (kV) or megavoltage (MV)?
What should be the frequency of imaging: Daily, alternate days, or weekly?
What should be the registration based on: Bone, soft tissues, or both?
How should the registration be performed: Automatic or manually?
Who should perform the registration: Therapist or oncologist?
What should be the action level: No action level, 3 mm, or 5 mm?
What if registration is unsatisfactory: re-position, re-image, still treat, or call physician?
Set-up errors, though undesirable are an inherent part of the radiation treatment process. They are defined as the difference between the actual and intended position with respect to radiation delivery. Set-up errors during radiotherapy fall under two main categories: Systematic errors and random errors. Systematic errors are those which occur during the planning process. They tend to be propagated during the course of treatment, hence assume greater importance than the random errors which occur during treatment execution. Set-up errors be managed and minimized, but not completely avoided.[13] The two broad categories of correction strategies are either offline or online correction.[9] In offline correction, images are acquired prior to treatment without applying any correction during the first few fractions. After few fractions are delivered (e.g., 2-5 fractions), the images are reviewed offline without the patient on the treatment couch. The systematic and random components of errors are calculated and correction is applied for the systematic component. This method is simple and since correction is done offline, it does not increase the ‘in-room’ time. However, one cannot correct random errors with offline approach and corrections based on images from first few days may not represent the subsequent errors during the course of radiotherapy. For online correction, images are acquired and correction applied with the patient still on the couch prior to treatment delivery. Both, the systematic and the random errors can be reduced by this method. This can be of great value in treatments with large dose per fraction and in treating targets lying close to a critical structure, irradiation of which may result in unacceptable toxicity. Online correction requires expertise, increased manpower and increases time spent per patient.
Virtually any anatomical site in the body and any tumor type can be targeted with IGRT. However, IGRT tends to be most useful in tumors where the surrounding normal tissue tolerance is lower than the dose needed for tumor control. It is also of great benefit in tumors which exhibit inter- or intra-fraction motion. A useful application of IGRT is for re-irradiation of progressive, recurrent, or residual tumors. A high dose of radiation may need to be