Abstract
The present study deals with the evaluation and assessment of the safety/toxic potential of Boswellia serrata, a well known Ayurvedic herb used to treat disorders of digestive system, respiratory ailments and bone related diseases. A repeated dose oral (90 days) toxicity study of Boswellia serrata was carried out. For this, 10 rats of each sex were treated with the Boswellia serrata at three different doses i.e. 100, 500 and 1000 mg/kg B. wt. /day. As a control, 10 rats of each sex were treated with corn oil only which was the vehicle. Two groups consisting of five male and five female rats were kept as control recovery and high dose recovery group which were treated with the vehicle (corn oil) and the Boswellia serrata at the dose of 1000 mg/kg B. wt. Animals of control recovery and high dose recovery groups were further observed for 28 days without any treatment. From this study, it was found that the rats treated with high dose of the Boswellia serrata gained their body weight with much less rate than that of the control group. However, during the recovery period, the loss in body weight gain as observed during the study period exhibits a reversible effect on the metabolic activity and recovered. The results also indicate that Boswellia serrata is relatively safe in rat up to the dose of 500 mg/kg B.wt. as no adverse impact on health factors was observed. Thus, the No observed adverse effect level is 500 mg/kg B. wt.
Keywords: Boswellia serrata, NOAEL, safety, sub-chronic, toxicity, wistar rat
INTRODUCTION
Boswellia serrata (Frankincense) is a moderate to large branching tree (growing to a height of 12 feet) found in India, Northern Africa and the Middle East. The barks of Boswellia serrata consists majorly of a gummy oleo-resin.[1] Boswellia serrata is a natural and safe NSAID (Non steroidal anti-inflammatory drugs) used to treat a variety of health problems.[2] The oleo resin of Boswellia serrata has been widely used in Ayurvedic medicines against various diseases that include Arthritis,[3] Inflammation,[2] Colitis,[4] Asthma,[5] Psoriasis,[6] Hyperlipidemia[7] and Cancers.[8] Boswellia serrata contains Boswellic acids (β-boswellic acid, 3-acetyl-b-boswellic acid, 11-keto-b-boswellic acid and 3-acetyl-11-keto-β-boswellic acid) oils, terpenoids and sugars.[9] The Boswellic acids are specific, non redox inhibitors of 5-lipoxygenase without affecting 12-lipoxygenase and cyclooxygenase activities.[10–12] Though some studies related to the safety profile of Boswellia serrata were reported on experimental animals as well as on primates but they were not sufficient to explain the systemic effect. The present study was conducted to establish the safety of this herb by conducting a repeated dose 90 day oral toxicity study in accordance to OECD Guideline No. 408 and WHO Guidelines[13,14]
The objective of the study was to evaluate the systemic toxic effect of the Boswellia serrata in wistar rats and also to determine the No Observed Adverse Effect Level (N. O. A. E. L). It will also provides information on health hazards likely to arise from a repeated oral exposure of the test substance, as Boswellia serrata is being used repeatedly for a variety of ailments.
MATERIALS AND METHODS
Test substance
Boswellia serrata, the test substance was provided by M/s Arjuna Natural Extracts Ltd., Kerala. It was in the form of cream color granules. The test substance was finely ground and was suspended in corn oil which served the role of vehicle for the dosing. The active ingredient in the herbal product was checked by HPLC method by the company which is mentioned in the certificate of analysis. The concentration in the formulation was determined by HPLC method showing 100 % homogeneity in the solution.
Animals and their treatment
Rats used for the study were bred at the animal house facility of Shriram Institute for Industrial Research, Delhi. For the study, 5 to 8 weeks old wistar rats weighing between 100 to 140 grams were used. Prior to starting the experiment, necessary approvals were taken from IAEC (Institutional animal ethics committee) and CPCSEA (Committee for the Purpose of Control and Supervision of Experimental Animals) for conducting the study. The animals were housed (3 rats each cage) in an air conditioned room (12-15 air changes per hour) at the temperature 22 ± 3 °C and 30-70 % relative humidity with a 12 hour light/ dark cycle. They were provided with standard laboratory animal diet (Amrut feed Ltd) and filtered water ad-libitum. The animals were acclimatized for five days prior to the initiation of experiment. The various group of experimental animals used in the study are summarized in Table 1.
Table 1.
