Abstract
Objective:
We attempted to find the possibility of determining the minimum inhibitory concentration and minimum bactericidal concentration needed for nano-silver base inorganic anti-microbial agent (Novaron® AG 300, AG 1100) against Streptococcus mutans in vitro using broth dilution assay.
Materials and Methods:
An ampoule of freeze-dried S. mutans NCTC reference strain was revived, and the colony-forming units (CFU) were calculated. The MIC and MBC was determined by broth dilution assay using different concentrations of Novaron® AG 300 and Novaron® AG 1100 against 1 × 105 CFU/ml of S. mutans.
Results:
The MIC and MBC of Novaron® AG 300 and Novaron® AG 1100 against S. mutans were found to be 40 μg/ml.
Conclusions:
Novaron® has anti-bacterial effect against S. mutans. Further studies are needed to explore the applicability of these silver-supported anti- microbial agents in clinical dentistry.
Keywords: Broth dilution assay, MBC, MIC, novaron®, S. Mutans, silver-supported anti-microbial agent
Introduction
Scientific research has provided overwhelming evidence that dental caries is a specific bacterial infection linked with certain host factors.[1] There is always a tendency for the accumulation of the dental plaque on the surfaces of the restorative materials including the composite resin and glass ionomer cement, hence increasing the risk of secondary caries beneath these restorations. Plaque accumulation on composite resin is related to its surface roughness and free energy, which is related to resin type, filler size, and the percentage of filler.[2,3] Composite resins tend to accumulate more bacteria or plaque than other restorative materials in vitro[4] and in vivo.[5] Attention has now been directed towards increasing their anti-microbial and biologic properties.[6] Anti-microbial effects have been attributed to the presence and release of fluorides in restorative materials such as glass ionomers and fluoride-releasing composites, though this has been shown to be clinically effective only in the former.[6] However, the leaking of fluoride ions weakens the matrix, and the short duration and sudden “burst” of fluoride release has shown it to be clinically unsuccessful in preventing decalcification.[7–9] In some clinical trials, it has been found that fluoride - releasing bonding systems did not significantly reduce the incidence of enamel decalcification; 50% of the patients and 13.5% of the teeth had post-treatment decalcification.[10] Hence, the addition of certain agents that result in the inactivation of the bacteria is a direct strategy to eradicate further spread of the dental caries.
Silver has a long history of use in medicine as an anti-microbial agent.[11] Silver in the form of silver nitrate and along with sulfonamide to form silver sulfadiazine has been used in the management of wounds and burns. Silver ions (Ag+(aq)) are generally recognized as the bioactive agent, supplied for clinical applications from numerous silver-containing formulations comprising silver salts, silver oxide, metallic silver, silver chelates, and silver particles.[12,13] Silver ions form metal-organic complexes and insoluble compounds with sulfhydryl groups (e.g., cysteine residues) in cell walls of bacteria and fungi, generally inactivating essential enzymes responsible for energy metabolism and electron transport. Silver ions also block the electron transport chain functions most sensitively between cytochrome reductase and cytochrome oxidase and less sensitively between nicotinamide adenine dinucleotide (NADH) and succinate dehydrogenase.[14,15]
Silver, in the form of silver amalgam, have been in use for the restoration of carious lesions, though its anti-bacterial effect is minimal and, if noted, has been attributed to the presence of copper, mercury, and zinc rather than metallic silver.[16] Silver diamine fluoride has been used effectively since 1969 for Arresting Caries Treatment (ACT).[17] Nanotechnology modulates metals into their nano size, hence changing dramatically their chemical, physical, and optical properties. Silver nanoparticles show efficient anti-microbial efficacy because of their large surface area, hence providing better contact with micro-organisms.[18,19] Thomas V et al.[20] synthesized chitosan/silver nanoparticles films, which demonstrated excellent anti-bacterial action against model bacteria, Escherichia coli, and Bacillus spp. These films can be used as anti-microbial packaging materials, as wound dressings, and can also be grafted onto various implants.