delivered to previously irradiated tissues for achieving durable tumor control. To achieve a favorable therapeutic index in this setting, the volume of normal uninvolved tissues should be kept as low as possible, which can be with daily image-guidance. There has been a considerable increase in the use of image-guidance over the past decade. In a survey[14] of practicing radiation oncologists in the United States, >90% respondents reported using some form of image-guidance before radiation delivery, of which around 20% used it routinely in clinical practice. The most commonly used IGRT modalities were MV planar, volumetric imaging, and kV planar imaging. Image-guided radiation therapy was applied in almost all disease sites, most commonly prostate, head-neck, and central nervous system.
Commercial availability and selection of IGRT systems
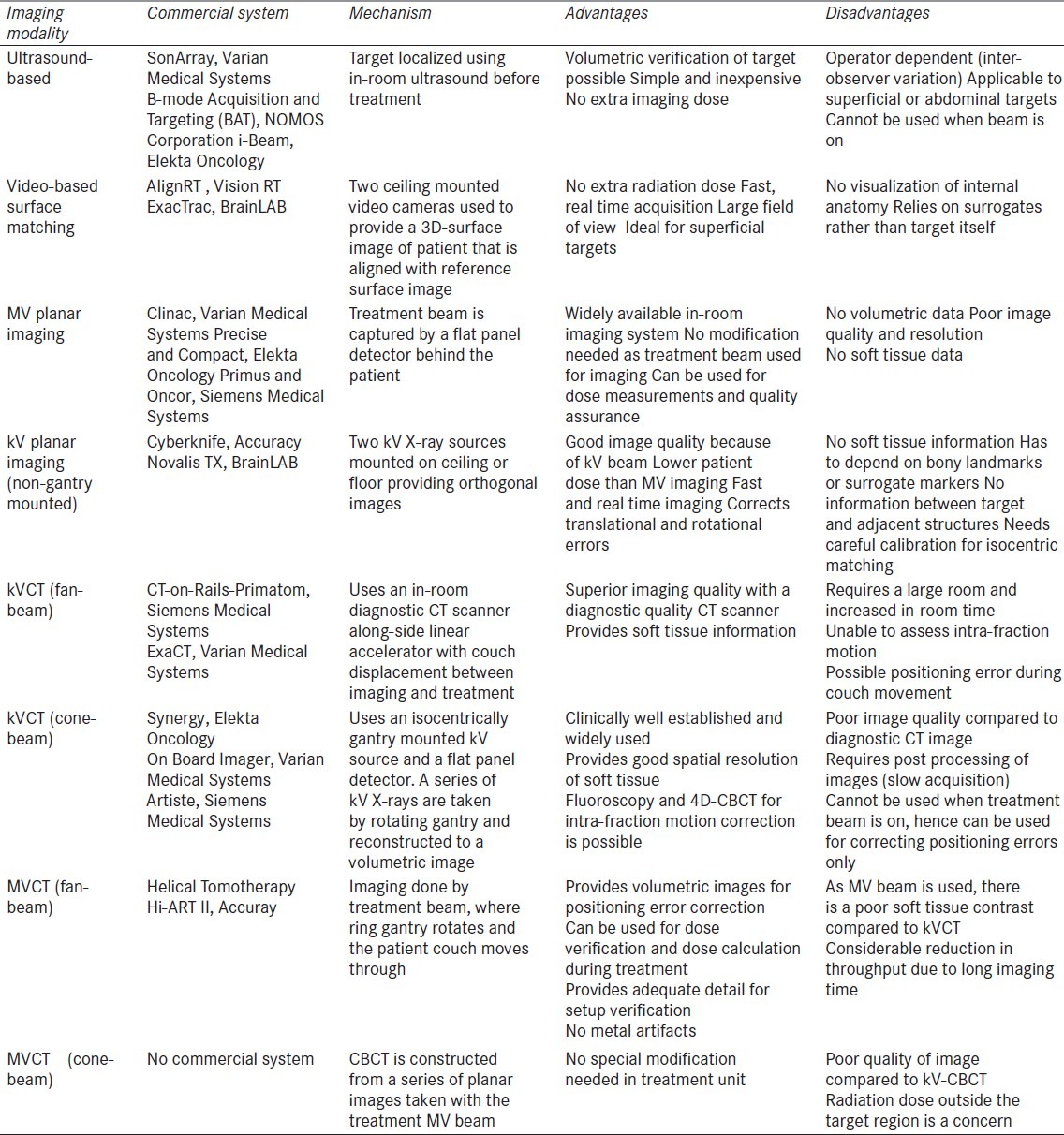
Ultrasound was the first imaging modality to be used for volumetric in-room imaging[5] for genitourinary and gynecological tumors. Later on, CT scans (cone-beam or fan-beam) were introduced for in-room volumetric verification that used either kV or MV X-rays [Figure 2] for image reconstruction.[5,7] A detailed description of commercially available IGRT systems is beyond the scope of this article. The advantages and disadvantages of several contemporary IGRT solutions are summarized in Table 1. The reader is referred to exhaustive reviews[5,7,10,15,16] of commercially available IGRT systems for more complete information. The manufacturers and vendors are pushing and promoting their own version of IGRT and claim to be better than their rival products, both in terms of price and performance. Given the plethora of IGRT solutions available commercially, selection of IGRT system needs to be appropriate for the given clinical context and setting. The drivers for selection include i) clinical objectives (dose escalation, normal tissue sparing), ii) desired level of geometric precision (hypofractionation, conventional fractionation), iii) management of uncertainties (reliability of fiducials, correction strategies, action levels), iv) resource availability (staff, finances, infrastructure), v) logistic and administrative aspects (efficiency, workflow, capacity), and vi) desire of adaptive delivery. It is also necessary to have a consistent and standardized terminology to define the fundamentals of IGRT, as confusing terminology could actually hold back implementation and further innovation.
Figure 2.
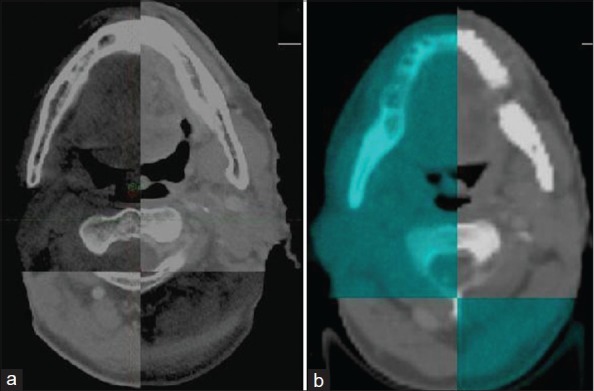
Images for volumetric verification on contemporary computed-tomography (CT) based IGRT systems in head neck cancer. Note the better image quality with kilovoltage cone-beam CT (a) compared to megavoltage CT (b)
Table 1.
