Abstract
The conjunctiva is a mucous membrane that covers the sclera and lines the inside of the eyelids. Throughout the conjunctiva are goblet cells that secrete mucins to protect the eye. Chronic inflammatory diseases such as allergic conjunctivitis and early dry eye lead to increased goblet cell mucin secretion into tears and ocular surface disease. The purpose of this study was to determine the actions of the inflammatory mediators, the leukotrienes and the proresolution resolvins, on secretion from cultured rat and human conjunctival goblet cells. We found that both cysteinyl leukotriene (CysLT) receptors, CysLT1 and CysLT2, were present in rat conjunctiva and in rat and human cultured conjunctival goblet cells. All leukotrienes LTB4, LTC4, LTD4, and LTE4, as well as PGD2, stimulated goblet cell secretion in rat goblet cells. LTD4 and LTE4 increased the intracellular Ca2+ concentration ([Ca2+]i), and LTD4 activated ERK1/2. The CysLT1 receptor antagonist MK571 significantly decreased LTD4-stimulated rat goblet cell secretion and the increase in [Ca2+]i. Resolvins D1 (RvD1) and E1 (RvE1) completely reduced LTD4-stimulated goblet cell secretion in cultured rat goblet cells. LTD4-induced secretion from human goblet cells was blocked by RvD1. RvD1 and RvE1 prevented LTD4- and LTE4-stimulated increases in [Ca2+]i, as well as LTD4 activation of ERK1/2. We conclude that cysteinyl leukotrienes stimulate conjunctival goblet cell mucous secretion with LTD4 using the CysLT1 receptor. Stimulated secretion is terminated by preventing the increase in [Ca2+]i and activation of ERK1/2 by RvD1 and RvE1.
An increase in goblet cell mucin production causes pathogenesis in several tissues. In the lung, an increase in mucin production occurs in asthma, chronic obstructive pulmonary disease, and cystic fibrosis (1). In the upper airways, an increase in goblet cell mucin production occurs in chronic rhinosinusitis (2). Goblet cells and their mucin are also upregulated in the intestine in the setting of inflammatory bowel disease, Crohn’s disease, and ulcerative colitis (3). In the ocular surface (cornea and conjunctiva), an excess in goblet cell mucin production is deleterious to the ocular surface and occurs in early dry eye and allergic conjunctivitis (4).
Conjunctival goblet cell secretion is induced by stimulation of the afferent sensory nerves in the cornea and conjunctiva that by a reflex arc activate efferent parasympathetic and sympathetic nerves that surround the goblet cells (5). Activation of parasympathetic nerves releases acetylcholine and vasoactive intestinal peptide (VIP) to cause goblet cell secretion, whereas the role of sympathetic nerves is unknown. Acetylcholine and VIP interact with muscarinic acetylcholine receptors type 2 and 3 (MAchR2 and MAchR3) (6) and VIP receptors type 2 (VIPAC2) (7), respectively. Activation of these receptors increases the intracellular [Ca2+] concentration ([Ca2+]i) and activates ERK1/2, also known as p44/p42 MAPK (8, 9). Activation of ERK1/2 causes fusion of secretory granules and release of their contents into the tear film. Cholinergic agonists activate protein kinase C in addition to increasing the [Ca2+]i, and both of these cellular mediators transactivate the epidermal growth factor (EGF) receptor that initiates the signaling cascade culminating in the stimulation of ERK1/2 activity.
Production of cysteinyl leukotrienes LTC4, LTD4, and LTE4 from arachidonic acid initiates the inflammatory response (10). Inflammation is actively terminated with the production of resolvins. Resolvins are products of omega-3 fatty acids that are biosynthesized by switching of the enzymes that produce leukotrienes to synthesize the proresolving resolvins. Resolvins possess potent actions in actively controlling the resolution of inflammatory exudates in multiple different models of inflammation (for recent reviews, see Refs. 11, 12). Resolvin E1 (RvE1) is derived from eicosapentaenoic acid (13) and is a potent mediator that: 1) reduces polymorphonuclear neutrophil infiltration; 2) promotes resolution; 3) reduces colitis; 4) regulates dendritic cell function and IL-12 production; 5) protects from osteoclast-mediated bone destruction (reviewed in Ref. 14); and 6) regulates IL-23, IFN-γ, and lipoxin A4 to resolve allergic airway inflammation (14, 15). Resolvin D1 (RvD1) is derived from docosahexaenoic acid (16) and is another powerful compound that: 1) stops neutrophil recruitment in peritonitis and the dorsal skin air pouch of mice (16), 2) protects from ischemia-reperfusion–induced kidney and lung damage and loss of function (17, 18), and 3) protects against neovascularization in retinopathy (12, 14). In the eye, RvE1 and RvD1 protect against retinal and corneal neovascularization (19, 20).
In this study, we have investigated the action of the chemical mediators cysteinyl leukotrienes and resolvins on conjunctival goblet cell secretion using rat and human conjunctival goblet cells in primary culture. We report that cysteinyl leukotrienes stimulated goblet cell secretion that is regulated by resolvins.
Materials and Methods
Animals
Male Sprague Dawley rats weighing between 125 and 150 g were obtained from Taconic Farms (Germantown, NY). Rats were anesthetized with CO2 for 1 min, decapitated, and the nictitating membranes and fornix removed from both eyes. All experiments conformed to the U.S. Department of Agriculture Animal Welfare Act (2007) and were approved by the Schepens Eye Research Institute Animal Care and Use Committee.
Human material
Human conjunctival tissue was obtained from patients during ocular surgery using a protocol that adhered to the tenets of the Declaration of Helsinki. The protocol was approved by the Schepens Eye Research Institute Human Studies Internal Review Board. The tissue, which was normally discarded during surgery, was donated by three patients (two male and one female patient; age range, 58–73 y; average age, 65 y). The surgeries performed on the patients were pars plana vitrectomy, pars plana vitrectomy with membrane peeling, and scleral buckle. Tissue was placed in PBS solution containing penicillin-streptomycin (300 μg/ml).
Cell culture
Goblet cells from rat and human conjunctiva were grown in organ culture as described previously (21–23). Pieces of minced tissue were placed in RPMI 1640 medium supplemented with 10% FBS, 2 mM glutamine, and 100 μg/ml penicillin-streptomycin. The tissue plug was removed after nodules of cells were observed. As described previously, cells were identified as goblet cells by the following characteristics: 1) morphology by light microscopy both bright-field and histochemical staining with Alcian blue/periodic acid–Schiff’s reagent (indicates secretory product); 2) positive staining with the lectin Ulex europaeus agglutinin type I (UEA-I) and Abs to MUC5AC (both stain secretory product) and cytokeratin 7 (stains cell body); and 3) negative staining with Ab to cytokeratin 4 and lectin Griffonia Bandeiraea simplicifolia (indicates stratified squamous cells). First-passage goblet cells were used in all experiments.
Secretion
First-passage, cultured goblet cells were plated in 24-well plates and grown to confluence. Cells were serum starved for 2 h before use, preincubated with antagonists for 30 min, and then stimulated with agonists for 0–4 h in the presence of serum-free RPMI 1640 supplemented with 0.5% BSA. Goblet cell secretion was measured using an enzyme-linked lectin assay (ELLA) with the lectin UEA-I that detects rat conjunctival goblet cell mucins. The media were collected and analyzed for the amount of lectin-detectable glycoconjugates, which include the mucin MUC5AC and indicate goblet cell secretion. The standards and supernatant were spotted onto Nunc microplates and dried overnight at 60°C. The ELLA was performed according to a protocol from Pierce, using UEA-I conjugated to HRP. The UEA-I was then detected using Amplex Red (Invitrogen, Carlsbad, CA), which when oxidized by peroxidase in the presence of hydrogen peroxide produces a highly fluorescent molecule. The fluorescence was quantified on a fluorescent ELISA reader (model FL600; Bio-Tek, Winooski, VT) with an excitation wavelength of 530 nm and an emission wavelength of 590 nm. The cells were removed and sonicated, and the cell homogenate analyzed for the total amount of protein by using the Bradford protein assay. Glycoconjugate secretion was normalized to total protein in the homogenate. Bovine submaxillary mucin was used for the standard curve. Glycoconjugate secretion was expressed as fold increase over basal that was set to 1.