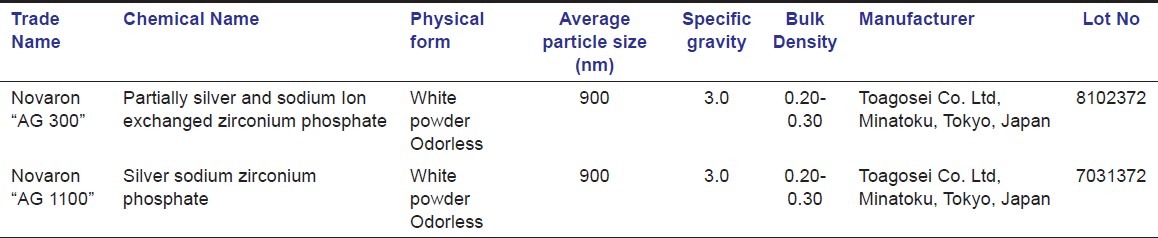
Novaron® is a silver-supported inorganic anti-microbial agent (SSAM) and exhibits an excellent anti-microbial efficacy to wide variety of micro-organisms.[21] There is a lacunae of its action against cariogenic bacteria in the literature limited to studies exclusively in Peoples Republic of China[22,23] and Japan[24] only. Novaron® is designed and approved under the voluntary standard of SIAA (The Society of Industrial Technology for Anti-microbial Articles) in Japan [Table 1]. It has uniform fine particles, low moisture absorption capability, and superior heat resistance. It can be easily mixed into textiles, films, and molded plastic products and has found usage in medical environments, medical devices, plastics, fabrics, paints, and polymeric food contact material approved by the U.S. Food and Drug Administration. In addition, it has high physical and chemical stability along with superior discoloration resistance during processing or use.[21] The objective of the present study was to determine the minimum inhibitory and minimum bactericidal concentration of Novaron®, if any, against Streptococcus mutans using broth dilution assay.
Table 1.
Properties of Nanosilver particles used in this study
Materials and Methods
This study was partially carried out in the Dept of Pedodontics and Preventive Dentistry, K. D. Dental College and Hospital, Department of Microbiology, Rajiv Academy for Pharmacy, Dept. of Microbiology, Dindyal Upadhay Vetenary and Agricultural Science University, Mathura, Uttar Pradesh, INDIA. Ethical committee clearance was obtained prior to the beginning of this study.
Revival of S. mutans
An ampoule of freeze-dried S. mutans reference strain (National Collection of Type Culture NCTC – 10499, IMTECH, Chandigarh, India) was revived by adding 0.4 ml of the Muller Hinton Broth under sterile conditions (As per the instructions from Institute of Microbiology Testing and Technology (IMTECH) Chandigarh, India). The suspension was inoculated in Muller Hinton broth (Himedia, Mumbai) and on Mitis Salivarius Bacitracin (MSB) (Himedia, Mumbai) agar slants and plates and were incubated under anaerobic conditions (in an anaerobic gas jar with anaerobic gas pack (Himedia Mumbai), which releases 5% carbon-dioxide creating an anaerobic conditions) at 37°C for 24 hours and 48 hours, respectively. The growth of the S. mutans was confirmed after incubation by observing the colony characteristics under microscope and by biochemical tests.
Determining the Colony-Forming Units
The revived S. mutans in Muller Hinton broth was centrifuged at 11,000 rpm for 5 minutes, and the supernatant liquid was discarded, and to the bacterial precipitate, 20 ml of sterile normal saline was added and further centrifuged. Again, the supernatant liquid was discarded, and 20 ml of sterile normal saline was added. The bacterial precipitate thus obtained was washed in normal saline and considered to be viable but non-growing. This was maintained as an undiluted stock solution.
From this undiluted stock solution, l ml was serially diluted utilizing Miles and Misra's method[25] of serial dilution in 10 test tubes of 9 ml normal saline.
Tube-1 containing 9 ml of sterile media; 1 ml of the undiluted bacterial suspension added to yield a total volume of 10 ml.
![]()
Tube- 2 containing 9 ml of sterile media; 1 ml of the 1:10 diluted bacterial suspension added to yield a total volume of 10 ml
![]()
Utilizing this method,[25] 10 dilutions were made, which made the stock bacterial suspension to be diluted up to 10-10. From each of these serial dilutions, 0.2 ml was mixed with liquid MSB Agar (MS agar with 0.5% sucrose and 1 unit/ml Bacitracin) and poured into sterile culture petri plates. The plates were incubated for 72 hrs at 37°C in anaerobic gas jars containing CO2 gas packs. The plates containing between 30 and 300 colonies were counted, and the colony-forming unit (CFU) was calculated using the formula,
CFU = Number of Colonies Counted/[Amount plated (in ml) × the dilution].
