Abstract
Cancer is not a single disease but a group of complex genetic diseases of aged cells. Chemoprevention of cancer is the attempt to use natural and synthetic compounds to intervene in the early stages of cancer, before invasive disease begins. Consuming a diet rich in plant foods can provide a milieu of phytochemicals and non-nutritive plant substances that possess health-protective effects. Some phytochemicals derived in spices and herbs as well as other plants possess substantial cancer preventive properties. Thus the cancer chemo preventive potential of naturally occurring phytochemicals is of great interest because of their preventive role and as they are not perceived as “medicine”. During the course of present study Trigonella foenum graecum (L.) seed- TFGS (commonly called fenugreek) extract was given at pre-initiational, post-initiational, promotional and throughout the experiment along with 7,12-dimethylbenz [a] anthracene DMBA and 12-O-tetradecanoylphorbol-13-acetate TPA treatment in Swiss albino mice. A significant reduction of papillomas in DMBA + TPA + TFGS (400 mg/kg. body wt.) treated group was found to be effective in decreasing the rate of tumor incidence in comparison to control. Furthermore, cumulative number of papillomas, tumor yield and tumor burden were also found to be reduced. The TFGS extract treatment before DMBA and TPA application (i.e. Pre initiation) were more effective than that of treatment during, and /or after DMBA treatment, however TFGS extract treatment was most effective when treated throughout all the stages of tumorigenesis. The TFGS treatment also showed a modulatory influence on mouse hepatic antioxidant defense system (GSH and LPO level).
Keywords: Cancer, chemoprevention, DMBA, phytochemicals, TPA, Trigonella foenum graecum
INTRODUCTION
Research has demonstrated that cancer is a largely avoidable disease.[1] It is estimated that more than two-thirds of cancer may be prevented through lifestyle modification.[2–4] Chemoprevention is the attempt to use natural and synthetic compounds to intervene in the early stages of cancer, before invasive disease begins.[5] Natural dietary agents including fruits, vegetables, and spices have drawn a great deal of attention from both the scientific community and the general public due to their various health promoting effects including suppression of cancers, many of them have been used as traditional medicines for thousands of years.[6,7] Some phytochemicals derived in spices and herbs as well as other plants possess substantial cancer preventive properties.[8–11] Chemopreventive agents can be grouped into two major classes: blocking agents and suppressing agents. Blocking agents prevent carcinogenic compounds from reaching or reacting with critical target sites by preventing the metabolic activation of carcinogens or tumor promoters by enhancing detoxification systems and by trapping reactive carcinogens.[12,13] Suppressing agents prevent the evolution of the neoplastic process in cells that would otherwise become malignant.
There have been two major diet-related prevention strategies that have evolved to combat cancer, i.e., cancer chemoprevention and dietary cancer prevention, with appreciable overlap existing between them. Generally, cancer chemoprevention is recognized as the pharmacologic intervention with synthetic or naturally occurring chemicals to prevent, inhibit or reverse carcinogenesis or prevent the development of invasive cancer.[14,15]
Dietary epidemiologic studies have provided initial leads for the identification of numerous naturally occurring chemopreventive agents and laboratory studies have identified many potential agents that suppress carcinogenesis in animal models. So, dietary prevention is considered as the change in food consumption patterns necessary to decrease cancer development.[16] A diet rich in plant foods may provide protection against several chronic diseases including cancers.[17] Differences among individuals, including inherited genetic susceptibility, could also contribute to inconsistent epidemiologic associations between dietary factors and specific cancers.[18,19]
Trigonella foenum graecum (L.), commonly called fenugreek, is an aromatic leguminous plant native to many Asian, Middle Eastern, and European countries.[20] Fenugreek belongs to the subfamily Papilionacae of the family Leguminosae (bean family, Fabaceae). Research has shown that the seeds can inhibit cancer of the liver, lower blood cholesterol levels and also have an antidiabetic effect. The seed can be cooked or sprouted or even eaten raw.[21–24] Fenugreek seeds are a good source of many essential elements such as iron, phosphorus and sulphur.[25] These seeds and leaves have been used extensively in various medicinal preparations.
In the present study attempt has been made to study the chemomodulatory potential of Trigonella foenum graecum (L.) against 7, 12-dimethylbenz (a) anthracene (DMBA) and 12-O-tetradecanoylphorbol-13-acetate (TPA) induced mouse skin papillomagenesis. The intermediate biomarkers for the study are changes in lipid peroxidation (LPO), the status of the antioxidants such as reduced glutathione (GSH) in the liver.
