Abstract
Aqueous extract of the date palm (Phoenix dactylifera L.) pits was prepared and its antigenotoxic activity was evaluated against N-Nitroso-N-methylurea (NMU) induced mutagenic effect in mice, using chromosome aberration (CA), micronuclei (MN) and DNA fragmentation assays as experimental end points in male mice. Date pits extract (DPE) was given orally to mice at the dose 25 mg/25 g mouse for successive five days in a week up to four consecutive weeks. NMU was used as mutagen and was given intraperitoneal (i.p) injection at single dose 80 mg/kg b.w., 24 hr after last dose of DPE in pre-treatment regimen and 24 hr before the first dose of DPE in the post-treatment regimen. Mice were scarified after one, two and seven days after the end of treatment. The results have shown that pre-and post-treatment regimens of DPE were significantly restored the DNA damage induced by NMU, as revealed by lowering of the occurrence of CAs and MN in bone marrow cells and inhibition of hepatic DNA fragmentation. These findings suggested that DPE produced their inhibitory activity either by desmutagenic or bioantimutagenic manner in pre-and post-treatment regimens respectively.
Keywords: Chromosome aberration assay, date palm pits, DNA fragmentation assay, micronucleus assay, N-Nitro-N-methylurea
INTRODUCTION
Fruit seeds have not generally received much attention as antioxidant sources and this could be due to their lack of popularity and lack of commercial applications. However, there are considerably higher ratios of by-products arising from fruit-processing plants and derived products have experienced growing worldwide popularity.[1]
The date palm (Phoenix dactylifera L.) has been an important crop in Egypt and Middle Eastern countries. As per recent information, date production of Egypt alone represented about 20% of the total world production for 2008.[2,3] In ancient Egypt the pollen grains of the date palm have been used to improve fertility in women. For Muslims all over the world date are of religious importance and are mentioned in many places in the Holy Quran. They are customarily used to break the day long fast during the holy month of Ramadan.[4] In Egypt 2006; productive date palms were estimated at 11,888,020 million trees and produced 1,328,720 tons of dates.[2] Therefore, utilization of date by-products, particular seeds, is very important to date cultivation and to increase the national income to this sector which supports over one million people. Recently, date pit powders are marketed and are a source of choice to people preferring a non-caffeinated coffee with coffee-related flavor.[5]
Experimental studies have shown that feeding rats with the aqueous extract of date flesh or pits exhibit gastroprotective, hepatoprotective and nephroprotective effects in rodents.[6–8] The date kernels have also been reported to exhibit anti-aging properties and significantly reduce skin wrinkles.[9] What's more, date pits were grind and added to the feed of domesticated animals to enhance growth and this was ascribed to an increase in the plasma level of estrogens or testosterone.[5,10,11]
N-Nitroso compounds (NOC) represent a major class of important chemical carcinogens and mutagens which have been implicated as a hazard to human health.[12,13] The presence of these compounds and their precursors in the environment, in certain occupational settings, in diet and also due to the use of tobacco products, cosmetics, pharmaceutical products as well as their endogenous formation in the human body from dietary components, may be potential risk factors in cancer.[14] NOC are divided into two categories: nitrosamines, which require activation to exert their genotoxic effect, and nitrosamide include N-Nitro-N-methylurea (NMU) which spontaneously decompose to form alkylating agent.[15]
NMU induces various cancers in animal models include: squamous cell carcinomas of the forestomach, sarcomas and gliomas of the brain, adeno-carcinomas of the pancreas, leukemia, and lymphomas.[16] The presence of characteristic ras mutations might be a possible fingerprint of nitroso compounds.[17] In 30% of rat colon carcinomas, NMU was reported to induce G to A transitions in codons 12 and 13 of K-ras.[18] Besides, exposure to NMU during pre-implantation, post-implantation, organogenesis or by paternal exposure results in cleft palate and skeletal defects, increased fetal resorption, and fetal growth retardation.[19,20] The increasing appreciation of the importance of NOC as potential human carcinogens stimulated intense research on protective dietary factors in chemical carcinogenesis.
To the best of our knowledge, there are no published data on antigenotoxic activity of date palm pits in mammalian systems. Accordingly, the present investigation is a novel attempt to spotlight on the efficacy of the aqueous date pits extract for reducing DNA damage induced by NMU in male mice.
MATERIALS AND METHODS
Experimental animals
All experiments were performed with male Swiss mice aged 10-14 weeks and weighing 25-30 g. Animals were supplied by Animal House of the National Research Center, Dokki, Cairo, Egypt. Mice acclimated for a period of one week before the beginning of the experiment. The animals were housed in stainless steel polypropylene hanging cages with wood chip bedding, maintained in an experimental room under controlled conditions of temperature, humidity and light. The animals were kept on standard solid pellet diet and water ad libitum.
Chemicals
N-Nitroso-N-methyl urea (NMU; CAS 684-93-5) was purchased from Sigma-Aldrich Chemical Company (St. Louis, MO, USA) dissolve in distilled water. Mice received a single intraperitoneal dose of 80 mg NMU/kg b.wt.