Contemporary image-guided radiation therapy systems
Quality assurance (QA) in IGRT
Image-guided radiation therapy entails complex interaction of different systems that must be monitored through a comprehensive QA process or set of procedures to ensure safety, reliability, and quality.[7,10] This end-to-end QA test starts with the CT-simulation, extend through treatment planning, patient positioning, and finally the treatment step. It can generally be set up as a simple 2-step process. The first step is to position test markers in space using the IGRT system, and the second step is to irradiate and image these markers with the treatment beam, with the two steps being connected through the treatment planning system. For IGRT systems that use the treatment beam for imaging also, the alignment between the imaging and treatment system is inherent. However, for systems with a separate imaging source, it is imperative to document and demonstrate that both the treatment delivery and image-guidance systems properly communicate with each other and that their frames of reference are geometrically related to lie within a pre-specified tolerance. For all IGRT systems, it is important to avoid unnecessary image distortion and artifacts due to poorly calibrated detector arrays. Several commercial IGRT systems come with specialized and dedicated QA phantoms and guidelines for quality control, which can enhance the accuracy and precision of the system.
Evidence Profile
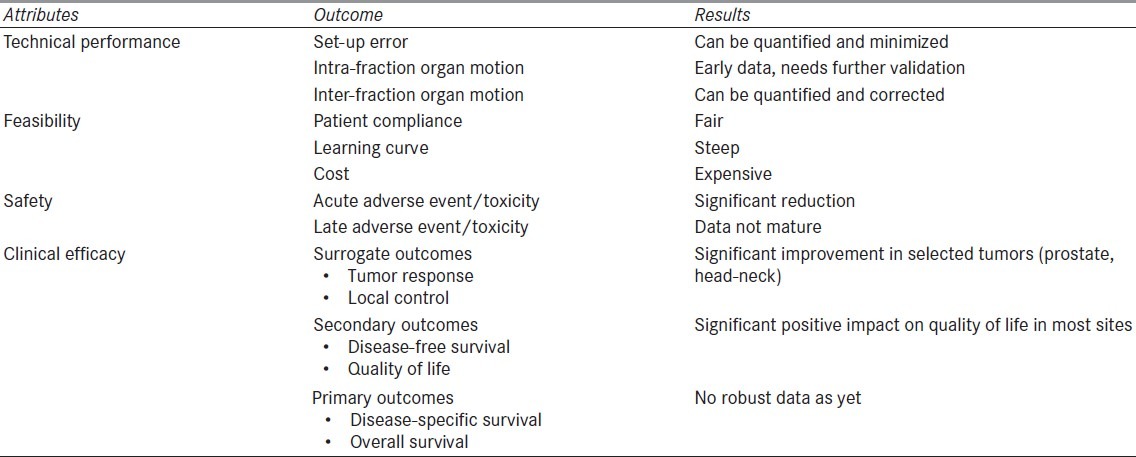
Image-guided radiation therapy has been introduced in clinical practice only recently and is not backed by solid scientific evidence. The indexed scientific literature comprises mainly of uncontrolled studies or small case series evaluating technical performance or safety. Although, IGRT has a fairly strong theoretical and technical rationale, rigorous evaluations of clinical impact proving the theoretically expected benefits are still lacking. The evidence profile of IGRT including attributes of technology, outcomes of interest and their results are depicted in Table 2.
Table 2.
