Abstract
Dental resin composite that are tooth-colored materials have been considered as possible substitutes to mercury-containing silver amalgam filling. Despite the fact that dental resin composites have improved their physico-chemical properties, the concern for its intrinsic toxicity remains high. Some components of restorative composite resins are released in the oral environment initially during polymerization reaction and later due to degradation of the material. In vitro and in vivo studies have clearly identified that these components of restorative composite resins are toxic. But there is a large gap between the results published by research laboratories and clinical reports. The objective of this manuscript was to review the literature on release phenomenon as well as in vitro and in vivo toxicity of dental resin composite. Interpretation made from the recent data was also outlined.
Keywords: Biocompatibility, dental resin composite, release phenomenon, toxicity
INTRODUCTION
The use of dental resin composites in dentistry is ubiquitous and during the past decades composite restorations have proved to be satisfying alternate for amalgam to restore traumatized and decayed teeth.[1] Despite their growing popularity there are concerns that resin composites may be toxic based on the fact that they may release components.[2] The initial release of free monomers may occur during the monomer –polymer conversion and the long-term release of leachable substances is generated by erosion and degradation over time.[3] In addition, ion release and proliferation of bacteria located at the interface between the restorative material and dental tissues are also implicated in the tissue response.[4] Molecular mechanisms involve glutathione depletion and reactive oxygen species (ROS) production as key factors leading to pulp or gingival cell apoptosis.[5]
In the present work, information about the release phenomenon and the toxicity of dental resin composite has been reviewed and discussed.
Routes of absorption
The possible routes of systemic intake of chemical substances released from resin based composites can be through (i) oral mucosa directly, (ii) diffusion to pulp via dentinal tubules,[2] (iii) absorption of volatile components in lungs[6,7] and (iv) ingestion of released components in the gastrointestinal tract.[8]
Causes of degradation of polymer
Saliva components
The major component of saliva is water. Since resin composite is a polar material, water molecules can easily penetrate into the polymer network allowing the diffusion of unbound or uncured monomers and/or additives from the material network.[9] Several studies have shown that water sorption follows Fickian diffusion kinetics.[10,11] Therefore, one might expect a typical polymeric dental material to become saturated with its aqueous environment within one to two months after placement.[12]
Polymers may be degraded in aqueous solutions through two primary mechanisms: Passive hydrolysis and enzymatic reaction.[13] The extent of the enzymatic degradation is probably related to the extent of cure of the resin, because ester groups may be more available for attack in more loosely crosslinked networks. However, the composition of the monomers producing the network is of primary importance in determining the extent of degradation, especially when enzymes are responsible.[12]
Chewing forces
During exposure to oral environment, biodegradation of resin composite materials can also be induced by fatigue, which is caused by relatively weak repetitive loads such as ordinary chewing force. A continuous application of mechanical and environmental loads leads to progressive degradation and crack initiation and growth, resulting in catastrophic failure of the resins. This process is further assisted by pre-existing voids introduced during the material processing and residual stresses.[14]
Thermal changes
Routine eating and drinking may induce changes in intraoral temperature. These temperature changes produce an antagonistic environment for the materials as they have a different coefficient of thermal expansion compared to natural tooth. Thermal fluctuations encountered in vivo can induce surface stresses due to the high thermal gradients near the surface which in turn can lead to degradation of these materials.[15]
Chemical dietary changes
Daily intake of foods and drinks can also affect dental materials by the their direct effect or their capacity to change the intraoral pH values.[16]
Oral microbes
An interaction between oral microbes and the polymer network also may occur,[12] though little current information exists regarding this possibility. An in vitro study[17] has shown that bacteria can colonize on surfaces of composite resin and that the surface roughness of the material increased after incubation with bacteria suggesting some surface degradation. It is likely that this surface degradation is a result of acids produced by the bacteria.
In general, these factors are responsible for degradation of polymer collectively. For example, effect of water in saliva may be compounded with chewing forces or thermal changes.
Consequences of degradation of polymer
Immediate release of polymeric material
Unbound monomers and/or additives are eluted by solvents or polymer degradation within the first hours after initial polymerization. The origin of this release phenomenon from free surfaces is due to the degree of the polymerization conversion that is significantly lower than 100% in the bulk volume, but it is by far lower at the tooth/material interface or on the polymer free surface produced by addition reaction. In this case, owing to inhibition of the polymerization reaction produced by air oxygen and/or oxygen absorbed on the free surfaces, the degree of conversion decreases up to 20%. Accordingly, the larger the material surface the larger the toxicity effect.[18] It has also been shown that the free surface of composite resins exposed to oxygen during curing produces a nonpolymerized surface layer that also contains formaldehyde, which by itself is an additional factor of cell toxicity.[19]
Due to the efforts of the industry the percentage of unbound monomers has been decreased during the past 10 years but the problem is still not eradicated. Until now, there is no total conversion during polymerization. It may be expected that at the end of initial polymerization, most of the monomers will react with the polymer network and the quantity of residual monomers is less than a tenth of the remaining methacrylic groups, which therefore have been evaluated as no more than 1.5-5%. However, this is enough to contribute to major cytotoxic effects.[20]
With the exception of very few reports there is a general consensus that resin composite restorative materials are cytotoxic,[19,21,22] especially after mixing. At early intervals, resin-containing materials are more cytotoxic than at later intervals. However, long-term effects should also be taken into consideration.
Release of polymeric material overtime
The chemical characteristics of leachable substances determine the diffusion through the polymer network. Leachable components are released due to degradation or erosion over time. Chemical degradation is caused by hydrolysis or enzymatic catalysis. Unspecific esterases and human saliva derived esterase may readily catalyze the biodegradation of commercial resinous materials.[22,23] Interactions between resin monomers and commercial composite resins with human saliva-derived esterases and pseudocholinesterase occur in the oral cavity and they contribute to the degradation of composite resins. Water or other solvents enter the polymer leading to the release of biodegradation products namely oligomers and monomers. This form of erosion leads to weight loss of the polymer. Softening of the 2,2-bis [4-(2’-hydroxy-3’- methacryloxypropoxy)phenyl]-propane (Bis-GMA) matrix allows the solvents to penetrate more easily and expand the polymer network a process that facilitates the long-term diffusion of unbound monomers.[24,25]
Release of other substances (filler particles or ions)
Composite resins release ions such as fluoride, strontium, and aluminum. Some other ions are implicated in the color of the restorative material and these metal elements may interfere with the biocompatibility of the resin because they are implicated in the Fenton reaction producing reactive oxygen species (ROS) that are cytotoxic. The concentration of F- and Sr2+ ions is too low to be cytotoxic. In contrast, Cu2+, Al3+, and Fe2+ are present in toxic concentrations. The cytotoxic cascade was shown to be enhanced by metals such as aluminum and iron present in various amounts in composite resins and RM-GIC.[5,20,26]
Types of studies done for measuring release of material
In the majority of studies the release of ingredients was analyzed by high performance liquid chromatography (HPLC). Fewer studies used GC/MS (gas chromatography/mass spectrometry) or LC/MS (liquid chromatography/mass spectrometry). Typically, a flat cylindrical shaped resin based specimen was incubated in a solvent after polymerization and after a certain period the quantity of predetermined eluates in the solvent was analyzed. Most frequently the release was determined within one week (short term); only few studies researched the long term release. The solvents that were used to incubate the sample could be divided in two main groups: (i) water or aqueous mixtures, such as cell culture media, human or artificial saliva or water based buffer solutions (ii) organic solvents, usually ethanol or methanol based, including their aqueous mixtures.[8] In the majority of the studies, an incubation medium belonging to the first group was used. In most cases, the release in organic solvents was higher than in water or aqueous mixtures. In a recent study,[8] a statistically significant difference between the released amount of BPA, BisGMA and HEMA in water based and organic media was found. There were proportionally more measurements below the detection limit in water based solutions than in organic solvents for BisGMA, HEMA, triethyleneglycoldimethacrylate (TEGDMA) and 1,6-bis (2’- methacryloxyethoxycarbonylamino)-2, 4, 4-trimethylhexane (UDMA).