Preparation of date pits extract (DPE)
Date fruits were purchased from a local market in Dokki, Cairo, Egypt. The pits were manually separated from the flesh rip fruit and washed clear of any flesh by water. The dried date pits were oven dried for seven days at 60°C and then finely ground into powder using a stainless-steel blender. The water extract of the date pit of 100 g was made by adding distilled water to coarsely pounded date pit (1:2 ratio, weight to volume), and kept in water bath 100°C for 6 h, repeatedly for five times. The resulting brownish and dark extract were filtered and then stored in fridge in dark place.
Mode of administration of DPE
Mice were given DPE at the dose of 25 mg/25 g mouse by oral gavage for successive five days in a week up to four consecutive four weeks.
Treatment schedule
Two kinds of treatment pre- and post-treatment were given to mice on the basis of the time of administration of DPE to evaluate its preventive and therapeutic potential respectively. Mice were randomly assigned to five main experimental groups as shown below in Table 1.
Table 1.
Experimental design of the current study
At the end one, two and seven day of the last treatment schedule, all animals were injected with colchicine 2h before killing by cervical dislocation. Then all animals were dissected and the samples of bone marrow and liver tissues of each animal were collected for different analysis.
Chromosome aberrations (CAs) assay
Bone marrow preparation for metaphase cells was made according to the standard technique.[21] In brief, mice were i.p injected with colchicine 2 h before scheduled killing by cervical dislocation. Bone marrow cells were collected from both femurs by flushing with 0.56% KCl and incubated for 20 min at 37°C. Cells were centrifuged and pellet was dispersed in fresh and chilled Carnoy's fixation. The slides were prepared by dropping the cells on clean chilled slides; air dried to stain in 5% buffered Giemsa (pH. 6.8). A total 100 well spread metaphase per animal were examined by light microscope at 2500 X magnification for chromosome aberrations.
Micronucleus (MN) assay
The assay was performed according to the standard protocols described by Schmid.[22] Briefly, bone marrow cells were gently flushed out in fetal calf serum, centrifuged at 1000 rpm for 5 min and the supernatant was decanted. Cell pellets were dispersed in 0.25 ml, smeared on clean slides, fixed in 70% methanol, air dried and stained with May-Grunwald/Giemsa protocol. The combination of May–Grunwald and Giemsa was stained PCEs bluish in color. Micronuclei are generally round or almonds shaped, have sharp borders and are generally 5-20% the size of the PCE. For the determination of the frequency of micronucleated polychromatic erythrocytes (MNPCEs), a total 2000 polychromatic erythrocytes (PCE) per animal were analyzed by light microscopy at 1000 X magnification.
Diphenylamine (DPA) assay
DNA fragmentation in liver tissue was carried out according to Wu et al.[23] with some modification. Briefly, about 50 mg of liver tissue were homogenized in lysis buffer pH 8.0 (10mM Tris base, 1mM EDTA and 0.2% triton X-100) and incubated on ice for 20 min. The cell lysate were centrifuged at 12.000 rpm for 30 min at 4°C. The supernatant containing small DNA fragments was separated. The supernatant and pellet were re-suspended in1N and 0.5N of perchloric acid respectively. Then samples heated at 90°C for 20 min and centrifuged at 11.000 rpm for 10 min to remove proteins. Supernatant fractions were reacted with diphenylamine (DPA) for 16-20 hr at room temperature and the developing blue color was measured at 600 nm. DNA fragmentation in samples [(fragmented DNA in supernatant)/fragmented DNA in supernatant + intact DNA in pellet) ×100] were expressed as percentage of total DNA appearing in the supernatant fraction.
Statistical analysis
The results were expressed as means%±standard Error. All data were computerized using the Statistical Package for Social Sciences (SPSS, version 11). The results were statistically analyzed by using one-way analysis of variance (ANOVA) followed by Duncan's multiple range test (DMRT) for comparison between different treatment groups. Statistical significance was set at P < 0.05.
The suppression of mutagenicity was calculated according to Hu et al.[24] using the following formula
Reduction (%) =[(% aberrant cells in group A–% aberrant cells in group B)/(% aberrant cells in group A)]×100
A: represents groups treated with NMU alone
B: represents groups treated with NMU + DPE
RESULTS
Chromosome aberrations (CAs) assay
Data tabulated in Table 2 and Figure 1 pointed up that NMU induced different types of structural and numerical CAs in mouse bone marrow cells. The percentage of CAs reached 22.00, 14.40 and 10.80% after one, two and seven day post-treatment with NMU respectively compared with the control group (3.00%). Such values represent 7.3, 4.8 and 3.5 fold increases as compared with that value of the control group after three treatment periods respectively. This finding implies that, NMU is mutagenic agent in somatic cells. However, mice administrated DPE alone show no significant change in the percentage of CAs at one, two and seven days and their values were near normal value.
Table 2.