Immunohistochemistry
For immunofluorescence microscopy of intact conjunctiva, the eyes were enucleated with the lids intact and fixed in 4% formaldehyde in PBS for overnight at 4°C. Eyes were embedded in paraffin. Sections (6 μm) were placed on slides and kept at −20°C until use. For immunohistochemistry of cultured cells, first-passage cells were grown on glass coverslips and then fixed in methanol before use. Tissue sections and cultured cells were processed and viewed for immunofluorescence as described previously. Abs used included anti-cysteinyl leukotriene (CysLT)1 receptor (rabbit polyclonal Ab used at 1:200, sc-25448; Santa Cruz Biotechnology, Santa Cruz, CA), anti-CysLT2 receptor (goat polyclonal Ab used at 1:200, sc-27097; Santa Cruz Biotechnology), anti-human ChemR23 (rabbit polyclonal Ab used at 1:100, sc-66829; Santa Cruz Biotechnology), and anti-human GPR32 (rabbit polyclonal Ab used at 1:50, GTX71225; Gene Tex, Irvine, CA). UEA-I conjugated to FITC (Sigma-Aldrich, St. Louis, MO) was used at a dilution of 1:500 and identified goblet cell secretory product. DAPI was in the mounting medium and indicated cell nuclei. Secondary Abs were conjugated to either Cy2 or Cy3 (Jackson ImmunoResearch Laboratories, West Grove, PA) and were used at a dilution of 1:150. Negative control experiments included use of isotype controls (sc-3888 [anti-rabbit] and sc-3887 [anti-goat]; Santa Cruz Biotechnology).
Western blotting
Pieces of rat conjunctiva and goblet cells cultured in eight-well plates were homogenized in RIPA buffer. The homogenate was centrifuged at 2000 × g for 30 min at 4°C. Sample buffer (4X) was added to the homogenate and protein separated by NaDodSO4-PAGE (SDS-PAGE) using a 10% gel and processed for Western blotting as described previously. Primary Abs were the same as those used for immunofluorescence experiments and were diluted to 1:500. Secondary Ab was from Millipore (Billerica, MA) and was used at a dilution of 1:5000. Immunoreactive bands were visualized by the ECL method. Negative control experiments included omission of the primary Ab.
Measurement of [Ca2+]i
Cultured goblet cells were seeded onto glass-bottom 35-mm petri dishes (MatTek, Ashland, MA) and allowed to attach overnight at 37°C. Cells were then incubated for 1 h with 8 μM fura 2-AM (Invitrogen, Carlsbad, CA) in buffer containing 119 mM NaCl, 4.8 mM KCl, 1.0 mM CaCl2, 1.2 mM MgCl2, 1.2 mM KH2PO4, and 25 mM NaHCO3 supplemented with 10 mM HEPES, 5.5 mM glucose, 250 μM sulfinpyrazone, and 0.5% BSA (KRB-HEPES). Cells were stimulated with agonists alone or preincubated with RvD1 or RvE1 for 0.5 h and then stimulated with agonist. Fluorescent images of cells were recorded and analyzed with a digital fluorescence imaging system (InCyte Im2, Intracellular Imaging). Peak [Ca2+]i was calculated by subtracting the basal values (before the addition of agonist) from the peak calcium value.
Measurement of ERK1/2 activity
Goblet cells were grown in 12-well plates. Cells were serum starved for 2 h and then stimulated with LTD4 (10−11 M) for 0–10 min. In other experiments, cells were preincubated with RvD1 or RvE1 for 0.5 h before stimulation with LTD4 (10−11 M) for 5 min. The reaction was terminated by removal of buffer and addition of ice-cold RIPA buffer (10 mM Tris-HCl pH 7.4, 150 mM NaCl, 1% deoxycholic acid, 1% Triton X-100, 0.1% SDS, 1 mM EDTA, and protease inhibitors). The cells were scraped and proteins were separated by SDS-PAGE. Western blot was performed with Abs directed against phosphorylated (phospho-; active) ERK1/2 (Tyr204) and total ERK2 (Santa Cruz Biotechnology, Santa Cruz, CA). Immunoreactive bands were digitally scanned and analyzed using ImageJ (National Institutes of Health). The amount of phospho-ERK1/2 in each sample was standardized to the amount of total ERK2. Basal values were set to 1.
Solutions and chemicals
PBS used for immunofluorescence microscopy contained 145 mM NaCl, 7.3 mM Na2HPO4, and 2.7 mM NaH2PO4 (pH 7.2). RIPA used for Western blotting contained 10 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1% deoxycholic acid, 1% Triton X-100, 0.1% SDS, and 1 mM EDTA. Leukotrienes, PGD2, montelukast, MK571, and MK886 were purchased from Cayman Chemical (Ann Arbor, MI). RvD1 and RvE1 were obtained from Cayman Chemical, and their physical properties were monitored according to published criteria (24, 25). Chemerin was obtained from R&D Systems (Minneapolis, MN).
Statistical analysis
Results were expressed as the fold increase above basal or the percentage of inhibition of the net stimulation by agonist alone. Results are presented as mean ± SEM. Data were analyzed by Student t test. A p value <0.05 was considered statistically significant.
Results
Cysteinyl leukotrienes LTD4 and LTE4 stimulate conjunctival goblet cell secretion
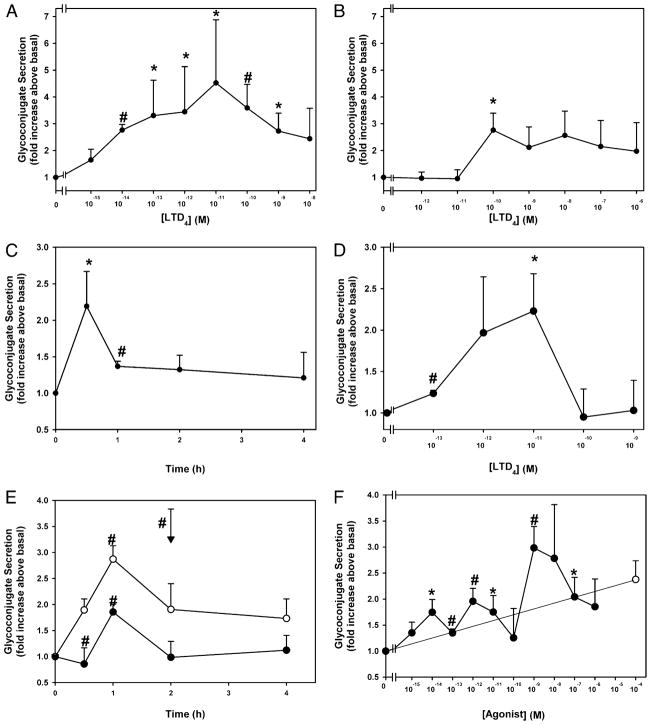
One of the hallmarks of inflammation is the generation of LTD4 and LTE4 by activated mast cells. Cultured rat goblet cells were incubated for 0.5 h with increasing concentrations of LTD4. LTD4 significantly increased secretion at 10−14 to 10−9 M, and 10−11 M caused a maximum increase in secretion of 4.5 ± 2.4-fold that decreased with increasing stimulus concentration (Fig. 1A). A second incubation time of 2 h was chosen because this is the time that a known agonist of conjunctival goblet cell secretion, the cholinergic agonist carbachol, caused a significant increase in secretion (26). When incubated for 2 h, LTD4 at only 10−10 M stimulated secretion, which was a 2.8 ± 0.6 increase (Fig. 1B). When the time dependency of goblet cells was examined, LTD4 at 10−11 M induced a rapid peak of secretion at 0.5 h that decreased by 1 h but remained elevated for 2 and 4 h (Fig. 1C). The time course of secretion is consistent with results from the two concentration-response experiments in which secretion was decreased at almost all concentrations of LTD4 used at 2 compared with 0.5 h. The effect of LTD4 on human conjunctival goblet cells was investigated. LTD4 was incubated for 0.5 h with cultured human goblet cells. LTD4 at 10−11 M caused a maximum increase in secretion of 2.2 ± 0.5-fold that decreased with increasing concentration (Fig. 1D). LTD4 is a potent and effective stimulus of conjunctival goblet cell secretion in both human and rat conjunctival goblet cells.