The dilution with 1 × 105 CFU/ml was used as inoculum for the rest of the study (equivalent to 0.5 McFarland diluted to the ratio of 1:20 using normal saline).[26,27]
Determining the MIC and MBC of Novaron®
Two grades of nanosilver particles (Novaron® AG 300 and Novaron® AG 1100) were obtained from Toagosei Co. Ltd, Tokyo, Japan and utilized in this study [Table 1]. Nanosilver particles were suspended in normal saline and ultrasonicated for 20 minutes to result in a uniform suspension. Two-fold dilutions of both the grades Novaron® were obtained in accordance with Humberto et al.[28] viz: 10, 20, 40, 80, and 160 μg/ml and utilized in this study. Each of 0.2 ml of the bacterial suspensions from the stock solution was inoculated into the corresponding tubes containing different concentrations of Novaron® and same amount of Muller Hinton (MH) broth. To these test tubes, phenol red indicator was added such that the volume of solution in each test tube was adjusted to 4 ml. The methodology also included a positive control (tubes containing inoculum and nutrient media, devoid of nanoparticles) and a negative control (tubes containing Ag nanoparticles and nutrient media, devoid of inoculum). The test tubes with only muller hinton broth served as a blank control. All the test tubes were incubated in an anaerobic gas jar with anaerobic gas pack (Himedia Mumbai) (which releases carbon-dioxide) creating an anaerobic conditions at 37°C for 24 hrs and were observed for change in color and pH. The entire procedure was repeated for 6 sets of test tubes.
Results
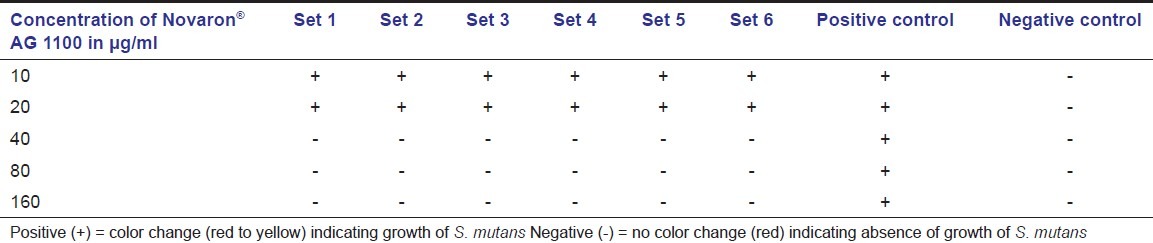
After 24 hours of incubation under anaerobic conditions, change in the color from red to yellow was observed for all the test tubes containing 10 and 20 μg/ml of nanosilver particles, indicating acid production because of growth of the bacteria. This color change was observed for both the grades of nanosilver particles; but, at the concentration of 40 μg/ml and above, no color change was observed indicating inhibition of bacterial growth [Table 2 and 3]. There was a mean fall in the pH from 7.2 to 6.0 ± 0.2 in the tubes that showed change in the color due to acid production by S. mutans. The change in the color and drop in pH was observed in the positive control test tubes also, indicating the microbial growth while the negative control group and blank control showed no change in the color and pH for all the test tubes. The suspensions from the test tubes with the lowest concentration of Novaron® AG 300 and AG 1100 that did not showed any change in color and pH were inoculated on MSB agar. After incubation, no growth of S. mutans was observed, confirming bactericidal concentration. Thus, 40 μg/ ml of Novaron® was considered as minimum bactericidal concentration (MBC). Further dilutions were made between 20 and 40 μg/ml and tested for MIC against S. mutans. After incubation, change in the color from red to yellow as well as fall in the pH was observed for all the test tubes containing the dilutions from 21 to 39 μg/ml. Based on these observations, both MIC and MBC were confirmed as 40 μg/ml for both the grades of Novaron® against S. mutans.
Table 2.
Bacterial growth in different concentrations of Novaron® AG 300
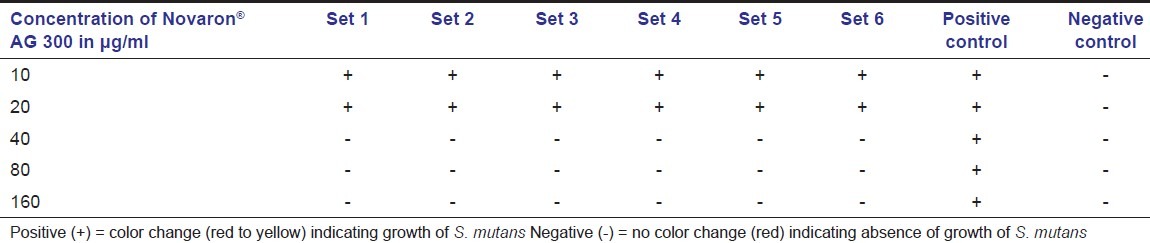
Table 3.