Experimental groups
Parameters studied
All experimental animals were examined, once daily, for clinical signs, symptoms and for mortality. Detailed clinical observations (eye abnormalities and apparent functional changes) were made for each animal once before the start of dose administration and thereafter weekly till termination of the study. Body weight of each animal was recorded before initiation (Day zero) and weekly thereafter up to the termination of the study. Body weight of each of the fasted animals was taken, to calculate the organ weight ratio just before their sacrifice. At the end of the treatment and of the recovery period, all animals were kept for fasting overnight before collecting their blood for Hematology and Clinical Chemistry examination. Blood samples were collected via orbital sinus under light CO2 anesthesia. Hematology and Clinical Chemistry parameters were determined by using Beckman Coulter hematology analyzer and Hitachi 902 Clinical Chemistry auto analyzer system, respectively. At the end of the study, all animals from each group were sacrificed for gross and histopathological examination. A detailed gross pathological examination was conducted for external surfaces and orifices. All Organs were weighed individually immediately after necropsy and they were preserved in 10% Formalin for histopathological examinations. The tissues were processed, embedded in paraffin, sectioned and stained with hematoxylin and eosin.
Statistical analysis
All data was expressed as Mean ± S.D. The data of weekly body weight, body weight gain, hematology and clinical Chemistry were compared by ANOVA. Organ weight and percentile organ weight was analyzed by Student's t-test. A 95% confidence level was used to determine statistically significant differences.
RESULTS
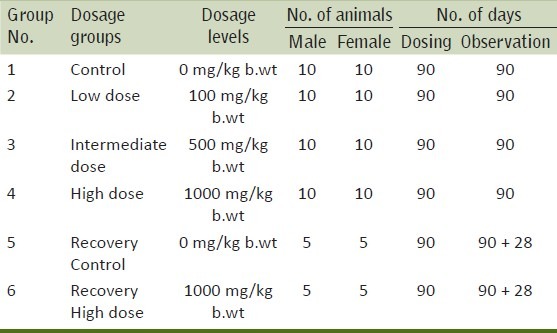
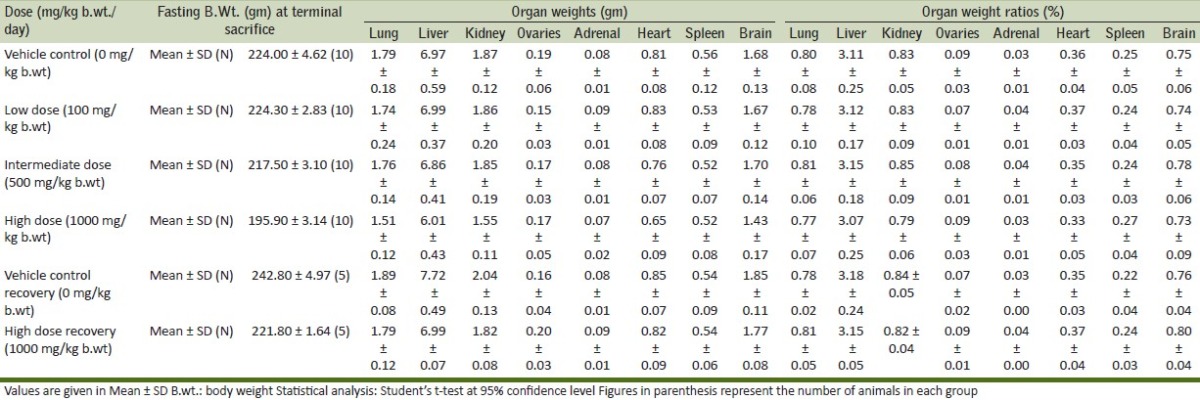
It is evident that there were no clinical signs or symptoms to indicate that the animals were adversely affected by the doses or treatment given throughout the period of the study. This observation is further supplemented by the fact that all the animals were alive at the end of the study. The results of the body weight of all animals during the study are given in Table 2. There was no effect on the mean and percentile body weights of the animals of low dose and intermediate dose groups when compared to their control counterparts, whereas, a significant decrease was observed in the body weight gain of high dose group animals. Evaluation of various hematological parameters like WBC count, RBC count, hematocrit and platelet count of test and recovery group animals did not reveal any changes when compared with the control group animals. As all the parameters fell within the normal range except Hemoglobin which shows slightly higher values in high dose group [Table 3]. The Biochemical parameters like glucose, BUN, GOT, GPT, total protein and creatinine of the test and recovery group animals were comparable to the control counterparts except cholesterol and triglycerides which shows lower values in test groups as compared to control but it was non-significant [Table 4]. Statistical analysis of the weights of organs like lung, liver, kidneys, ovaries, adrenals, heart, spleen and brain did not show any deviations in the test and recovery group animals when compared to their control counterparts [Tables 5 and 6]. Macroscopically and microscopically, no significant changes were noticed in any treated and control group animals. All the changes noticed were incidental and are common findings of the laboratory.