FIGURE 1.
Effect of time and concentration of LTD4 and LTE4 on glycoconjugate secretion from cultured rat and human conjunctival goblet cells. Cultured rat conjunctival goblet cells were serum starved for 2 h before stimulation with increasing concentrations of LTD4 for 0.5 (A) (n = 5) or 2 h (B) (n= 5), or LTD4 (10−11 M) for 0–4 h (C) (n = 4). Cultured human conjunctival goblet cells were incubated with increasing concentrations of LTD4 for 0.5 h (D) (n = 3). Cultured rat conjunctival goblet cells were serum starved for 2 h before stimulation with LTE4 (10−11 M, open circles, and 10−12 M, closed circles) for 0–4 h or the cholinergic agonist carbachol (10−4 M, inverted triangle) for 2 h (E) (n = 5), or increasing concentrations of LTE4 (closed circles) for 1 h or carbachol (10−4 M, open circles) for 2 h (F) (n = 5). The amount of glycoconjugate secretion was measured by ELLA. Data are mean ± SEM. *p < 0.05 from no addition, #p < 0.01 from no addition.
LTD4 produced a rapid burst of secretion that declined to a lower constant level for 4 h. Because LTD4 is rapidly metabolized to LTE4, LTE4 could be a less effective stimulus than LTD4, and this could account for the decrease in secretion with time. LTE4 at 10−12 M significantly stimulated secretion with a maximum 1.9 ± 0.3-fold, whereas LTE4 at 10−11 M also significantly increased secretion 2.9 ± 0.3-fold, both at 1 h of stimulation (Fig. 1E). Secretion then decreased to plateau for 3 and 4 h of stimulation. When incubated for 0.5 h, maximum secretion of 3.0 ± 0.4-fold was induced by 10−9 M LTE4 (Fig. 1F). The cholinergic agonist carbachol at 10−4 M stimulated secretion to the same extent as LTE4 (Fig. 1E, 1F). Thus, LTE4 induces conjunctival goblet cell secretion but is less effective and followed a slower time course than LTD4. Therefore, the metabolism of LTD4 to LTE4 did not account for the decrease in secretion obtained with LTD4 at longer incubation times. Both LTD4 and LTE4 are potent and effective stimuli of conjunctival goblet cell secretion.
LTB4, LTC4, and PGD2 stimulate conjunctival goblet cell mucous secretion
To determine the effect of the inflammatory mediators LTB4, LTC4, and PGD2 on conjunctival goblet cell secretion, cultured goblet cells were incubated for 2 h in the presence of increasing concentrations (10−12 to 10−6 M) of each mediator. A 2-h incubation time was chosen because this is the time that the cholinergic agonist carbachol caused a significant increase in secretion (26). LTB4 stimulated secretion a maximum of 2.3 ± 0.9 at 10−10 M (Fig. 2A). LTC4 similarly induced secretion a maximum of 2.6 ± 0.6-fold at 10−8 M, a 100-fold greater concentration than LTB4 (Fig. 2B). For both compounds, secretion remained increased as the concentration of LT increased. PGD2 stimulated secretion by 2.2 ± 0.8-fold at 10−11 M, but secretion decreased as the concentration of PGD2 increased (Fig. 2C). These data suggest that the proinflammatory products of both neutrophils and mast cells cause goblet cells to secrete. Because LTD4 and LTE4 were the most effective stimuli of secretion, these compounds were further characterized.
FIGURE 2.
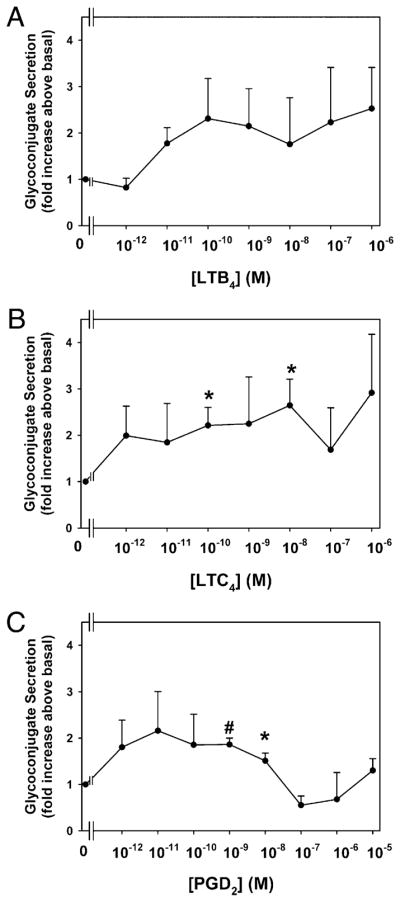
Effect of leukotrienes and PGD2 on glycoconjugate secretion from cultured rat conjunctival goblet cells. Cultured rat conjunctival goblet cells were serum starved for 2 h before stimulation with LTB4 (A), LTC4 (B), or PGD2 (C) for 2 h. The amount of glycoconjugate secretion was determined with ELLA. Data are mean ± SEM from four to five individual experiments. *p < 0.05 from no addition, #p < 0.01 from no addition.
Cysteinyl leukotriene receptors are present on conjunctival goblet cells
The cysteinyl leukotrienes LTD4 and LTC4 interact with their receptors CysLT1 and CysLT2. LTD4 binds CysLT1 receptor more effectively than LTC4, and both LTC4 and LTD4 bind CysLT2 receptor equally effectively (27). To determine the presence of CysLT1 and CysLT2 receptors, homogenates prepared from rat conjunctiva and cultured conjunctival goblet cells were subjected to Western blotting analysis using Abs specific to these receptors. In rat conjunctiva and goblet cells, CysLT1 receptor appeared as a single band at 41 kDa and CysLT2 receptor at 35 kDa (Fig. 3A). Rat conjunctival sections were analyzed by immunohistochemistry with anti-CysLT1 receptor Ab and the lectin UEA-I that stains goblet cell secretory granules. In the rat, conjunctival goblet cells occur as clusters with the cell body reaching to the basement membrane and the secretory granules abutting the tear film. Multiple layers of stratified squamous cells surround the goblet cells. In the rat, CysLT1 receptor was detected on the basolateral membranes of clusters of filled goblet cells (Fig. 3B). CysLT1 receptor was also present on the plasma membranes of stratified squamous cells. CysLT2 receptor immunoreactivity was found diffusely distributed in the cytoplasm of both goblet and stratified squamous cells (Fig. 3C). Incubation with the isotype control Abs showed no apparent immunoreactivity (Fig. 3D, 3E).
FIGURE 3.
Identification of cysteinyl leukotriene receptors in rat conjunctival epithelium and cultured rat conjunctival goblet cells. The presence of CysLT1 and CysLT2 receptors was determined by Western blot analysis and is shown in A. Each lane represents a separate animal. Fluorescence micrographs show localization of CysLT1 and CysLT2 receptors (B, C). CysLT receptors are shown in red, and UEA-I, which indicates goblet cell secretory granules, is shown in green. Arrows indicate basolateral membranes of goblet cells. Arrowhead indicates stratified squamous cells. Anti-rabbit isotype control is shown in D, whereas anti-goat isotype control is shown in E. Fluorescence micrographs show localization of CysLT1 and CysLT2 in cultured rat goblet cells in the presence (F–K) and absence (L–Q) of a permeabilization compound Triton X-100. Anti-rabbit isotype control is shown in R, whereas anti-goat isotype control is shown in S. CysLT receptors are shown in red, UEA-I indicating goblet cell secretory granules is shown in green, and DAPI indicating cell nuclei is shown in blue. Original magnification ×200. Micrographs are representative of three separate animals.
