Abstract
Background:
Propolis as a natural remedy has maintained its popularity over long periods of time. The aim of this study was to determine the chemical composition in terms of total phenolic compounds and flavonoids present in Chinese propolis and to carry out an in vitro evaluation of its antimicrobial activity and the minimal inhibitory concentrations for Porphyromonas gingivalis (Pg) and Aggregatibacter actinomycetemcomitans (Aa).
Materials and Methods:
From the ethanolic extract of propolis (EEP), total phenol content was determined by the Folin–Ciocalteau method, flavones and flavonols by the modified aluminum chloride colorimetric method, and flavanones by the 2.4-dinitrophenylhydrazine (2,4-DNP) method. Agar well diffusion assay was used to evaluate the antimicrobial potential of propolis against Pg and Aa. The minimum inhibitory concentration of propolis against the two bacteria was determined using serial tube dilution technique.
Results:
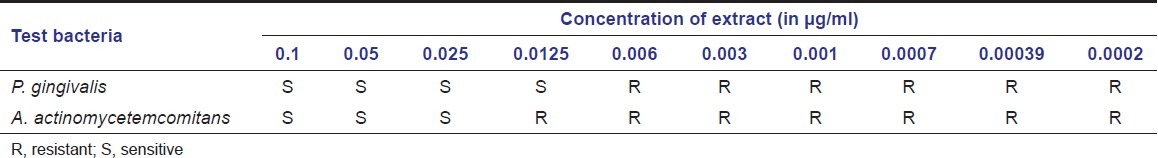
The total concentration of phenol in the EEP was 19.44%, flavones and flavonols 2.616%, and flavanones 16.176%. The inhibitory zone depicting antimicrobial activity ranged from 18 to 25 mm for Pg and from 12 to 14 mm for Aa. The concentration range of Chinese propolis that is sensitive to inhibit the growth of Pg was 0.1–0.0125 μg/ml and for Aa it was 0.1–0.025 μg/ml.
Conclusion:
These data suggest that Chinese propolis has potent antimicrobial activity against the two periodontopathogens, suggesting its possible use as a natural alternative to the widely used synthetic antibiotics for periodontal therapy.
Keywords: Aggregatibacter actinomycetemcomitans, antimicrobial, flavonoids, minimal inhibitory concentration, phenolic compounds, Porphyromonas gingivalis, propolis
Introduction
Detection of clay tablet of early civilization period in the excavation revealed the practice of gingival massage combined with various herbal medications for periodontal problems by Babylonians and Assurians.[1] Currently there are various popular therapeutic antimicrobial products in the market, but the search and screening for the development of natural remedies with wide range of pharmaceutical properties without the side effects of synthetic medications for the treatment of oral diseases is still ongoing.[2]
Among the various natural products, propolis has received greater attention due to its broad-spectrum antimicrobial activity against a wide range of pathogenic microorganisms. Propolis, sometimes also referred to as “bee glue,” is the generic name for the resinous substance collected by honeybees (Apis mellifera) from various plant sources.[3] The word propolis is derived from the Greek words “pro” meaning “in defense of” and “polis” meaning “city,” referring to the defense of the city or the beehive. It is a strongly adhesive substance collected and used by bees to seal holes in their honeycombs and protect the entrance against intruders.[4,5]
Propolis not only has a strong antibacterial,[4,6–11] antifungal,[12] antiviral,[13,14] antioxidant action,[15] but also has immunity enhancing,[16] pain and inflammation relieving, and wound repair accelerating effects.[3] The first systematic investigation on antibacterial properties of propolis was made by Kivalkina in 1948,[5] and since then, the antibacterial effect of propolis has been demonstrated in a variety of Gram-positive and Gram-negative bacteria.[6,17]
Among the oral diseases, periodontitis is the most prevalent disease of the adult population throughout the world. Though it is caused by complex microbiota, Porphyromonas gingivalis (Pg) and Aggregatibacter actinomycetemcomitans (Aa) are considered to be the major pathogens for initiation and progression of destruction of tooth supporting structures.[18] Due to inaccessibility of instruments to certain anatomical areas and the tissue penetrable nature of pathogenic bacteria, the use of antimicrobials alone or as adjunct to conventional mechanical therapy becomes a routine in the management of periodontal diseases.[19] Hence, the development of natural form of therapies for the treatment of diseases of the oral cavity is of great relevance, as the administration of systemic antimicrobials has been reported to cause the development of multiresistant microorganisms, interbacterial transfer of resistance determinants, and various side effects.[2] Though propolis has shown great potential against the bacteria of dentistry, these types of data are limited.