Table 2.
Body weights and body weight gain in rats after 90 days oral exposure
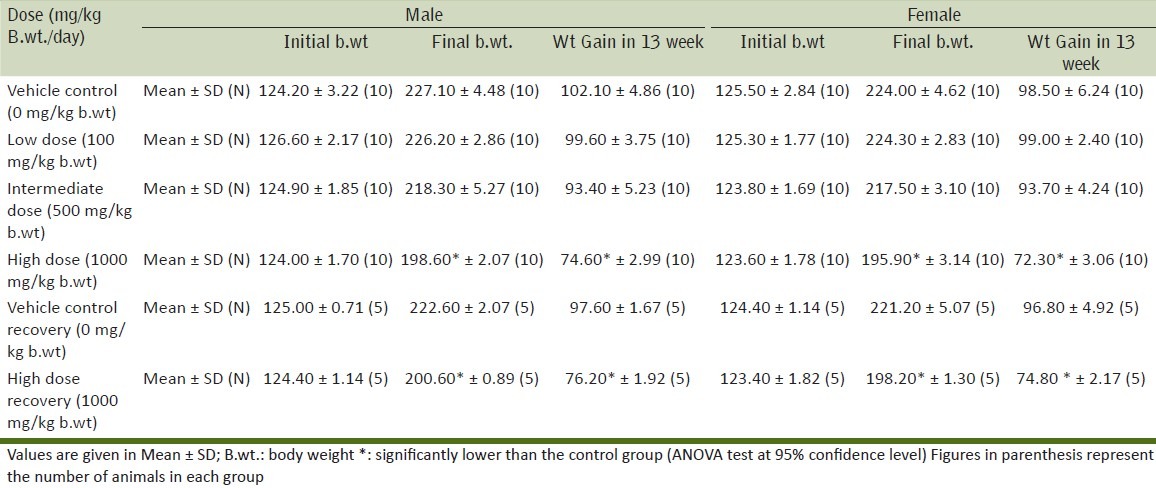
Table 3.
Effect of Boswellia serrata on Hematological parameters after 90 days oral exposure
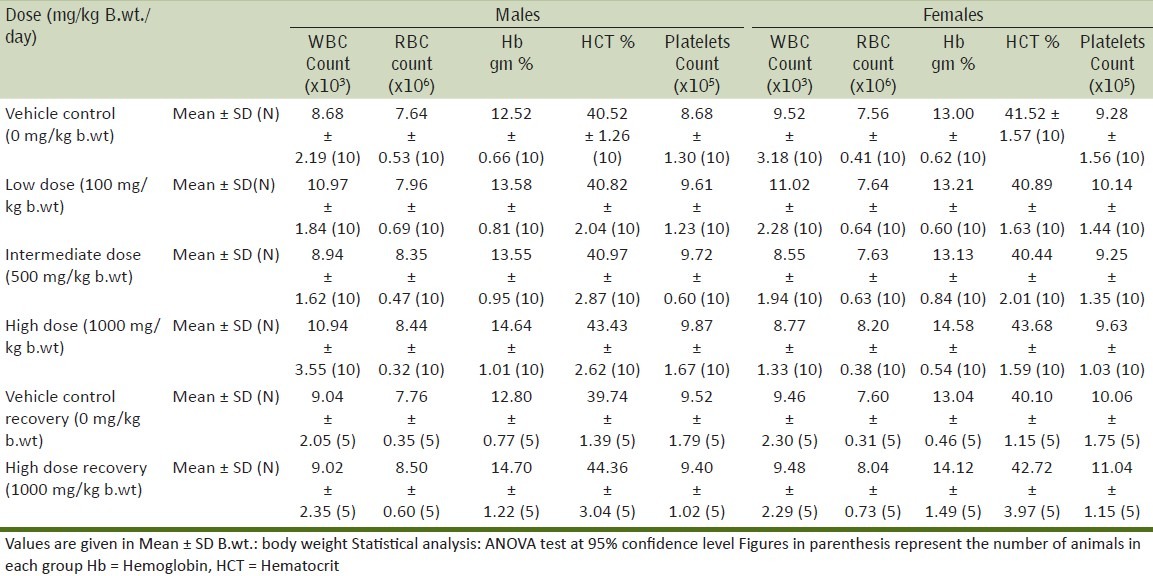
Table 4.