MATERIALS AND METHODS
Animals
Random-bred male Swiss albino mice (8-9 weeks old) were obtained from the animal facility (JNU, New Delhi). The animals were maintained in the animal house at temperature of 24 ± 3°C and a light: dark exposure period of 12 hours: 12 hours. The animals were housed in polypropylene cages and fed with standard mice feed (Hindustan Lever Ltd., India). Tap water was provided ad libitum.
Chemicals
5,5-dithiobis-2-nitrobenzoic acid (DTNB), reduced glutathione (GSH), thiobarbituric acid (TBA), sodium dodecyl sulphate (SDS), 1,1,3,3-tetramethoxy propane (TMP), n-butanol, pyridine, meta phosphoric acid (MPA) were obtained from Qualigens, Himedia Laboratories Ltd., India and Sigma Chemicals Co., USA.7,12-dimethylbenz (a) anthracene (DMBA), 12-O-tetradecanoylphorbol-13-acetate (TPA), colchicine, fetal calf serum (FCS), methanol, acetic acid, saline, may-grunwald and giemsa stain powder were procured from Sigma Chemical Co., USA.
Preparation of Trigonella foenum graecum (L.) seed extracts
Trigonella foenum graecum (L.) seeds ( TFGS ) were collected locally and identified at Herbarium, Department of Botany, University of Rajasthan, Jaipur, India (RUBL 20658). Seeds were air-dried in shade without direct exposure to sun rays and powdered. The extract of seeds of Trigonella foenum graecum (L.) (TFGS) was prepared using Soxhlet apparatus with methanol for 36 hours at 70°C.
Experimental design
Seed extract tolerance study
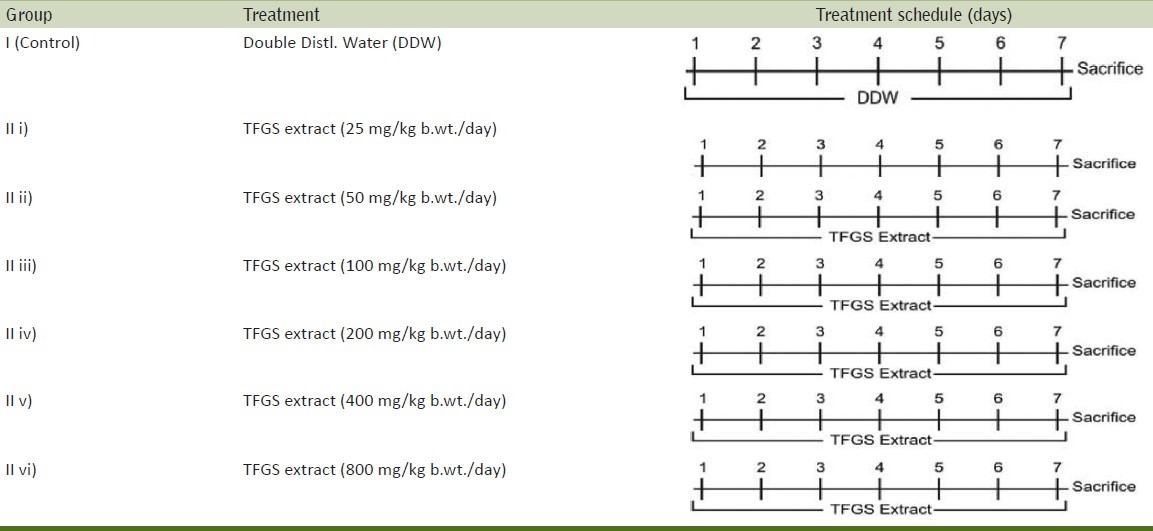
Mice for tolerance study were divided into two groups, group I served as control and group II as treatment group, which was further subdivided into several subgroups with nine animals in each group. Animals of group II were given 25, 50, 100, 200, 400, 800 mg/kg. body weight/day of TFGS in double distilled water by oral gavages for seven consecutive days. All these animals were observed regularly till seven days and no toxic effect were observed in terms of sickness, mortality, morbidity and behaviour in animals treated with different doses (25, 50, 100, 200, 400, 800 mg/ kg. b.wt. / day) of TFGS extract. This suggests that extracts of TFGS can be tolerated by mice up to 800 mg/ kg. b. wt./day.
Experiments were designed to test the modulatory influence of TFGS seed extracts on mouse hepatic lipid peroxidation (LPO) level and reduced glutathione (GSH) content, as follows.
Depending upon the increase of GSH level and decrease in LPO content 400mg/kg. b.wt. of TFGS was selected [Table 1].
Table 1.