Evidence profile for image-guided radiation therapy : Attributes of technology and outcomes of interest
The advent of IGRT has provided a wake-up call to the radiation oncology community to re-focus their attention on the targeting problem - something that has been neglected for too long. The initial data for image-guidance was mainly generated in prostate cancer using planar X-ray or ultrasound with markers implanted into prostate.[17,18] The first clinical outcome report of IGRT[19] highlighted the influence of systematic errors on biochemical control in radical radiotherapy for prostate cancer. The authors demonstrated significant correlation between rectal-filling-induced displacements of the prostate at the time of initial CT-planning with reduced biochemical control. Subsequently, there has been a rapid increase in the use of volumetric imaging that has now expanded to virtually all anatomic sites. Although, IGRT could be useful in most anatomic sites, its benefit would be most evident in two scenarios. Firstly, it could potentially allow safe reduction in set-up margins with resultant reduction in toxicity in sites with demonstrable, quantifiable, and correctable inter- and/or intra-fraction motion (prostate, lung, head and neck cancers). Secondly, in parallel, IGRT could allow dose escalation in sites where a radiotherapy dose-response relationship exists beyond the current practice (prostate, lung, head and neck cancers), but has been hindered due to concerns regarding safe delivery. There has been improved local control, reduced rectal toxicity and safe dose escalation in prostate cancer with the use of image-guided IMRT.[20–22] Similar encouraging outcomes have been reported for other pelvic and upper gastro-intestinal tumors.[23–25] Recently, successful reduction in planning target volume (PTV) margins (from 5mm to 3mm) has been achieved with daily image-guidance in head-neck cancers without any detriment in loco-regional control or survival.[26] A large PTV margin has been necessary in thoraco-abdominal tumors to encompass the entire target volume all through the respiratory cycle, resulting in excess normal tissue toxicity and inability to escalate dose. Respiratory-gated IGRT has improved local control with reduction in radiation-induced esophagitis, pneumonitis, and cardiac toxicity.[27–29]
While the technical performance, feasibility, and safety of IGRT are fairly well established now, there is remarkably little data on its clinical efficacy as discussed above. The best and most robust way to generate the highest quality evidence in this regard is to conduct large randomized controlled trials (RCTs) in various disease sites using appropriate endpoints. Although the conduct of such controlled trials is difficult, time-consuming, and expensive, it is pertinent to note that currently there are at least four ongoing RCTs registered at clinicaltrials.gov that are evaluating this technology in a randomized setting in prostate, cervix, head and neck, and non-small cell lung cancers, respectively. In the absence of large randomized data, the next best way to evaluate technology critically is via testing of hypotheses in prospective single-arm studies (preferably in multi-institutional, co-operative group setting) with pre-defined objectives and endpoints. It is heartening to note that apart from the four RCTs mentioned above, there are over 35 other prospective studies evaluating IGRT in various anatomic sites across the world that should generate quality evidence to guide clinical practice in the future.
Cost-benefit analyses
Image-guided radiation therapy is a technically advanced process that requires investment in special equipments, human resources and expertise. The potential benefits of IGRT are better local control rates and toxicity profiles resulting in improved disease-free survival, overall survival and quality of life. These benefits are multi-faceted and have to be viewed from several perspectives.[11] The paucity of available data on clinical effectiveness makes a comprehensive economic evaluation, considering both costs and outcomes, unfeasible at present. Cost-benefit analysis for IGRT requires far more assumptions than for most cancer interventions but an estimate of the potential scale of the benefit can be estimated easily (bearing in mind that radiotherapy on average produces around a 15% absolute survival improvement for every patient treated). For example, if the increase in the tumor control probability for a protocol that images daily online instead of weekly offline, is estimated to be 2% (with resultant 1% improvement in the 5-year overall survival), online IGRT may result in substantial cost savings in mortality alone. This ignores other benefits from dose escalation and savings in radiation morbidity. In prostate cancer, over the past decade, IGRT has enabled a 2-5 fold reduction in clinically relevant rectal toxicity despite increased total doses. The main estimated ‘cost’ of daily IGRT is an increase in linear accelerator time of 2-5 min per patient for every image-guided fraction resulting in reduced throughput.