Preventive and therapeutic effect of DPE on NMU induced chromosomal aberrations (CAs) in mouse bone marrow cells
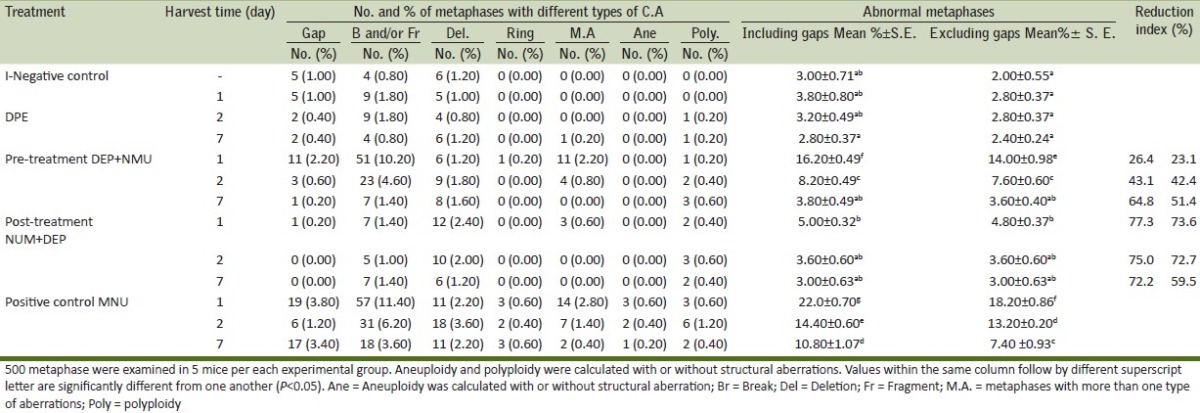
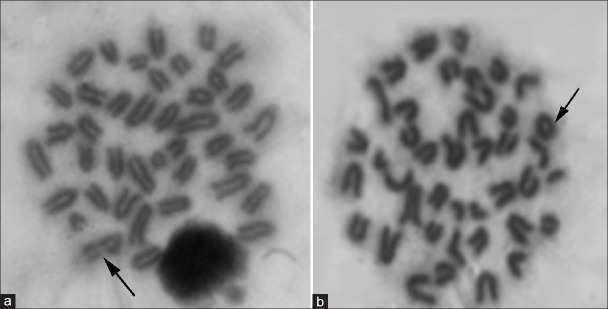
Figure 1.
Metaphases plates from mouse bone marrow cells after i.p. treatment with NMU showing a) break, b) Aneuploidy with ring chromosome.
In view of DPE pretreatment experiments, the results illustrated that DPE was significantly minimized the incidence of CAs at three treatment periods even after excluding gap compared with that observed in the corresponding groups treated with NMU only. The suppression rate reached 26.4, 43.1 and 64.8% after one, two and seven day from the end of the treatment, respectively indicating that DPE had moderate protective effect.
On the other hand, the results of DPE post-treatment experiments showed that the frequency of CAs was significantly lowered after one, two and seven days from the end of treatment with DPE compared with the corresponding groups treated NMU only. The reduction rate reached 77.3, 75.0 and 72.2% at the three treatment periods respectively suggesting that DPE had potential therapeutic effect.
Micronucleus (MN) assay
A remarkable and statistically significant increase in the frequencies of MNPCE was found in groups of mice treated with NMU alone at the three treatment periods compared with the negative control [Table 3, Figure 2].
Table 3.
Preventive and therapeutic effect of DPE on NMU induced Micronucleated polychromatic erythrocytes (MNPCE) in mouse bone marrow cells
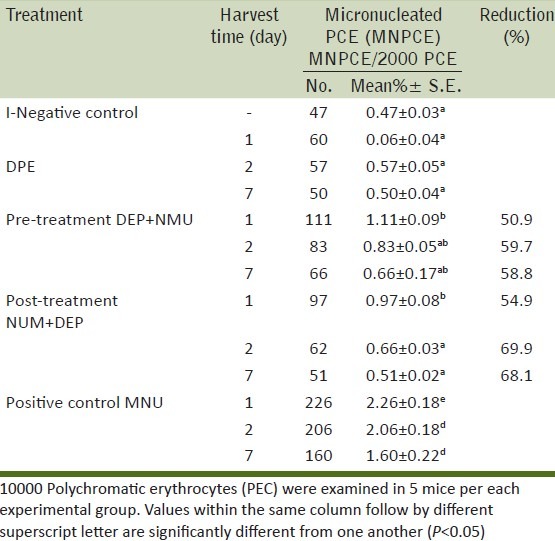
Figure 2.