Experimental design for seed extract tolerance study
Mouse skin papilloma model
Animals were assorted into control and experimental groups. The animals were marked and body weight was taken. The hair on the dorsal region of the body (back) was removed, three days before the commencement of the experiment and only those animals in the resting phase of the hair cycle were selected for the experiments. Mice were shaved before each treatment to allow a better distribution of the chemical.
Two stage skin carcinogenesis models were used as reported earlier in our lab.[26] For the induction of tumors/papillomas, the two-stage protocol consisting of initiation with a single topical application of the carcinogenDMBA, followed by three times a week treatment with a promoter TPA, was standardized.
Experiments were designed to see the effect of TFGS extract on DMBA / TPA induced skin papillomagenesis. All the animals were divided into two groups and each group was given separate treatments, as follows [Figure 1].
Figure 1.
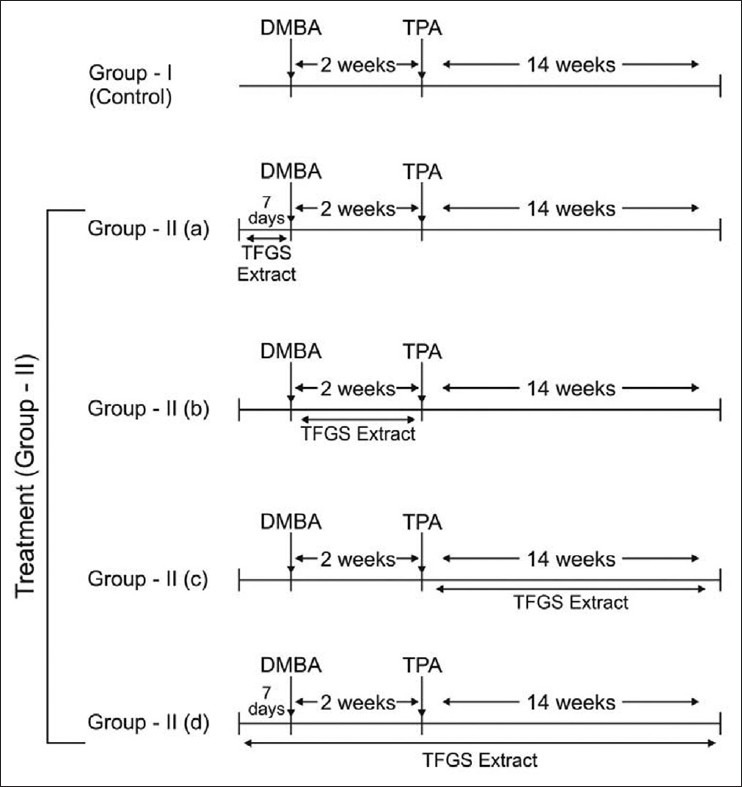
Dose application pattern in control and treatment group
Parameters studied for skin tumor model system
General parameters
-
Tumor Rate / Tumor Incidence
The number of mice carrying at least one tumor, expressed as percent incidence.
-
Tumor yield
Total number of tumors per group and the mean number of tumors per mouse.
Diameter of each tumor
Weight of tumors of each animal at the termination of the experiment.
-
Tumor burden
The average number of tumors per tumor bearing mouse.
Average latent period
It is the time lag between the application of the promoting agent and the appearance of 50% of tumors. The average latent period was computed by multiplying the number of tumors appearing each week by the time in weeks after the application of the promoting agent and dividing the sum by the total number of tumors.
![]()
Where,
F is the number of tumors appearing in each week.
X is the number of weeks.
n is the total number of tumors.
Biochemical study
-
Preparation of Homogenate for Biochemical Studies
Animals were killed by cervical dislocation and the entire liver was then perfused immediately with cold 0.9% NaCl and thereafter carefully removed, trimmed free of extraneous tissue. It was then weighed and blotted dry. For assaying reduced glutathione it was homogenized in ice-cold Tris- KCl buffer (pH 7.4) to yield a 10% (w/v) homogenate. A 0.5 ml aliquot of this homogenate was used for assaying reduced glutathione. For assaying lipid peroxidation this tissue was homogenized in ice-cold 1.15% KCl to yield a 10% (w/v) homogenate. A 0.8 ml aliquot of this homogenate was used for assaying lipid peroxidation.
-
Reduced Glutathione (GSH) Assay
Reduced glutathione was estimated as total nonprotein sulphydryl group by the method as described by Moron et al.[27] Homogenates were precipitated immediately with 0.1 ml of 25% trichloroacetic acid and the precipitate was removed after centrifugation. Free -SH groups were assayed in a total 3 ml volume by adding 2 ml of (0.6 mM) DTNB and 0.9 ml prepared 0.2 M Sodium phosphate buffer (pH 8.0), to 0.1 ml of the supernatant and absorbance was read at 412 nm using a UV-VIS Systronics spectrophotometer. GSH was used as a standard to calculate μmole of - SH content / gm tissue.
-
Lipid Peroxidation (LPO) Assay
Lipid peroxidation in the liver was estimated spectrophotometrically by thiobarbituric acid reactive substances (TBARS) method, as described by Ohkhawa et al.[28] and is expressed in terms of malondialdehyde (MDA) formed per mg of tissue. In brief, 0.8 ml of homogenate was mixed with 0.2 ml of 8.1% Sodium dodesylsulphate (SDS) to which 1.5 ml of 20% acetic acid was added. Then 1.5 ml of 0.6% TBA was added and placed in a water bath for 1 hr at 80°C, cooled in ice and mixed with 5 ml mixture of n-butanol and pyridine (15:1). It is then centrifuged at room temperature for 10 min at 3,000 rpm. The absorbance of the clear supernatant was measured against blank of distilled water at 532 nm.
Statistical analysis
Statistical significance of difference between control and experimental groups was determined by student's t-test and chi-square test.
RESULTS
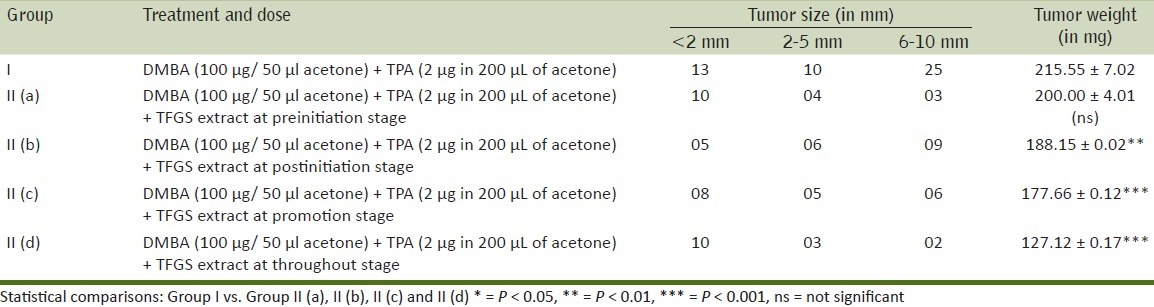
Findings of present investigations are depicted in Tables 2–6. In the control group (Group I), in which a single topical application of DMBA was followed, two weeks later, by repeated application (three times in a week) of TPA, skin papillomas appeared in all the animals (100% tumor incidence). A significant reduction in tumor incidence (60 ± 1.13, 59.92 ± 1.06, 60.66 ± 11.06, 56.65 ± 2.41) at pre, post, promotional and throughout stages of treatment respectively were observed in animals of TFGS extracts treated groups as compared to control group where it is 100% [Table 4].The cumulative number of papillomas during observation period of 16 weeks was significantly reduced in TFGS extract treated group (i.e. 17,20,19,15) at pre, post, promotional and throughout stages of treatment respectively as compared to control group where it is 48 [Table 3]. Whereas average latent period was significantly increased from 9.87 ± 0.15 weeks in control group to 11.58 ± 0.19, 10.44 ± 0.25, 11.11 ± 0.19, 11.96 ± 0.65 weeks in the treated groups [Table 4]. The average weight of tumors (in mg) was also reduced in pre, post, promotional and throughout stages of treatment with TFGS extracts [Table 5].
Table 2.
Modulatory influence of Trigonella foenum graecum (L.) (TFGS) extract on mouse hepatic antioxidant status
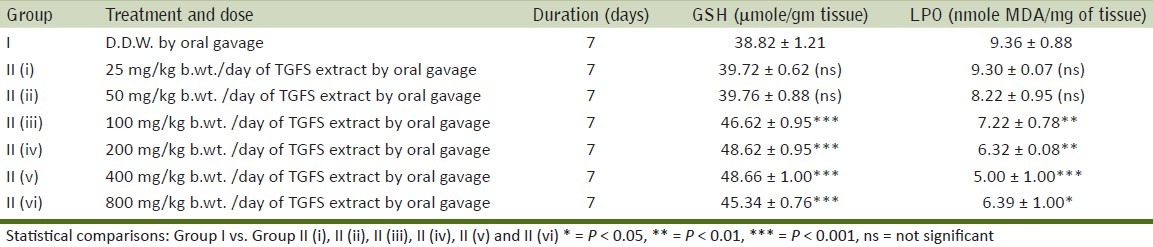
Table 6.
Modulatory influence of TFGS extract on mouse hepatic antioxidant status

Table 4.
Average latent period, tumor burden, tumor incidence recorded after initiation by DMBA followed two weeks later by TPA treatment (three times a week) for 14 weeks with / without TFGS treatment
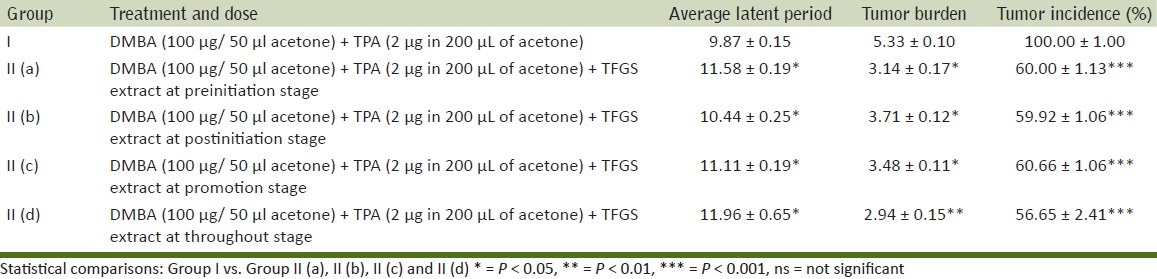
Table 3.
Cumulative number of papillomas recorded after initiation by DMBA followed two weeks later by TPA treatment (three times a week) for 14 weeks with / without TFGS treatment
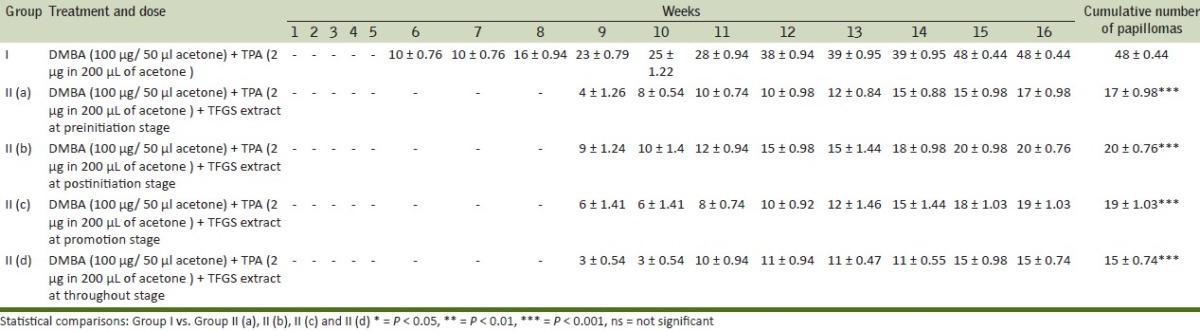
Table 5.
Tumor size (in mm) and tumor weight (in mg) recorded after initiation by DMBA followed two weeks later by TPA treatment (three times a week) for 14 weeks with / without TFGS treatment
The treatment of TFGS (400 mg/kg. b.wt/day) is more effective when given from seven days before the DMBA treatment till the end of the experiment (the level of reduced glutathione -GSH in this group is 35.29 ± 2.09 μmole/ gm and that of LPO is 18.52 ± 1.49 nmole/mg of tissue) followed by the animals treated with TFGS in the preinitiation (there was a significant increase in the level of reduced glutathione (GSH) in this group is 30.46 ± 3.65 μmole/gm and significant decrease in the level of LPO is 25.98 ± 2.68 nmole/mg of tissue) and promotion stages of papillomagenesis. The level of reduced glutathione (GSH) in the control group is 7.05 ± 1.72 μmole/gm and that of LPO is 36.03 ± 3.49 nmole/mg of tissue [Table 6].
DISCUSSION
The present investigation demonstrates the chemopreventive action of Trigonella foenum graecum (L.) seed extract (TFGS) in Swiss albino mice. The drug tolerance study carried out with different doses of TFGS extract in Swiss albino mice has shown most suitable results in terms of increase in GSH level and decrease in LPO at 400 mg/kg. b.wt. dose level. So the rest of the study has been carried out with that particular dose. The TFGS extracts were given at different stages of DMBA and TPA induced skin papillomagenesis to observe the time period at the process of carcinogenesis when the treatment is going to be most effective.
During the course of present study TFGS extract was given at pre-initiational, post-initiational, promotional and throughout the experiment along with DMBA and TPA treatment.
TFGS administered orally at a dose of 400 mg/kg. b.wt. showed a reduction in tumor rate/tumor incidence, tumor yield, cumulative number of papillomas, average weight of tumors remarkably compared to control, with a maximum reduction in the throughout treatment group.
All these observation are reflection of chemopreventive activity of the methanolic extract of TFGS on DMBA induced skin papillomagenesis in Swiss albino mice.
The TFGS extract treatment before DMBA and TPA application (i.e. preinitiation) were more effective than that of treatment during, and /or after DMBA treatment, however TFGS extract treatment was most effective when treated throughout all the stages of tumorigenesis.
This leads to the supposition that the inhibition of tumorigenesis by the seed extract might have been executed either by preventing the formation of active carcinogens from their precursors or by augmenting detoxification process, preventing promotional events in the mouse skin through free radical scavenging mechanism.
The chemopreventive activity of the methanolic extract of TFGS may be due to the rich chemical constituents (such as, saponins, flavonoids, alkaloids, galactomannans) that are present in the seed working synergistically at various stages of angiogenesis.
Diosgenin [(25R)-5-spirosten-3β-ol], a steroid sapogenin constituent of fenugreek seeds suppresses proliferation, osteoclastogenesis and inhibits invasion through inhibition of necrosis factor NF-κB-regulated gene expression and Tumor Necrotic Factor (TNF)-induced activation of AKT.[29,30]
Flavonoids, a group of about 4000 naturally occuring polyphenolic compounds were shown to inhibit various TPA3-induced phenomena, such as protein kinase C, and protein phosphorylation,[31] all of which are believed to represent nonspecific markers of tumor promotion[32].
Seeds of fenugreek contain the alkaloid, trigonelline (0.38%, methyl betaine of nicotinic acid). It yields nicotinic acid on heating with hydrochloric acid at 260–270°C.[33] Trigonelline has shown potential for use in cancer therapy.[34]
Antioxidant potential of plants is known to be closely linked with their cancer chemoprevention properties. Many types of chemoprotectors against cancer evoke large inductions of phase II enzymes of xenobiotic metabolism and increase glutathione levels in animal tissues.
In the present study the significant increase in GSH level and decrease in LPO in the group with TFGS extract treatment before DMBA and TPA application (preinitiation) satisfies the same.
Glutathione, often regarded as the first line of defence against oxidative stress, is the most important cellular thiol that acts as a substrate for several transferases, peroxidases and other enzymes that prevent the detrimental effects of oxygen free radicals.[35,36]
The elevated level of GSH protects cellular proteins against oxidation through glutathione redox cycle and also detoxifies reactive oxygen species directly and/or neutralizes reactive intermediate species generated from exposure to xenobiotics including chemical carcinogens.[37] GSH has been endowed with an important function in maintaining the reduced state of cellular environment, in addition to its conjugating ability owing to nucleophilic center and its involvement in detoxification of xenobiotics that cause toxicity and carcinogenicity. Such a mechanism would decrease the level of reactive electrophiles available to bind DNA, reducing the likelihood of DNA damage and possible induction of carcinogenic process.[38]
Lipid peroxidation products modify the physical characteristics of biological membranes.[39] Incorporation of LOOH, changes the physical structure of the membrane by decreasing the fluidity and increasing the permeability. Furthermore, the decreased lipid peroxidation which is measured by thiobarbituric acid reactive substances (TBARS) in the liver homogenate of TFGS treated mice is correlated well with the induction of antioxidant enzymes above basal level. A wide range of plant products are source of antioxidants and act as modifiers of the carcinogenic process, appear to be the right approach for modifying cancer risk in the population.[40]
The present investigation demonstrated the chemopreventive action in Swiss albino mice against DMBA and TPA, which may be due to the immunomodulatory, antiinflammatory, pharmacological properties of Trigonella foenum graecum (L.) and the mechanism underlying the antiinflammatory action of its seed may be due to the presence of steroidal compounds including two steroidal saponins which, on hydrolysis, give two steroidal sapogenins (diosgenin and gitogenin), flavonoids,galactomannan.[41]
Hence, fenugreek is suggested as a promising protective medicinal herb for complementary therapy in cancer patients under chemotherapeutic interventions.
So it is well accepted that to reduce the occurrence of cancer, one promising approach is its prevention, especially by chemical intervention through minor nutritional dietary constituents.[42]
ACKNOWLEDGEMENTS
The author is thankful to the Department of Zoology, University of Rajasthan, Jaipur and The IIS University, Jaipur for providing facilities to conduct this study.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Shukla S, Gupta S. Apigenin: A promising molecule for cancer prevention. Pharm Res. 2010;27:962–78. doi: 10.1007/s11095-010-0089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oliveria SA, Christos PJ, Berwick M. The role of epidemiology in cancer prevention. Proc Soc Exp Biol Med. 1997;216:142–50. doi: 10.3181/00379727-216-44164. [DOI] [PubMed] [Google Scholar]
- 3.Martinez M, Giovanucci E. Diet and the prevention of cancer. Cancer Metastasis Rev. 1997;16:357–76. doi: 10.1023/a:1005860413247. [DOI] [PubMed] [Google Scholar]
- 4.Washington, DC: American Institute for Cancer Research; 1997. World Cancer Research Fund. Food, nutrition and the prevention of cancer: A global perspective. [DOI] [PubMed] [Google Scholar]
- 5.Greenwald P, Kelloff G, Burch-Whitman C. Chemoprevention. CA Cancer J Clin. 1995;45:31–49. doi: 10.3322/canjclin.45.1.31. [DOI] [PubMed] [Google Scholar]
- 6.Lampe JW. Health effects of vegetables and fruit: Assessing mechanisms of action in human experimental studies. Am J Clin Nutr. 1999;70:475S–90S. doi: 10.1093/ajcn/70.3.475s. Review. [DOI] [PubMed] [Google Scholar]
- 7.Aggarwal BB, Shishodia S. Molecular target of dietary agents for prevention and therapy of cancer. Biochem Pharmacol. 2006;71:1397–421. doi: 10.1016/j.bcp.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 8.Surh YJ. Anti-tumor promoting potential of selected spice ingredients with antioxidative and anti-inflammatory activities: A short review. Food Chem Toxicol. 2002;40:1091–7. doi: 10.1016/s0278-6915(02)00037-6. [DOI] [PubMed] [Google Scholar]
- 9.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–80. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 10.Lai PK, Roy J. Antimicrobial and chemopreventive properties of herbs and spices. Curr Med Chem. 2004;11:1451–60. doi: 10.2174/0929867043365107. [DOI] [PubMed] [Google Scholar]
- 11.Aggarwal BB, Kunnumakkara AB, Harikumar KB, Tharakan ST, Sung B. Potential of spice derived phytochemicals for cancer prevention. Planta Med. 2008;74:1560–9. doi: 10.1055/s-2008-1074578. [DOI] [PubMed] [Google Scholar]
- 12.Kelloff GJ, Boone CW, Crowell JA, Steele VE, Lubet R, Sigman CC. Chemopreventive drug development: Perspectives and progress. Cancer Epidemiol Biomarkers Prev. 1994;3:85–98. [PubMed] [Google Scholar]
- 13.Wattenberg LW. Chemoprevention of cancer. Prev Med. 1996;25:44–5. doi: 10.1006/pmed.1996.0015. [DOI] [PubMed] [Google Scholar]
- 14.Mayne S, Lippman S. Cancer prevention: Chemopreventive agents. In: DeVita V, Hellman S, Rosenberg S, editors. Cancer: Principles and Practices of Oncology. Vol. 5. Philadelphia, PA: Lippincott-Raven Publishers; 1997. pp. 585–99. [Google Scholar]
- 15.Kelloff GJ, Hawk ET, Karp JE, Crowell JA, Boone CW, Steele VE, et al. Progress in clinical chemoprevention. Semin Oncol. 1997;24:241–52. [PubMed] [Google Scholar]
- 16.Goodman G. Cancer prevention: Contrasting dietary modification with intervention agents. Encycl Cancer. 1997;1:199–206. [Google Scholar]
- 17.Singletary K. Diet, natural products and cancer chemoprevention. J Nutr. 2000;130:465S–66S. doi: 10.1093/jn/130.2.465S. [DOI] [PubMed] [Google Scholar]
- 18.Slattery ML, O’Brien E, Mori M. Disease heterogeneity: Does it impact our ability to detect dietary associations with breast cancer? Nutr Cancer. 1995;24:213–20. doi: 10.1080/01635589509514410. [DOI] [PubMed] [Google Scholar]
- 19.Barone M, Sofano K, Tullio ND, Licino R, Albano F, Dileo A. Dietary, endocrine and metabolic factors in tje development of colorectal cancer. J Gastrointest Cancer. 2012;43:13–9. doi: 10.1007/s12029-011-9332-7. [DOI] [PubMed] [Google Scholar]
- 20.Chevallier A. New York (NY): Dorling Kindersley Publishing, Inc; 2000. Encyclopedia of herbal medicine; p. 271. [Google Scholar]
- 21.Hedrick UP. New York: Dover Publications; 1972. Sturtevant's edible plants of the world. ISBN 0-486-20459-6. [Google Scholar]
- 22.Harrison S, Wallis M, Masefield G. Oxford University Press; 1975. The oxford book of food plants. [Google Scholar]
- 23.Lust J. Bantam books. USA: Benedict Lust Publication; 1983. The Herb Book. [Google Scholar]
- 24.Grieve . A modern herbal. In: Leyel CF, editor. Great Britain: Penguin Publisher; 1984. [Google Scholar]
- 25.Phillips R, Foy N. London: Pan Books Ltd; 1990. Herbs. ISBN 0-330-30725-8. [Google Scholar]
- 26.Qiblawi S, Kumar A. Chemopreventive action by an extract from Brassica compestris (var sarason) on 7, 12-dimethylbenz (a) anthracene induced skin papillomagenesis in mice. Phytother Res. 1999;13:261–3. doi: 10.1002/(SICI)1099-1573(199905)13:3<261::AID-PTR441>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 27.Moron MA, Depierre JW, Mannervik B. Levels of GSH, GR and GST, in rat lung and liver. Biochem Biophys Acta. 1979;582:67–78. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- 28.Ohkhawa H, Ohishi N, Yogi K. Assay for lipid peroxidation in animal tissue by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 29.Shishodia S, Aggarwal BB. Diosgenin inhibits osteoclastogenesis, invasion, and proliferation throughthe downregulation of Akt, IkappaB kinase activation and NF-kappaB-regulated gene expression. Oncogene. 2006;25:1463–7. doi: 10.1038/sj.onc.1209194. [DOI] [PubMed] [Google Scholar]
- 30.Patel K, Godewar M, Tahilyani V, Patel DK. A Review on pharmacological and analytical aspects of diosgenin: A concise report. Nat Prod Bioprospect. 2012;2:46–52. [Google Scholar]
- 31.Levy J, Teuerstein L, Marbach M, Radian S, Sharoni Y. Tyrosine protein kinase activity in the DMBA-induced rat mammary tumor: Inhibition by quercetin. Biochem Biophys Res Commun. 1984;725:1227–33. doi: 10.1016/s0006-291x(84)80264-8. [DOI] [PubMed] [Google Scholar]
- 32.Afaq F, Katiyar SK. Dietary phytochemicals and chemoprevention of solar ultraviolet radiation induced skin cancer. In: Sarkar FH, editor. Neutraceuticals and Cancer. 1st Edn. USA: Springer Publishers; 2012. pp. 259–321. [Google Scholar]
- 33.Mazza G, Oomah BD, editors. Herbs, botanicals and teas. Lancaster, USA: Technomic Publishing Co; 2000. Chemistry and pharmacology of fenugreek. [Google Scholar]
- 34.Gupta M, Shaw BP. Uses of medicinal plants in Panchakarma Ayurvedic therapy. Indian J Traditional Knowledge. 2009;8:372–8. [Google Scholar]
- 35.Thiele JJ, Schroeter C, Hsieh SN, Podda M, Packer L. The antioxidant network of the stratum corneum. Curr Probl Dermatol. 2001;29:26–42. doi: 10.1159/000060651. [DOI] [PubMed] [Google Scholar]
- 36.Mandelker L. Oxidative stress, free radicals, and cellular damage. Oxidat Stress Appl Basic Res Clin Pract. 2011;5:1–17. [Google Scholar]
- 37.Ketterer B. Glutathione S-Transferase and prevention of cellular free radical damage. Free Radic Res. 1998;28:647–58. doi: 10.3109/10715769809065820. [DOI] [PubMed] [Google Scholar]
- 38.Seo KW, Kim GJ, Park M, Kim TW, Kim HJ. Effects of phenethylisothiocyanate on the expression of glutathione S transferase and hepatotoxicity induced by acetaminophen. Xenobiotica. 2000;30:535–43. doi: 10.1080/004982500237532. [DOI] [PubMed] [Google Scholar]
- 39.Ursini F, Maiorino M, Sevanian A. Membrane hydroperoxides. In: Sies H, editor. Oxidative stress: Oxidants and antioxidants. London: Harcourt Brace Jovanovich; 1991. pp. 319–36. [Google Scholar]
- 40.Krishnaswamy K, Raghuramulu N. Bioactive phytochemicals with emphasis on dietary practices. Indian J Med Res. 1998;108:167–81. [PubMed] [Google Scholar]
- 41.Raju J, Patlolla JM, Swamy MV, Rao CV. Diosgenin, a steroid saponin of Trigonella foenum graecum (fenugreek), inhibits azoxymethane-induced aberrant crypt foci formation in f344 rats and induces apoptosis in ht-29 human colon cancer cells. Cancer Epidemiol Biomarkers Prev. 2004;13:1392–8. [PubMed] [Google Scholar]
- 42.Wattenberg L. Chemoprevention of cancer. Cancer Res. 1985;45:1–6. [PubMed] [Google Scholar]