Pitfalls and Limitations
That IGRT will improve the quality radiotherapy is taken as axiomatic. However, data demonstrating improvement in outcomes in a clinically meaningful and measurable way are relatively sparse. If treatment margins are already larger than set-up uncertainties, and if such margins result in acceptable toxicity, what is the worthwhile gain with IGRT? Given the uncertainties in target volume delineation, it would be naïve to reduce field margins based on the assumption that IGRT reduces errors. Over-zealous reduction of set-up margins may lead to geographical misses and sub-optimal tumor control. Image-quality of contemporary IGRT systems is significantly inferior to diagnostic CT. Longer scan acquisition times typical of current generation IGRT systems also leads to significant breathing artifacts. Current IGRT systems are not truly capable of real-time monitoring; hence intra-fractional errors are poorly managed. The most appropriate technique of image-registration is yet to be defined. Most commercially available IGRT systems allow rigid registration ignoring soft-tissue deformation. Rotational correction is generally not possible in most commercial systems, although newer systems with robotic couch allow six-degrees of freedom. Another important concern is extra dose delivered through daily imaging that may potentially increase the risk of second malignancy. Image-guided radiation therapy increases the complexity of radiation planning and delivery process, mandating stringent quality assurance at every level for effective and safe treatment.
Adaptive Radiotherapy: Future of IGRT?
Image-guidance involves aligning the patient to match with the anatomy at initial CT-simulation. However, the initial planning CT is perhaps the most biased representation of the patient's anatomy and a single plan designed before treatment is insufficient to describe actual dose delivered often leading to suboptimal treatment. Changes during the course of radiotherapy such as tumor regression or change of patient contours due to weight loss cannot be corrected entirely by repositioning of patient. Applying the original plan to the now altered anatomy can significantly alter dose-distribution and dose-volume statistics to the target volumes, as well as OARs (potential under-dosage of target volumes and/or over-dosage of OARs).[30,31] Adaptive radiotherapy is defined as ‘changing the original radiation treatment plan (by modifying either beam apertures or intensity patterns) during a course of fractionated radiotherapy to account for the temporal changes in anatomy (weight loss, tumor shrinkage, internal organ motion) or changes in tumor biology or function (hypoxia, proliferation)’. It aims to adapt to the change in patient contour or tumor volume by modification of the dose prescription, target volumes, and/or the treatment plan. The potential of adaptive delivery has been most aptly demonstrated in head-neck radiotherapy. Medial translation of the parotid glands from tumor regression and patient weight loss tend to bring the parotids into higher dose regions thereby increasing mean parotid dose.[32,33] Though the resultant increase in mean parotid dose may seem small and insignificant, parotid being a radiosensitive gland, even small changes in dose can have a large impact (decrease of salivary function at a rate of 5% per 1Gy increase in mean parotid dose). By adaptation, the difference between the planned and actual dose delivered to the parotid glands can be minimized. Similar promising data is emerging for urological and gynecological cancers as well.[34–36] Another novel method of adaptive planning is to make multiple plans for predictable change in the shape and size of target volumes with treatment being executed with the ‘plan of the day’ that most fits the changing anatomy [Figure 3]. Technical implementation and preliminary data of this novel approach has recently been reported.[36] The anticipated benefits of adaptive radiotherapy are highly intuitive and desirable, but there are significant barriers and challenges to its widespread adoption. First, it is unclear when and how often adaptive re-planning should be done. Attempts are underway to identify the optimal re-planning schedule, which must take into account the technical difficulties and the time required to create a new plan. Secondly, new technologies such as deformable image-registration and robust auto-segmentation in conjunction with higher computational power will be necessary to facilitate easy re-planning. The additional resource-burden imposed by such adaptive strategies with modest clinical benefits would need appropriate justification for policy-makers and administrators. Adaptive planning is customized to each individual patient and may result in non-uniform target delineation and volume modification guidelines. This will make the comparison of inter-institutional data and the deduction of real clinical impact of adaptive strategy difficult and challenging.
Figure 3.