The nature of the matrix monomer(s) can also significantly influence the release of components. It has been reported that UDMA-based composite resin is less water-soluble than materials containing Bis-GMA.[27]
Microbial effects
Several micro-organisms are associated with caries lesions, especially mutans streptococci (S. mutans, S sobrinus) and lactobacilli. Bacteria and their by-products may also cause pulpal irritation.The presence and consequently, the effects of bacteria located at the interface between the resin and the dental tissues probably constitute important factors.[28] EGDMA and TEDGMA promote the proliferation of cariogenic micro-organisms such as Lactobacillus acidophilus and Streptococcus sobrinus. As a confirmation, TEDGMA stimulates the growth of S.mutans and S.salivarius in a pH-dependent manner.[29] This provides an explanation for the secondary caries lesions that develop beneath resin-containing restorative materials. In addition, bacterial exotoxins have noxious effects on pulp cells after diffusion throughout dentin tubules.
For years, some publications refuted any direct cytotoxic effects of the resin monomers upon the dental pulp and laid the blame on bacterial contamination.[30,31] This is still a matter of controversy and a few reports still consider that the pulp reaction to adhesive systems is generally minimal.[32,33] They incriminate the colonization by bacteria of the wide interface between the composite (or adhesive) and cavity walls. Bacteria may produce acids that can be responsible for the pulp reaction.[30,31] This was considered true in the early 1980s when important volume shrinkage followed chemo-polymerization and produced gaps wider than 10 μm.[31] This interval was decreased by the layer by layer photopolymerization technology. Improvements of resin-containing materials have reduced the shrinkage. New adhesive technologies lead to the formation of a hybrid layer and diminish the interface to less than 1 μm.[33] However, this is still a large gap for many micro-organisms such as lactobacilli that are less than 0.1 μm in diameter and therefore, the microbial parameter cannot be ignored. Some authors have emphasized the importance of hemorrhage control and its interference with bacterial contamination.[34] However, the major issues today seem to be the short-term and long-term release of unbound toxic free monomers[20–22] rather than the release of acids and toxins by bacteria.
General and local toxicity of dental resin components
In vitro studies
A widely used method for measuring cytotoxicity of composite resin is the MTT test, although succinic dehydrogenase (SDI) and alkaline phosphatase responses have also been used.[35] Depending on the cell lines used and the method of evaluation the results may vary.[36] As an example, human pulp fibroblasts (not cloned) and human gingival fibroblasts tested with the MTT test and lactate dehydrogenase activity assay (LDH) gave different results and MTT was more sensitive than LDH.[37] Despite methodological differences altogether the results underline the cytotoxic effects of the monomers released by resin-containing restorative materials. In vitro toxic reactions, produced only by component release have been shown.[38] The change in the chemical structure of the composite and the variation in the ratio of the filler and monomer produces a significant effect on the element. Release and cytotoxicity level of the material. It has been reported[39] that the flowable materials of the traditional composites were more toxic than the standard ones.
Previously, Geurtsen et al.[21] investigated cytotoxic effects of 35 single monomers and additives of composite resins in permanent 3T3 cells and three primary human oral fibroblast cultures. ED50 values varied significantly, from 0.0465 mM to > 5 mM. The tested inhibitor 2,6-di-t-butyl-4-methyl phenol (BHT), the photostabilizer 2-hydroxy-4-methoxy benzophenone (HMBP), the initiator diphenyliodonium chloride (DPICL) and the contaminant triphenyl-stibane (TPSb) exhibited severe cytotoxic effects. Within the groups of (co)monomers and (co)initiators, high or moderate cytotoxic reactions were observed whereas the evaluated decomposition/reaction products caused only moderate or slight effects. The most important photo-initiator, camphoroquinone, which was found in significant amounts in aqueous extracts from resin based materials revealed moderate cytotoxic effects. This was confirmed by Atsumi et al.[40] with permanent human submandibular-duct cells. Analysis of the data from various reports taken together indicates that for most of the severely cytotoxic resin components less toxic alternatives are available. In particular, the cytotoxicity of TEGDMA may be of great clinical interest.
TEGDMA is hydrophilic and interferes with oral tissues. The compound can penetrate membranes and reacts with intracellular molecules. Specifically, glutathione–TEGDMA adducts are formed a mechanism reducing cellular detoxifying potency.[38] A current study[41] has demonstrated that TEGDMA induces significant intracellular glutathione level (GSH) depletion and causes severe cytotoxicity in cultures of human periodontal ligament fibroblasts (HPLF). Glutathione, even in high concentrations had no protective effect against TEGDMA-induced cytotoxicity. Therefore, the investigated hypothesis that exogenous GSH may prevent or reduce TEGDMA-associated cytotoxicity in HPLF has to be rejected.
Significant toxic effects of monomers were reported on isolated human gingival fibroblast. The cytotoxicity of the monomers increased as follows: HEMA<TEGDMA <UDMA<BisGMA.[42] Monomers released from resin composites were also found to be toxic to HGFs and immortalised human keratinocytes. The cytotoxicity ranking was BisGMA>UDMA>TEGDMA. However, they cannot induce IL-1β release from these cells by themselves.[43] Using the human pulp fibroblasts, toxicity was also reported by BisGMA in vitro.[44]
On the other hand in a recent study,[45] the researchers concluded that regardless of the light-activation time the experimental composite resin presented mild to no toxic effects to the odontoblast-like MDPC-23 cells. However, intense cytotoxic effects occurred when the resin-based material was not light-cured.