Immunoreactivity to CysLT1 and CysLT2 receptors was observed with punctate staining in the cytoplasm of all cultured rat goblet cells labeled with UEA-I (Fig. 3F–K). When cells were incubated in the absence of Triton X-100 to prevent permeabilization of cells and demonstrate cell surface binding, both CysLT1 and CysLT2 receptors were present on the cell surface (Fig. 3L–Q). Incubation with the isotype control Abs showed no apparent immunoreactivity (Fig. 3R, 3S).
Localization of CysLT1 and CysLT2 receptors in human cultured goblet cells was similar to that seen in rat cultured goblet cells where it was detected in plasma membranes and stress fibers (Supplemental Fig. 1A–F). Incubation with isotype control Abs showed no apparent immunoreactivity (Supplemental Fig. 1G, 1H).
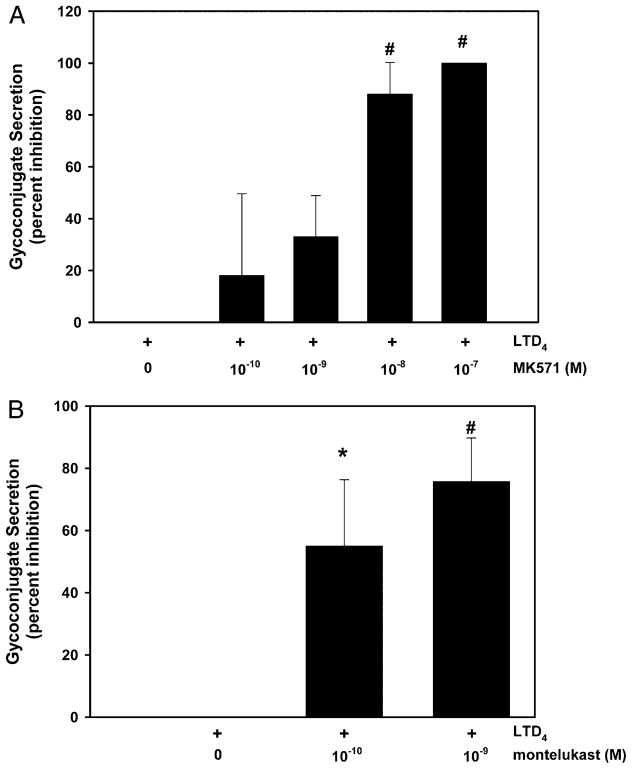
CysLT1 receptor antagonists block LTD4-stimulated conjunctival goblet cell secretion
Cultured rat goblet cells were preincubated for 0.5 h with the CysLT1 receptor antagonists MK571 and montelukast, and then stimulated for 0.5 h with 10−11 M LTD4. LTD4 significantly stimulated secretion by 2.1 ± 0.4-fold, respectively (data not shown). Secretion stimulated by 10−11 M LTD4 was decreased in a concentration-dependent manner by 10−10 to 10−7 M MK571. The action of MK571 was significant at 10−8 and 10−7 M MK571, with MK571 at 10−7 M completely blocking secretion (Fig. 4A). Montelukast also significantly inhibited secretion stimulated by LTD4 10−11 M (Fig. 4B). Both concentrations of montelukast were effective, with a maximum inhibition of 55 ± 22% and 76 ± 14% obtained with 10−10 and 10−9 M montelukast, respectively. LTD4 uses CysLT1 receptors to stimulate conjunctival goblet cell secretion.
FIGURE 4.
Effect of CysLT1 antagonists on LTD4-stimulated glycoconjugate secretion from cultured rat conjunctival goblet cells. Cultured rat conjunctival goblet cells were preincubated for 0.5 h with increasing concentrations of MK571 (A) or montelukast (B) before stimulation with LTD4 (10−11 M) for 0.5 h. The amount of glycoconjugate secretion was measured by ELLA. Data are mean ± SEM from three (MK571) or four (montelukast) independent experiments. *p < 0.05 from LTD4 alone, #p < 0.01 from LTD4 alone.
LTD4 and LTE4 increase [Ca2+]i in conjunctival goblet cells
Rat conjunctival goblet cells containing the Ca2+-sensitive dye fura 2 were stimulated with LTD4. LTD4 increased the [Ca2+]i in a concentration-dependent manner with a maximum increase of 104 ± 19 nM occurring at 10−9 M, a 100-fold greater concentration than stimulates secretion (Fig. 5). LTE4 also increased the [Ca2+]i to a maximum of 514 ± 58 nM at 10−9 M, the same concentration that was maximal for secretion (Fig. 5). Comparison of the Ca2+ responses to LTD4 and LTE4 demonstrated that the response to LTE4 was significantly greater than the response to LTD4. The cholinergic agonist carbachol at 10−4 M increased [Ca2+]i to 328 ± 60 nM, a value between those obtained in response to LTD4 and LTE4 (Fig. 5, inset), though not significantly different from either one.
FIGURE 5.
Effect of LTD4. LTE4 and chemerin on peak [Ca2+]i in cultured rat conjunctival goblet cells. Cultured rat conjunctival goblet cells containing the calcium-sensitive dye fura 2 were stimulated with increasing concentrations of LTE4 (closed circles), LTD4 (inverted triangles), and chemerin (open circles). The effect of the cholinergic agonist carbachol (Cch) used at a concentration (10−4 M) maximum for increasing [Ca2+]i is shown in the inset. Data are mean ± SEM from three independent experiments. *p < 0.05 from no addition, #p < 0.01 from no addition, ‡p < 0.01 from LTE4.
Because chemerin, in addition to RvE1, can activate the ChemR23 receptor, we determined whether chemerin increases the [Ca2+]i in cultured goblet cells. Chemerin from 10−9 to 10−7 M did not increase the [Ca2+]i in the same experiments in which both LTD4 and LTE4 were effective (Fig. 5).
To determine whether the leukotriene-induced increase in [Ca2+]I was mediated by the CysLT1 receptor, cultured goblet cells were preincubated for 30 min with MK571. LTD4 at 10−9 M increased the [Ca2+]i by 104 ± 19 nM (Supplemental Fig. 2A). The presence of MK571 at 10−9 to 10−7 M blocked the LTD4 increase in [Ca2+]i. MK571 at 10−8 M was most effective, almost completely blocking the response. LTE4 at 10−7 M increased [Ca2+]i by 493 ± 45 nM (Supplemental Fig. 2B). MK571 was less effective in blocking the LTE4 Ca2+ response. Only 10−8 M MK571 was effective, inhibiting the Ca2+ response by ~60%.
Both LTD4 and LTE4 increase [Ca2+]i in conjunctival goblet cells; LTD4 works through the CysLT1 receptor, and a component of the LTE4 response could be through this receptor as well.
LTD4 activates ERK1/2 in conjunctival goblet cells
Conjunctival goblet cells were stimulated for 0–10 min with LTD4 and phospho-ERK1/2 activity measured. LTD4 at 10−11 M activated ERK1/2 a maximum of 2.0 ± 0.3-fold at 5 min of stimulation (Fig. 6A, 6B). LTD4 activates ERK1/2 in addition to increasing the [Ca2+]i in conjunctival goblet cells; both of these signaling components are the major mechanism by which goblet cell secretion is stimulated in the conjunctiva.
FIGURE 6.

Effect of LTD4 on activation ERK1/2 in cultured rat conjunctival goblet cells. Cultured rat conjunctival goblet cells were stimulated for 0–10 min with LTD4 (10−11 M), and the amount of phospho-ERK1/2 (pERK1/2) and total ERK2 was determined by Western blotting analysis. One representative Western blot is shown in A. Three independent experiments were analyzed by densitometry, and the mean ± SEM is shown in B. *p < 0.05 from no additions, #p < 0.01 from no addition.