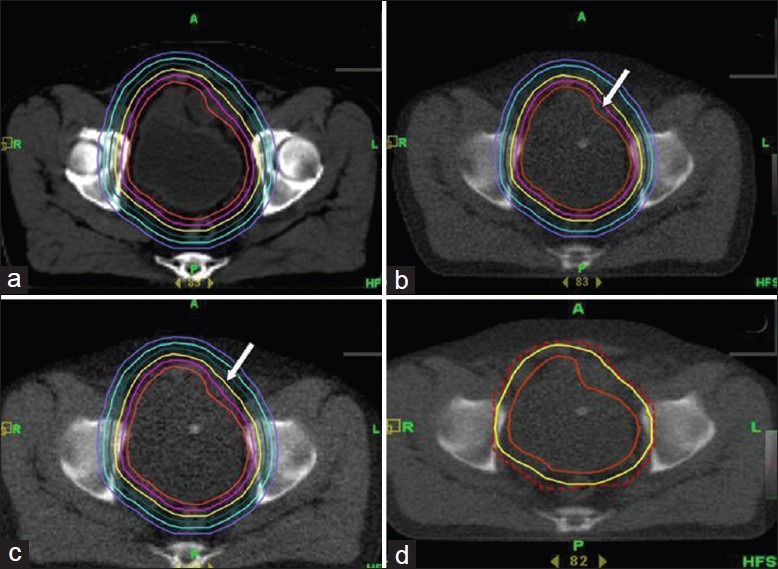
Adaptive radiotherapy for bladder cancer. Clinical target volume is expanded by 5mm incremental margins isotropically (a) to generate six planning target volumes (PTVs) with a separate radiotherapy plan for every PTV. Verification CT prior to treatment delivery showing changing PTV (arrow) due to change in bladder filling (b). The plan with appropriate margin encompassing the entire bladder safely (arrow) is selected (c) and treatment delivered. Immediate post-treatment imaging (d) confirms adequate coverage of the PTV (solid yellow line) by prescription isodose (red dashed line)
Conclusions
The integration of various imaging modalities within the treatment room for IGRT has vastly improved the management of geometric uncertainties in contemporary radiotherapy practice. Image-guidance should be considered as a necessary and natural corollary to high-precision radiotherapy that was long overdue. Image-guided radiation therapy not only provides accurate information on patient and tumor position on a quantitative scale, it also gives an opportunity to verify consistency of planned and actual treatment geometry including adaptation to daily variations and deviations resulting in improved dose delivery. Increasing the precision and accuracy of radiation delivery through IGRT is likely to reduce toxicity with potential for dose escalation and improved tumor control resulting in favourable therapeutic index. The radiation oncology community needs to leverage this technology to generate high-quality evidence to support the widespread adoption of IGRT in contemporary radiotherapy practice.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Reco C, Clifton Ling C. Broadening the scope of image-guided radiotherapy (IGRT) Acta Oncol. 2008;47:1193–200. doi: 10.1080/02841860802241956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verellen D, Ridder MD, Storme G. A (short) history of image-guided radiotherapy. Radiother Oncol. 2008;86:4–13. doi: 10.1016/j.radonc.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 3.Bernier J, Hall EJ, Giaccia A. Radiation oncology: A century of achievements. Nat Rev Cancer. 2004;4:737–47. doi: 10.1038/nrc1451. [DOI] [PubMed] [Google Scholar]
- 4.Intensity Modulated Radiation Therapy Collaborative Working Group. Intensity-modulated radiotherapy: Current status and issues of interest. Int J Radiat Oncol Biol Phys. 2001;51:880–914. doi: 10.1016/s0360-3016(01)01749-7. [DOI] [PubMed] [Google Scholar]
- 5.Dawson LA, Jaffray DA. Advances in image-guided radiation therapy. J Clin Oncol. 2007;25:938–46. doi: 10.1200/JCO.2006.09.9515. [DOI] [PubMed] [Google Scholar]
- 6.Van Herk M. Different styles of image-guided radiotherapy. Semin Radiat Oncol. 2007;17:258–67. doi: 10.1016/j.semradonc.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Korreman S, Rasch C, McNair H, Verellen D, Oelfke U, Maingon P, et al. The European Society of Therapeutic Radiology and Oncology-European Institute of Radiotherapy (ESTRO-EIR) report on 3D CT-based in-room image guidance systems: A practical and technical review and guide. Radiother Oncol. 2010;94:129–44. doi: 10.1016/j.radonc.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Michalski J, Purdy JA, Gaspar L, Souhami L, Ballow M, Bradley J, et al. Radiation Therapy Oncology Group. Research Plan 2002-2006. Image-Guided Radiation Therapy Committee. Int J Radiat Oncol Biol Phys. 2001;51:60–5. [PubMed] [Google Scholar]
- 9.Van Herk M. Errors and margins in radiotherapy. Semin Radiat Oncol. 2004;14:52–64. doi: 10.1053/j.semradonc.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Verellen D, De Ridder M, Tournel K, Duchateau M, Reynders T, Gevaert T, et al. An overview of volumetric imaging technologies and their quality assurance for IGRT. Acta Oncol. 2008;47:1271–8. doi: 10.1080/02841860802244182. [DOI] [PubMed] [Google Scholar]