Effect on clinical chemistry parameters after 90 days oral exposure
Table 5.
Effect of Boswellia serrata on organ weights and organ weight ratios-males
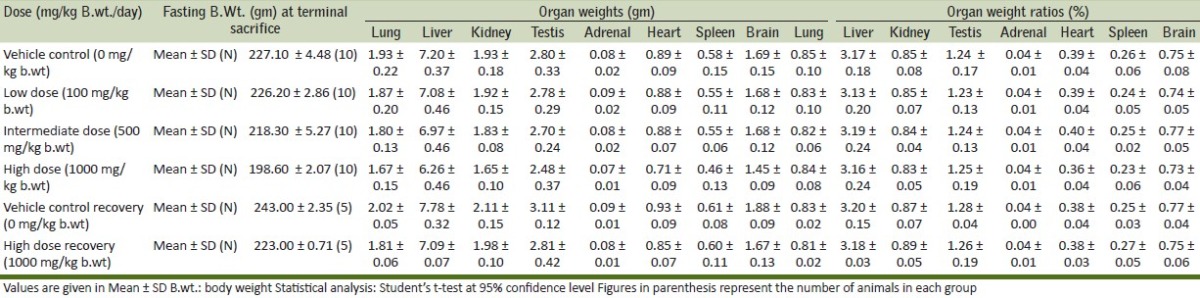
Table 6.
Effect of Boswellia serrata on organ weights and organ weight ratios-females
DISCUSSION
Boswellia serrata has been used extensively in Ayurveda for arthritis and to provide an overall sense of well-being. Boswellia serrata, an Ayurvedic herb; it also promotes healthy cholesterol and triglyceride levels and provides broad health and immunomodulating benefits. The present study results indicate that Boswellia serrata did not produce any toxic signs and symptoms or mortality therefore indicative of its safety.
Based on the observations and results obtained from various studies, it can be said that the Boswellia serrata given to the animals did not show any mortality as well as any adverse impact on the health of animals. As reported in the literature, the metabolic rate of both male and female rats is governed by the functions of liver. The body weight is expected to rise with time at standard laboratory rate. Any deviations on either side i.e. increase or decrease in body weight of the animals would be ascribed to the functioning of the organs mainly the liver. A significant decrease in body weight gain was noticed in the animals of high dose group [Table 2]. The reason for this decrease might be that as Boswellia serrata is a rich source of guggalsterones, which helps in (gugglesterones) stimulating the thyroid, leading to metabolic up-regulation, an increase in thyroid efficieny, increased caloric burn and therefore possible weight loss.[15] This all may lead to the reduction in the biosynthesis of cholesterol and may stimulate cholesterol's conversion to bile acids and increased faecal excretion without having any effect on the Biochemical and Hematological parameters, organ weights and Histopathology. The exact underlying mechanism of the decreased body weight gain needs to be further clarified. From the results of haematological parameters, it is evident that all the animals were found to show higher values of haemoglobin with the treatment. This indicates that with the administration of Boswellia serrata, animals showed an improvement in major haematological parameters. Similarly, the data of cholesterol and triglyceride suggest that the functioning of liver improved with the increasing dose of Boswellia serrata. It may be said that the Boswellia serrata acts positively for the health factors of animals both females and males. Now, it may be noted that for almost all the parameters, the trend was reversed as soon as the dosing of Boswellia serrata was stopped. This can be understood from the values of critical parameters which were found to be similar to the ones observed for control animals. This can therefore be said that the effect of dosing the animals with the Boswellia serrata is reversible and the animal starts to exhibit similar parameters of health as observed with the control animals, once the treatment is over.
As there was a decrease in the body weight gain of the animals of high dose group (1000 mg/kg B. wt.), therefore it may be concluded that the NOAEL i.e. No observed Adverse Effect Level of Boswellia serrata came out as 500 mg/kg B. wt. /day since no reduction in the body weight gain was observed at 100 and 500 mg/kg B. wt.