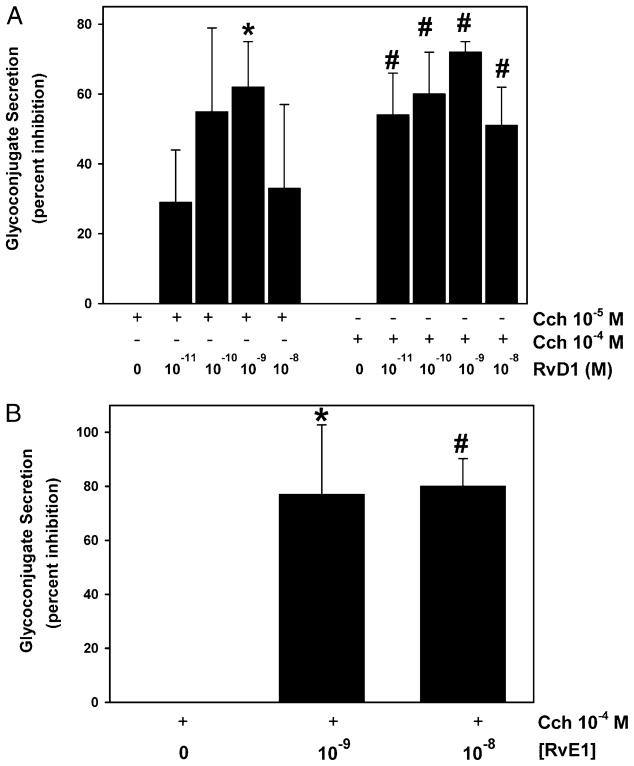
Cholinergic agonists do not generate leukotrienes, but their stimulation of secretion is blocked by resolvins
Cholinergic agonists mediate parasympathetic stimulation of conjunctival goblet cell secretion (7). To determine whether cholinergic agonists cause secretion by producing leukotrienes, cultured rat goblet cells were incubated with MK886, an inhibitor of 5-lipoxygenase–activating protein (FLAP), the accessory protein of 5-lipoxygenase that is responsible for leukotriene synthesis (28). Cells were incubated for 0.5 h with MK886 (10−6 and 10−5 M) and then stimulated with carbachol for 2 h. MK886 alone did not significantly alter goblet cell secretion (data not shown). Carbachol 10−5 and 10−4 M stimulated goblet cell secretion 1.8 ± 0.5- and 1.9 ± 0.5-fold, respectively. Neither concentration of MK886 altered secretion stimulated by carbachol at any concentration (data not shown).
Carbachol at 10−5 and 10−4 M significantly increased secretion 2.1 ± 0.2- and 2.5 ± 0.3-fold, respectively (data not shown). RvD1 decreased cholinergic agonist-stimulated secretion in a concentration-dependent manner. RvD1 at 10−9 M caused a maximum inhibition of 62 ± 13 and 72 ± 3% at carbachol concentrations of 10−5 and 10−4 M, respectively (Fig. 7A). In separate experiments, carbachol increased secretion by 1.5 ± 0.3-fold, which was decreased by a maximum of 83 ± 10% with RvE1 at 10−8 M (Fig. 7B).
FIGURE 7.
Effect of addition of resolvins on cholinergic agonist-stimulated glycoconjugate secretion from cultured rat conjunctival goblet cells. Cultured rat conjunctival goblet cells were serum starved for 2 h and preincubated for 0.5 h with increasing concentrations of RvD1 before stimulation with the cholinergic agonist carbachol (Cch; 10−5 and 10−4 M) for 2 h (A, n = 3). Cultured rat conjunctival goblet cells were serum starved for 2 h and preincubated for 0.5 h with increasing concentrations of RvE1 before stimulation with the cholinergic agonist Cch (10−4 M) for 2 h (B) (n = 5). The amount of glycoconjugate secretion was measured by ELLA. Data are mean ± SEM. *p < 0.05 from Cch alone, #p < 0.01 from Cch alone.
Cholinergic agonists do not produce leukotrienes in conjunctival goblet cells, but both resolvins terminated secretion stimulated by this agonist.
RvE1 and RvD1 reduce LTD4-stimulated conjunctival goblet cell secretion
Cultured goblet cells were preincubated with no addition or increasing concentrations of the resolvins RvE1 and RvD1 for 0.5 h and then stimulated with LTD4 at 10−11 M for 0.5 h. Neither RvE1 nor RvD1 alone caused goblet cell secretion during the 0.5-h stimulation (data not shown). LTD4 significantly induced secretion 2.7 ± 0.4-fold (data not shown). RvE1 and RvD1 blocked LTD4-stimulated secretion in a concentration-dependent manner (Fig. 8A, 8B). RvE1 and RvD1 at 10−9 and 10−8 M completely blocked secretion.
FIGURE 8.
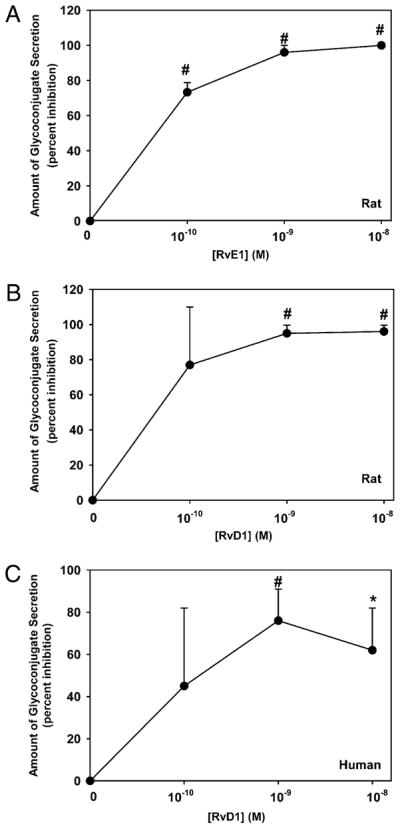
Effect of RvE1 and RvD1 on LTD4-stimulated glyco-conjugate secretion from cultured rat and human conjunctival goblet cells. Cultured rat conjunctival goblet cells were serum starved for 2 h and preincubated for 0.5 h with increasing concentrations of RvE1 (A) or RvD1 (B) before stimulation with LTD4 (10−11 M) for 0.5 h. Cultured human conjunctival goblet cells were serum starved for 2 h and preincubated for 0.5 h with increasing concentrations of RvD1 (C) before stimulation with LTD4 (10−11 M) for 0.5 h. The amount of glycoconjugate secretion was measured by ELLA. Data are mean ± SEM from six (rat) or three (human) independent experiments. *p < 0.05 from agonist alone, #p < 0.01 from agonist alone.
Experiments analogous to those performed on rat goblet cells were carried out with human conjunctival goblet cells. LTD4 significantly stimulated secretion 2.0 ± 0.3-fold (data not shown). In the human cells, RvD1 inhibited LTD4-stimulated secretion a maximum of 74 ± 15% at 10−9 M (Fig. 8C).
Goblet cell mucin secretion stimulated by LTD4 can be completely blocked by both types of resolvins in rat goblet cells. In human goblet cells, RvD1 terminates LTD4-stimulated secretion.
RvD1 blocks LTD4-stimulated secretion at all times tested
Conjunctival goblet cells were preincubated with RvD1 at 10−9 M (Supplemental Fig. 3A) and 10−8 M (Supplemental Fig. 3B) for 0.5 h and then stimulated with LTD4 (10−11 M) for 0.5, 1, and 4 h. RvD1 itself slightly, but significantly, stimulated goblet cell secretion at almost all times and concentrations used (Supplemental Fig. 3A, 3B). As demonstrated in Fig. 1C, LTD4-stimulated secretion was maximum at 0.5 compared with 1 and 4 h. RvD1 at 10−9 and 10−8 M almost completely blocked LTD4-stimulated secretion for up to 4 h (Supplemental Fig. 3A, 3B).