Bacterial growth in different concentrations of Novaron® AG 1100
Discussion
In an attempt to evaluate the feasibility of imparting long-term anti-microbial effect to the restorative materials, various anti-microbial agents have been tried and approved for intra-oral use. The modification of filling and bonding materials by the addition of anti-microbial agents such as chlorhexidine,[29,30] triclosan,[31] and cetylpyridinium chloride[32] has been attempted. It was concluded that these agents, when added in minute amounts, could impart an anti-bacterial trait to the dental materials without significantly affecting their physical properties.
Silver is a metal known for its broad-spectrum anti-microbial activity against Gram-positive and Gram-negative bacteria, fungi, protozoa, and certain viruses, including antibiotic-resistant strains.[33–35] The anti-microbial properties of silver are related to its oxidized form, a form of silver that is not necessarily present at the surface coated with metallic silver.[36] Polymers that release silver in the oxidized form have shown strong anti-bacterial activity and would act as reservoirs of silver and are capable of releasing silver ions (SI) for extended periods.[37] Many authors concluded that[38–51] the anti-microbial activity of silver is dependent on SI, which binds strongly to electron donor groups in biological molecules containing sulfur, oxygen, or nitrogen. This may result in defects in the bacteria cell wall so that the cell contents are lost.[41] A complex formation between SI and proteins may disturb the metabolism of bacterial cells and their power functions, such as permeability and respiration.[38]
Silver in the form of nanoparticles have found renewed acceptance while several studies have demonstrated that silver ions are toxic to bacteria including oral streptococci.[52] The efficacy of nanoparticles is primarily related to the fact they attack more than one site in the bacteria, hence reducing the chance of developing bacterial resistance. This property of nanosilver particles has been exploited in medicine, dental materials, coating of stainless steel, textile fabrics, water treatment, and sunscreen lotions.[18] Though the mechanism of action of nanosilver particles is not fully understood, it is attributed to the release of silver ion once the nanoparticles enter the bacterial cell. They attack the respiratory chain and cell division, leading to cell death. Also, suggested mechanism is that the oxygen is changed into oxygen-free radicals by the action of light energy in air or water as a result of the catalytic action of silver, which leads to structural damage in the bacteria.
Kong and Jang[53] compared the anti-bacterial properties of polymethyl methacrylate (PMMA) nanofibre containing silver nanoparticles with silver sulfadiazine and AgNO3 at the same silver concentration against E. coli and S. aureus. The silver/PMMA nanofibre had a faster kill rate than silver sulfadiazine and AgNO3. Ahn et al.[54] incorporated silica nanofillers and silver nanoparticles in orthodontic adhesives. Even with an increased surface roughness due to the incorporation of silver nanoparticles, the adhesives produced a significant reduction in the adhesion of cariogenic streptococci, regardless of the silver added (250 ppm and 500 ppm). The bond strength of the orthodontic adhesives was not affected and, because the anti-microbial effect was maintained after saliva coating, the silver was able to penetrate the saliva coating, which could bring beneficial clinical implications. Jain et al.[55] concluded that silver nanoparticles could have successful therapeutic use as a part of the anti-microbial gel for topical use. Standard anti-microbial sensitivity tests carried out in Muller-Hinton agar plates were used to evaluate the anti-microbial activity of the silver nanoparticles containing gel against bacterial cultures of Escherichi coli (ATCC 117), Pseudomonas aeruginosa (ATCC 9027), Staphylococcus aureus (ATCC 6538), and Streptococcus epidermidis (ATCC 12228). Gram-negative bacteria were killed more effectively (3 log decrease in 5-9 h) than Gram-positive bacteria (3 log decrease in 12 h). The anti-microbial gel also exhibited good anti-fungal activity (50% inhibition at 75 μg/mL with anti-fungal index 55.5% against Aspergillus niger and MIC of 25 μg/mL against Candida albicans). Acute dermal toxicity studies on gel formulation (S-gel) in Sprague- Dawley rats showed complete safety for topical application.
Hernandez-Sierra et al.[56] used nanoparticles of silver, zinc oxide, and gold of an average size of 25 nm, 125 nm, and 80 nm, respectively, to demonstrated bacteriostatic and bactericidal effects on S. mutans. They concluded that nanoparticles of silver, as compared with those of gold and zinc oxide, required a lower concentration (MIC on average 4.86 ± 2.71 μg/mL) to inhibit the growth of S. mutans strains compared to the zinc oxide (MIC on average 500 ± 306.18 μg/mL) and gold (MIC on average 197 μg/mL). Hernandez-Sierra et al.[57] also evaluated the bactericidal and bacteriostatic effects of silver nanoparticles, in addition to the Gantrez S-27 copolymer, on S mutans. The mixture was obtained by preparing 98 microg/mL of silver nanoparticles (10 (3) mol) with Gantrez S-27 2% in distilled water. The results showed an average MIC of 6.12 microg/mL and MBC of 6.12 microg/mL. Espinosa et al.[58] evaluated 3 sizes of silver nanoparticles to estimate the MIC for S. Mutans and concluded that nanoparticles with the lowest MIC 66.87 ± 0 (μg/ml) was 8.4 nm in diameter, followed by 16.1 nm with MIC 108.33 ± 77.22 (μg/mL) and 98 nm with MIC 222.92 ± 77.22 (μg/mL), suggesting that anti-bacterial property of silver nanoparticles possibly depend on the size of the particles. However, silver nanoparticles remain a controversial research area in relation to their toxicity to biological systems. In particular, the oral toxicity of silver nanoparticles is of particular concern to ensure public and consumer health, and this arena should be investigated.