- 11.Baumann M, Hölscher T, Zips D. The future of IGRT - cost benefit analysis. Acta Oncol. 2008;47:1188–92. doi: 10.1080/02841860802304556. [DOI] [PubMed] [Google Scholar]
- 12.Dawson LA, Sharpe MB. Image-guided radiotherapy: Rationale, benefits, and limitations. Lancet Oncol. 2006;7:848–58. doi: 10.1016/S1470-2045(06)70904-4. [DOI] [PubMed] [Google Scholar]
- 13.Van Herk M, Barrilot I, Bel A, Bijhold J, Bruce A, de Jaeger K, et al. Geometric uncertainties in conformal radiotherapy-and how to deal with them. Brussels: Proceedings of the 6th International Workshop on Electronic Portal Imaging; 2000. pp. 5–7. [Google Scholar]
- 14.Simpson DR, Lawson JD, Nath SK, Rose BS, Mundt AJ, Mell LK. A survey of image-guided radiation therapy use in the United States. Cancer. 2010;116:3953–60. doi: 10.1002/cncr.25129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verellen D, De Ridder M, Linthout N, Tournel K, Soete G, Storme G. Innovations in image-guided radiotherapy. Nat Rev Cancer. 2007;7:949–60. doi: 10.1038/nrc2288. [DOI] [PubMed] [Google Scholar]
- 16.Murphy MJ, Balter J, Balter S, BenComo JA, Jr, Das IJ, Jiang SB, et al. The management of imaging dose during image-guided radiotherapy: Report of the AAPM Task Group 75. Med Phys. 2007;34:4041–63. doi: 10.1118/1.2775667. [DOI] [PubMed] [Google Scholar]
- 17.Schiffner DC, Gottschalk AR, Lometti M, Aubin M, Pouliot J, Speight J, et al. Daily electronic portal imaging of implanted gold seed fiducials in patients undergoing radiotherapy after radical prostatectomy. Int J Radiat Oncol Biol Phys. 2007;67:610–9. doi: 10.1016/j.ijrobp.2006.09.042. [DOI] [PubMed] [Google Scholar]
- 18.Bohrer M, Schröder P, Welzel G, Wertz H, Lohr F, Wenz F, et al. Reduced rectal toxicity with ultrasound-based image guided radiotherapy using BAT (B-mode acquisition and targeting system) for prostate cancer. Strahlenther Onkol. 2008;184:674–8. doi: 10.1007/s00066-008-1837-z. [DOI] [PubMed] [Google Scholar]
- 19.De Crevoisier R, Tucker SL, Dong L, Mohan R, Cheung R, Cox JD, et al. Increased risk of biochemical and local failure in patients with distended rectum on the planning CT for prostate cancer radiotherapy. Int J Radiat Oncol Biol Phys. 2005;62:965–73. doi: 10.1016/j.ijrobp.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 20.Martin JM, Bayley A, Bristow R, Chung P, Gospodarowicz M, Menard C, et al. Image guided dose escalated prostate radiotherapy: Still room to improve. Radiat Oncol. 2009;4:50. doi: 10.1186/1748-717X-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pervez N, Small C, MacKenzie M, Yee D, Parliament M, Ghosh S, et al. Acute toxicity in high-risk prostate cancer patients treated with androgen suppression and hypofractionated intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;76:57–64. doi: 10.1016/j.ijrobp.2009.01.048. [DOI] [PubMed] [Google Scholar]
- 22.Gill S, Thomas J, Fox C, Kron T, Rolfo A, Leahy M, et al. Acute toxicity in prostate cancer patients treated with and without image-guided radiotherapy. Radiat Oncol. 2011;6:145. doi: 10.1186/1748-717X-6-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salama JK, Mundt AJ, Roeske J, Mehta N. Preliminary outcome and toxicity report of extended-field, intensity-modulated radiation therapy for gynecologic malignancies. Int J Radiat Oncol Biol Phys. 2006;65:1170–6. doi: 10.1016/j.ijrobp.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 24.Fuller CD, Dang ND, Wang SJ, Desai P, Choi M, Thomas CR, Jr, et al. Image-guided intensity-modulated radiotherapy (IG-IMRT) for biliary adenocarcinomas: Initial clinical results. Radiother Oncol. 2009;92:249–54. doi: 10.1016/j.radonc.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perkins CL, Fox T, Elder E, Kooby DA, Staley CA, 3rd, Landry J. Image-guided radiation therapy (IGRT) in gastrointestinal tumors. JOP. 2006;7:372–81. [PubMed] [Google Scholar]