RvD1 and RvE1 receptors are present on rat conjunctival goblet cells
The receptors mediating the proresolution actions of RvD1 and RvE1 were recently identified. RvD1 can activate the orphan receptor GPR32 and ALX/FPR2, a lipoxin A4 receptor (29). Using immunohistochemistry, we detected GPR32 on both goblet and stratified squamous cells in the intact conjunctiva. Notably, GPR32 was localized on the basal and lateral membranes of the goblet cells (Supplemental Fig. 4A). In cultured rat goblet cells, GPR32 was localized in a distinct area of the cytoplasm (Supplemental Fig. 4D). This localization does not correspond to the secretory vesicles (identified by binding of the lectin UEA-I; Supplemental Fig. 4E) and was not in the nucleus (Supplemental Fig. 14F).
RvE1 binds the receptor ChemR23 to activate specific signaling pathways that resolve inflammation (30). ChemR23 is an orphan receptor that can also be activated by the polypeptide chemerin (31). ChemR23 was detected in rat conjunctiva by immunohisto-chemistry. This receptor, similarly to GPR32, was located on both stratified squamous and goblet cells (Supplemental Fig. 4B). In particular, GPR32 localized to the lateral membranes of the goblet cells. In cultured rat goblet cells, ChemR23 was found in a punctuate pattern within the cytosol (Supplemental Fig. 4G–I). In the presence of isotype control Ab, there was minimal diffuse staining in the conjunctival epithelium and goblet cells in culture (Supplemental Fig. 4C, 4J).
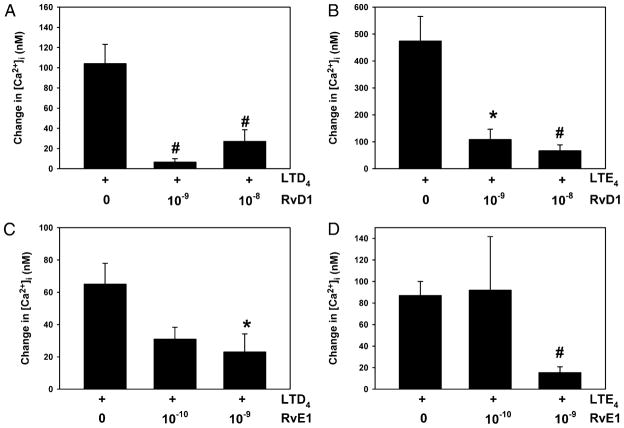
RvD1 and RvE1 block LTD4- and LTE4-stimulated increase in [Ca2+]i
Fura 2-containing cultured goblet cells were preincubated for 0.5 h with RvD1 (10−9 and 10−8 M) before stimulation with LTD4 at 10−9 M, the concentration that gave a maximum increase in [Ca2+]i. LTD4 increased the [Ca2+]i by 104 ± 19 nM (Fig. 9A). RvD1 at both concentrations almost completely attenuated the LTD4 increase in [Ca2+]i. Similar results were obtained for LTE4 (10−7 M), whose increase in [Ca2+]i of 474 ± 92 nM was substantially prevented by both concentrations of RvD1 (Fig. 9B). In similar experiments, goblet cells were preincubated with RvE1 (10−10 and 10−9 M) before stimulation with LTD4. LTD4 increased the [Ca2+]i by 66 ± 13 nM (Fig. 9C). RvD1 at 10−9 M blocked the increase in [Ca2+]i by almost 70%. Similar results were obtained for LTE4 (10−7 M), whose increase in [Ca2+]i of 87 ± 13 nM was inhibited by 85% by the RvE1 at 10−9 M (Fig. 9D).
FIGURE 9.
Effect of RvD1 and RvE1 on LTD4- and LTE4-simulated increase in peak [Ca2+]i in cultured rat conjunctival goblet cells. Cultured rat conjunctival goblet cells containing the calcium-sensitive dye fura 2 were preincubated for 0.5 h with RvD1 (10−9 and 10−8 M) before stimulation with LTD4 (10−9 M, A) or LTE4 (10−7 M, B). Cultured rat conjunctival goblet cells were preincubated for 0.5 h with RvE1 (10−10 and 10−9 M) before stimulation with LTD4 (10−9 M, C) or LTE4 (10−7 M, D). Data are mean ± SEM from three independent experiments. *p < 0.05 from leukotriene alone, #p < 0.01 from leukotriene alone.
RvD1 and RvE1 attenuate LTD4 activation of ERK1/2
Western blotting analysis was used to determine the effect of RvD1 on LTD4 stimulation of ERK1/2 activation. Goblet cells were preincubated for 0.5 h with RvD1 from 10−10 to 10−8 M before stimulation for 5 min with LTD4 at 10−11 M, the concentration that gave maximum stimulation of secretion. RvD1 did not increase phospho-ERK1/2 activity, whereas LTD4 increased it by 2.1 ± 0.3-fold (Fig. 10A, 10B). RvD1 at 10−9 and 10−8 M almost completely decreased the activation of ERK1/2 by LTD4. Similar experiments were performed with RvE1. RvE1 did not increase phospho-ERK1/2 activity, whereas LTD4 increased it by 2.0 ± 0.2-fold (Fig. 10C, 10D). RvE1 at 10−9 and 10−8 M decreased by ~80% the activation of ERK1/2 by LTD4.
FIGURE 10.
Effect of RvD1 and RvE1 on leukotriene D4-simulated activation of ERK1/2 in cultured rat conjunctival goblet cells. Cultured rat conjunctival goblet cells were preincubated for 0.5 h with RvD1 (10−10 to 10−8 M) before stimulation with LTD4 (10−11 M) for 5 min, and the amount of phospho-ERK1/2 (pERK) and total ERK2 was determined by Western blotting analysis. One representative Western blot is shown in A. Three independent experiments were analyzed by densitometry, and the mean ± SEM is shown in B. Cultured rat conjunctival goblet cells were preincubated for 0.5 h with RvE1 (10−10 to 10−8 M) before stimulation with LTD4 (10−11 M) for 5 min, and the amount of phospho-ERK1/2 and total ERK2 was determined by Western blotting analysis. One representative Western blot is shown in C. Three independent experiments were analyzed by densitometry, and the mean ± SEM is shown in D. *p < 0.05 from no additions, #p < 0.01 from no addition, ‡p < 0.05 from leukotriene alone, §p < 0.01 from no addition.
Thus, RvD1 and RvE1 each block LTD4-stimulated goblet cell secretion by preventing the increase in the [Ca2+]i and the activation of ERK1/2, the two major mechanisms by which conjunctival goblet cell secretion is stimulated.
Discussion
A hallmark of conjunctival inflammation that occurs in ocular allergy is an influx of mast cells and neutrophils that release histamine, leukotrienes, proteases, PGs, and cytokines (32, 33). Similarly, in dry eye disease, inflammation of the ocular surface includes cytokine production and infiltration of T cells and neutrophils (34). We now show that cysteinyl leukotrienes LTC4, LTD4, and LTE4 produced in the conjunctiva during ocular allergy, dry eye disease, or other inflammatory diseases of the ocular surface stimulate goblet cell mucous secretion that can contribute to the excess mucous seen in these diseases. The chronic inflammation in these ocular surface diseases damages the cornea and conjunctiva, causing chronic pain from exposed nerve endings. In this study, we demonstrated that cysteinyl leukotriene-stimulated goblet cell secretion was completely blocked by the proresolution resolvins RvD1 and RvE1. Thus, resolution of inflammation by the production of proresolution mediators, namely, RvD1 and RvE1, can terminate excess goblet cell mucous secretion allowing the ocular surface to repair. These results also support the hypothesis that resolution of inflammation is an active process.