A latest research[46] found that human dental pulp cells expressed mainly carboxylesterase-2 (CES2) and smaller amounts of CES1A1 and CES3 isoforms. Exposure to BisGMA stimulated CES isoforms expression of pulp cells, and this event was inhibited by catalase. Exogenous addition of porcine esterase prevented BisGMA- and DBA-induced cytotoxicity. Interestingly, inhibition of CES by bis(p-nitrophenyl) phosphate (BNPP) and CES2 by loperamide enhanced the cytotoxicity of BisGMA and DBA. Addition of porcine esterase or N-acetyl-l-cysteine prevented BisGMA-induced prostaglandin E(2) (PGE(2) and PGF(2α) production. In contrast, addition of BNPP and loperamide but not mevastatin, enhanced BisGMA-induced PGE(2) and PGF(2α) production in dental pulp cells. These results suggest that BisGMA may induce the cytotoxicity and prostanoid production of pulp cells, leading to pulpal inflammation or necrosis via reactive oxygen species production. Expression of CES, especially CES2, in dental pulp cells can be an adaptive response to protect dental pulp against BisGMA-induced cytotoxicity and prostanoid release. Resinmonomers are the main toxic components and the ester group is crucial for monomertoxicity.
An another newer study[47] investigated cytotoxic and genotoxic effects of urethane dimethacrylate (UDMA), often used as a monomer at 1mM and TEGDMA a typical co-monomer, at 5mM singly and in combination. Experiments were conducted on Chinese hamster ovary cells. Cell viability, apoptosis and cell cycle were assessed by flow cytometry, whereas DNA damage was evaluated by plasmid conformation test and comet assay. Both compounds decreased the viability of the cells, but did not induce strand breaks in an isolated plasmid DNA. However, both substances either singly or in combination damaged DNA in CHO cells as evaluated by comet assay. Both compounds induced apoptosis but a combined action of them led to a decrease in the number of apoptotic cells. The combined action of UDMA and TEGDMA in the disturbance of cell cycle was lesser compared to the action of each compound individually. Individually, though UDMA and TEGDMA may induce cytotoxic and genotoxic however, a combination of both does not produce a significant increase in these effects.
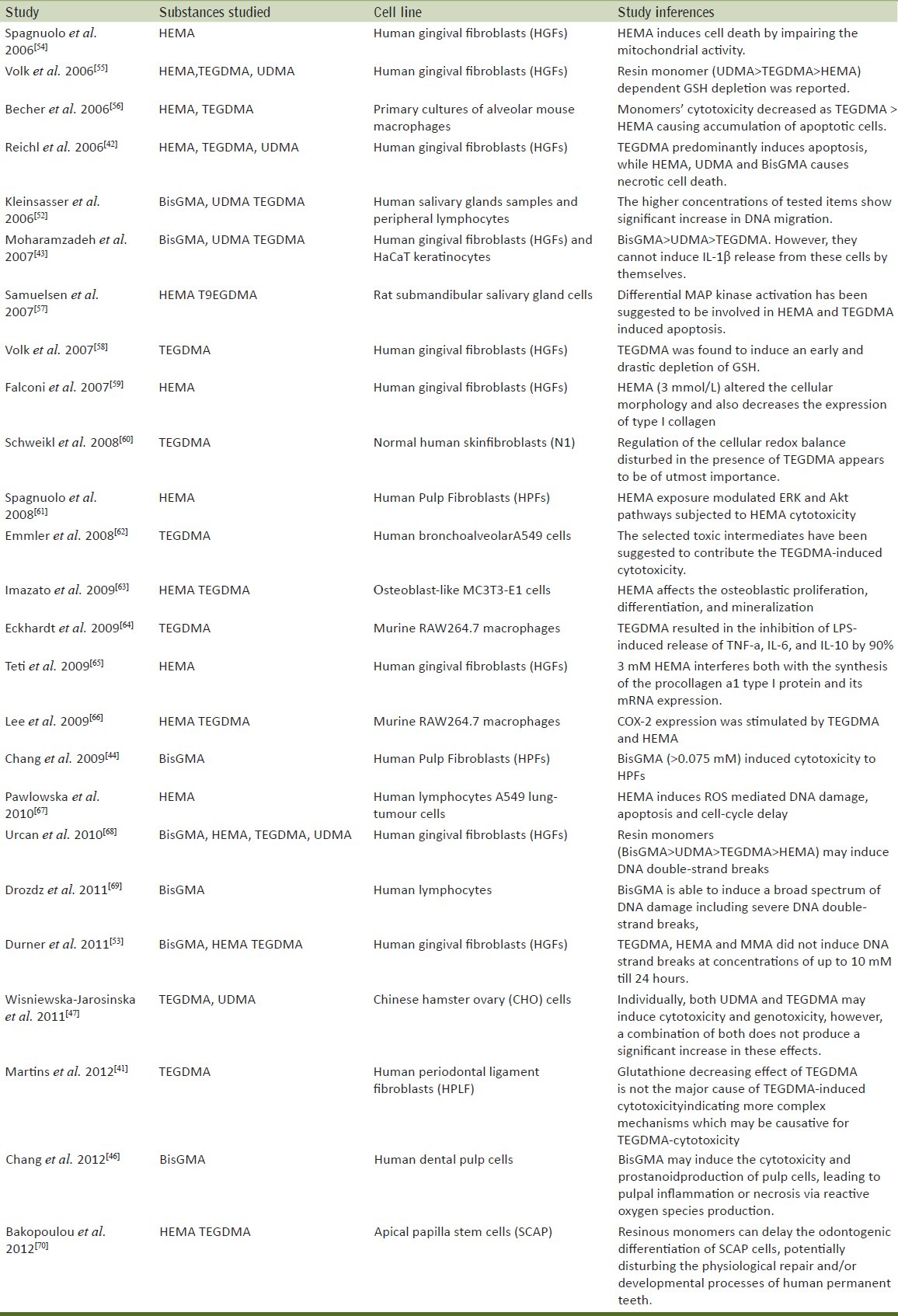
Evaluation of the mutagenicity has shown that TEGDMA causes large DNA deletions in mammalian cells (genotoxicity).[48] TEGDMA, HEMA, and GMA induce an increase of the number of mutants by a factor of 2 to 8[49] and the formation of micronuclei.[50] Using the Comet assay (alkaline single cell microgel electrophoresis assay), TEGDMA, UDMA, and Bis-GMA induce significant but minor enhancement of DNA migration a possible sign for limited genotoxic effects.[51] Newer datagive evidence of a possible risk factor for tumor initiation in human salivary glands.[52] On the contrary, more recent research tested the hypothesis that realistic concentrations (and/or worst case concentrations/situations) of BisGMA, TEGDMA, HEMA and MMA found in elution experiments can cause DNA strand breaks in human gingival fibroblasts (HGF). Such DNA damage was compared with that resulting from ionizing radiation coming from natural sources, dental radiography or tumor therapy. TEGDMA, HEMA and MMA did not induce DNA strand breaks at concentrations of up to 10 mM. About 24 hours after incubation with 0.25 mM BisGMA significantly more DNA strand breaks were found in HGF compared to controls. DNA strand breaks caused by 0.25 mM BisGMA, correspond to DNA strand breakage caused by irradiation with 4Gy, only used in the high single-dose irradiation tumor therapy. But 0.25 mM BisGMA is more than 100-fold higher than that concentration found in worst case calculations.[53] A summary of recent studies have been mentioned in Table 1.[41–44,46,47,52–70]
Table 1.