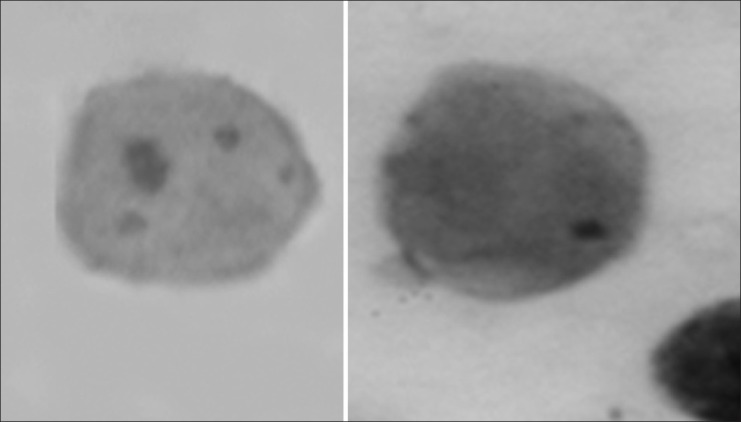
Difference in size of micronuclei from mouse bone marrow cells after i.p. treatment with NMU
Nevertheless, no significant difference in the induction of micronuclei was observed between the groups treated with DPE alone and the negative control. Its percentage reached 0.06±0.04, 0.57±0.05, 0.50±0.04 at the three tested periods one, two and seven days respectively compared with 0.47±0.03 for the control group.
As shown in Table 3, the mean percentage of MNPCE reached 1.11±0.09, 0.83±0.05 and 0.66±0.17 in pre-treatment groups and reached 0.97±0.08, 0.66±0.03 and 0.51±0.02 in post-treatment groups at the three treatment periods one, two and seven day, respectively compared with 2.26±0.18, 2.26±0.18 and 1.60±0.22 for the corresponding groups treated with the NMU only. These results imply that, DPE was significantly minimized the frequency of MNPCE induced by NMU.
DNA fragmentation assay
The pre-and post-treatment effect of DPE against NMU-induced DNA damage was evaluated by measuring the level of genomic DNA fragmentation using the diphenylamine assay [Table 4]. NMU treatment only caused a significant increase in the percentage of liver genomic DNA fragmentation which reached 21.29, 19.46 and 17.35% at three treatment periods one, two and seven days, respectively compared with the control group (6.09%). However, there was no significant change in liver genomic DNA fragmentation in DPE groups compared with the control group.
Table 4.
Preventive and therapeutic effect of DPE on NMU induced genomic DNA fragmentation in mouse hepatocytes
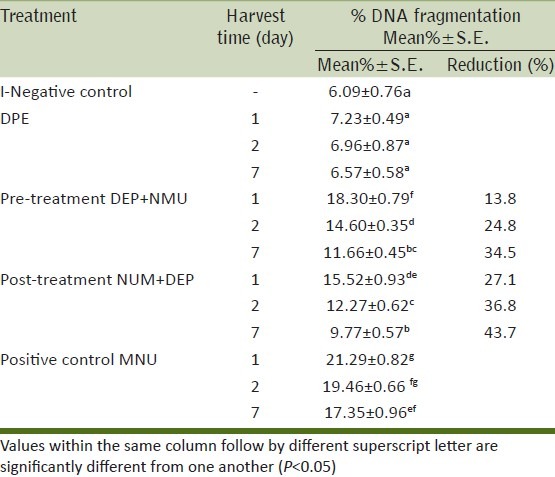
The administration of DPE prior or after NMU was effective in blunting liver genomic DNA fragmentation to as low as 13.8, 24.8 and 34.5% in pre-treatment and 27.1, 36.8 and 43.7% in post-treatment groups at three treatment periods one, two and seven days, respectively.
DISCUSSION
NMU is a well-known direct action mutagen and inflicts DNA damage.[25] Several studies have shown that NMU is genetoxic “in vitro” and “in vivo” through induction of sister chromatid exchange, micronuclei, structural chromosomal aberration, aneuploidy and comet assay.[25–29] Our results confirmed the genotoxicity of NMU by showing an increase in the incidence of CAs, MNPCE and DNA fragmentation over the vehicle control group. In fact, NMU has a half life of <1 h under physiological conditions and its genotoxic effect occurs within a very short period of its mechanism.[30] To further understand mechanism of NMU, Stephanou et al.[26] reported that NMU is alkylating agent able to react with DNA and other biomolecules in most tissues. This reaction takes place by a transfer of methyl group to nitrogen bases of DNA resulting in potential altered bas pairing. Methylation at the O6 position of guanine is considered to be an important lesion, especially G→A transition during DNA replication, with significant role in mutagenesis and carcinogenesis.[31,32] O6-methylguanine (O6MeG) base lesion is known to be signal for apoptosis.[33] Frequency of O6MeG is well correlated with the induction of different mutations in E. coli, mammalian cell cultures as well as in murine germ cells.[34] Apart from methylating DNA bases, NMU enhances peroxidation of lipids, Thereby, generate clastogenic free radical species.[35]
With regard to the genotoxicity of date pits extract (DPE), the results illustrated that DPE by itself didn’t induce any significant difference in the percentage of all tested assays as compared to control suggesting absence of its genotoxic effect. Similar findings were obtained by Vayalil[36] who proven that fruit extract possessed antimutagenic properties in the Ames mutagenicity assay. The extract caused a dose-dependent inhibition of benzo (a) pyrene-induced mutagenecity on Salmonella tester strains TA-98 and TA-100 with metabolic activation.
Analysis of CAs was reflected that efficacy of DPE in reducing the frequency of different types of CAs induced by MNU in bone marrow cells in particular aneuploidy and ring chromosome. This finding implies that DPE have antimutagenic and protect the cells from the aneugenic effect of MNU.
