Abstract
During the present investigation the effect of α-tocopherol (100 μmolL-1) in prevention of testicular toxicity induced by atrazine in goat Capra hircus have been analyzed. Vitamin E (α-tocopherol) at dose level 100 μmolL-1 provides attenuation over the histopathological changes generated by pesticide atrazine (100 nmolml-1). Small pieces (approximately 1mm3) of testicular tissue were divided into three groups (one control group + two experimental groups). Experimental group (A) was supplemented with 100 nmolml-1 concentration of atrazine and experimental group (B) was supplemented with 100 nmolml-1 atrazine and 100 μmolL-1 concentrations of vitamin E (α-Tocopherol) and harvesting was carried out after 1, 4 and 8 hrs of exposure. Control was run along with all the experimental groups. In the experimental group (A) treated with atrazine at dose level 100 nmolml-1, revealed histomorphological alterations in the seminiferous tubule. After one hour of exposure duration small vacuoles in cytoplasm of the Sertoli cells and spermatogonia were observed. Chromolysis at pycnosis were also noticed in the spermatogonia and spermatids. In the experimental group (B) exposed with atrazine and simultaneously supplemented with Vitamin E also showed degeneration but it was milder as compared with experimental group treated with atrazine without antioxidant. Atrazine exposure induced a decline in diameter of spermatocytes from 10.51 ± 0.2052 μm in control to 7.915 ± 0.2972, 7.5 ± 0.211 and 7.14 ± 0.225 μm after exposure of 1, 4 and 8 hrs respectively but in case of atrazine supplemented with vitamin E [experimental group (B)], there was less decline in cell diameter that was 8.5 ± 0.1865, 8.1 ± 0.1201 and 7.8 ± 0.2066μm after exposure of 1, 4 and 8 hrs respectively. The result demonstrated that vitamin E delays the degenerative changes induced by atrazine.
Keywords: α-tocopherol, antioxidant, atrazine, capra hircus, goat, pesticide, testis
INTRODUCTION
Recently, there has been a great concern among the scientific community, policy makers and general public regarding the reproductive potential and health hazards of a wide range of environmental chemicals.[1] Out of these environmental toxicants, some chemical act as endocrine disruptors with the potential to alter hormone action within the body.[2] Endocrine disrupters, may be present in the environment naturally (phytoestrogens) or may be man-made, are ubiquitous because of their production in large quantities and easily transported through the atmosphere and their slow degradable activity in the environment.[3] Man-made endocrine disruptors vary in potency and in the level of exposure required to produce an adverse effects in the animals. Different endocrine disruptors may not be released individually, into the environment at levels that would exert substantial risks, but the effects of chronic low levels of exposure of the endocrine disrupting chemicals are of concern.[4] It has been reported that DDT present in the environment was the reason of broken eggs of predatory birds, which results in a sharp decrease in their number around the Great Lakes.[5] Unabated pollution of the environment is considered to be a major reason for the decline of human semen quality over the years.[6] Atrazine, the agricultural pesticide, once one of the most widely used pesticide all over the world, is now recognized as an endocrine disruptor and to have disrupting effects on the reproductive systems of mammals.[7–11] More dramatically, it has been shown that fertile men in an agricultural area of Missouri have lower sperm counts (about 40%) than men in three urban US areas, and also have higher concentrations of three currently used pesticides in urine.[12] It has been reported that environmental toxicants such as lindane, di-n-butylphthalate nonylphenol, methoxychlor, and dioxin induced oxidative stress in the testes and epididymal sperm of rats and thus results in decrease in reproductive potential in animals.[13–17] Endocrine disrupting chemicals disturb the pro-oxidant/anti-oxidant system of the cells leading to the generation of oxygen free radical and reactive oxygen species (ROS).[18] Mammalian spermatozoa, being rich in membrane bound polyunsaturated fatty acids (PUFA), are more susceptible to ROS attack, and makes them more vulnerable targets of ROS-producing polychlorinated biphenyls.[19] In spermatozoa, several antioxidant systems as glutathione peroxidase, superoxide dismutase and catalase[20–22] are operating for their normal functioning. In addition, it has been observed in earlier study that alpha-tocopherol is found in various amounts in testicular cells of rat in the order of Sertoli cells greater than pachytene spermatocytes greater than round spermatids, with a factor of 4 in the alpha-tocopherol content between Sertoli cells and round spermatids.[23] Antioxidant therapy appears to be efficient not only in vitro but also in vivo. In fact, a growing body of evidence points out supplemental intake of vitamin A (the retinoids and carotenoids).[24] Mixtures of carotenoids or associations with others antioxidants (e.g. vitamin E) can increase their ability to protect against lipid peroxidation.[25] The protecting effects of high doses level 20 and 40 mg/kg body wt/day of vitamin C (L-ascorbic acid) in modulating the genotoxicity of pesticides in murine bone marrow cells,[26–28] and in primary spermatocytes have been observed.[29] Positive effect of vitamin C (ascorbic acid) in preventing the degenerating effects of endosulphan in Capra hircus in vitro have been documented.[30]
Keeping in view the effect of pesticides on reproductive potential of animals it is necessary to seek the protective measures to reduce the hazardous effect of the pesticides. In the light of the background information the present study was designed to evaluate the antioxidant attenuation of atrazine induced histopathological changes in testicular tissue of goat Capra hircus in vitro.