Summary of recent studies on cytotoxic effects of substances released by dental resin composite
In vivo experimental studies
Rodents are used mainly for the determination of adverse systemic effects to resin-based restorative materials. Various aspects can be investigated including acute oral toxicity (LD50 = lethal dose for 50% of the test animals after oral uptake) acute inhalation toxicity, reproductive toxicity, alterations in systemic organs, and cardiovascular effects. It should be recognized that resin-based materials release components in relatively small amounts. Therefore, acute oral toxicity is of less value for the assessment of the biocompatibility of resin-based dental materials.[22]
The clearance, distribution, and elimination of TEGDMA have been extensively studied.[71,72] About 4% of the 14C-TEGDMA injected in the jugular vein of guinea pigs was found in different tissues such as muscle, kidney, skin, blood, and liver after 24hours, whereas 61.9% was exhaled. Exhalation seems to be the major route of elimination.[71] Gastric, intradermal, and intravenous administration of 14C-TEGDMA establish that most is excreted in 1 day, and the peak equivalent TEGDMA level in mouse and guinea pig is 1,000-fold less than known toxic levels.[72] The same researchers in their further study,[73] have shown that after the administration of the radioactive monomer either in the gastric tube or after intradermal injection, the uptake was almost completed within 1 day. Low fecal (<1%) and urine (about 15%) elimination was noted, whereas between 60% and 65% was exhaled. In addition, 14C-pyruvate seems to be formed as a toxic 14C-TEGDMA-intermediate. Confirming previous findings, despite the high doses administered in this experimentation, after 24 hours, the doses found in tissues were 100,000-fold less than known toxic levels.[73]
It has been also shown that both Bis-GMA and TEGDMA have reproductive toxic effects in female mice when they were administreted at doses of 25 and 100 μg/kg intragastrically for 28 days.[74]
Many factors affect the toxicity of the components leached from the resin. In general, the monomers are more cytotoxic after 24 hours of aging and become less toxic with aging.[75] The removal of the leachable components from polymerized composites using organic solvents completely decreased their cytotoxicity.[76] In a recent study,[77] the silorane based (FiltekTMSilorane) and the methacrylate-based nanoparticle (FiltekTM Supreme XT) resins presented tissue response similar to that of the control group (empty tube) after implantation in the subcutaneous connective tissue of isogenic mice.
Interpretation from recent data
The results obtained in a recent in vitro investigation[45] (mild to no toxic effects to the odontoblast-like MDPC-23 cells after light activation in contrast to intense cytotoxic effects when the resin-based material was not light-cured) suggested that any factor that limits or undermines the polymerization of resin materials such as low light intensity, short light-curing time, longer distance between material surface and light source, may contribute to increase significantly their cytotoxic effects to the pulp cells. Therefore, it is mandatory that the manufacturers’ instructions of use are strictly followed regarding the adequate photoactivation of resin materials, which is inversely related to cytotoxicity.
The most notorious compound that may leach from resin based materials is no doubt BPA, which is known to act as an estrogen receptor agonist and thus may cause so-called ‘endocrine disruption’.[8] Composites may contain BPA as an impurity from the synthesis process of BisGMA, but there are also indications that BPA may be released from composites following degradation of BisGMA. A recent meta-analysis[8] on release of BPA from resin composite computed that one full crown restoration of a molar (with surface area 573 mm2) may release in worst case [release per surface-organic solution-maximum measured value (231 nmol/mm2)] 132.36 μmol after 24 hours (however, measured for Scotchbond Multipurpose a dental adhesive that is never used in large quantities) or on average 57.38 nmol [release per surface-organic solution-geometric mean (0.1 nmol/mm2)]. Typical BPA migration levels from BPA-based food-contact materials are assumed to be less than 10 μg/Kg food (=43.8 nmol),[78] indicating that the 24 hours release of BPA from dental materials may be relevant in patients with multiple large restorations and that resin-based dental materials may represent an relevant source of BPA next to contaminated food, especially in case of large and/or multiple restorations. However, the Tolerable Daily Intake proposed by EFSA (European Food Safety Agent) was determined to be 0.05 mg BPA/kg bodyweight/day, which is equal to a limit of 220 nmol/kg bodyweight/day. This indicated that the level of BPA released from resin-based dental materials may be safe,[78,79] but more research is warranted especially regarding the safety of composite restorations in children. However, at present no regulatory or professional organization has expressed concern about health effects of bisphenols in dental materials.[80]
A concentration-dependence was observed in a newer study[70] for HEMA and TEGDMA during long-term exposure, the effects of HEMA were evident at much lower concentrations compared to TEGDMA. The same study concluded that persistent exposure to low concentrations of eluted resin substances may have chronic negative effects on pulp cells. Even if not causing acute cytotoxicity (such as pulp inflammation and/or necrosis) the continuous release of such substances may significantly compromise dental pulp homeostasis and repair, therefore jeopardizing the pulps’ vitality and prognosis of the restored teeth.
In a further recent research,[81] a series of homologous dimethacrylates 1,4-bis [(2’-hydroxy-3’- methacryloxypropoxy)-phthaloxy] -butane (PhA-14), 1, 5-bis [-(2’- hydroxy-3’- methacryloxypropoxy)- phthaloxy]- pentane (PhA-15) and 1,6-bis [-(2’- hydroxy-3’- methacryloxypropoxy) -phthaloxy]- hexane (PhA-16) was obtained and evaluated as possible dental resins. The values of degree of conversion are within 77% for the Bis-GMA/TEGDMA copolymer and 89% for the acetylated dimethacrylate 1,5-bis [-(2’- acetoxy-3’- methacryloxypropoxy) -phthaloxy]-pentane (Acet-PhA-15)/TEGDMA, respectively. Among the TEGDMA based copolymers all new compositions exhibit higher degree of conversion than the 50 / 50 wt.%Bis-GMA copolymer whereas their curing shrinkages are fairly comparable. Therefore, it was expected that unreacted monomer units should not be present and should not constitute a great concern for subsequent leaching and toxicological effects in the body.