The micronucleus (MN) test is able to assess both the clastogenic and aneugenic properties of a test compound. Micronuclei are cytoplasmic chromatin-containing bodies formed when a centric chromosome fragments or intact chromosomes lag during anaphase and fail to become incorporated into daughter cell nucleus during cell division.[37] In the present study, micronuclei analysis showed that NMU induced micronuclei in mouse bone marrow cells. This is due to clastogenic and aneugenic effect of NMU. These activities are largely documented in previous reports.[25,26,29] On the other hand, DPE have proven an efficacy in reduction of number of micronuclei induced by NMU. This reduction is marker of enhanced DNA repair in the cells or due to cell death or apoptosis of heavy DNA damage.[38]
Apoptosis is a normal event that occurs both during and after development. This phenomenon occurs in cells injured by certain levels of toxic agents.[39] The diphenylamine assay is a very useful method for measuring apoptosis by determining the percentage of fragmentation of DNA into oligosomal-sized fragments. Measure of soluble DNA released from apoptotic nuclei into the cytoplasm constitutes a quantitative measure of cellular response.[40] Hepatocytes were chosen as indicators of apoptosis because of their high metabolic capacity which enables a highly sensitive test system for antimutagenicity and detoxification properties.[41]
The present findings show that, DPE reduced the DNA fragmentation in mouse hepatoctes indicating its hepatoprotective effect. These results are consistent with Al-Qarawi et al.[6] who reported that pre- and post-treatment with aqueous extract of date flesh or pits significantly reduced CCl4 induced hepatoxicity in mice by elevation of bilirubin concentration, hepatic marker enzymes and concomitantly increased the level of antioxidant enzymes.
Basically, there are two main groups of protective mechanisms for DNA, desmutagenic and bio-antimutagenic according to mode of action. Desmutagenesis could be detected with pre-treatment, while bio-antimutagenicity could be better detected with post-treatment.[42] The two regimens treatment used in the present study could lead to a better manner of understanding the antimutagenicity mechanisms of DPE.
Our data have shown that pretreatment regimen was significantly minimized the genotoxicity of NMU in all examined assays however the genotoxicity was not recovered to the normal control value. These findings indicated that DPE interacted extracellulary with NMU prior to attack DNA in desmutagenic manner. This view supported by De Flora et al.[43] who reported that desmutagenic agents are compounds that act directly on mutagens, or on their precursors, in order to inactivate them; they can bind to the mutagenic compound in an irreversible way, inactivating it chemically through a direct link or inhibiting activation by the modulation of the phase I and II enzymes.
It is well documented that, the mutagenic and carcinogenic actions of alkylating agents may be related to their ability to form O6-methylguanine (O6MeG).[26] O6MeG is repaired by protein termed O6-methylguanine DNA methyltransferase (MGMT).[44] MGMT directly repair O6MeG lesion in DNA by transferring methyl group from a base lesion to a cysteine residue, thereby restoring the integrity of the DNA without creating additional DNA damage,[45] cells which unable to repair O6MeG because of lack of expression of MGMT are highly sensitive to O6MeG agents compared to MGMT competent cells.[46] In contract, over expression of MGMT has been shown to render tissues more resistant to the carcinogenic effects of alkylating agents. Post-treatment experiments illustrated that DPE has more pronounced effect in reduction genotoxic effect induced by NMU in all examined assays but the genotoxicity was nearly recovered to the normal control value. These results indicated DPE acts intracellulary by the DNA repair damage induced by NMU in a bioantimutagenic manner. This means that DPE have the ability of anti-alkylation damage and improves the activity of MGMT. This view supported by De Flora[43] who reported that the bioantimutagenic agents act on the physiological mechanisms of DNA protection and repair, reverting the mutagenic effects and preventing the fixation of mutations.
It is noteworthy; two regimens of DPE treatment were resulted in an earlier recovery of bone marrow cells and hepatocytes compared to mice treated with NMU only. This reflected that DPE had effect on the acceleration of cell recovery by restitution of damage parts of DNA. Nevertheless, DPE had more pronounced inhibitory rate in post-treatment compared with pre-treatment regimens in all examined assay.