ACKNOWLEDGEMENTS
The authors are thankful to the Arjuna Natural Extracts Ltd for providing the drug and Director, Shriram Institute for Industrial Research, Delhi for their kind support and guidance for this research work.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Alternative Medicine Review. 2008;13(number 2) [Google Scholar]
- 2.Singh GB, Atal CK. Pharmacology of an extract of Salai guggal ex-Boswellia serrata, A new non steroidal anti-inflammatory agent. Agents Actions. 1986;18:407–12. doi: 10.1007/BF01965005. [DOI] [PubMed] [Google Scholar]
- 3.Sharma ML, Bani S, Singh GB. Anti-arthritic activity of boswellic acids in bovine serum albumin (BSA)-induced arthritis. Int J Immunopharmacol. 1989;11:647–52. doi: 10.1016/0192-0561(89)90150-1. [DOI] [PubMed] [Google Scholar]
- 4.Gupta I, Parihar A, Malhotra P, Gupta S, Ludtke A, Safayhi H, et al. Effects of gum resin of Boswellia serrata in patients with chronic colitis. Planta Med. 2001;67:391–5. doi: 10.1055/s-2001-15802. [DOI] [PubMed] [Google Scholar]
- 5.Gupta I, Gupta V, Parihar A, Gupta S, Ludtke R, Safayhi H, et al. Effects of Boswellia serrata gum resin in patients with bronchial asthma: Results of a double-blind, placebo controlled, 6-week clinical study. Eur J Med Res. 1998;3:511–4. [PubMed] [Google Scholar]
- 6.Chopra RN, Nayar SL, Chopra IC. New Delhi: Council of Industrial and Scientific Research; 1956. Glossary of Indian medicinal plants; p. 39. [Google Scholar]
- 7.Pandey RS, Singh BK, Yamini B. Tripathi, Extract Of Gum Resins Of Boswellia serrata L. Inhibits Lipopolysaccheriode Induced Nitric Oxide Production In Rat Macrophages Along With Hypolipidemic Property. Indian J Exp Biol. 2005;43:509–6. [PubMed] [Google Scholar]
- 8.Shao Y, Ho CT, Chin CK, Badmaev V, Ma W, Huang MT. Inhibitory activity of Boswellic acids from Bosweliia serrata against human leukemia HL-60 cells in culture. Planta Med. 1998;64:328–31. doi: 10.1055/s-2006-957444. [DOI] [PubMed] [Google Scholar]
- 9.Safayhi H, Mack T, Sabieraj J, Anazodo MI, Subramanian LR, Ammon HP. Boswellic acids: Novel, specific, nonredox inhibitors of 5-lipoxygenase. J Pharmacol Exp Ther. 1992;261:1143–6. [PubMed] [Google Scholar]
- 10.Ammon HP, Safayhi H, Mack T, Sabieraj J. Mechanism of antiinflammatory actions of curcumin and boswellic acids. J Ethnopharmacol. 1993;38:113–9. doi: 10.1016/0378-8741(93)90005-p. [DOI] [PubMed] [Google Scholar]
- 11.Wildfeuer A, Neu IS, Safayhi H, Metzger G, Wehrmann M, Vogel U, et al. Effects of boswellic acids extracted from a herbal medicine on the biosynthesis of leukotrienes and the course of experimental autoimmune encephalomyelitis. Arzneimittelforschung. 1998;48:668–74. [PubMed] [Google Scholar]
- 12.Safayhi H, Boden SE, Schweizer S, Ammon HP. Concentration-dependent potentiating and inhibitory effects of Boswellia extracts on 5-lipoxygenase product formation in stimulated PMNL. Planta Med. 2000;66:110–3. doi: 10.1055/s-2000-11136. [DOI] [PubMed] [Google Scholar]
- 13.OECD Guideline (1998). No. 408 for Testing of Chemicals. “Repeated Dose day Oral Toxicity Study in Rodents”. 1998 Sep 21; [Google Scholar]
- 14.Geneva: WHO; 2000. WHO. General Guidelines for Methodologies on Research and evaluation of Traditional Medicine. [Google Scholar]
- 15.Safayhi H, Sailer ER, Amnon HPT. 5-lipoxygenase inhibition by acetyl-11-keto-b-boswellic acid. Phytomedicine. 1996;3:71–2. doi: 10.1016/S0944-7113(96)80013-4. [DOI] [PubMed] [Google Scholar]