Dry eye and allergic conjunctivitis are chronic inflammatory diseases of the cornea and conjunctiva (ocular surface). Dry eye affects 5 million individuals in the United States alone and is more prevalent in women than men (35). Allergy including seasonal allergic conjunctivitis, vernal keratoconjunctivitis, giant papillary conjunctivitis (also known as contact lens-induced papillary conjunctivitis), and atopic keratoconjunctivitis affects ~20% of the population (36). Hallmarks of these diseases are symptoms of ocular pain and discomfort, and signs of ocular surface inflammation that generate inflammatory cytokines and matrix metalloproteinases. These inflammatory mediators lead to death of the surface cells of the corneal and conjunctival epithelia (4). Conjunctival goblet cells are potential targets of the inflammatory mediators produced during dry eye disease and allergic conjunctivitis as an increase in tear mucus accompanies these diseases (4). In the initial stages of dry eye and in allergic conjunctivitis, ocular surface damage and irritation of the afferent sensory nerves leads to the neural reflex stimulation of conjunctival goblet cell secretory product that includes the large gel forming mucin MUC5AC.
An increase in ocular surface mucus appears to be correlated with damaging inflammation of the ocular surface in the setting of early dry eye and allergic conjunctivitis, and the proinflammatory leukotrienes play a pivotal role in allergic and hypersensitivity reactions and goblet cell mucous production in the airway (10). Yet, there is limited information on the effect of inflammatory mediators on conjunctival goblet cell mucin secretion. In the only published study, an in vivo one in the guinea pig conjunctiva, platelet-activating factor and histamine, but not LTD4 or LTE4, increased goblet cell secretion (37). Until now goblet cells in culture have not been available; thus, in this report, we are able to address the role of goblet cell secretion uncomplicated by effects on the other cell type of the conjunctiva and independently of changes in proliferation and differentiation. Goblet cells cultured from the upper airways or lung have not been used for investigation of inflammation. In vivo studies on the effect of leukotrienes and resolvins on goblet cells in these two tissues cannot differentiate between acute and chronic effects, and cannot distinguish between effects on cell proliferation, secretion, and differentiation as can the present studies in the conjunctiva.
Use of rat goblet cells is an excellent model for human conjunctiva. In previous studies, we compared human and rat goblet cells, and found that they were similar in their stimulation of proliferation by EGF, activation of ERK1/2 by EGF and cholinergic agonists, and the signaling pathways activated by EGF to stimulate proliferation (21, 38). The cysteinyl leukotriene LTD4 is also an effective mucin stimulus in human goblet cells as in rat goblet cells, and the proresolution mediator RvD1 blocks secretion in both human and rat goblet cells.
Cysteinyl leukotrienes work by activating CysLT1 or CysLT2 receptors. We found that both receptors were present in conjunctival goblet cells. LTD4 works by activating the CysLT1 receptors as two CysLT1 receptor antagonists blocked induced mucous secretion. The role of CysLT2 receptor was not tested. Strategies that block CysLT1 receptors such as topical or systemic application of CysLT1 receptor antagonists should be beneficial in treatment of ocular allergy. In one 12-patient clinical trial of oral montelukast in patients with vernal conjunctivitis and asthma, a significant and persistent reduction of ocular signs and symptoms was reported (39). Further evaluation of the use of CysLT1 receptor antagonists in ocular allergy is warranted.
CysLT1 or CysLT2 receptors were also detected on conjunctival stratified squamous cells. Stratified squamous cells secrete electrolytes and water in response to β-adrenergic or purinergic P2Y agonists. Activation of cysteinyl leukotrienes receptors could stimulate electrolyte and water secretion or modify agonist-stimulated secretion, thereby increasing tear production. Conjunctival epithelium differs from the other goblet cell-containing epithelia including upper and lower airways, intestine, and colon, in that conjunctival epithelium expresses abundant cysteinyl leukotriene receptors, whereas the other epithelia contain little or no expression of these receptors (40, 41).
Both LTD4 and LTE4 stimulated conjunctival goblet cell mucous secretion. Of interest, LTE4 appeared to be a potent stimulus for [Ca2+]i mobilization and secretion in these cells. Hence the potential and capacity for LTC4 and LTD4 conversion in this tissue to LTE4 is of interest for further studies along these lines. LTE4 was also recently reported to be a potent agonist for mast cell activation (42). Together, these results and those of this study document the potent activity of LTE4, which was considered earlier to be an inactive member of the SRS-A/leukotriene family (10).
Active resolution of inflammation is mediated by poly-unsaturated fatty acids (PUFA), the precursors of the resolvins. Results from several clinical studies have shown that systemic or topical PUFA are important for the health of the ocular surface and can improve dry eye disease (14–17). The mechanisms underlying the improvement of dry eye with omega-3 fatty acids have not been investigated. PUFA are also important in the retina because increased dietary intake of PUFA reduces pathological retinal angiogenesis (19). These studies suggest that resolvins might be beneficial to treat ocular surface disease.
The proresolution mediators RvD1 and RvE1 both reduced LTD4-stimulated conjunctival goblet cell secretion, which occurs by exocytosis. Goblet cell secretion is stimulated by increasing the [Ca2+]i and activating ERK1/2 (9). Both LTD4 and LTE4 increase the [Ca2+]i, and LTD4 activated ERK1/2 and likely stimulated goblet cell secretion by these mechanisms. RvD1 activates the GPR32 receptor that is present in conjunctival goblet cells and prevents the increase in [Ca2+]i stimulated by LTD4 and LTE4, and the activation of ERK1/2 by LTD4. It is also possible that RvD1 works through the ALX/FPR2 receptor, but this was not determined in the present system. We suggest that these inhibitory actions prevent stimulation of goblet cell secretion by the leukotrienes. RvE1 acts via the ChemR23 receptor (28) that is also present in conjunctival goblet cells to block the increase in [Ca2+]i and inhibit the activation of ERK1/2.
The proresolution mediators RvD1 and RvE1 inhibited cholinergic agonist-stimulated goblet cell secretion in addition to their actions on leukotriene-induced secretion. Cholinergic agonists play a role in the normal goblet cell secretory response to changes in the external environment such as mechanical, thermal, chemical, and microbial stimuli. The neural regulation of goblet cell secretion functions to protect the ocular surface from the environment. The neural regulation of secretion could also play a role in the inflammatory response as histamine released during allergic inflammation can interact with histamine receptors (usually H4 receptors) on sensory nerves and activate them to stimulate the efferent parasympathetic nerves. Furthermore, leukotrienes and cholinergic agonists use the same signaling pathways to stimulate goblet cell secretion as both types of agonists increase [Ca2+]i and activate ERK1/2. Thus, resolvins appear to block the same cellular signaling pathway to prevent cholinergic and leukotriene-induced goblet cell secretion.
Mucin production is regulated by controlling the number of goblet cells (proliferation), the rate of mucin synthesis, and the rate of mucin secretion. In the lung, the leukotrienes appear to increase mucin production by increasing the number of goblet cells by stimulating goblet cell proliferation and differentiation. Resolvins decreased the number of filled goblet cells, but the mucin production mechanism used by resolvins was not investigated (43). In contrast, in our study, we found that, in the conjunctiva, leukotrienes increase mucin production by stimulating mucin secretion. Using cultured goblet cells, we were able to study secretion, without change in goblet cell number or other chronic effects that could alter the steady-state mucin production. We showed that leukotrienes stimulate conjunctival goblet cell secretion and resolvins block the stimulation. Similarly in the colon, omega-3 PUFA restored the number of filled goblet cells and the maturity of their mucins in a rat model of experimental ulcerative colitis (44). Thus, resolvins affect different cellular processes to block mucin production in the lung compared with the conjunctiva and return the tissue to its normal predisease state.
Resolvins block many physiological processes including reducing corneal angiogenesis, decreasing neutrophil infiltration, blocking cytokine production, inhibiting osteoclast bone growth and reabsorption, and decreasing airway mucous production (15, 45, 46). Based on these cellular actions, resolvins were able to reduce inflammation in animal models of a variety of chronic inflammatory diseases including corneal angiogenesis, asthma, colitis, obesity-induced insulin resistance, periodontal disease, atherosclerosis, and kidney reperfusion injury (15, 18, 45, 47, 48). Thus, resolvins should reduce allergic inflammation in ocular allergy by blocking goblet cell secretion as demonstrated in this study, and by reducing the accompanying neutrophil infiltration, increase vascular permeability and cytokine production.