The mechanism by which date pits enables to suppress genotoxic effect of NMU is not certain. However, the earlier studies reported that date pits contain higher quantity of protein (5.1 g/100 g) and fat (9.0 g/100 g) such as oleic, linoleic, lauric, palmitic, myristic and stearic when compared to date flesh. It is also high in dietary fiber (73.1g/100 g), phenolics (3942 mg/100 g), antioxidants (80,400 μmol/100 g) and trace elements such as potassium, manganese, calcium, phosphorus, sodium, iron, aluminum, cadmium, chloride, lead, sulfur, and fluoride in various proportions.[47,48] It is logical to assume that cumulatively the presence of all these components in the date pits may have been responsible for reduction of DNA binding and methylation with NMU. It is a well know that 1-alkane diazotic acid is the central inactive metabolite of NMU, which is formed directly from hydrolysis of NMU[28] and is transformed to a free radical through the microsomal cytochrome P-450-dependent enzymes, resulting in activation of NMU toxicity.[35] Therefore, the antigenotoxicity of date pits is due to its ability to scavenge the alkyl radical or inhibit the aromatase activity of cytochrome P-450 or blocking the reaction between methane diazonium ion and DNA. Indeed, “in vitro” studies have shown that the aqueous extract of date fruit was a potent scavenger of superoxide and hydroxyl radicals and to inhibit iron-induced lipid peroxidation and protein oxidation in the rat brain homogenate in a concentration-dependent manner.[36] Animal studies have also shown that oral feeding of p-coumaric acid present in date increases the expression of antioxidant enzyme genes in rat cardiac tissue.[49]
CONCLUSIONS
Our data illustrated that DPE produced their inhibitory activity either by pre-treatment or by post-treatment. These results suggested that DPE interacted with intact NMU extracellulary, in a desmutagenic manner, and act intracellulary by affect the DNA repair damage induced by NMU in a bioantimutagenic manner. The observed antimutagenic activity may be attributed to the presence of mineral and various photochemical of diverse chemical structure. Also, these findings suggested that date by-products are considered as an inexpensive source of natural antioxidant and can be exploited economical in pharmaceutical industries.
Therefore, further studies should be identifying the active compounds of DPE and the application other “in vivo”” and “in vitro” genotoxic end-points to contribute to understanding of mechanism of action of the chemopreventive strategies of DPE.
ACKNOWLEDGMENT
This work is supported by National Research Center, Dokki, Cairo, Egypt. The authors thank Prof. Dr. Aida I. El-makawy for critical reading of the manuscript and her helpful suggestions.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Soong Y, Barlow PJ. Antioxidant activity and phenolic content of selected fruit seeds. Food Chem. 2004;88:411–7. [Google Scholar]
- 2.Part III: Plant Production, Statistics Division. Vol. 28. Arab Organization for Agricultural Development: ANS Publisher; 2008. Arab Organization for Agricultural Development (AOAD). Arab Agricultural Statistics Yearbook. [Google Scholar]
- 3.El-Juhany LI. Degradation of date palm trees and date production in Arab countries: causes and potential rehabilitation. Aust J Basic and Appl Sci. 2010;4:3998–4010. [Google Scholar]
- 4.Al-Shahib W, Marshall RJ. The fruit of the date palm: Its possible use as the best food for the future. Int J Food Sci Nutr. 2003;54:247–59. doi: 10.1080/09637480120091982. [DOI] [PubMed] [Google Scholar]
- 5.Baliga MS, Baligab BRV, Kandathilc SM, Bhatd HP, Vayalile PK. A review of the chemistry and pharmacology of the date fruits (Phoenix dactylifera L.) Food Res Inter. 2011;44:1812–22. [Google Scholar]
- 6.Al-Qarawi AA, Mousa HM, Ali BEH, Abdel-Rahman H, El-Mougy SA. Protective effect of extracts from Dates (Phoenix dactylifera L.) on carbon tetrachloride–induced hepatotoxicity in rats. Intern J Appl Res Vet Med. 2004;2:176–80. [Google Scholar]
- 7.Al Qarawi AA, Abdel-Rahman H, Ali BH, Mousa HM, El-Mougy SA. The ameliorative effect of dates (Phoenix dactylifera L.) on ethanol-induced gastric ulcer in rats. J Ethnopharmacol. 2005;98:313–7. doi: 10.1016/j.jep.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 8.Al Qarawi AA, Abdel-Rahman H, Mousa HM, Ali BH, El-Mougy SA. Nephroprotective Action of Phoenix dactylifera in gentamicin-induced nephrotoxicity. Pharmaceut Biol. 2008;46:227–30. [Google Scholar]