On basis of the ongoing researches, manufacturers are introducing the advanced materials which are focused on decreasing the toxicity along with maintaining/improving the physical characteristics of the dental composite resin. The recent finding[77] of similar tissue response among the tested resins [siloranebased (FiltekTMSilorane) and the methacrylate-based nanoparticle (FiltekTM Supreme XT)] and the control group (empty tube) after implantation in the subcutaneous connective tissue of isogenic mice is encouraging.
It should also be noted that expiration dates have a significant effect on the cytotoxicity of the composite materials as the researchers found that expired composites presented lower cell viability than the non-expired composites over the three-day time period; the difference in survival rates between the expired and non-expired groups was statistically significant.[82]
The latest concept derived from a current study states that the shade of the composite has an influence on its cytotoxicity and this cytotoxicity is also influenced by the light curing unit used. The researchers observed that composites of the darker shade (C2) had a higher cytotoxicity, which varied with the Light Curing Unit employed. The study further suggested that fast curing with a high-power unit may be beneficial for composites with regard to minimizing release of toxic substances. Especially the influence of the composite shade on cytotoxicity can be minimized by polymerization with the SML high-intensity light curing unit.[83]
The dentist should always check whether the curing light and irradiation distance used are adequate to polymerize the particular brand of resin used or not. Nevertheless, from a clinical point of view, LCUs and resin composites should be harmonized to one another for achieving maximal biocompatibility. The type of resin composite, light curing unit, curing tip distance and evaluation period factor had statistically significant cytotoxic effects on L–929 mouse fibroblast cells.[84]
CONCLUSION
Increasing concern arises regarding the safe clinical application of these materials due to their biodegradation under the oral environment. The number and diversity of processes by which composite resins may be degraded in the oral cavity are huge and are now recognized as a complex interplay of interactions. Causes for biodegradation comprise several factors such as saliva characteristics, chewing or thermal and chemical dietary changes.
Taken together all the searched data regarding toxicity of resin composites, one can conclude that the earlier literature showed the components released to be toxic, carcinogenic and mutagenic but the recent data reveals the improvement due to introduction of newer material as a result of subsequent researches. On clinical standpoint this review strongly suggests to follow the technical considerations and the manufacturer's instructions regarding the polymerization of resin materials such as light intensity, light-curing time, distance between material surface and light source, compatibility between light and brand of composite and shelf life of material. Cavity lining should be done in areas with deep dentin. Also, there should be no direct skin contact to the material.
The clinical consequences of biodegradation are still poorly understood. Assessing what may be the extent of the biological effects as a result of the long-term release of biodegradation products still requires extensive study. The gap that exists between the results published by research laboratories and clinical reports should be shortened. Further well-controlled clinical studies are necessary to improve the knowledge about materials biocompatibility in intraoral conditions including their potential to cause chronic local adverse effects or/and systemic side effects over time.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Sunnegårdh-Grönberg K, van Dijken JW, Funegård U, Lindberg A, Nilsson M. Selection of dental materials and longevity of replaced restorations in Public Dental Health clinics in northern Sweden. J Dent. 2009;37:673–8. doi: 10.1016/j.jdent.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 2.Reichl FX, Seiss M, Kleinsasser N, Kehe K, Kunzelmann KH, Thomas P, et al. Distribution and excretion of BisGMA in guinea pigs. J Dent Res. 2008;87:378–80. doi: 10.1177/154405910808700401. [DOI] [PubMed] [Google Scholar]
- 3.Goldberg M. In vitro and in vivo studies on the toxicity of dental resin components: A review. Clin Oral Investig. 2008;12:1–8. doi: 10.1007/s00784-007-0162-8. [DOI] [PubMed] [Google Scholar]
- 4.Lu Y, Liu T, Li H, Pi G. Histological evaluation of direct pulp capping with a self-etching adhesive and calcium hydroxide on human pulp tissue. Int Endod J. 2008;41:643–50. doi: 10.1111/j.1365-2591.2008.01396.x. [DOI] [PubMed] [Google Scholar]
- 5.Stanislawski L, Lefeuvre M, Bourd K, Soheili-Majd E, Goldberg M, Périanin A. TEGDMA-induced toxicity in human fibroblasts is associated with early and drastic glutathione depletion with subsequent production of oxygen reactive species. J Biomed Mater Res A. 2003;66:476–82. doi: 10.1002/jbm.a.10600. [DOI] [PubMed] [Google Scholar]
- 6.Rogalewicz R, Voelkel A, Kownacki I. Application of HS-SPME in the determination of potentially toxic organic compounds emitted from resin-based dental materials. J Environ Monit. 2006;8:377–83. doi: 10.1039/b517363a. [DOI] [PubMed] [Google Scholar]
- 7.Marquardt W, Seiss M, Hickel R, Reichl FX. Volatile methacrylates in dental practices. J Adhes Dent. 2009;11:101–7. [PubMed] [Google Scholar]
- 8.Van Landuyt KL, Nawrot T, Geebelen B, De Munck J, Snauwaert J, Yoshihara K, et al. How much do resin-based dental materials release? A meta-analytical approach. Dent Mater. 2011;27:723–47. doi: 10.1016/j.dental.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 9.León BL, Del Bel Cury AA, Rodrigues Garcia RC. Loss of residual monomer from resilient lining materials processed by different methods. Rev Odonto Ciênc. 2008;23:215–9. [Google Scholar]