Zhang FQ[23] et al. evaluated the biocompatibility of nano-silver base inorganic anti-bacterial agents and compared the cytotoxicity in vitro among 6 types of nano-silver base inorganic anti-bacterial agents. FUMAT T200-4, HN300, Novaron, Kangwang, MOD, and SR1000 were diluted to different concentrations, such as 100 g/L, 50 g/L, 25 g/L, and 12.5 g/L. They found that no cytotoxic effects were observed at or below the concentration of 25 g/L. FUMAT T200-4, Kongwang, and SR 1000, with the carrier of phosphate zirconium, had less cytotoxity than the others. Yong Soon Kim[59] et al. tested the oral toxicity of silver nanoparticles over a period of 28 days and suggested that silver nanoparticles do not induce genetic toxicity in male and female rat bone marrow in vivo. Nonetheless, the tissue distribution of silver nanoparticles did show a dose-dependent accumulation of silver content in all the tissues examined. In particular, a gender-related difference in the accumulation of silver was noted in the kidneys, with a two-fold increase in the female kidneys when compared with the male kidneys. Hernandez-Sierra et al.[60] evaluated the cytotoxicity of different concentration and sizes of silver nanoparticles with study design of 0-1000 μM silver nanoparticles. They concluded that silver nanoparticles of less than 20 nm increased cytotoxicity to human periodontal fibroblast in a dose- and time-dependent manner.
In this study, the minimum inhibitory and minimum bactericidal concentration of Novaron® was determined using the broth dilution assay and was observed to be 40 μg/ ml for both the grades of Novaron® . This study was in agreement with Tanagawa et al.[24] who concluded 40 μg/ml as the MIC for the Novaron® in their study. Further studies are needed to evaluate the feasibility of incorporation of these nanosilver particles in various restorative materials, their effect on the physical properties, and their clinical applicability.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared
References
- 1.Fitzgerald RJ, Keyes PH. Demonstration of the etiologic role of streptococci in test caries in the hamster. J Am Dent Assoc. 1960;61:9–19. doi: 10.14219/jada.archive.1960.0138. [DOI] [PubMed] [Google Scholar]
- 2.Svanberg M, Mjor IA, Orstavik D. Mutans streptococci in plaque from margins of amalgam, composite and glass ionomer restorations. J Dent Res. 1990;69:861–4. doi: 10.1177/00220345900690030601. [DOI] [PubMed] [Google Scholar]
- 3.Quiryen M, Bollen CM. The influence of surface roughness and surface-free energy on supra and subgingival plaque formation in man. A review of the literature. J Clin Periodontol. 1995;22:1–14. doi: 10.1111/j.1600-051x.1995.tb01765.x. [DOI] [PubMed] [Google Scholar]
- 4.Skjorland KK. Bacterial accumulation on silicate and composite materials. J Biol Buccale. 1976;4:315–22. [PubMed] [Google Scholar]
- 5.Weitman RT, Eames WB. Plaque accumulation on composite surfaces after various finishing procedures. J Am Dent Assoc. 1975;81:101–6. doi: 10.14219/jada.archive.1975.0294. [DOI] [PubMed] [Google Scholar]
- 6.Yap AU, Khor E, Foo SH. Fluoride release and antibacterial properties of new generation tooth coloured restoratives. Oper Dent. 1999;24:297–305. [PubMed] [Google Scholar]
- 7.Cooley RL, McCourt JW. Fluoride-releasing removable appliances. Quintessence Int. 1991;22:299–302. [PubMed] [Google Scholar]
- 8.Forsten L. Fluoride release from a glass ionomer cement. Scand J Dent Res. 1977;85:503–4. doi: 10.1111/j.1600-0722.1977.tb00586.x. [DOI] [PubMed] [Google Scholar]
- 9.Tveit AB, Gjerdet NR. Fluoride release from a fluoride-containing amalgam, a glass ionomer cement and a silicate cement in artificial saliva. J Oral Rehabil. 1981;8:237–41. doi: 10.1111/j.1365-2842.1981.tb00498.x. [DOI] [PubMed] [Google Scholar]
- 10.Banks PA, Burn A, O’Brien K. A clinical evaluation of the effectiveness of including fluoride into an orthodontic bonding adhesive. Eur J Orthod. 1997;19:391–5. doi: 10.1093/ejo/19.4.391. [DOI] [PubMed] [Google Scholar]
- 11.Brett DW. A discussion of silver as an antimicrobial agent: Alleviating the confusion. Ostomy Wound Manage. 2006;52:34–41. [PubMed] [Google Scholar]
- 12.Russell AD, Hugo WB. Antimicrobial activity and action of silver. Prog Med Chem. 1994;31:351–70. doi: 10.1016/s0079-6468(08)70024-9. [DOI] [PubMed] [Google Scholar]
- 13.Stickler DJ. Biomaterials to prevent nosocomial infections: Is silver the gold standard? Curr Opin Infect Dis. 2000;13:389–93. doi: 10.1097/00001432-200008000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Williams RL, Doherty PJ, Vince DG, Grashoff GJ, Williams DF. The biocompatibility of silver. Crit Rev Biocompat. 1989;5:221–43. [Google Scholar]
- 15.Bragg PD, Rainnie DJ. The effect of silver ions on the respiratory chain of Escherichia coli. Can J Microbiol. 1973;20:883–9. doi: 10.1139/m74-135. [DOI] [PubMed] [Google Scholar]
- 16.Morrier JJ, Suchett - Kaye G, Nguyen D, Rocca JP, Blanc-Benon J, Barsotti O. Antimicrobial activity of amalgams, alloys and their elements and phases. Dent Mater. 1998;14:150–7. doi: 10.1016/s0109-5641(98)00022-0. [DOI] [PubMed] [Google Scholar]
- 17.Yee R, Holmgren C, Mulder J, Lama D, Walker D, van Palenstein Helderman W. Efficacy of silver diamine flouride for arresting caries treatment. J Dent Res. 2009;88:644–7. doi: 10.1177/0022034509338671. [DOI] [PubMed] [Google Scholar]
- 18.Rai M, Yadav A, Gade A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv. 2009;27:76–83. doi: 10.1016/j.biotechadv.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Holister P, Weener JW, Romas Vas C, Harper T. Nanoparticles: Technology white papers 3. London: Sientific Ltd; 2003. pp. 2–11. [Google Scholar]
- 20.Thomas V, Sreedhar MM, Bajpai SK, Sreedhar B. Fabrication, characterization of chitosan/nanosilver film and its potential antibacterial application. J Biomater Sci Polym Edn. 2009;20:2129–44. doi: 10.1163/156856209X410102. [DOI] [PubMed] [Google Scholar]
- 21.Novaron®. [Last accessed on 2011 Dec 28]. Available from: http://www.hsingnan.com.tw/NOVARON.pdf .
- 22.She WJ, Zhang FQ. Comparison of the antibacterial activity on oral pathogens among six types of nano-silver base inorganic antibacterial agents. Shanghai Kou Qiang Yi Xue. 2003;12:356–8. [PubMed] [Google Scholar]
- 23.Zhang FQ, She WJ, Fu YF. Comparison of the cytotoxicity in vitro among six types of nano-silver base inorganic antibacterial agents. Zhonghua Kou Qiang Yi Xue Za Zhi. 2005;40:504–7. [PubMed] [Google Scholar]
- 24.Tanagawa M, Yoshida K, Matsumoto S, Yamada T, Atsuta M. Inhibitory effect of antibacterial resin composite against streptococcus mutans. Caries Res. 1999;33:366–71. doi: 10.1159/000016535. [DOI] [PubMed] [Google Scholar]
- 25.Miles AA, Misra SS, Irwin JO. Estimation of the bactericidal power of the blood. J Hyg (Lond) 1938;38:732–49. doi: 10.1017/s002217240001158x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bratthall D, Carlsson P. Clinical microbiology of saliva. In: Tenuovo JO, editor. Human saliva: Clinical, chemistry and microbiology. Boca Raton: CRC Press; 1989. pp. 204–23. [Google Scholar]
- 27.Leal SC, Mickenautsch S. Salivary streptococcus mutans count and caries outcome – a systematic review. J Minim Interv Dent. 2010;3:139–45. [Google Scholar]
- 28.Humberto HL, Ayala-Nunez NV, Liliana del Carmen IT, Cristina RP. Bactericidal effect of silver nanoparticles against multidrug-resistant bacteria. World J Microbiol Biotechnol. 2010;26:615–21. [Google Scholar]
- 29.Ribeiro I, Ericson D. In vitro antibacterial effect of chlorhexidine added to glass- ionomer cements. Scand J Dent Res. 1991;99:533–40. doi: 10.1111/j.1600-0722.1991.tb01066.x. [DOI] [PubMed] [Google Scholar]
- 30.Jedrychowski JR, Caputo AA, Kerper S. Antibacterial and mechanical properties of restorative materials combined with chlorhexidines. J Oral Rehabil. 1983;10:373–81. doi: 10.1111/j.1365-2842.1983.tb00133.x. [DOI] [PubMed] [Google Scholar]
- 31.Imazato S, Torii M, Tsuchitani Y. Antibacterial effect of composite incorporating triclosan against S.mutans. J Osaka Univ Dent She. 1995;35:5–11. [PubMed] [Google Scholar]
- 32.Al-Musallam TA, Evans CA, Drummond JL, Matasa CG, Wu CD. Antimicrobial properties of an orthodontic adhesive combined with cetylpyridinium chloride. Am J Orthod Dentofacial Orthop. 2006;129:245–51. doi: 10.1016/j.ajodo.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 33.Stobie N, Duffy B, McCormack DE, Colreavy J, Hidalgo M, McHale P, et al. Prevention of Staphylococcus epidermidis biofilm formation using a low-temperature processed silver-doped phenyltriethoxysilane sol-gel coating. Biomaterials. 2008;29:963–9. doi: 10.1016/j.biomaterials.2007.10.057. [DOI] [PubMed] [Google Scholar]
- 34.Balazs DJ, Triandafillu K, Wood P, Chevolot Y, van Delben C, Harms H, et al. Inhibition of bacterial adhesion on PVC endotracheal tubes by RF-oxygen glow discharge, sodium hydroxide and silver nitrate treatments. Biomaterials. 2004;25:2139–51. doi: 10.1016/j.biomaterials.2003.08.053. [DOI] [PubMed] [Google Scholar]
- 35.Melaiye A, Youngs WJ. Silver and its application as an antimicrobial agent. Expert Opin Ther Pat. 2005;15:125–30. [Google Scholar]
- 36.Weir E, Lawlor A, Whelan A, Regan F. The use of nanoparticles in anti-microbial materials and their characterization. Analyst. 2008;133:835–45. doi: 10.1039/b715532h. [DOI] [PubMed] [Google Scholar]
- 37.Hetrick EM, Schoenfisch MH. Reducing implant-related infections: Active release strategies. Chem Soc Rev. 2006;35:780–9. doi: 10.1039/b515219b. [DOI] [PubMed] [Google Scholar]
- 38.Panácek A, Kvítek L, Prucek R, Kolár M, Vecerová R, Pizúrová N, et al. Silver colloid nanoparticles: Synthesis, characterization, and their antibacterial activity. J Phys Chem B. 2006;110:16248–53. doi: 10.1021/jp063826h. [DOI] [PubMed] [Google Scholar]
- 39.Kawahara K, Tsuruda K, Morishita M, Uchida M. Antibacterial effect of silver–zeolite on oral bacteria under anaerobic conditions. Dent Mater. 2000;16:452–5. doi: 10.1016/s0109-5641(00)00050-6. [DOI] [PubMed] [Google Scholar]
- 40.Matsuura T, Abe Y, Sato Y, Okamoto K, Ueshige M, Akagawa Y. Prolonged antimicrobial effect of tissue conditioners containing silver-zeolite. J Dent. 1997;25:373–7. doi: 10.1016/s0300-5712(96)00050-4. [DOI] [PubMed] [Google Scholar]
- 41.Damm C, Münstedt H, Rösch A. The antimicrobial efficacy of polyamide 6/silvernano-and microcomposites. Mater Chem Phys. 2008;108:61–6. [Google Scholar]
- 42.Spadaro JA, Becker RO. Some specific cellular effects of electrically injected silver and gold ions. Bioelectrochem Bioenerg. 1975;3:49–57. [Google Scholar]
- 43.Duran N, Marcarto PD, De Souza GIH, Alves OL, Esposito E. Antibacterial effects of silver nanoparticles produced by fungal process on textile fabrics and their treatment. J Biomed Nanotechnol. 2007;3:203–8. [Google Scholar]
- 44.Butkus MA, Edling L, Labare MP. The efficacy of silver as a bactericidal agent: Advantages, limitations and considerations for future use. J Water Supply Res Technol AQUA. 2003;52:407–16. [Google Scholar]
- 45.Cho KH, Park JE, Osaka T, Park SG. The study of antimicrobial activity and preservative effects of nanosilver ingredient. Electrochim Acta. 2005;51:956–60. [Google Scholar]
- 46.Feng QL, Wu J, Chen GQ, Cui FZ, Kim TN, Kim JO. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J Biomed Mater Res. 2000;52:662–8. doi: 10.1002/1097-4636(20001215)52:4<662::aid-jbm10>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 47.Kim JS, Kuk E, Yu KN, Kim JH, Park SJ, Lee HJ, et al. Antimicrobial effects of silver nanoparticles. Nanomedicine. 2007;3:95–101. doi: 10.1016/j.nano.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 48.Lok CN, Ho CM, Chen R, He QY, Yu WY, Sun H, et al. Silver nanoparticles: Partial oxidation and antibacterial activities. J Biol Inorg Chem. 2007;12:527–34. doi: 10.1007/s00775-007-0208-z. [DOI] [PubMed] [Google Scholar]
- 49.Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramírez JT, et al. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16:2346–53. doi: 10.1088/0957-4484/16/10/059. [DOI] [PubMed] [Google Scholar]
- 50.Pal S, Tak YK, Song JM. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl Environ Microbiol. 2007;73:1712–20. doi: 10.1128/AEM.02218-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raffi M, Hussain F, Bhatti TM, Akhter JI, Hameed A, Hasan MM. Antibacterial characterization of silver nanoparticles against E.coli ATCC-15224. J Mater Sci Technol. 2008;24:192–6. [Google Scholar]
- 52.Sondi I, Salopek-Sondi B. Silver nanoparticles as antimicrobial agent: A case study of E.coli as a model for gram-negative bacteria. J Colloid Interface Sci. 2004;275:177–82. doi: 10.1016/j.jcis.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 53.Kong H, Jang J. Antibacterial properties of novel poly (methyl methacrylate) nanofiber containing silver nanoparticles. Langmuir. 2008;24:2051–6. doi: 10.1021/la703085e. [DOI] [PubMed] [Google Scholar]
- 54.Ahn SJ, Lee SJ, Kook JK, Lim BS. Experimental antimicrobial orthodontic adhesives using nanofillers and silver nanoparticles. Dent Mater. 2009;25:206–13. doi: 10.1016/j.dental.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 55.Jain J, Arora S, Rajwade JM, Omray P, Khandelwal S, Paknikar KM. Silver nanoparticles in therapeutics: Development of an antimicrobial gel formulation for topical use. Mol Pharm. 2009;6:1388–401. doi: 10.1021/mp900056g. [DOI] [PubMed] [Google Scholar]
- 56.Hernández-Sierra JF, Ruiz F, Pena D, Martinez-Gutierrez F, Martinez AE, Guillen Ade J, et al. The antimicrobial sensitivity of Streptococcus mutans to nanoparticles of silver, zinc oxide, and gold. Nanomedicine. 2008;4:237–40. doi: 10.1016/j.nano.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 57.Hernández-Sierra JF, Salas-López EK, Martínez-Gutiérrez F, Ruíz F, Pierdant-Pérez M, Mandeville P, et al. Bactericidal capacity of silver nanoparticles associated with Gantrez S-97 on Streptococcus mutans. J Clin Pediatr Dent. 2010;35:183–5. doi: 10.17796/jcpd.35.2.c61l421mj0655lgm. [DOI] [PubMed] [Google Scholar]
- 58.Espinosa-Cristobal LF, Martinez-Castanon GA, Martinez-Martinez RE, Loyola-Rodriguez JP, Patino-Marin N, Reyes-Macias JF, et al. Antibacterial effects of silver nanoparticles against Streptococcus mutans. Mater Lett. 2009;63:2603–6. [Google Scholar]
- 59.Kim YS, Kim JS, Cho HS, Rha DS, Kim JM, Park JD et al. Twenty eight day oral toxicity, genotoxicity, and gender-related tissue distribution of silver nanoparticles in sprague-dawley rats. Inhal Toxicol. 2008;20:575–83. doi: 10.1080/08958370701874663. [DOI] [PubMed] [Google Scholar]
- 60.Hernández-Sierra JF, Galicia-Cruz O, Salinas-Acosta A, Ruíz F, Pierdant-Pérez M, Pozos-Guillén AJ. In vitro cytotoxicity of silver nanoparticles on Human periodontal fibroblasts. J Clin Pediatr Dent. 2011;36:37–41. doi: 10.17796/jcpd.36.1.d677647166398886. [DOI] [PubMed] [Google Scholar]