- 9.Bauza E. Date palm kernel extract exhibits antiaging properties and significantly reduces skin wrinkles. Int J Tissue React. 2002;24:131–6. [PubMed] [Google Scholar]
- 10.Elgasim EA, Alyousif YA, Homeida AM. Possible hormonal activity of date pits and flesh fed to meat animals. Food Chem. 1995;52:149–50. [Google Scholar]
- 11.Ali BH, Bashir AK, Al Hadrami G. Reproductive hormonal status of rats treated with date pits. Food Chem. 1999;66:437–41. [Google Scholar]
- 12.Olajos EJ, Coulston F. Comparative toxicology of N-nitroso compounds and their carcinogenic potential to man. Ecotoxicol Environ Saf. 1978;2:317–67. doi: 10.1016/s0147-6513(78)80008-6. [DOI] [PubMed] [Google Scholar]
- 13.Gichner T, Velemínský J. Inhibitors of N-nitroso compounds-induced mutagenicity. Mutat Res. 1988;195:21–43. doi: 10.1016/0165-1110(88)90014-0. [DOI] [PubMed] [Google Scholar]
- 14.Coulston F, Olajos EJ. Toxicology of N-nitroso compounds. Toxicol Ecotoxicol Environ Saf. 1982;6:89–6. doi: 10.1016/0147-6513(82)90083-5. [DOI] [PubMed] [Google Scholar]
- 15.de KoK TM, van Mannen JMS. Evaluation of fecal mutagenicity and colorectal cancer risk. Mutat Res. 2000;463:53–101. doi: 10.1016/s1383-5742(00)00003-x. [DOI] [PubMed] [Google Scholar]
- 16.Musatov SA, Anisimov VN, André V, Vigreux C, Godard T, Sichel F. Effects of melatonin on N-nitroso-N-methylurea-induced carcinogenesis in rats and mutagenesis “in vitro” (Ames test and COMET assay) Cancer Lett. 1999;138:37–44. doi: 10.1016/s0304-3835(98)00365-6. [DOI] [PubMed] [Google Scholar]
- 17.Magee PN. The experimental basis for the role of nitroso compounds in human cancer. Cancer Surv. 1989;8:207–39. [PubMed] [Google Scholar]
- 18.Jacoby RF, Alexander RJ, Raicht RF, Brasitus TA. K-ras oncogene mutations in rat colon tumours induced by MNU. Carcinogenesis. 1992;13:45–9. doi: 10.1093/carcin/13.1.45. [DOI] [PubMed] [Google Scholar]
- 19.Nagao T, Morita Y, Ishizuka Y, Wada A, Mizutani M. Induction of fetal malformations after treatment of mouse embryos with methylnitrosourea at the preimplantation stages. Teratog Carcinog Mutagen. 1991;11:1–10. doi: 10.1002/tcm.1770110102. [DOI] [PubMed] [Google Scholar]
- 20.Wada A, Nagao T. Induction of congenital malformations in mice by paternal methylnitrosourea treatment. Congenit Anom (Kyoto) 1994;34:65–70. [Google Scholar]
- 21.Julian PR, Deam BJ, Galloway S, Holden H, Mcfee AF, Shelby M. Mammalian “in vivo” cytogenetic assays: analysis of chromosomes aberrations in bone marrow cells. Mutat Res. 1987;189:157–65. doi: 10.1016/0165-1218(87)90021-8. [DOI] [PubMed] [Google Scholar]
- 22.Schmid W. The micronucleus test. Mutat Res. 1975;31:9–12. doi: 10.1016/0165-1161(75)90058-8. [DOI] [PubMed] [Google Scholar]
- 23.Wu B, Iwakiri R, Ootani A, Tsunada S, Ujise T, Skata H, et al. Dietary corn oil promotes colon cancer by inhibiting mitochondria dependent apoptosis in azoxymethane-treated rats. Exp Biol Med (Maywood) 2004;299:1017–25. doi: 10.1177/153537020422901005. [DOI] [PubMed] [Google Scholar]
- 24.Hu Q, Xu J. Antimutagenicity of selenium-enriched rice on mice exposure to cyclophosphamide and mitomycin C. Cancer Lett. 2005;220:29–35. doi: 10.1016/j.canlet.2004.06.041. [DOI] [PubMed] [Google Scholar]
- 25.Vrzoc M, Petras ML. Comparison of alkaline single cell gel Comet/ and peripheral blood micronucleus assays in detecting DNA damage caused by direct and indirect acting mutagens. Mutat Res. 1997;381:31–40. doi: 10.1016/s0027-5107(97)00143-7. [DOI] [PubMed] [Google Scholar]
- 26.Stephanou G, Vlastos D, Vlachodimitropoulos D, Demopoulos NA. A comparative study on the effect of MNU on human lymphocyte cultures “in vitro” evaluated by 06-mdG formation, micronuclei and sister chromatid exchanges induction. Cancer Lett. 1996;109:109–14. doi: 10.1016/s0304-3835(96)04432-1. [DOI] [PubMed] [Google Scholar]
- 27.Heo MY, Sohn SJ, Au WW. Anti-genotoxicity of galangin as a cancer chemopreventive agent candidate. Mutat Res. 2001;488:135–50. doi: 10.1016/s1383-5742(01)00054-0. [DOI] [PubMed] [Google Scholar]
- 28.Sohn SJ, Huh IH, Au WW, Heo MY. Antigenotoxicity of galangin against N-methyl-N-nitrosourea. Mutat Res. 1998;402:231–6. doi: 10.1016/s0027-5107(97)00302-3. [DOI] [PubMed] [Google Scholar]