- 10.Braden M, Causton BE, Clarke RL. Diffusion in water in composite filling materials. J Dent Res. 1976;55:730–2. doi: 10.1177/00220345760550050501. [DOI] [PubMed] [Google Scholar]
- 11.Sideridou I, Achilias DS, Spyroudi C, Karabela M. Water sorption characteristics of light-cured dental resins and composites based on Bis-EMA/PCDMA. Biomaterials. 2004;25:367–76. doi: 10.1016/s0142-9612(03)00529-5. [DOI] [PubMed] [Google Scholar]
- 12.Ferracane JL. Hygroscopic and hydrolytic effects in dental polymer networks. Dent Mater. 2006;22:211–22. doi: 10.1016/j.dental.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Lenz R. Biodegradable polymers. In: Peppas NA, Langer RS, editors. Advances in polymer science (No 107: biopolymers) Berlin: Springer; 1993. pp. 1–40. [Google Scholar]
- 14.Drummond JL. Degradation, fatigue and failure of resin dental composite materials. J Dent Res. 2008;87:710–9. doi: 10.1177/154405910808700802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bettencourt AF, Neves CB, de Almeida MS, Pinheiro LM, Oliveira SA, Lopes LP, et al. Biodegradation of acrylic based resins: A review. Dent Mater. 2010;26:e171–80. doi: 10.1016/j.dental.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 16.Jepson NJ, McGill JT, McCabe JF. Influence of dietary simulating solvents on the viscoelasticity of temporary soft lining materials. J Prosthet Dent. 2000;83:25–31. doi: 10.1016/s0022-3913(00)70085-0. [DOI] [PubMed] [Google Scholar]
- 17.Willershausen B, Callaway A, Ernst CP, Stender E. The influence of oral bacteria on the surfaces of resin-based dental restorative materials: An in vitro study. Int Dent J. 1999;49:231–9. doi: 10.1111/j.1875-595x.1999.tb00527.x. [DOI] [PubMed] [Google Scholar]
- 18.Fano V, Shatel M, Tanzi ML. Release phenomena and toxicity in polymer-based dental restorative materials. Acta Biomed. 2007;78:190–7. [PubMed] [Google Scholar]
- 19.Schmalz G. The biocompatibility of non-amalgam dental filling materials. Eur J Oral Sci. 1998;106:696–706. doi: 10.1046/j.0909-8836.1998.eos10602ii05.x. [DOI] [PubMed] [Google Scholar]
- 20.Stanislawski L, Daniau X, Lauti A, Goldberg M. Factors responsible for pulp cell cytotoxicity induced by resin-modified glass ionomer cements. J Biomed Mater Res. 1999;48:277–88. doi: 10.1002/(sici)1097-4636(1999)48:3<277::aid-jbm11>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 21.Geurtsen W. Substances released from dental resin composites and glass ionomer cements. Eur J Oral Sci. 1998;106:687–95. doi: 10.1046/j.0909-8836.1998.eos10602ii04.x. [DOI] [PubMed] [Google Scholar]
- 22.Geurtsen W. Biocompatibility of resin-modified filling materials. Crit Rev Oral Biol Med. 2000;11:333–55. doi: 10.1177/10454411000110030401. [DOI] [PubMed] [Google Scholar]
- 23.Jaffer F, Finer Y, Santerre JP. Interactions between resin monomers and commercial composite resins with human saliva derived esterases. Biomaterials. 2002;23:1707–19. doi: 10.1016/s0142-9612(01)00298-8. [DOI] [PubMed] [Google Scholar]
- 24.Finer Y, Jaffer F, Santerre JP. Mutual influence of cholesterol esterase and pseudocholinesterase on the biodegradation of dental composites. Biomaterials. 2004;25:1787–93. doi: 10.1016/j.biomaterials.2003.08.029. [DOI] [PubMed] [Google Scholar]
- 25.Finer Y, Santerre JP. The influence of resin chemistry on a dental composite's biodegradation. J Biomed Mater Res A. 2004;69:233–46. doi: 10.1002/jbm.a.30000. [DOI] [PubMed] [Google Scholar]
- 26.Stanislawski L, Soheili-Majd E, Perianin A, Goldberg M. Dental restorative biomaterials induce glutathione depletion in cultured human gingival fibroblast: Protective effect of N-acetyl cysteine. J Biomed Mater Res. 2000;51:469–74. doi: 10.1002/1097-4636(20000905)51:3<469::aid-jbm22>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 27.Pearson GJ, Longman CM. Water sorption and solubility of resin-based materials following inadequate polymerization by a visible-light curing system. J Oral Rehabil. 1989;16:57–61. doi: 10.1111/j.1365-2842.1989.tb01317.x. [DOI] [PubMed] [Google Scholar]
- 28.Hansel C, Leyhausen G, Mai UE, Geurtsen W. Effects of various resin composite (co)monomers and extracts on two caries-associated micro-organisms in vitro. J Dent Res. 1998;77:60–7. doi: 10.1177/00220345980770010601. [DOI] [PubMed] [Google Scholar]
- 29.Khalichi P, Cvitkovitch DG, Santerre JP. Effect of composite resin biodegradation products on oral streptococcal growth. Biomaterials. 2004;25:5467–72. doi: 10.1016/j.biomaterials.2003.12.056. [DOI] [PubMed] [Google Scholar]
- 30.Bergenholtz G. Evidence for bacterial causation of adverse pulpal responses in resin-based dental restorations. Crit Rev Oral Biol Med. 2000;11:467–80. doi: 10.1177/10454411000110040501. [DOI] [PubMed] [Google Scholar]
- 31.Bergenholtz G, Cox CF, Loesche WJ, Syed SA. Bacterial leakage around dental restorations: Its effect on the dental pulp. J Oral Pathol. 1982;11:439–50. doi: 10.1111/j.1600-0714.1982.tb00188.x. [DOI] [PubMed] [Google Scholar]
- 32.Murray PE, Windsor LJ, Hafez AA, Stevenson RG, Cox CF. Comparison of pulp responses to resin composites. Oper Dent. 2003;28:242–50. [PubMed] [Google Scholar]
- 33.Murray PE, Hafez AA, Smith AJ, Cox CF. Identification of hierarchical factors to guide clinical decision making for successful long-term pulp capping. Quintessence Int. 2003;34:61–70. [PubMed] [Google Scholar]
- 34.Hashimoto M, Ito S, Tay FR, Svizero NR, Sano H, Kaga M, et al. Fluid movement across the resin-dentin interface during and after bonding. J Dent Res. 2004;83:843–8. doi: 10.1177/154405910408301104. [DOI] [PubMed] [Google Scholar]
- 35.Chen CC, Chen RC, Huang ST. Enzymatic responses of human deciduous pulpal fibroblasts to dental restorative materials. J Biomed Mater Res. 2002;60:452–7. doi: 10.1002/jbm.1291. [DOI] [PubMed] [Google Scholar]
- 36.Thonemann B, Schmalz G, Hiller KA, Schweikl H. Responses of L929 mouse fibroblasts, primary and immortalized bovine dental papilla-derived cell lines to dental resin components. Dent Mater. 2002;18:318–23. doi: 10.1016/s0109-5641(01)00056-2. [DOI] [PubMed] [Google Scholar]