Propolis is a complex of biologically active substances. In each sample of propolis, more than 80–100 chemical compounds are typically identified.[20] Raw propolis is composed of 50% resin, flavonoids, and related phenolic acids (known as the polyphenolic fraction), 30% wax, 10% essential oils, 5% pollen, and 5% various organic compounds.[21] Some of the principal phenolic esters and flavonoids like caffeic acid phenethyl ester, quercetine, baicalin, pinocembrin, naringin, galangin, and chrysin have been found to exert antimicrobial, antioxidant, and anti-inflammatory activities of propolis.[3,22] However, the precise composition of the identified substances varies, and it depends on the plant sources available to the bees, on the season, vegetation, and other factors.[5,17,23] Furthermore, individual samples of propolis not only have a different composition but also have a different concentration of active substances, which determines their different pharmacological action.[22,23]
Hence, the objectives of present study were to determine the chemical composition in terms of total phenolic compounds and flavonoids present in Chinese propolis and to carry out an in vitro evaluation of its antimicrobial activity and the minimal inhibitory concentrations for Pg and Aa.
Materials and Methods
Propolis powder of Chinese origin made by honey bees (A. mellifera) was obtained from Ecuadorian Rainforest, New Jersey, USA, in November 2010. It was certified to be free from any form of bacteria, yeast, or mold by the manufacturer after microbial analysis.
Chemicals
Ethanol, dimethyl sulfoxide (DMS), Folin–Ciocalteau reagent, Na2CO3 solution, gallic acid, aluminum chloride, quercetine, 2,4-dinitrophenylhydrazine (2,4-DNP), sulfuric acid, methanol, potassium hydroxide, and pinocembrin were obtained from S. D. Fine Chem Limited, Bangalore, Karnataka, India. Spectrophotometer was from Shimadzu Scientific Instruments, Columbia, MD, USA.
Preparation of ethanol extract of propolis for composition analysis[24]
One gram of propolis powder was added to 25 ml of 95% ethanol and allowed to mix on a magnetic mixture for 24 h at room temperature (37°C), and then the obtained solution was filtered with a Whatman no. 1 filter paper. The filtrate was then adjusted to 25 ml by adding 80% ethanol and stored in amber colored bottles.
Preparation of propolis extract for antimicrobial test
Ten grams of propolis powder was added to 100 ml of DMS (an inert solvent) and kept at a cool and dark place in an amber colored bottle.[25]
Quantification of total phenol content
Total phenol content in propolis was determined by the Folin-Ciocalteau method.[26] Four milliliters of Folin-Ciocalteau reagent was mixed with 15 ml of distilled water, 1 ml EEP, and 6 ml 20% Na2CO3 solution. The solution was then adjusted to a final volume of 50 ml by adding distilled water. After 2 h at room temperature, a change in color was observed and the absorbance was measured at 760 nm wavelength in a spectrophotometer. The obtained values were compared with a prepared standard calibration curve of gallic acid as a reference.
Quantification of total flavonoids
Determination of flavones and flavonols
The content of flavones and flavonols in propolis was determined by the modified aluminum chloride colorimetric method.[26] 0.5 ml of EEP was mixed with 1.5 ml 95% ethanol, 0.1 ml 10% aluminum chloride, 0.1 ml of 1 mol/l potassium acetate, and 2.8 ml water. A volume of 10% aluminum chloride was substituted by the same volume of distilled water in blank. After incubation at room temperature for 30 min, the absorbance of the reaction mixture was measured at 415 nm wavelength in a spectrophotometer. The obtained values were compared with a prepared standard calibration curve of quercetine as a reference.
Determination of flavanones
The content of flavanones in propolis was determined by using the 2,4-DNP method.[27] A mixture of 1 ml of EEP, 2 ml of 2,4-DNP reagent (1 g of 2,4-DNP dissolved in 2 ml of 96% sulfuric acid and adjusted to 100 ml with methanol), and 2 ml of methanol was heated over a water bath for 50 min at 50°C. After cooling to room temperature, the solution was mixed with 5 ml of 10% potassium hydroxide in 70% methanol and incubated for 2 min at room temperature. A 1-ml volume of this solution was mixed with 5 ml of methanol and centrifuged at 1610 g for 10 min. The absorbance of the supernatant was measured at 495 nm in a spectrophotometer. A blank solution was prepared in the same way, but using 1 ml of 96% ethanol instead of the test solution. The obtained values were compared with a prepared standard calibration curve of pinocembrin as a reference.
The total phenol and flavonoid contents in propolis were expressed in percentage and were corresponded to mean of three replicates.
Bacterial strains
The tested bacterial strains in this study were Pg ATCC 33277 and Aa ATCC 43718 (American Type Culture Collection, Manassas, VA, USA). Nutrient broth was used to obtain the viable growth of microbes from their freeze-dried form. After 48 h, turbidity in test tube confirmed the growth of microbes which was compared and adjusted to McFarland 0.5 turbidity standard (108 colony-forming units per milliliter).[28,29]
Determination of antimicrobial activity
Agar well diffusion assay was used to evaluate the antimicrobial potential of propolis.[30] Petri dishes containing 100 ml of brain heart infusion broth supplemented with 5 ml of 5% sheep blood were inoculated with approximately 100 μl of the respective microbial strain using swab technique. Wells of 8 mm diameter were cut into solidified agar media using a sterilized device. One hundred microliters of the propolis extract was poured in the wells and the plates were incubated at 37°C for 48 h. To ensure the consistency of all findings, the experiment was performed and repeated under strict aseptic conditions. The antibacterial activity of propolis extract was expressed in terms of the mean of diameter of inhibitory zone (in millimeters) produced by the extract at the end of incubation period.
Determination of minimum inhibitory concentration
Minimum inhibitory concentration (MIC) is defined as the lowest concentration of extract at which there will be no visible growth of the test organism. In the present study, MIC was determined using “serial tube dilution technique.” The MIC of propolis for Pg and Aa was conventionally determined in triplicate for each strain by the macrodilution broth method as described by the National Committee for Clinical Laboratory Standards (NCCLS).[31] Serial twofold dilutions of propolis extract were prepared in macrodilution tubes and inoculated with constant amount of test bacteria, and then all the test tubes were incubated at 37°C for 18–24 h. Each tube was mixed and examined for growth, comparing each tube to the control. For each test, DMS was used as the control solvent.
Statistical analysis
The data obtained were tabulated and graphs prepared.
Results
In the EEP, the total concentration of phenol was 19.44% and flavonoid was 18.792%. The total concentration of flavonoid was expressed as the sum of two independent colorimetric methods meant for the determination of flavones, flavonols (2.616%), and flavanones (16.176%).
Figures 1 and 2 show the antimicrobial activity of different dilutions of propolis extract against Pg and Aa after 48 h. It was effective against both the microorganisms. The zones of inhibition of propolis extract against Pg ranged from 18 to 25 mm and that against Aa was from 12 to 14 mm. The control sample (DMS) did not affect the growth of the microorganisms.
Figure 1.
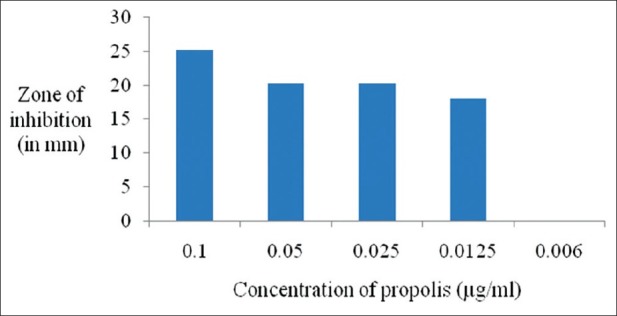
Antimicrobial activity of the propolis extract against P. gingivalis
Figure 2.
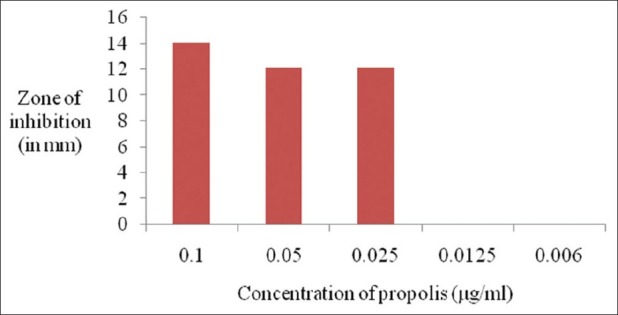
Antimicrobial activity of the propolis extract against A. actinomycetemcomitans
Table 1 shows the MIC of propolis extract against the organisms used in the study. The concentration range of Chinese propolis that is sensitive to inhibit the growth of Pg was 0.1–0.0125 μg/ml and of Aa was 0.1–0.025 μg/ml.
Table 1.
Minimal inhibitory concentration (MIC) of propolis extract
Discussion
Propolis as a natural remedy is used for its healing properties by ancient Egyptians, Greeks, Romans, etc.[32] It has received the attention of current clinicians and researchers also due to its diverse pharmacological activities and low toxicity.[33] As an anti-inflammatory agent, propolis has been shown to inhibit the synthesis of prostaglandins, activate the thymus gland, aid the immune system by promoting phagocytic activity, stimulate cellular immunity, and augment healing effects in epithelial tissues.[15,16] As the pharmacologic properties of propolis are not clearly known and some compounds of it give the synergic effect to the other compound activities, it is not possible to detect the exact antimicrobial activity of the components of propolis.[7,8] Presence of flavonoids and phenolic acids is found to be responsible for the antimicrobial activity of propolis.[5,9,34,35] These contents vary in the samples from different geographic areas[32] and are based on the local flora in the region from which propolis was collected.[22] Hence, the concentration of these active substances in Chinese propolis sample has been evaluated.
No difference was observed in their phenolic profile at different seasons.[10] The highest concentration of phenolic compound (1.64–1.53 g/100 ml) was found in samples of Propolis Ethanol Extract (PEE) collected from hives located near deciduous and mixed forests and the lowest concentrations (0.18 g/100 ml) from cultivated meadows far from forests.[22] The PEE often used in medicinal practice is of 1:10 ratio where the phenol content is expected to be not less than 2%.[22]
In propolis, flavonoids were found to kill or inhibit many bacterial strains, inhibit viral enzymes, scavenge free radicals, etc.[36,37] Though the concentration of flavonoids in propolis was similar during different seasons,[10,35] it showed variations in samples collected from same geographic areas.[38–40] In poplar type propolis, flavonoids account for a large part (around 50%) of phenolic compounds.[22] Significant correlation was found between the flavonoid content in propolis and MIC,[41] but flavonoid content below 1% showed no antibacterial activity.[32] The total flavonoid content obtained in Chinese propolis was 18.792%.
Variation in chemical composition due to seasonal and geographic changes brings non-significant change in their antibacterial activity.[10,11,42] On comparison between Lithuanian (L) and Czech (C) propolis samples, L-4 which had a very small content of propolis compound showed similar antimicrobial activity as did other PEE with a larger content of phenolic compounds (L-1, L-2, C-2, and C-5) or flavonoids. On the contrary, though L-5 and C-3 had similar concentration of phenolic compounds and flavonoids, their antimicrobial activity against gram-negative bacteria was different.[22] Similarly, German propolis showed the highest antimicrobial activity against Staphylococcus aureus and Escherichia coli, Australian propolis had the highest activity against Candida albicans, whereas French propolis was effective against all the pathogens but was less effective than either German or Australian propolis.[43] These results suggest that the antimicrobial effects vary for different fractions of propolis and microorganism species.[7]
The antimicrobial activity of propolis has been studied by several authors; however, few studies have investigated its activity toward oral pathogens.[44,45] Sonmez et al. tested the antibacterial activity of six propolis solutions from different geographic locations and found that all samples were active against various periodontopathogens including Pg test bacterial strain.[46] Another in vitro investigation also demonstrated the antimicrobial activity of Brazilian propolis against various periodontopathogens including Pg and Aa.[47] Santos et al. investigated the inhibitory activity of Brazilian propolis on Aa, Fusobacterium nucleatum, Pg, and Prevotella intermedia, and found that all of the assayed bacterial species were susceptible to propolis extract.[4]
The antimicrobial activity was measured in terms of diameter of the inhibitory zones in a soft agar layer. An inhibitory zone with a diameter less than 10 mm corresponded to lack of activity.[22] But Chinese propolis exhibited the maximum inhibitory zone of 25 mm for Pg and 14 mm for Aa at a concentration of 0.1 μg/ml. The MIC values for Pg and Aa are 0.0125 and 0.025 μg/ml, respectively. Increase in the dilution of ethanol extract of Chinese propolis reduced the antimicrobial activity, and beyond the MIC, it was ineffective to inhibit the growth of microorganisms. This is supported by a study where four times diluted Lithuanian and Czech propolis solutions inhibited the growth of all the 10 study microorganisms except gram-negative bacteria by Lithuanian sample, but when the dilution was increased to eight times, both the samples inhibited only few of the microorganisms.[22]
Black pigmented anaerobes were found to be the most sensitive group to propolis. The MIC of propolis was lower and inhibitory zone greater for Pg than Aa, showing its susceptibility to Chinese propolis. This result is in parallel with the studies testing the antimicrobial activity of various propolis solutions.[4,47]
It has been observed that propolis samples had a wide spectrum antimicrobial activity against methicillin-resistant S. aureus (MRSA) and vancomycin-resistant Enterococcus faecium (VREF).[7] Future evaluation of the effect of standardized propolis preparations having strong antimicrobial concentrations with low toxicity against pathogens responsible for aggressive or refractory periodontitis may throw more light on the management and prevention of the spread and development of similar antibiotic-resistant clinical conditions.
The effective concentrations of propolis solution which inhibited oral pathogens including Pg were found to be cytotoxic to gingival fibroblasts.[46] Hence, it is necessary to analyze if the obtained MIC of Chinese propolis is safe and effective to regenerative cells and other normal commensals to determine its best application as a promising natural medicine of future in different oral clinical conditions.
Acknowledgments
We thank Mr. Sanjoy Mondol, Lecturer, Bapuji Pharmacy College, for his assistance in determining the chemical composition of propolis and Dr. Kishor G. Bhat, Professor and HOD, Department of Microbiology, Maratha Mandal's Dental College, Belgaum, in assisting with the analysis of antimicrobial efficacy of propolis.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared
References
- 1.Guerini V. History of dentistry. Philadelphia: Lea and Febiger; 1909. [Google Scholar]
- 2.Walker CB. The acquisition of antibiotic resistance in the periodontal microflora. Periodontol 2000. 1996;10:79–88. doi: 10.1111/j.1600-0757.1996.tb00069.x. [DOI] [PubMed] [Google Scholar]
- 3.Burdock GA. Review of the biological properties and toxicity of bee propolis (propolis) Food Chem Toxicol. 1998;36:347–63. doi: 10.1016/s0278-6915(97)00145-2. [DOI] [PubMed] [Google Scholar]
- 4.Santos FA, Bastos EM, Uzeda M, Carvalho MA, Farias LM, Moreira ES, et al. Antibacterial activity of Brazilian propolis and fractions against oral anaerobic bacteria. J Ethnopharmacol. 2002;80:1–7. doi: 10.1016/s0378-8741(02)00003-x. [DOI] [PubMed] [Google Scholar]
- 5.Ghisalberti EL. Propolis. A review. Bee World. 1979;60:59–84. [Google Scholar]
- 6.Menezes H, Bacci M, Jr, Oliveira SD, Pagnocca FC. Anti-bacterial properties of propolis and products containing propolis from Brazil. Apidologie. 1997;28:71–6. [Google Scholar]
- 7.Kilic A, Baysallar M, Besirbellioglu B, Salih B, Sorkun K, Tanyuksel M. In vitro antimicrobial activity of propolis against methicillin-resistant Staphylococcus aureus. Ann Microbiol. 2005;55:113–7. [Google Scholar]
- 8.Kartal M, Yildiz S, Kaya S, Kurucu S, Topçu G. Antimicrobial activity of propolis samples from two different regions of Anatolia. J Ethnopharmacol. 2003;86:69–73. doi: 10.1016/s0378-8741(03)00042-4. [DOI] [PubMed] [Google Scholar]
- 9.Kujumgiev A, Bankova V, Ignatova A, Popov S. Antibacterial activity of propolis, some of its components and their analogs. Pharmazie. 1993;48:785–6. [PubMed] [Google Scholar]
- 10.Santos FA, Bastos EM, Maia AB, Uzeda M, Carvalho MA, Farias LM, et al. Brazilian propolis: Physicochemical properties, plant origin and antibacterial activity on periodontopathogens. Phytother Res. 2003;17:285–9. doi: 10.1002/ptr.1117. [DOI] [PubMed] [Google Scholar]
- 11.Kujumgiev A, Tsvetkova I, Serkedjieva Y, Bankova V, Christov R, Popov S. Antibacterial, antifungal and antiviral activity of propolis of different geographic origin. J Ethnopharmacol. 1999;64:235–40. doi: 10.1016/s0378-8741(98)00131-7. [DOI] [PubMed] [Google Scholar]
- 12.Ota C, Unterkircher C, Fantinato V, Shimizu MT. Antifungal activity of propolis on different species of Candida. Mycoses. 2001;44:375–8. doi: 10.1046/j.1439-0507.2001.00671.x. [DOI] [PubMed] [Google Scholar]
- 13.Huleihel M, Isanu V. Anti-herpes simplex virus effect of an aqueous extract of propolis. Isr Med Assoc J. 2002;4:923–7. [PubMed] [Google Scholar]
- 14.Amoros M, Sauvager F, Girre L, Cormier M. In vitro anti-viral activity of propolis. Apidologie. 1992;23:231–40. [Google Scholar]
- 15.Russo A, Longo R, Vanella A. Antioxidant activity of propolis: Role of caffeic acid phenethyl ester and galangin. Fitoterapia. 2002;73:S21–9. doi: 10.1016/s0367-326x(02)00187-9. [DOI] [PubMed] [Google Scholar]
- 16.Orsolić N, Basić I. Immunomodulation by water-soluble derivative of propolis: A factor of antitumor reactivity. J Ethnopharmacol. 2003;84:265–73. doi: 10.1016/s0378-8741(02)00329-x. [DOI] [PubMed] [Google Scholar]
- 17.Grange JM, Davey RW. Antibacterial properties of propolis (bee glue) J R Soc Med. 1990;83:159–60. doi: 10.1177/014107689008300310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zambon JJ. Periodontal diseases: Microbial factors. Ann Periodontol. 1996;1:879–925. doi: 10.1902/annals.1996.1.1.879. [DOI] [PubMed] [Google Scholar]
- 19.Systemic antibiotics in periodontics. J Periodontol. 1996;67:831–8. [PubMed] [Google Scholar]
- 20.Marcucci MC, Ferreres F, García-Viguera C, Bankova VS, De Castro SL, Dantas AP, et al. Phenolic compounds from Brazilian propolis with pharmacological activities. J Ethnopharmacol. 2001;74:105–12. doi: 10.1016/s0378-8741(00)00326-3. [DOI] [PubMed] [Google Scholar]
- 21.Pietta PG, Gardana C, Pietta AM. Analytical methods for quality control of propolis. Fitoterapia. 2002;73:S7–20. doi: 10.1016/s0367-326x(02)00186-7. [DOI] [PubMed] [Google Scholar]
- 22.Savickas A, Majiene D, Ramanauskiene K, Pavilonis A, Muselik J, Masteikova R, et al. Chemical composition and antimicrobial activity of Lithuanian and Czech Propolis. Biologija. 2005;4:59–63. [Google Scholar]
- 23.Bankova V. Recent trends and important developments in propolis research. Evid Based Complement Alternat Med. 2005;2:29–32. doi: 10.1093/ecam/neh059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alencar SM, Oldoni TL, Castro ML, Cabral IS, Costa-Neto CM, Cury JA, et al. Chemical composition and biological activity of a new type of Brazilian propolis: Red propolis. J Ethnopharmacol. 2007;113:278–83. doi: 10.1016/j.jep.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 25.Toker H, Ozan F, Ozer H, Ozdemir H, Eren K, Yeler H. A morphometric and histopathologic evaluation of the effects of propolis on alveolar bone loss in experimental periodontitis in rats. J Periodontol. 2008;79:1089–94. doi: 10.1902/jop.2008.070462. [DOI] [PubMed] [Google Scholar]
- 26.Woisky R, Salatino A. Analysis of propolis: Some parameters and procedures for chemical quality control. J Apic Res. 1998;37:99–105. [Google Scholar]
- 27.Cvek J, Medić-Sarić M, Jasprica I, Zubcić S, Vitali D, Mornar A, et al. Optimisation of an extraction procedure and chemical characterisation of Croatian propolis tinctures. Phytochem Anal. 2007;18:451–9. doi: 10.1002/pca.1001. [DOI] [PubMed] [Google Scholar]
- 28.Nanasombat S, Lohasupthawee P. Antibacterial activity of crude ethanolic extracts and essential oils of spices against salmonellae and other enterobacteria. KMITL Sci Tech J. 2005;5:527–38. [Google Scholar]
- 29.Norajit K, Laohakunjit N, Kerdchoechuen O. Antibacterial effect of five Zingiberaceae essential oils. Molecules. 2007;12:2047–60. doi: 10.3390/12082047. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Allen KL, Molan PC, Reid GM. A survey of the antibacterial activity of some New Zealand honeys. J Pharm Pharmacol. 1991;43:817–22. doi: 10.1111/j.2042-7158.1991.tb03186.x. [DOI] [PubMed] [Google Scholar]
- 31.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow anaerobically. Approved Standard. (4th ed) 2000;20(2) M7-A5. [Google Scholar]
- 32.Kosalec I, Pepeljnjak S, Bakmaz M, Vladimir-Knezević S. Flavonoid analysis and antimicrobial activity of commercially available propolis products. Acta Pharm. 2005;55:423–30. [PubMed] [Google Scholar]
- 33.Bankova V. Chemical diversity of propolis makes it a valuable source of new biologically active compounds. J Api Prod Api Med Sci. 2009;1:23–8. [Google Scholar]
- 34.Marcucci MC. Propolis: chemical composition, biological properties and therapeutic activity. Apidologie. 1995:83–99. [Google Scholar]
- 35.Park YK, Koo MK, Ikegaki M, Contado JL. Comparison of the flavonoid aglycone contents of Apis mellifera propolis from various regions of Brazil. Arq Biol Tecnol. 1997;40:97–106. [Google Scholar]
- 36.Middleton E, Jr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol Rev. 2000;52:673–751. [PubMed] [Google Scholar]
- 37.Havsteen BH. The biochemistry and medical significance of the flavonoids. Pharmacol Ther. 2002;96:67–202. doi: 10.1016/s0163-7258(02)00298-x. [DOI] [PubMed] [Google Scholar]
- 38.Pepeljnjak S, Jalsenjak I, Maysinger D. Flavonoid content in propolis extracts and growth inhibition of Bacillus subtilis. Pharmazie. 1985;40:122–3. [PubMed] [Google Scholar]
- 39.Kosalec I, Bakmaz M, Pepeljnjak S. Analysis of propolis from the continental and Adriatic regions of Croatia. Acta Pharm. 2003;53:275–85. [PubMed] [Google Scholar]
- 40.Kosalec I, Bakmaz M, Pepeljnjak S, Vladimir-Knezević S. Quantitative analysis of the flavonoids in raw propolis from northern Croatia. Acta Pharm. 2004;54:65–72. [PubMed] [Google Scholar]
- 41.Popova M, Bankova V, Butovska D, Petkov V, Nikolova-Damyanova B, Sabatini AG, et al. Validated methods for the quantification of biologically active constituents of poplar-type propolis. Phytochem Anal. 2004;15:235–40. doi: 10.1002/pca.777. [DOI] [PubMed] [Google Scholar]
- 42.Sforcin JM, Fernandes A, Jr, Lopes CA, Bankova V, Funari SR. Seasonal effect on Brazilian propolis antibacterial activity. J Ethnopharmacol. 2000;73:243–9. doi: 10.1016/s0378-8741(00)00320-2. [DOI] [PubMed] [Google Scholar]
- 43.Hegazi AG, Abd El Hady FK, Abd Allah FA. Chemical composition and antimicrobial activity of European propolis. Z Naturforsch C. 2000;55:70–5. doi: 10.1515/znc-2000-1-214. [DOI] [PubMed] [Google Scholar]
- 44.Park YK, Koo MH, Abreu JA, Ikegaki M, Cury JA, Rosalen PL. Antimicrobial activity of propolis on oral microorganisms. Curr Microbiol. 1998;36:24–8. doi: 10.1007/s002849900274. [DOI] [PubMed] [Google Scholar]
- 45.Steinberg D, Kaine G, Gedalia I. Antibacterial effect of propolis and honey on oral bacteria. Am J Dent. 1996;9:236–9. [PubMed] [Google Scholar]
- 46.Sonmez S, Kirilmaz L, Yucesoy M, Yücel B, Yilmaz B. The effect of bee propolis on oral pathogens and human gingival fibroblasts. J Ethnopharmacol. 2005;102:371–6. doi: 10.1016/j.jep.2005.06.035. [DOI] [PubMed] [Google Scholar]
- 47.Gebara EC, Lima LA, Mayer MP. Propolis antimicrobial activity against periodontopathic bacteria. Braz J Microbiol. 2002;33:365–9. [Google Scholar]