- 26.Chen AM, Farwell DG, Luu Q, Donald PJ, Perks J, Purdy JA. Evaluation of the Planning Target Volume in the Treatment of Head and Neck Cancer with Intensity-Modulated Radiotherapy: What Is the Appropriate Expansion Margin in the Setting of Daily Image Guidance? Int J Radiat Oncol Biol Phys. 2010;81:943–9. doi: 10.1016/j.ijrobp.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 27.Baumann P, Nyman J, Hoyer M, Wennberg B, Gagliardi G, Lax I, et al. Outcome in a Prospective Phase II Trial of Medically Inoperable Stage I Non-Small-Cell Lung Cancer Patients Treated With Stereotactic Body Radiotherapy. J Clin Oncol. 2009;27:3290–6. doi: 10.1200/JCO.2008.21.5681. [DOI] [PubMed] [Google Scholar]
- 28.Fakiris AJ, McGarry RC, Yiannoutsos CT, Papiez L, Williams M, Henderson MA, et al. Stereotactic body radiation therapy for early-stage non-small-cell lung carcinoma: Four-year results of a prospective phase II study. Int J Radiat Oncol Biol Phys. 2009;75:677–82. doi: 10.1016/j.ijrobp.2008.11.042. [DOI] [PubMed] [Google Scholar]
- 29.Andolino DL, Johnson CS, Maluccio M, Kwo P, Tector AJ, Zook J, et al. Stereotactic body radiotherapy for primary hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2011;81:e447–53. doi: 10.1016/j.ijrobp.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 30.Barker JL, Jr, Garden AS, Ang KK, O’Daniel JC, Wang H, Court LE, et al. Quantification of volumetric and geometric changes occurring during fractionated radiotherapy for head-and-neck cancer using an integrated CT/linear accelerator system. Int J Radiat Oncol Biol Phys. 2004;59:960–70. doi: 10.1016/j.ijrobp.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 31.Castadot P, Lee JA, Geets X, Grégoire V. Adaptive radiotherapy of head and neck cancer. Semin Radiat Oncol. 2010;20:84–93. doi: 10.1016/j.semradonc.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 32.Bhide SA, Davies M, Burke K, McNair HA, Hansen V, Barbachano Y, et al. Weekly volume and dosimetric changes during chemoradiotherapy with intensity-modulated radiation therapy for head and neck cancer: A prospective observational study. Int J Radiat Oncol Biol Phys. 2010;76:13608. doi: 10.1016/j.ijrobp.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 33.Barker JL, Jr, Garden AS, Ang KK, O’Daniel JC, Wang H, Court LE, et al. Quantification of volumetric and geometric changes occurring during fractionated radiotherapy for head-and-neck cancer using an integrated CT/linear accelerator system. Int J Radiat Oncol Biol Phys. 2004;59:960–70. doi: 10.1016/j.ijrobp.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 34.Ahunbay EE, Peng C, Holmes S, Godley A, Lawton C, Li XA. Online adaptive replanning method for prostate radiotherapy. Int J Radiat Oncol Biol Phys. 2010;77:1561–72. doi: 10.1016/j.ijrobp.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 35.Park SS, Yan D, McGrath S, Dilworth JT, Liang J, Ye H, et al. Adaptive Image-guided Radiotherapy (IGRT) Eliminates the Risk of Biochemical Failure Caused by the Bias of Rectal Distension in Prostate Cancer Treatment Planning: Clinical Evidence. Int J Radiat Oncol Biol Phys. 2012;83:947–52. doi: 10.1016/j.ijrobp.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 36.Murthy V, Master Z, Adurkar P, Mallick I, Mahantshetty U, Bakshi G, et al. “Plan of the day” adaptive radiotherapy for bladder cancer using helical tomotherapy. Radiother Oncol. 2011;99:55–60. doi: 10.1016/j.radonc.2011.01.027. [DOI] [PubMed] [Google Scholar]