- 37.Issa Y, Watts DC, Brunton PA, Waters CM, Duxbury AJ. Resin composite monomers alter MTT and LDH activity of human gingival fibroblasts in vitro. Dent Mater. 2004;20:12–20. doi: 10.1016/s0109-5641(03)00053-8. [DOI] [PubMed] [Google Scholar]
- 38.Geurtsen W, Leyhausen G. Chemical-Biological interactions of the resin monomer triethyleneglycol-dimethacrylate (TEGDMA) J Dent Res. 2001;80:2046–50. doi: 10.1177/00220345010800120401. [DOI] [PubMed] [Google Scholar]
- 39.Al-Hiyasat AS, Darmani H, Milhem MM. Cytotoxicity evaluation of dental resin composites and their flowable derivatives. Clin Oral Inves. 2005;9:21–5. doi: 10.1007/s00784-004-0293-0. [DOI] [PubMed] [Google Scholar]
- 40.Atsumi T, Murata J, Kamiyanagi I, Fujisawa S, Ueha T. Cytotoxicity of photosensitizerscamphorquinone and 9-fluorenone with visible light irradiation on a human submandibular-duct cell line in vitro. Arch Oral Biol. 1998;43:73–81. doi: 10.1016/s0003-9969(97)00073-3. [DOI] [PubMed] [Google Scholar]
- 41.Martins CA, Leyhausen G, Geurtsen W, Volk J. Intracellular glutathione: A main factor in TEGDMA-induced cytotoxicity? Dent Mater. 2012;28:442–8. doi: 10.1016/j.dental.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 42.Reichl FX, Esters M, Simon S, Seiss M, Kehe K, Kleinsasser N, et al. Cell death effects of resin-based dental material compounds and mercurials in human gingival fibroblasts. Arch Toxicol. 2006;80:370–7. doi: 10.1007/s00204-005-0044-2. [DOI] [PubMed] [Google Scholar]
- 43.Moharamzadeh K, Van Noort R, Brook IM, Scutt AM. Cytotoxicity of resin monomers on human gingival fibroblasts and Ha Ca T keratinocytes. Dent Mater. 2007;23:40–4. doi: 10.1016/j.dental.2005.11.039. [DOI] [PubMed] [Google Scholar]
- 44.Chang MC, Lin LD, Chan CP, Chang HH, Chen LI, Lin HJ, et al. The effect of BisGMA on cyclooxygenase-2 expression, PGE2 production and cytotoxicity via reactive oxygen species- and MEK/ERK-dependent and -independent pathways. Biomaterials. 2009;30:4070–7. doi: 10.1016/j.biomaterials.2009.04.034. [DOI] [PubMed] [Google Scholar]
- 45.Aranha AM, Giro EM, Hebling J, Lessa FC, Costa CA. Effects of light-curing time on the cytotoxicity of a restorative composite resin on odontoblast-like cells. J Appl Oral Sci. 2010;18:461–6. doi: 10.1590/S1678-77572010000500006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chang MC, Lin LD, Chuang FH, Chan CP, Wang TM, Lee JJ, et al. Carboxylesterase expression in human dental pulp cells: Role in regulation of BisGMA-induced prostanoid production and cytotoxicity. Acta Biomater. 2012;8:1380–7. doi: 10.1016/j.actbio.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 47.Wisniewska-Jarosinska M, Poplawski T, Chojnacki CJ, Pawlowska E, Krupa R, Szczepanska J, et al. Independent and combined cytotoxicity and genotoxicity of triethylene glycol dimethacrylate and urethane dimethacrylate. Mol Biol Rep. 2011;38:4603–11. doi: 10.1007/s11033-010-0593-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schweikl H, Spagnuolo G, Schmalz G. Genetic and cellular toxicology of dental resin monomers. J Dent Res. 2006;85:870–7. doi: 10.1177/154405910608501001. [DOI] [PubMed] [Google Scholar]
- 49.Schweikl H, Schmalz G, Rackebrandt K. The mutagenic activity of unpolymerized resin monomers in Salmonella typhimurium and V79 cells. Mutat Res. 1998;415:119–30. doi: 10.1016/s1383-5718(98)00067-9. [DOI] [PubMed] [Google Scholar]
- 50.Schweikl H, Schmalz G, Spruss T. The induction of micronuclei in vitro by unpolymerized resin monomers. J Dent Res. 2001;80:1615–20. doi: 10.1177/00220345010800070401. [DOI] [PubMed] [Google Scholar]
- 51.Kleinsasser NH, Wallner BC, Harréus UA, Kleinjung T, Folwaczny M, Hickel R, et al. Genotoxicity and cytotoxicity of dental materials in human lymphocytes as assessed by the single cell microgel electrophoresis (comet) assay. J Dent. 2004;32:229–34. doi: 10.1016/j.jdent.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 52.Kleinsasser NH, Schmid K, Sassen AW, Harréus UA, Staudenmaier R, Folwaczny M, et al. Cytotoxic and genotoxic effects of resin monomers in human salivary gland tissue and lymphocytes as assessed by the single cell microgel electrophoresis (Comet) assay. Biomaterials. 2006;27:1762–70. doi: 10.1016/j.biomaterials.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 53.Durner J, Dębiak M, Bürkle A, Hickel R, Reichl FX. Induction of DNA strand breaks by dental composite components compared to X-ray exposure in human gingival fibroblasts. Arch Toxicol. 2011;85:143–8. doi: 10.1007/s00204-010-0558-0. [DOI] [PubMed] [Google Scholar]
- 54.Spagnuolo G, D’Anto V, Cosentino C, Schmalz G, Schweikl H, Rengo S. Effect of Nacetyl- L-cysteine on ROS production and cell death caused by HEMA in human primary gingival fibroblasts. Biomaterials. 2006;27:1803–9. doi: 10.1016/j.biomaterials.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 55.Volk J, Engelmann J, Leyhausen G, Geurtsen W. Effects of three resin monomers on the cellular glutathione concentration of cultured human gingival fibroblasts. Dent Mater. 2006;22:499–505. doi: 10.1016/j.dental.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 56.Becher R, Kopperud HM, Al RH, Samuelsen JT, Morisbak E, Dahlman HJ, et al. Pattern of cell death after in vitro exposure to GDMA, TEGDMA, HEMA and two compomer extracts. Dent Mater. 2006;22:630–40. doi: 10.1016/j.dental.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 57.Samuelsen JT, Dahl JE, Karlsson S, Morisbak E, Becher R. Apoptosis induced by the monomers HEMA and TEGDMA involves formation of ROS and differential activation of the MAP-kinases p38, JNK and ERK. Dent Mater. 2007;23:34–9. doi: 10.1016/j.dental.2005.11.037. [DOI] [PubMed] [Google Scholar]
- 58.Volk J, Leyhausen G, Dogan S, Geurtsen W. Additive effects of TEGDMA and hydrogenperoxide on the cellular glutathione content of human gingival fibroblasts. Dent Mater. 2007;23:921–6. doi: 10.1016/j.dental.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 59.Falconi M, Teti G, Zago M, Pelotti S, Breschi L, Mazzotti G. Effects of HEMA on type I collagen protein in human gingival fibroblasts. Cell Biol Toxicol. 2007;23:313–22. doi: 10.1007/s10565-006-0148-3. [DOI] [PubMed] [Google Scholar]