We conclude that the cysteinyl leukotrienes acting via CysLT1 receptors are potent stimuli of conjunctival goblet cell mucous secretion, and the proresolution mediators RvE1 and RvD1 terminate this action by preventing the increase in [Ca2+]i and activation of ERK1/2. Because resolvins stimulate resolution of inflammation and are not immunosuppressive (14), they may be useful alternatives to prolonged steroid use in the eye. Our results support the notion that goblet cell mucous secretion is an important component of ocular allergy and early dry eye, and termination of this secretion occurs by active resolution of inflammation.
Supplementary Material
Acknowledgments
We thank Dr. Nan Chiang for helpful discussions and reading of the manuscript.
This work was supported by National Institutes of Health Grants EY EY019470 (to D.A.D.) and GM38765 (to C.N.S.).
Abbreviations used in this article
- [Ca2+]i
intracellular Ca2+ concentration
- CysLT
cysteinyl leukotriene
- EGF
epidermal growth factor
- ELLA
enzyme-linked lectin assay
- LTB4
leukotriene B4
- LTC4
leukotriene C4
- LTD4
leukotriene D4
- LTE4
leukotriene E4
- PUFA
polyunsaturated fatty acid
- RvD1
resolvin D1
- RvE1
resolving E1
- UEA-I
Ulex europaeus agglutinin type I
Footnotes
The online version of this article contains supplemental material.
Disclosures
Most of the experiments and results reported in this article were initiated by the authors before licensing of patents for clinical development. Resolvins are biotemplates for stable analogs, and patents on these are awarded and assigned to the Brigham and Women’s Hospital, of which C.N.S. is the inventor. These analog patents are licensed for clinical development. C.N.S. retains founder stock in Resolvyx Pharmaceuticals.
References
- 1.Rogers DF. The airway goblet cell. Int J Biochem Cell Biol. 2003;35:1–6. doi: 10.1016/s1357-2725(02)00083-3. [DOI] [PubMed] [Google Scholar]
- 2.Kim DH, Chu HS, Lee JY, Hwang SJ, Lee SH, Lee HM. Up-regulation of MUC5AC and MUC5B mucin genes in chronic rhinosinusitis. Arch Otolaryngol Head Neck Surg. 2004;130:747–752. doi: 10.1001/archotol.130.6.747. [DOI] [PubMed] [Google Scholar]
- 3.Gersemann M, Becker S, Kübler I, Koslowski M, Wang G, Herrlinger KR, Griger J, Fritz P, Fellermann K, Schwab M, et al. Differences in goblet cell differentiation between Crohn’s disease and ulcerative colitis. Differentiation. 2009;77:84–94. doi: 10.1016/j.diff.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Lemp M, Baudoiin C, Baum J, Dogru M, Foulks G, Kinoshita S, Laibson P, McCulley J, Murube J, Pflugfelder SC, et al. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5:75–92. doi: 10.1016/s1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- 5.Dartt DA. Control of mucin production by ocular surface epithelial cells. Exp Eye Res. 2004;78:173–185. doi: 10.1016/j.exer.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Ríos JD, Forde K, Diebold Y, Lightman J, Zieske JD, Dartt DA. Development of conjunctival goblet cells and their neuroreceptor subtype expression. Invest Ophthalmol Vis Sci. 2000;41:2127–2137. [PubMed] [Google Scholar]
- 7.Ríos JD, Zoukhri D, Rawe IM, Hodges RR, Zieske JD, Dartt DA. Immunolocalization of muscarinic and VIP receptor subtypes and their role in stimulating goblet cell secretion. Invest Ophthalmol Vis Sci. 1999;40:1102–1111. [PubMed] [Google Scholar]
- 8.Dartt DA, Rios JD, Kanno H, Rawe IM, Zieske JD, Ralda N, Hodges RR, Zoukhri D. Regulation of conjunctival goblet cell secretion by Ca(2+)and protein kinase C. [Published erratum appears in 2001. Exp Eye Res. 2000;72:357. doi: 10.1006/exer.2000.0915. [DOI] [PubMed] [Google Scholar]; Exp Eye Res. 71:619–628. doi: 10.1006/exer.2000.0915. [DOI] [PubMed] [Google Scholar]
- 9.Kanno H, Horikawa Y, Hodges RR, Zoukhri D, Shatos MA, Rios JD, Dartt DA. Cholinergic agonists transactivate EGFR and stimulate MAPK to induce goblet cell secretion. Am J Physiol Cell Physiol. 2003;284:C988–C998. doi: 10.1152/ajpcell.00582.2001. [DOI] [PubMed] [Google Scholar]
- 10.Samuelsson B, Dahlén SE, Lindgren JA, Rouzer CA, Serhan CN. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 1987;237:1171–1176. doi: 10.1126/science.2820055. [DOI] [PubMed] [Google Scholar]
- 11.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Serhan CN. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu Rev Immunol. 2007;25:101–137. doi: 10.1146/annurev.immunol.25.022106.141647. [DOI] [PubMed] [Google Scholar]
- 13.Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med. 2000;192:1197–1204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Serhan CN. Systems approach with inflammatory exudates uncovers novel anti-inflammatory and pro-resolving mediators. Prostaglandins Leukot Essent Fatty Acids. 2008;79:157–163. doi: 10.1016/j.plefa.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haworth O, Cernadas M, Yang R, Serhan CN, Levy BD. Resolvin E1 regulates interleukin 23, interferon-gamma and lipoxin A4 to promote the resolution of allergic airway inflammation. Nat Immunol. 2008;9:873–879. doi: 10.1038/ni.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac RL. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kasuga K, Yang R, Porter TF, Agrawal N, Petasis NA, Irimia D, Toner M, Serhan CN. Rapid appearance of resolvin precursors in inflammatory exudates: novel mechanisms in resolution. J Immunol. 2008;181:8677–8687. doi: 10.4049/jimmunol.181.12.8677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duffield JS, Hong S, Vaidya VS, Lu Y, Fredman G, Serhan CN, Bonventre JV. Resolvin D series and protectin D1 mitigate acute kidney injury. J Immunol. 2006;177:5902–5911. doi: 10.4049/jimmunol.177.9.5902. [DOI] [PubMed] [Google Scholar]
- 19.Connor KM, SanGiovanni JP, Lofqvist C, Aderman CM, Chen J, Higuchi A, Hong S, Pravda EA, Majchrzak S, Carper D, et al. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat Med. 2007;13:868–873. doi: 10.1038/nm1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aguirre SA, Huang W, Prasanna G, Jessen B. Corneal neovascularization and ocular irritancy responses in dogs following topical ocular administration of an EP4-prostaglandin E2 agonist. Toxicol Pathol. 2009;37:911–920. doi: 10.1177/0192623309351724. [DOI] [PubMed] [Google Scholar]
- 21.Shatos MA, Gu J, Hodges RR, Lashkari K, Dartt DA. ERK/ p44p42 mitogen-activated protein kinase mediates EGF-stimulated proliferation of conjunctival goblet cells in culture. Invest Ophthalmol Vis Sci. 2008;49:3351–3359. doi: 10.1167/iovs.08-1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shatos MA, Hodges RR, Bair JA, Lashkari K, Dartt DA. Stimulatory role of PKCalpha in extracellular regulated kinase 1/2 pathway in conjunctival goblet cell proliferation. Invest Ophthalmol Vis Sci. 2009;50:1619–1625. doi: 10.1167/iovs.08-2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shatos MA, Ríos JD, Horikawa Y, Hodges RR, Chang EL, Bernardino CR, Rubin PA, Dartt DA. Isolation and characterization of cultured human conjunctival goblet cells. Invest Ophthalmol Vis Sci. 2003;44:2477–2486. doi: 10.1167/iovs.02-0550. [DOI] [PubMed] [Google Scholar]
- 24.Arita M, Bianchini F, Aliberti J, Sher A, Chiang N, Hong S, Yang R, Petasis NA, Serhan CN. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med. 2005;201:713–722. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun YP, Oh SF, