Abstract
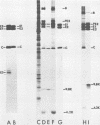
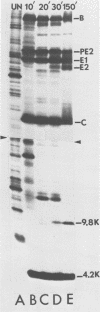
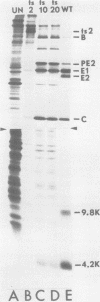
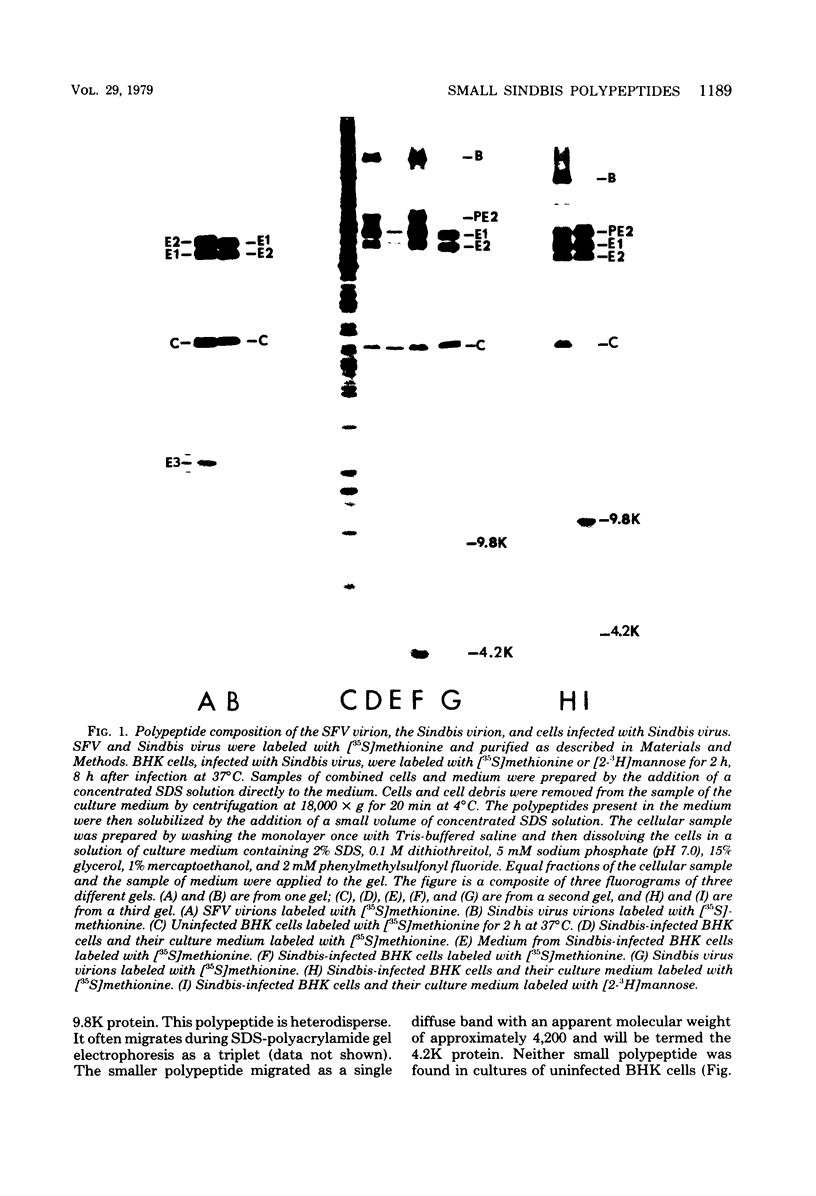
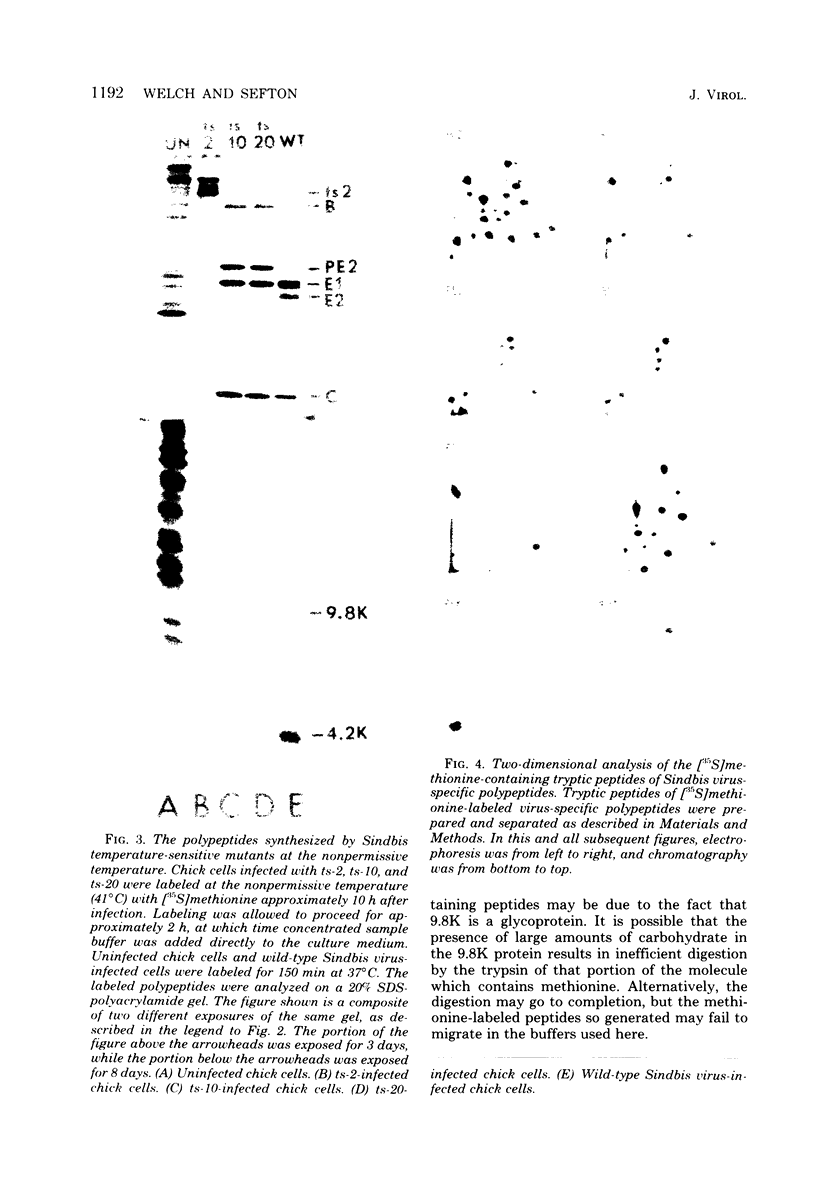
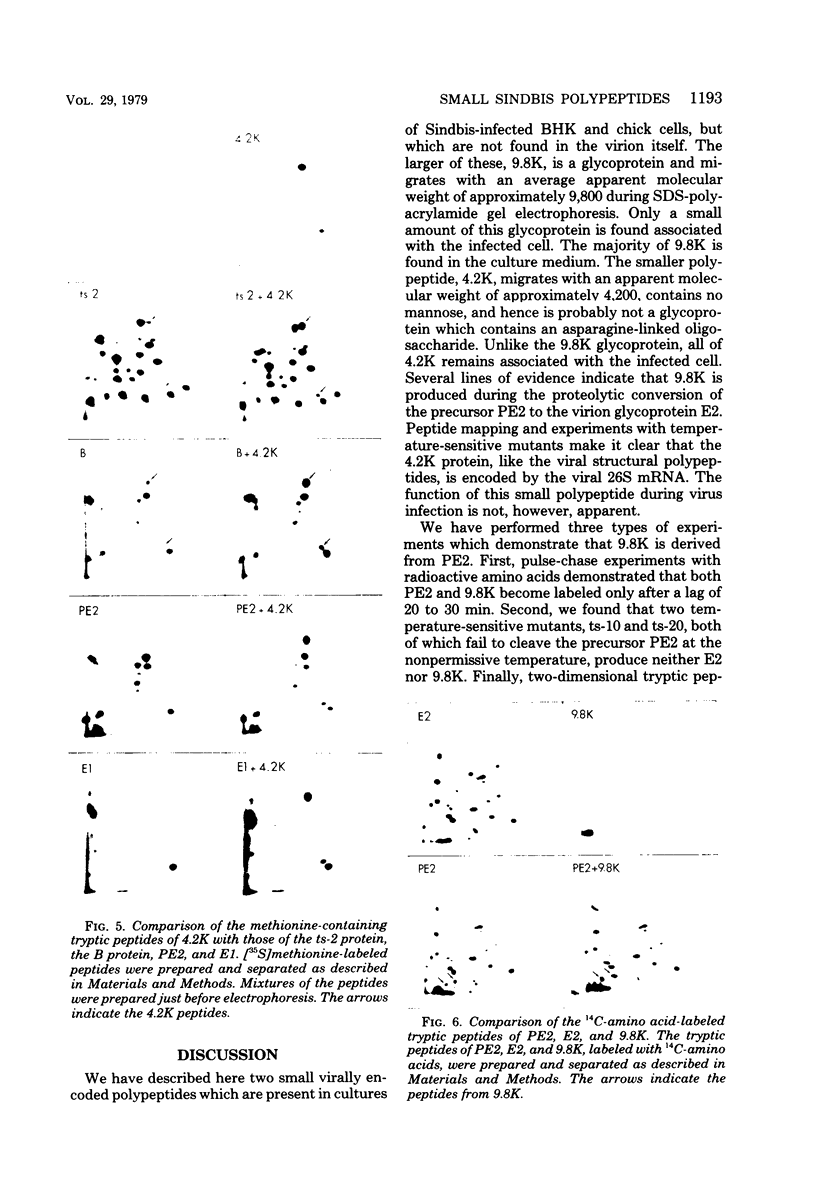
We have identified and characterized two small virus-specific polypeptides which are produced during infection of cells with Sindbis virus, but which are not incorporated into the mature virion. The larger of these is a glycoprotein with an approximate molecular weight of 9,800 and is found predominantly in the medium of infected cells. Three independent lines of evidence demonstrate conclusively that this 9,800-dalton glycoprotein is produced during the proteolytic conversion of the precursor polypeptide, PE2, to the virion glycoprotein E2. This small glycoprotein is therefore analogous to the virion glycoprotein E3 of the very closely related alphavirus, Semliki Forest virus. The 9,800-dalton glycoprotein of Sindbis virus, unlike the E3 glycoprotein of Semliki Forest virus, is not, however, present in the viral particle. The other virus-specific polypeptide is 4,200 daltons in size, does not appear to be a glycoprotein, and is neither incorporated into the mature virus nor released into the culture medium. The gene for this small polypeptide is present in the viral 26S mRNA (the mRNA which encodes all the viral structural polypeptides) and appears to be located in the portion of the mRNA which encodes the two viral glycoproteins. The possibility that this 4,200-dalton polypeptide functions as a signal peptide during the synthesis of the viral membrane glycoproteins is discussed.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beemon K., Hunter T. Characterization of Rous sarcoma virus src gene products synthesized in vitro. J Virol. 1978 Nov;28(2):551–566. doi: 10.1128/jvi.28.2.551-566.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Stedman J. D. Efficient fluorography of 3H and 14C on thin layers. Anal Biochem. 1978 Aug 15;89(1):247–256. doi: 10.1016/0003-2697(78)90747-9. [DOI] [PubMed] [Google Scholar]
- Bracha M., Schlesinger M. J. Defects in RNA+ temperature-sensitive mutants of Sindbis virus and evidence for a complex of PE2-E1 viral glycoproteins. Virology. 1976 Oct 15;74(2):441–449. doi: 10.1016/0042-6822(76)90350-0. [DOI] [PubMed] [Google Scholar]
- Burge B. W., Pfefferkorn E. R. Complementation between temperature-sensitive mutants of Sindbis virus. Virology. 1966 Oct;30(2):214–223. doi: 10.1016/0042-6822(66)90097-3. [DOI] [PubMed] [Google Scholar]
- Cancedda R., Villa-Komaroff L., Lodish H. F., Schlesinger M. Initiation sites for translation of sindbis virus 42S and 26S messenger RNAs. Cell. 1975 Oct;6(2):215–222. doi: 10.1016/0092-8674(75)90012-4. [DOI] [PubMed] [Google Scholar]
- Clegg J. C. Sequential translation of capsid and membrane protein genes of alphaviruses. Nature. 1975 Apr 3;254(5499):454–455. doi: 10.1038/254454a0. [DOI] [PubMed] [Google Scholar]
- Garoff H., Simons K., Renkonen O. Isolation and characterization of the membrane proteins of Semliki Forest virus. Virology. 1974 Oct;61(2):493–504. doi: 10.1016/0042-6822(74)90285-2. [DOI] [PubMed] [Google Scholar]
- Gibson W. Polyoma virus proteins: a description of the structural proteins of the virion based on polyacrylamide gel electrophoresis and peptide analysis. Virology. 1974 Dec;62(2):319–336. doi: 10.1016/0042-6822(74)90395-x. [DOI] [PubMed] [Google Scholar]
- Jones K. J., Waite M. R., Bose H. R. Cleavage of a viral envelope precursor during the morphogenesis of Sindbis virus. J Virol. 1974 Apr;13(4):809–817. doi: 10.1128/jvi.13.4.809-817.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Schlesinger M. J., Schlesinger S., Burge B. W. Identification of a second glycoprotein in Sindbis virus. Virology. 1972 Feb;47(2):539–541. doi: 10.1016/0042-6822(72)90298-x. [DOI] [PubMed] [Google Scholar]
- Schlesinger M. J., Schlesinger S. Large-molecular-weight precursors of sindbis virus proteins. J Virol. 1973 Jun;11(6):1013–1016. doi: 10.1128/jvi.11.6.1013-1016.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger S., Schlesinger M. J. Formation of Sindbis virus proteins: identification of a precursor for one of the envelope proteins. J Virol. 1972 Nov;10(5):925–932. doi: 10.1128/jvi.10.5.925-932.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M. Immediate glycosylation of Sindbis virus membrane proteins. Cell. 1977 Apr;10(4):659–668. doi: 10.1016/0092-8674(77)90099-x. [DOI] [PubMed] [Google Scholar]
- Simmons D. T., Strauss J. H. Translation of Sindbis virus 26 S RNA and 49 S RNA in lysates of rabbit reticulocytes. J Mol Biol. 1974 Jun 25;86(2):397–409. doi: 10.1016/0022-2836(74)90027-8. [DOI] [PubMed] [Google Scholar]
- Simons K., Keränen S., Käriänen L. Identification of a precursor for one of the Semliki forest virus membrane proteins. FEBS Lett. 1973 Jan 15;29(2):87–91. doi: 10.1016/0014-5793(73)80532-0. [DOI] [PubMed] [Google Scholar]
- Strauss J. H., Jr, Burge B. W., Darnell J. E. Sindbis virus infection of chick and hamster cells: synthesis of virus-specific proteins. Virology. 1969 Mar;37(3):367–376. doi: 10.1016/0042-6822(69)90220-7. [DOI] [PubMed] [Google Scholar]