MATERIALS AND METHODS
Mature goat (Capra hircus) testis were collected from slaughter houses around Kurukshetra (29°6’N, 76°50’E), Haryana, India. The material was brought to the Reproductive Physiology Laboratory in normal saline during year 2009 at 4°C. After decapsulation, the testis was cut into small pieces (approximately 1mm3) for culture.
After washing three times with the normal saline the testicular tissue were cultured by Sharma et al.,[30] method. The tissue was divided into three groups (one control group + two experimental groups). Experimental group (A) was supplemented with 100 nmolml-1 concentration of atrazine [Structure 1] and experimental group (B) was supplemented with 100 nmolml-1 atrazine and 100 μmolL-1 concentrations of vitamin E (α-Tocopherol) [Structure 2] and harvesting was carried out after 1, 4 and 8 hours of exposure. The culture plates were kept at 39°C in 5% CO2 level in CO2 incubator for specified duration. Tissues from all the groups were processed for the histomorphological studies. Paraffin embedded tissue from all experimental and control was cut at 5 μm thickness and after dewaxing in cleansing agent xylene, the sections were processed through decreasing grades of alcohol and stained with haematoxylene. After that the sections were gradually dehydrated up to the 70% alcohol and finally stained with eosin, after further dehydration up to absolute alcohol the sections were cleared with xylene and finally mounted with the help of DPX.[31]
Structure 1.
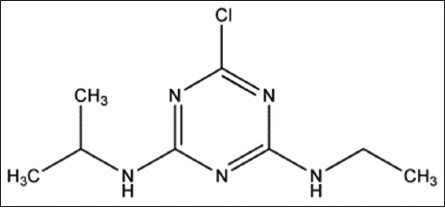
Atrazine. IUPAC Name: 6-chloro-N-ethyl-N’-isopropyl-1,3,5-triazine-2,4-diamine. Chemical formula: C8H14ClN5. Molecular mass: 215.7 g/mol
Structure 2.
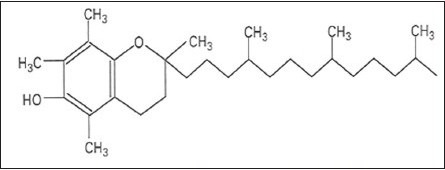
α-Tocopherol. Chemical formula: C29 H50 O2. Molecular Mass: 430.69 g/mol
RESULTS
During the present investigation 100 μmolL-1 concentration of α-tocopherol in rescue of degenerative changes induced by atrazine in testicular tissue of goat Capra hircus have been analyzed in vitro. Testicular tissue sections (5 μm) stained with haematoxylene and eosin revealed normal histoarchetecture of the seminiferous tubules in control group. Under light microscope, seminiferous tubules were packed with loose connective tissue having blood vessels. Normal Leydig cells were present outside the seminiferous tubules. Sertoli cells and all types of the germ cells were present in the seminiferous tubules and were arranged in the specific manner. Spermatogonia were present at the basal portion and next to these spermatocytes occupy the middle potion. Spermatids form the next layer and then mature elongated spermatozoa were present toward the lumen of the seminiferous epithelium. Sertoli cells were having broad basal portion having round or oval nuclei resting on the basal lamina and its cytoplasmic part with its irregular extensions scattered in between the germ cells up to the lumen of the seminiferous tubules [Figure 1].
Figure 1.
