Abstract
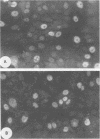
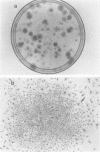
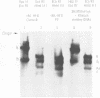
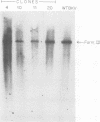
After infection of permissive human fetal brain cells by BK human papovavirus (BKV), the vast majority of the cells were killed by the virus, but rare survivors were recovered after frequent medium changes. These surviving cells grew and formed visible colonies after 5 to 6 weeks and were thereafter established as permanent cell lines. These cells, designated as BK-HFB cells, were persistently infected and shed BKV. Morphologically, they were small polygonal cells and had transformed growth properties. Their plating efficiency on solid substrates or in semisolid medium was high, and they were tumorigenic in athymic nude mice. Cloning experiments in medium containing BKV antiserum revealed that BKV did not persist in the cultures in a simple carrier state. All cloned cell lines were initially T-antigen negative and virus-free. However, every clone began to release BKV and again became persistently infected within 3 weeks after removal of BKV antiserum. After rigorous antibody treatment, four of seven clones still released virus spontaneously upon removal of antiserum; three clones have remained virus-free and are apparently cured. Although these cloned cell lines are T- and V-antigen negative when grown in antiserum-containing medium, they retain "free" or episomal BKV genomes; integrated viral DNA was not detected in any of the clones. These free genomes are indistinguishable from prototype BKV DNA and are found in much larger amounts in virus-shedding cell lines.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Botchan M., Topp W., Sambrook J. The arrangement of simian virus 40 sequences in the DNA of transformed cells. Cell. 1976 Oct;9(2):269–287. doi: 10.1016/0092-8674(76)90118-5. [DOI] [PubMed] [Google Scholar]
- Daya-Grosjean L., Monier R. Presence of free viral DNA in simian virus 40-transformed nonproducer cells. J Virol. 1978 Aug;27(2):307–312. doi: 10.1128/jvi.27.2.307-312.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulbecco R., Vogt M. SIGNIFICANCE OF CONTINUED VIRUS PRODUCTION IN TISSUE CULTURES RENDERED NEOPLASTIC BY POLYOMA VIRUS. Proc Natl Acad Sci U S A. 1960 Dec;46(12):1617–1623. doi: 10.1073/pnas.46.12.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIRARDI A. J., JENSEN F. C., KOPROWSKI H. SV40-INDUCED TRANFORMATION OF HUMAN DIPLOID CELLS: CRISIS AND RECOVERY. J Cell Physiol. 1965 Feb;65:69–83. doi: 10.1002/jcp.1030650110. [DOI] [PubMed] [Google Scholar]
- Gardner S. D., Field A. M., Coleman D. V., Hulme B. New human papovavirus (B.K.) isolated from urine after renal transplantation. Lancet. 1971 Jun 19;1(7712):1253–1257. doi: 10.1016/s0140-6736(71)91776-4. [DOI] [PubMed] [Google Scholar]
- Girardi A. J., Weinstein D., Moorhead P. S. SV40 transformation of human diploid cells. A parallel study of viral and karyologic parameters. Ann Med Exp Biol Fenn. 1966;44(2):242–254. [PubMed] [Google Scholar]
- Holland J. J., Villarreal L. P. Persistent noncytocidal vesicular stomatitis virus infections mediated by defective T particles that suppress virion transcriptase. Proc Natl Acad Sci U S A. 1974 Aug;71(8):2956–2960. doi: 10.1073/pnas.71.8.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howley P. M., Khoury G., Byrne J. C., Takemoto K. K., Martin M. A. Physical map of the BK virus genome. J Virol. 1975 Oct;16(4):959–973. doi: 10.1128/jvi.16.4.959-973.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. S. Defective interfering viruses. Annu Rev Microbiol. 1973;27:101–117. doi: 10.1146/annurev.mi.27.100173.000533. [DOI] [PubMed] [Google Scholar]
- Ketner G., Kelly T. J., Jr Integrated simian virus 40 sequences in transformed cell DNA: analysis using restriction endonucleases. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1102–1106. doi: 10.1073/pnas.73.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACPHERSON I., MONTAGNIER L. AGAR SUSPENSION CULTURE FOR THE SELECTIVE ASSAY OF CELLS TRANSFORMED BY POLYOMA VIRUS. Virology. 1964 Jun;23:291–294. doi: 10.1016/0042-6822(64)90301-0. [DOI] [PubMed] [Google Scholar]
- Magnusson G., Nilsson M. G. Multiple free viral DNA copies in polyoma virus-transformed mouse cells surviving productive infection. J Virol. 1977 Jun;22(3):646–653. doi: 10.1128/jvi.22.3.646-653.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major E. O., Di Mayorca G. Malignant transformation of BHK21 clone 13 cells by BK virus--a human papovavirus. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3210–3212. doi: 10.1073/pnas.70.11.3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutchan J. H., Pagano J. S. Enchancement of the infectivity of simian virus 40 deoxyribonucleic acid with diethylaminoethyl-dextran. J Natl Cancer Inst. 1968 Aug;41(2):351–357. [PubMed] [Google Scholar]
- Näse L. M., Kärkkäinen M., Mäntyjärvi R. A. Transplantable hamster tumors induced with the BK virus. Acta Pathol Microbiol Scand B. 1975 Aug;83(4):347–352. doi: 10.1111/j.1699-0463.1975.tb00112.x. [DOI] [PubMed] [Google Scholar]
- Portolani M., Barbanti-Brodano G., Placa M. L. Malignant transformation of hamster kidney cells by BK virus. J Virol. 1975 Feb;15(2):420–422. doi: 10.1128/jvi.15.2.420-422.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad I., Zouzias D., Basilico C. Nonintegrated viral DNA in rat cells doubly transformed by SV40 and polyoma virus. Virology. 1978 Mar;85(1):328–331. doi: 10.1016/0042-6822(78)90439-7. [DOI] [PubMed] [Google Scholar]
- Prasad I., Zouzias D., Basilico C. State of the viral DNA in rat cells transformed by polyoma virus. I. Virus rescue and the presence of nonintergrated viral DNA molecules. J Virol. 1976 May;18(2):436–444. doi: 10.1128/jvi.18.2.436-444.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preble O. T., Youngner J. S. Temperature-sensitive viruses and the etiology of chronic and inapparent infections. J Infect Dis. 1975 Apr;131(4):467–473. doi: 10.1093/infdis/131.4.467. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- SHEIN H. M., ENDERS J. F. Multiplication and cytopathogenicity of Simian vacuolating virus 40 in cultures of human tissues. Proc Soc Exp Biol Med. 1962 Mar;109:495–500. doi: 10.3181/00379727-109-27246. [DOI] [PubMed] [Google Scholar]
- Sekellick M. J., Marcus P. I. Persistent infection. I Interferon-inducing defective-interfering particles as mediators of cell sparing: possible role in persistent infection by vesicular stomatitis virus. Virology. 1978 Mar;85(1):175–186. doi: 10.1016/0042-6822(78)90422-1. [DOI] [PubMed] [Google Scholar]
- Shah K. V., Daniel R. W., Strandberg J. D. Sarcoma in a hamster inoculated with BK virus, a human papovavirus. J Natl Cancer Inst. 1975 Apr;54(4):945–950. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Takemoto K. K., Martin M. A. Transformation of hamster kidney cells by BK papovavirus DNA. J Virol. 1975 Jan;17(1):247–253. doi: 10.1128/jvi.17.1.247-253.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto K. K., Mullarkey M. F. Human papovavirus, BK strain: biological studies including antigenic relationship to simian virus 40. J Virol. 1973 Sep;12(3):625–631. doi: 10.1128/jvi.12.3.625-631.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida S., Watanabe S., Aizawa T., Kato K., Furuno A. Induction of papillary ependymomas and insulinomas in the Syrian golden hamster by BK virus, a human papovavirus. Gan. 1976 Dec;67(6):857–865. [PubMed] [Google Scholar]
- Upcroft P., Skolnik H., Upcroft J. A., Solomon D., Khoury G., Hamer D. H., Fareed G. C. Transduction of a bacterial gene into mammalian cells. Proc Natl Acad Sci U S A. 1978 May;75(5):2117–2121. doi: 10.1073/pnas.75.5.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt M., Bacheler L. T., Boice L. Proposed structure of two defective viral DNA oligomers produced in 3T3 cells transformed by the ts-a mutant of polyoma virus. J Virol. 1976 Mar;17(3):1009–1026. doi: 10.1128/jvi.17.3.1009-1026.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R. C., Wu R. BK virus DNA: cleavage map and sequence analysis. Proc Natl Acad Sci U S A. 1978 May;75(5):2150–2154. doi: 10.1073/pnas.75.5.2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zouzias D., Prasad I., Basilico C. State of the viral DNA in rat cells transformed by polyma virus. II. Identification of the cells containing nonintegrated viral DNA and the effect of viral mutations. J Virol. 1977 Oct;24(1):142–150. doi: 10.1128/jvi.24.1.142-150.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]