- 29.Okada E, Fujiishi Y, Yasutake N, Ohyama W. Detection of micronucleated cells and gene expression changes in glandular stomach of mice treated with stomach-targeted carcinogens. Mutat Res. 2008;657:39–42. doi: 10.1016/j.mrgentox.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 30.Lu SJ, Archer MC. Ha-ras oncogene activation in mammary glands of N-methyl-N-nitrosourea-treated rats genetically resistant to mammary adenocarcinogenesis. Proc Natl Acad Sci USA. 1992;89:1001–5. doi: 10.1073/pnas.89.3.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ellison SK, Dogliotti E, Connors DT, Basu KA, Essigman MG. Site-specific mutagenesis by 06- alkylguanine located in the chromosomes of mammalian cells: Influence of the mammalian O6-alkylguanine-DNAalkyl transferase. Proc Natl Acad Sci USA. 1989;86:8620–4. doi: 10.1073/pnas.86.22.8620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lewandowska J, Bartoszek A. DNA methylation in cancer development diagnosis and therapy multiple-opportunities for genotoxic agent to act as methyl disruptor remediators. Mutagenesis. 2011;26:475–87. doi: 10.1093/mutage/ger019. [DOI] [PubMed] [Google Scholar]
- 33.Meikrantz W, Bergom MA, Memisoglu A, Samson L. O6-Alkylguanine DNA lesions trigger apoptosis. Carcinogenesis. 1998;19:369–72. doi: 10.1093/carcin/19.2.369. [DOI] [PubMed] [Google Scholar]
- 34.Van Zeeland AA. Molecular dosimetry of alkylating agents: quantitative comparison of genetic effects on the basis of DNA adduct formation. Mutagenesis. 1988;3:179–91. doi: 10.1093/mutage/3.3.179. [DOI] [PubMed] [Google Scholar]
- 35.Zakhidov ST, Marshak TL, Smirnova OB, Paranyushkina LP. Modifying cytogenetic effects of gossypol and some derivatives. Izv Akad Nauk Ser Biol. 1994;4:694–700. [PubMed] [Google Scholar]
- 36.Vayalil PK. Antioxidant and antimutagenic properties of aqueous extract of date fruit (Phoenix dactylifera L. Arecaceae) J Agric Food Chem. 2002;50:610–7. doi: 10.1021/jf010716t. [DOI] [PubMed] [Google Scholar]
- 37.Bonassi S, Znaor A, Ceppi M, Lando C, Chang WP, Holland N, et al. An increased micronucleus in peripheral blood lymphocytes predicts the risk of cancer in humans. Carcinogenesis. 2007;28:625–31. doi: 10.1093/carcin/bgl177. [DOI] [PubMed] [Google Scholar]
- 38.Vukicevic V, Kampfinger K, Stopper H. Influence of altered apoptosis in human lymphoblastoid cell lines on micronucleus frequency. Toxicol Lett. 2004;147:187–95. doi: 10.1016/j.toxlet.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 39.Hale AJ, Smith CA, Sutherland LC, Stoneman VEA, Longthorne VL, Culhane AC. Apoptosis: Molecular regulation of cell death. Eur J Biochem. 1996;236:1–26. doi: 10.1111/j.1432-1033.1996.00001.x. [DOI] [PubMed] [Google Scholar]
- 40.Gercel-Taylor C. Diphenylamine assay of DNA fragmentation “in vivo” models, imaging and molecular regulator. In: Blumenthel RD, editor. Chemosensitivity, “In vivo” models, imaging and molecular regulator. Vol. 2. New Jersey: Humana press; 2005. pp. 79–82. [DOI] [PubMed] [Google Scholar]
- 41.Eckl PM, Bresgen N. The cultured primary hepatocyte and its application in toxicology. J Appl Biomed. 2003;1:117–26. [Google Scholar]
- 42.De Flora S. Mechanisms of inhibitors of mutagenesis and carcinogenesis. Mutat Res. 1998;402:151–9. doi: 10.1016/s0027-5107(97)00292-3. [DOI] [PubMed] [Google Scholar]
- 43.De Flora S, Lynnette R, Ferguson LR. Overview of mechanisms of cancer chemopreventive agents. Mutat Res. 2005;591:8–15. doi: 10.1016/j.mrfmmm.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 44.Margison GP, Santibanez-Koref MF. O6-alkylguanine-DNA alkyltransferase: role in carcinogenicity and chemotherapy. Bioessays. 2002;24:255–66. doi: 10.1002/bies.10063. [DOI] [PubMed] [Google Scholar]
- 45.Kaina B, Christmann M. DNA repair in resistance to alkylating anticancer drugs. Int J Clin Pharmacol Ther. 2002;40:354–67. doi: 10.5414/cpp40354. [DOI] [PubMed] [Google Scholar]
- 46.Kaina B, Fritz G, Mitra S, Coquerelle T. Transfection and expression of human O6-methylguanine-DNA methyltransferase (MGMT) cDNA in Chinese hamster cells: the role of MGMT in protection against the genotoxic effects of alkylating agents. Carcinogenesis. 1991;12:1857–67. doi: 10.1093/carcin/12.10.1857. [DOI] [PubMed] [Google Scholar]
- 47.Al Farsi MA, Lee CY. Nutritional and functional properties of dates: a review. Crit Rev Food Sci Nutr. 2008;48:877–87. doi: 10.1080/10408390701724264. [DOI] [PubMed] [Google Scholar]
- 48.Nehdi I, Omri S, Khalila MI, Al-Resayes SI. Characteristics and chemical composition of date palm (Phoenix canariensis) seeds and seed oil. Ind Crop Pro. 2010;32:360–5. [Google Scholar]
- 49.Yeh CT, Ching LC, Yen GC. Inducing gene expression of cardiac antioxidant enzymes by dietary phenolic acids in rats. J Nutr Biochem. 2009;20:163–71. doi: 10.1016/j.jnutbio.2008.01.005. [DOI] [PubMed] [Google Scholar]