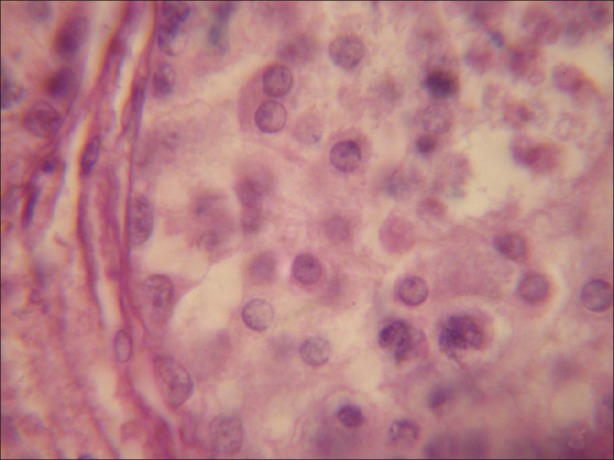
Testicular tissue exhibiting normal structure of seminiferous tubule, having normal Leydig cells, Sertoli cells and different types of germ cells. (×1000)
In the experimental group (A) treated with atrazine with dose level 100 nmolml-1, revealed histomorphological alterations in the seminiferous tubule. After one hour of exposure duration small vacuoles in cytoplasm of the Sertoli cells and spermatogonia were observed. Chromolysis were also noticed in the spermatogonia and spermatids. Pycnosis were also visible in some of the germ cells present in the seminiferous tubule [Figure 2]. As the exposure duration of atrazine increased up to four hours these atretogenic changes in different germ cells and somatic cells were enhanced. Large vacuoles of varied sizes and shapes were clearly visible in Sertoli cells, spermatogonia and spermatids. Various small vesicles were also noticed in nuclei of various germ cells and somatic cells. Hyalinization and fragmented nuclei were clearly visible. Degenerating Leydig cells were also observed in the testicular sections. Basal lamina was also detached from the underlying cells in few portions of seminiferous tubule. As the exposure duration further increased from 4 to 8 hrs these changes were further increased in the time dependent manner. Chromolysis, hyalinization and pycnosis were frequently observed. Basal lamina was detached at more places from the underlying cells. Frequency of fragmented nuclei was enhanced with the increase in exposure duration. Pinching off the nuclear material was noticed in germ cells. Vacuoles were of very large size and dislodging of germ cells in the seminiferous tubules was observed [Figure 3].
Figure 2.
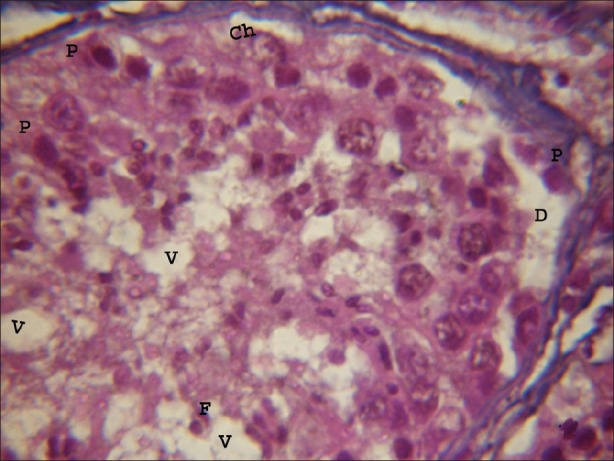
Light microphotograph of testicular portion treated with atrazine (100 nmolml-1) without antioxidant after 1 hour, showing initiation of degeneration in all types of cells. Pycnosis (P) and chromolysis (Ch) were clearly visible. Vacuoles of varying sizes and shapes were observed. Basal lamina was detached due to the exposure of atrazine (D). (×1000)
Figure 3.
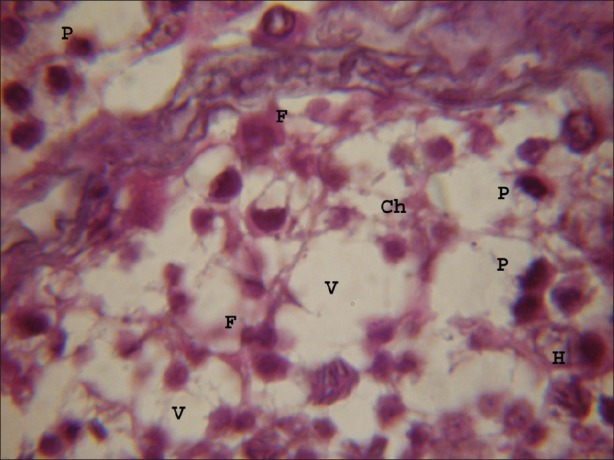
Testicular portion treated with atrazine at dose level 100 nmolml-1, chromolysis (Ch), fragmentation (F) of nuclear material, hyalinization (H) were frequently observed after 8 hours. Vacuoles of varying sizes and shapes were observed. Small vesicles were observed in nuclei of germ cells and Somatic cells. (×1000)
Due to the supplementation of vitamin E at dose level 100 μmolL-1 along with the exposure of atrazine [experimental group (B)], antioxidant exerts prevention in degenerating changes induced by atrazine in testicular tissue. After 1 hr of exposure duration there were very small changes in the histopathology of seminiferous tubules. Very few pycnotic nuclei, condensed nuclei were observed due to the supplementation of vitamin E [experimental group (B)]. After 4 hrs of exposure duration changes in germ cells and somatic cells were noticed but these degeneration changes were milder as compared with the experimental group (A) treated with atrazine. As the exposure duration increased up to 8 hours the number of fragmented nuclei, hyalinization and chromolysis were reduced due to the vitamin E supplementation. These observations demonstrated that the atretogenic changes induced by atrazine were delayed due to the supplementation of α-tocopherol [Figures 4–6].
Figure 4.
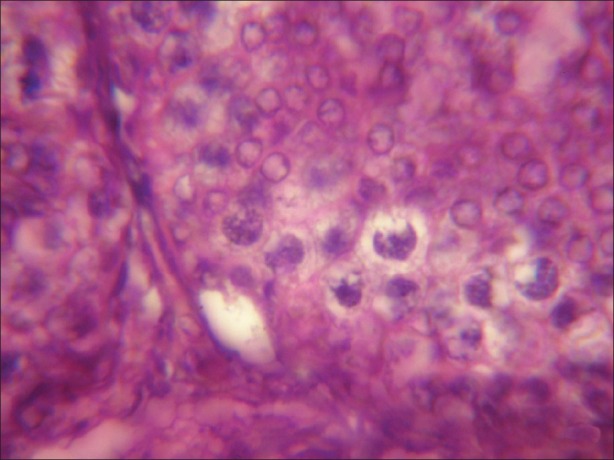
Microphotograph of testicular tissue treated with atrazine (100 nmolml-1) and simultaneously supplemented with antioxidant (α-tocopherol) showing reduction in degenerating changes induced by atrazine after 1 hours of exposure. (×1000)
Figure 6.
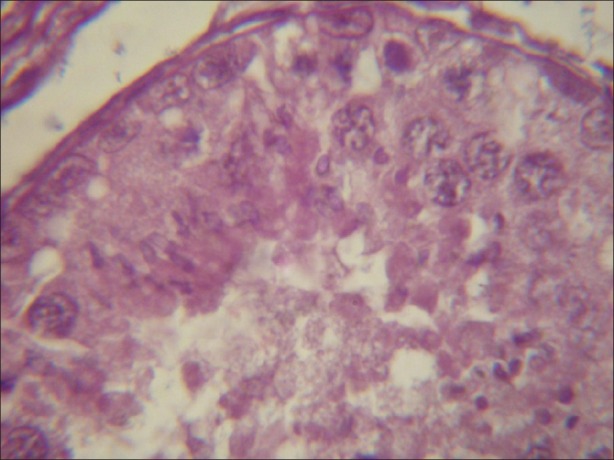
Light micrograph of testicular tissue treated with atrazine supplemented with antioxidant revealing degeneration as compared with the tissue treated with atrazine without a-tocopherol after 8 hours. (×1000)
Figure 5.
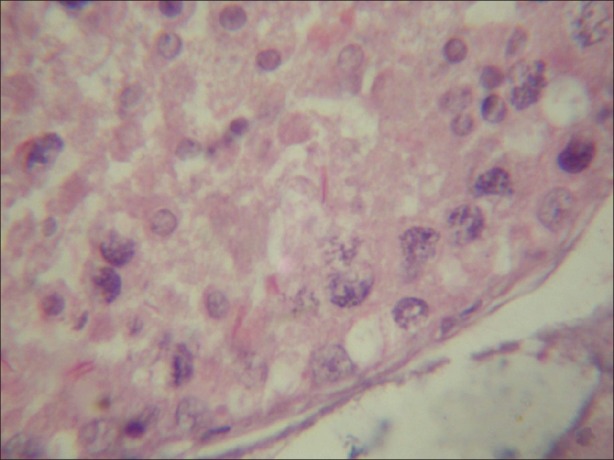
Light micrograph of testicular tissue treated with atrazine (100 nmolml-1) and simultaneously supplemented with antioxidant (α-tocopherol) (100 nmolL-1) showing decreasing number of chromolysis, condensation and fragmentation. (×1000)
Atrazine at dose level 100 nmolml-1 [experimental group (A)] induced a decline in diameter of spermatocyte from 10.51 ± 0.2052 μm in control to 7.915 ± 0.2972, 7.5 ± 0.211 and 7.14 ± 0.225 μm after exposure of 1, 4 and 8 hrs respectively but in case of atrazine supplemented with vitamin E [experimental group (B)], there was less decline in cell diameter that was 8.5 ± 0.1865, 8.1 ± 0.1201 and 7.8 ± 0.2066μm after exposure of 1, 4 and 8 hrs respectively [Figure 7]. There was also decline in nuclear diameter of spermatocyte from 7.10 ± 0.2395 in control group to 5.63 ± 0.2649, 5.30 ± 0.217 and 4.87 ± 0.225μm after exposure of atrazine [experimental group (A)] at 1, 4 and 8 hrs respectively, whereas in case of atrazine with vitamin E supplementation [experimental group (B)], there was less decline in nuclear diameter that was 6.3 ± 0.1319, 5.9 ± 0.1388 and 5.5 ± 0.1845 μm after exposure duration of 1, 4 and 8 hrs respectively [Figure 8]. The variations recorded were statistically significant (P ≤ 0.05). In atrazine treated group [experimental group (A)], atretic spermatocytes were enhanced from 22% in control to 48% after 1 hr, 28 to 58% after 4 hrs and 32 to 66 % after 8 hrs of exposure duration [Figure 9]. Chi-square values between control and atrazine treated groups were 7.42, 6.98 and 11.563 after 1, 4 and 8 hrs of exposure durations and all the variations recorded were statistically significant (χ2 0.05). In the experimental group treated with atrazine and simultaneously supplemented with vitamin E [experimental group (B)] there was decline in atretic cell percentage as compared with atretic spermatocytes noticed in testicular tissue treated with endosulphan without antioxidant [experimental group (A)]. This decline in atretic spermatocytes was from 48% in atrazine treated group [experimental group (A)] to 35% in atrazine supplemented with vitamin E [experimental group (B)] was observed after 1 hr of exposure duration, from 58 to 44% after 4 hours of exposure duration, and from 66 to 52% after exposure duration of 8 hours [Figure 9]. Chi-square values were 3.48, 3.92 and 4.06 after 1, 4, and 8 hrs of exposure duration respectively and all the variations recorded were statistically significant (χ2 0.05).
Figure 7.
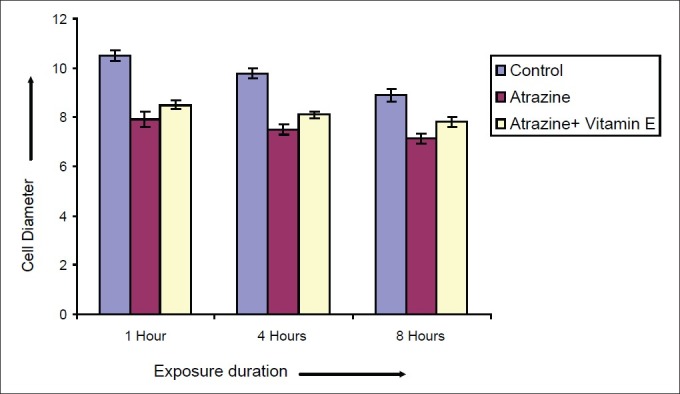
Effect of vitamin E on diameter of Spermatocytes after varying exposure durations
Figure 8.
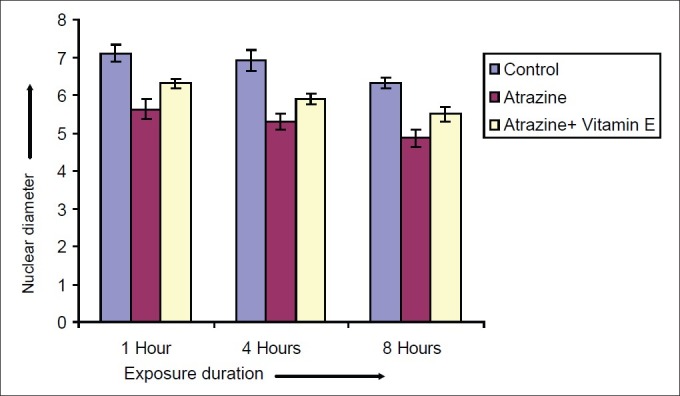
Effect of vitamin E on nuclear diameter of Spermatocytes after varying exposure durations
Figure 9.
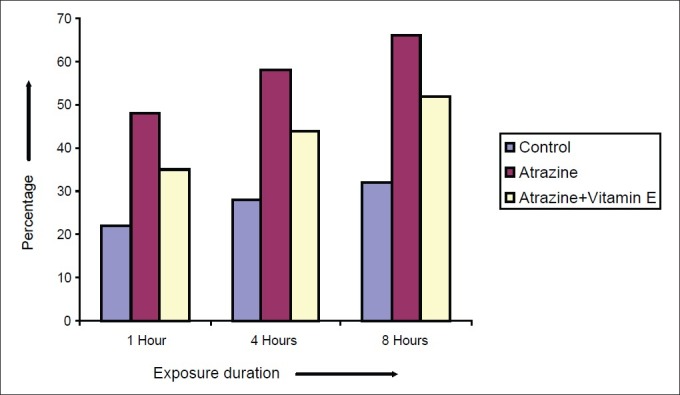
Effect of vitamin E on atretic percentage of Spermatocytes after varying exposure durations
DISCUSSION
The results of the present investigation have clearly demonstrated that atrazine induced atretogenic changes like chromolysis, pycnosis, fragmentation, hyalinization and condensation were dramatically diminished when the atrazine culture was supplemented with 100 μmolL-1 concentrations of vitamin E (a-Tocopherol). Thereby indicating protective effect of vitamin E on atrazine damage inducing potential on the cells. These results of the present finding strongly support the earlier findings wherein glutathione administration in the infertile patients induced the protecting effect on sperm motility and improved morphology.[32] The high frequency of atretic spermatocytes observed during present study is possibly due to the oxidative stress generated by pesticide exposure. The reduction in percentage of atretic spermatocytes from 58 to 44% after 4 hrs of exposure duration, and from 66 to 52% after exposure duration of 8 hrs after the supplementation of vitamin E along with atrazine as antioxidant, have the potential to circumvent the free radicals generated due to this oxidative stress. The present findings tangibly support the observations of Mediratta et al,[33] who have reported that sub-chronic lindane exposure increased MDA level in serum indicative of an elevation in free oxygen radical generation by pesticides. Organochlorine pesticide induced oxidative stress as well as immune suppression in rats is similar to the oxidative stress imposed by pesticide exposure.[34,35] Antioxidant supplementation with ascorbic acid produced significant amelioration up to the control level in morphological degeneration due to the pesticides.[36] Ascorbic acid exhibited beneficial effects in detoxifying the effects of endosulfan in the spermatological parameters rabbits and other mammals.[30,36]
The results of the present investigation strongly advocate the findings of El-Demerdash et al,[34] who have observed the role of alpha-tocopherol (Vitamin E), β-carotene and their combination as antioxidants against the toxicity of fenvalerate insecticide. The results of the present study that vitaminE supplementation along with the atrazine induced protection from the damage caused by the pesticide support strongly that Vitamin E, β-carotene and/or their combination did not cause any changes in the investigated parameters, but improved semen quality and exert protection against the toxic effect of fenvalerate.[37] Lucesoli and Fraga[38] have observed the effect of vitamin E supplementation on oxidative stress produced by chronic iron intoxication. The present observations strongly supports the earlier study demonstrated the ameliorating effects vitamin C (L-ascorbic acid) in modulating the genotoxicity of pesticides.[26] The results of the present investigation also support the findings of Semercioz et al,[39] who have used Melatonin, as free radical scavenger, thereby suggesting that the atrazine induces its toxic manifestations possibly through free radical generation and scavengers thereof like vitamin E (100 μmolL-1) ameliorate these toxic effects.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Pocar P, Brevini TA, Fischer B, Gandolfi F. The impact of endocrine disruptors on oocyte competence. Reproduction. 2003;125:313–25. doi: 10.1530/rep.0.1250313. [DOI] [PubMed] [Google Scholar]
- 2.Damstra T, Barlow S, Bergman A, Kavlock R, Van Der Kraak G. Global assessment of the state-of-the-science of endocrine disruptors. International Programme on Chemical Safety. 2002. WHO/PCS/EDC/02.2 http://www.who.int/ipcs/publications/new_issues/endocrine_disruptors/en/
- 3.Hollander D. Environmental effects on reproductive health: The endocrine disruption hypothesis. Fam Plann Perspect. 1997;29:82–6. Available from: http://www.guttmacher.org/pubs/journals/2908297.html . [PubMed] [Google Scholar]
- 4.Colborn T, vom Saal FS, Soto AM. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ Health Perspect. 1993;101:378–84. doi: 10.1289/ehp.93101378. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1519860/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Safe SH, Foster WG, Lamb JC, Newbold RR, Van Der Kraak G. Estrogenicity and endocrine disruption. CAST. 2000. pp. 1–16. Available from: http://www.cast-science.org/websiteUploads/publicationPDFs/endocrine.pdf .
- 6.Skakkebaek NE, Negro-Villar A, Michal F, Fathalla M. Impact of the environment on reproductive health.Report and Recommendations of a WHO International Workshop. Dan Med Bull. 1991;38:425–6. [PubMed] [Google Scholar]
- 7.Ashby J, Tinwell H, Stevens J, Pastoor T, Breckenridge CB. The effects of atrazine on the sexual maturation of female rats. Regul Toxicol Pharmacol. 2002;35:468–73. doi: 10.1006/rtph.2002.1571. [DOI] [PubMed] [Google Scholar]
- 8.Rayner JL, Wood C, Fenton SE. Exposure parameters necessary for delayed puberty and mammary gland development in Long-Evans rats exposed in utero to atrazine. Toxicol Appl Pharmacol. 2004;195:23–34. doi: 10.1016/j.taap.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Kniewald J, Jakominic M, Tomljenovic A, Simic B, Romac P, Vranesic D, Kniewald Z. Disorders of male rat reproductive tract under the influence of atrazine. J Appl Toxicol. 2000;20:61–8. [PubMed] [Google Scholar]
- 10.Friedmann AS. Atrazine inhibition of testosterone production in rat males following peripubertal exposure. Reprod Toxicol. 2002;16:275–9. doi: 10.1016/s0890-6238(02)00019-9. [DOI] [PubMed] [Google Scholar]
- 11.Trentacoste SV, Friedmann AS, Youker RT, Breckenridge CB, Zirkin BR. Atrazine effects on testosterone levels and androgen-dependent reproductive organs in peripubertal male rats. J Androl. 2001;22:142–8. Available from: http://www.andrologyjournal.org/cgi/content/abstract/22/1/142 . [PubMed] [Google Scholar]
- 12.Swan SH, Kruse RL, Liu F, Barr DB, Drobnis EZ, Redmon JB, et al. Semen quality in relation to biomarkers of pesticide exposure. Environ Health Perspect. 2003;111:1478–84. doi: 10.1289/ehp.6417. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1241650/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chitra KC, Sujatha R, Latchoumycandane C, Mathur PP. Effect of lindane on antioxidant enzymes in epididymis and epididymal sperm of adult rats. Asian J Androl. 2001;3:205–8. [PubMed] [Google Scholar]
- 14.Chitra KC, Latchoumycandane C, Mathur PP. Effect of nonylphenol on the antioxidant system in epididymal sperm of rats. Arch Toxicol. 2002;76:545–51. doi: 10.1007/s00204-002-0372-4. [DOI] [PubMed] [Google Scholar]
- 15.Farombi EO, Abarikwu SO, Adedara IA, Oyeyemi MO. Curcumin and kolaviron ameliorate di-n-butylphthalate-induced testicular damage in rats. Basic Clin Pharmacol Toxicol. 2007;100:43–8. doi: 10.1111/j.1742-7843.2007.00005.x. [DOI] [PubMed] [Google Scholar]
- 16.Latchoumycandane C, Mathur PP. Induction of oxidative stress in the rat testis after short-term exposure to the organochlorine pesticide methoxychlor. Arch Toxicol. 2002;76:692–8. doi: 10.1007/s00204-002-0388-9. [DOI] [PubMed] [Google Scholar]
- 17.Latchoumycandane C, Chitra KC, Mathur PP. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) induces oxidative stress in the epididymis and epididymal sperm of adult rats. Arch Toxicol. 2003;77:280–4. doi: 10.1007/s00204-003-0439-x. [DOI] [PubMed] [Google Scholar]
- 18.Harris C, Lee E, Hiranruengchok R, McNutt TL, Larson SJ, Akeila S, et al. Characteristics of glutathione redox and antioxidant status in post implantation rat embryos: Response to oxidative stress. Toxicology. 1996;30:2–2. [Google Scholar]
- 19.Lenzi A, Gandini L, Picardo M, Tramer F, Sandri G, Panfili E. Lipoperoxidation damage of spermatozoa polyunsaturated fatty acids (pufa): Scavenger mechanisms and possible scavenger therapies. Front Biosci. 2000;5:1–15. doi: 10.2741/lenzi. Available from: http://www.bioscience.org/2000/v5/e/lenzi/fulltext.htm . [DOI] [PubMed] [Google Scholar]
- 20.Jeulin C, Soufir JC, Weber P, Laval-Martin D, Calvayraec R. Catalase activity in human spermatozoa and seminal plasma. Gamete Res. 1989;24:185–96. doi: 10.1002/mrd.1120240206. [DOI] [PubMed] [Google Scholar]
- 21.Alvarez JG, Storey BT. Role of glutathione peroxidase in protecting mammalian spermatozoa from loss of motility caused by spontaneous lipid peroxidation. Gamete Res. 1989;23:77–90. doi: 10.1002/mrd.1120230108. [DOI] [PubMed] [Google Scholar]
- 22.Mennella MR, Jones R. Properties of spermatozoal superoxide dismutase and lack of involvement of superoxides in metal-ion-catalysed lipid-peroxidation and reactions in semen. Biochem J. 1980;191:289–97. doi: 10.1042/bj1910289. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1162218/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoganathan T, Eskild W, Hansson V. Investigation of detoxification capacity of rat testicular germ cells and sertoli cells. Free Radic Biol Med. 1989;7:355–9. doi: 10.1016/0891-5849(89)90121-4. [DOI] [PubMed] [Google Scholar]
- 24.Paiva SAR, Russell RM. Beta;-carotene and other carotenoids as antioxidants. J Am Coll Nutr. 1999;18:426–33. doi: 10.1080/07315724.1999.10718880. Available from: http://www.jacn.org/cgi/content/full/18/5/426 . [DOI] [PubMed] [Google Scholar]
- 25.Mascio PD, Murphy ME, Sies H. Antioxidant defense systems: The role of carotenoids, tocopherols, and thiols. Am J Clin Nutr. 1991;53:194S–200S. Available from: http://www.ajcn.org/content/53/1/194S.short . [PubMed] [Google Scholar]
- 26.Khan PK, Sinha SP. Dose-dependent minimisation of cytogenetic toxicity of endosulfan by vitamin C. Cytologia. 1992;57:217–21. [Google Scholar]
- 27.Khan PK, Sinha SP. Antimutagenic efficacy of higher doses of vitamin C. Mutat Res. 1993;298:157–61. doi: 10.1016/0165-1218(93)90036-d. [DOI] [PubMed] [Google Scholar]
- 28.Khan PK, Sinha SP. Impact of higher doses of vitamin C in modulating pesticide genotoxicity. Teratog Carcinog Mutagen. 1994;14:175–81. doi: 10.1002/tcm.1770140404. [DOI] [PubMed] [Google Scholar]
- 29.Khan PK, Sinha SP. Vitamin C mediated amelioration of pesticide genotoxicity in murine spermatocytes. Cytobios. 1994;80:199–204. [PubMed] [Google Scholar]
- 30.Sharma RK, Fulia A, Chauhan PK. Protective effect of ascorbic acid in endosulphan induced testicular toxicity in goat in vitro. J Biol Sci. 2010;10:624–30. [Google Scholar]
- 31.Pearse AGE. London: Churchill; 1968. Histochemistry: Theoritical and Applied. [Google Scholar]
- 32.Lenzi A, Culasso F, Gandini L, Lombardo F, Dondero F. Placebo-controlled, double-blind, cross-over trial of glutathione therapy in male infertility. Hum Reprod. 1993;8:1657–62. doi: 10.1093/oxfordjournals.humrep.a137909. [DOI] [PubMed] [Google Scholar]
- 33.Mediratta PK, Tanwar K, Reeta KH, Mathur R, Benerjee BD, Singh S, et al. Attenuation of the effect of lindane on immune responses and oxidative stress by Ocimum sanctum seed oil (OSSO) in rats. Indian J Physiol Pharmacol. 2008;52:171–7. [PubMed] [Google Scholar]
- 34.Koner BC, Banerjee BD, Ray A. Organochlorine pesticide induced oxidative stress and immune suppression in rats. Indian J Exp Biol. 1998;36:395–8. [PubMed] [Google Scholar]
- 35.Banerjee BD, Seth V, Ahmed RS. Pesticides induced oxidative stress: Perspectiveness and trends. Rev Environ Health. 2001;16:1–40. doi: 10.1515/reveh.2001.16.1.1. [DOI] [PubMed] [Google Scholar]
- 36.Ata A, Hatipoglu FS, Yildiz-Gulay O, Gulay MS. Protective Role of Ascorbic Acid on Subacute Sperm Toxicity in Male New Zealand White Rabbits Treated with Endosulfan. Drug Chem Toxicol. 2007;30:181–95. doi: 10.1080/01480540701374896. Available from: http://www.informaworld.com/smpp/title~db=all~content=t713597244~tab=issueslist~branches=30 - v30 . [DOI] [PubMed] [Google Scholar]
- 37.El-Demerdash FM, Yousef MI, Kedwany FS, Baghdadi HH. Role of and alpha;-tocopherol and and beta;-carotene in ameliorating the fenvalerate-induced changes in oxidative stress, hemato-biochemical parameters & semen quality of male rats. J Environ Sci Health B. 2004;39:443–59. doi: 10.1081/pfc-120035929. [DOI] [PubMed] [Google Scholar]
- 38.Lucesoli F, Fraga CG. Oxidative stress in testes of rats subjected to chronic iron intoxication and and alpha;-tocopherol supplementation. Toxicology. 1999;132:179–86. doi: 10.1016/s0300-483x(98)00152-8. [DOI] [PubMed] [Google Scholar]
- 39.Semercioz A, Onur R, Ogras S, Orhan I. Effects of melatonin on testicular tissue nitric oxide level and antioxidant enzyme activities in experimentally induced left varicocele. Neuro Endocrinol Lett. 2003;24:86–90. [PubMed] [Google Scholar]