- 60.Schweikl H, Hiller KA, Eckhardt A, Bolay C, Spagnuolo G, Stempfl T, et al. Differential gene expression involved in oxidative stress response caused by triethylene glycol dimethacrylate. Biomaterials. 2008;29:1377–87. doi: 10.1016/j.biomaterials.2007.11.049. [DOI] [PubMed] [Google Scholar]
- 61.Spagnuolo G, D’Antò V, Valletta R, Strisciuglio C, Schmalz G, Schweikl H, et al. Effect of 2-hydroxyethyl methacrylate on human pulp cell survival pathways ERK and AKT. J Endod. 2008;34:684–8. doi: 10.1016/j.joen.2008.02.040. [DOI] [PubMed] [Google Scholar]
- 62.Emmler J, Seiss M, Kreppel H, Reichl FX, Hickel R, Kehe K. Cytotoxicity of the dental composite component TEGDMA and selected metabolic by-products in human pulmonary cells. Dent Mater. 2008;24:1670–5. doi: 10.1016/j.dental.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 63.Imazato S, Horikawa D, Nishida M, Ebisu S. Effects of monomers eluted from dental resin restoratives on osteoblast-like cells. J Biomed Mater Res B Appl Biomater. 2009;88:378–86. doi: 10.1002/jbm.b.31067. [DOI] [PubMed] [Google Scholar]
- 64.Eckhardt A, Gerstmayr N, Hiller KA, Bolay C, Waha C, Spagnuolo G, et al. TEGDMA-induced oxidative DNA damage and activation of ATM and MAP kinases. Biomaterials. 2009;30:2006–14. doi: 10.1016/j.biomaterials.2008.12.045. [DOI] [PubMed] [Google Scholar]
- 65.Teti G, Mazzotti G, Zago M, Ortolani M, Breschi L, Pelotti S, et al. HEMA down-regulates procollagen alpha1 type I in human gingival fibroblasts. J Biomed Mater Res A. 2009;90:256–62. doi: 10.1002/jbm.a.32082. [DOI] [PubMed] [Google Scholar]
- 66.Lee DH, Kim NR, Lim BS, Lee YK, Ahn SJ, Yang HC. Effects of TEGDMA and HEMA on the expression of COX-2 and i NOS in cultured murine macrophage cells. Dent Mater. 2009;25:240–6. doi: 10.1016/j.dental.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 67.Pawlowska E, Poplawski T, Ksiazek D, Szczepanska J, Blasiak J. Genotoxicity and cytotoxicity of 2-hydroxyethyl methacrylate. Mutat Res. 2010;696:122–9. doi: 10.1016/j.mrgentox.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 68.Urcan E, Scherthan H, Styllou M, Haertel U, Hickel R, Reichl FX. Induction of DNA double-strand breaks in primary gingival fibroblasts by exposure to dental resin composites. Biomaterials. 2010;31:2010–4. doi: 10.1016/j.biomaterials.2009.11.065. [DOI] [PubMed] [Google Scholar]
- 69.Drozdz K, Wysokinski D, Krupa R, Wozniak K. Bisphenol A-glycidyl methacrylate induces a broad spectrum of DNA damage in human lymphocytes. Arch Toxicol. 2011;85:1453–61. doi: 10.1007/s00204-010-0593-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bakopoulou A, Leyhausen G, Volk J, Koidis P, Geurtsen W. Effects of resinous monomers on the odontogenic differentiation and mineralization potential of highly proliferative and clonogenic cultured apical papilla stem cells. Dent Mater. 2012;28:327–39. doi: 10.1016/j.dental.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 71.Reichl FX, Durner J, Kunzelmann KH, Hickel R, Spahl W, Hume WR, et al. Biological clearance of TEGDMA in guinea pigs. Arch Toxicol. 2001;75:22–7. doi: 10.1007/s002040000159. [DOI] [PubMed] [Google Scholar]
- 72.Reichl FX, Durner J, Hickel R, Kunzelmann KH, Jewett A, Wang MY, et al. Distribution and excretion of TEGDMA in guinea pigs and mice. J Dent Res. 2001;80:1412–5. doi: 10.1177/00220345010800050501. [DOI] [PubMed] [Google Scholar]
- 73.Reichl FX, Durner J, Hickel R, Spahl W, Kehe K, Walther U, et al. Uptake, clearance and metabolism of TEGDMA in guinea pigs. Dent Mater. 2002;18:581–9. doi: 10.1016/s0109-5641(01)00094-x. [DOI] [PubMed] [Google Scholar]
- 74.Homa Darmani AS, Alhiyasat B. The effect of Bis-GMA and TEG-DMA on female mouse fertility. Dent Mat. 2006;22:353–8. doi: 10.1016/j.dental.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 75.Bouillaguet S, Shaw L, Gonzalez L, Kerjci I. Long term cytotoxicity of resin-based dental restorative materials. J Oral Rehab. 2002;29:7–13. doi: 10.1046/j.1365-2842.2002.00804.x. [DOI] [PubMed] [Google Scholar]
- 76.Rathbun MA, Carig RG, Hancks CT, Filisko FE. Cytotoxicity of Bis-GMA dental composite before and after leaching in organic solvents. J Biomed Mater Res. 1991;25:443–57. doi: 10.1002/jbm.820250403. [DOI] [PubMed] [Google Scholar]
- 77.Castañeda ER, Silva LA, Gaton-Hernández P, Consolaro A, Rodriguez EG, Silva RA, et al. Filtek™ Silorane and Filtek™ Supreme XT resins: Tissue reaction after subcutaneous implantation in isogenic mice. Braz Dent J. 2011;22:105–10. doi: 10.1590/s0103-64402011000200003. [DOI] [PubMed] [Google Scholar]
- 78.European Union. 2010. [Accessed Jun 07,2012]. Available from: http://www.bisphenol-a-europe.org .
- 79.European Union Risk Assessment Report: 4, 4’ Isopropylidenediphenol (Bisphenol-A) 2010. [Accessed Jun 23, 2012]. Available from: http://publications.jrc.ec.europa.eu/repository/
- 80.Myers DE, Hutz RJ. Current status of potential bisphenol toxicity in dentistry. Gen Dent. 2011;59:262–5. [PubMed] [Google Scholar]
- 81.Podgórski M. Structure-property relationship in new photo-cured dimethacrylate-based dental resins. Dent Mater. 2012;28:398–409. doi: 10.1016/j.dental.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 82.Bal BT, Sönmez N, Bavbek B, Akçaboy C. Cytotoxicity of composite resins before and after expiration date. Preliminary report. N Y State Dent J. 2011;77:31–5. [PubMed] [Google Scholar]
- 83.Sigusch BW, Pflaum T, Völpel A, Gretsch K, Hoy S, Watts DC, et al. Resin-composite cytotoxicity varies with shade and irradiance. Dent Mater. 2012;28:312–9. doi: 10.1016/j.dental.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 84.Ergun G, Egilmez F, Cekic-Nagas I. The cytotoxicity of resin composites cured with three light curing units at different curing distances. Med Oral Patol Oral Cir Bucal. 2011;16:e252–9. doi: 10.4317/medoral.16.e252. [DOI] [PubMed] [Google Scholar]


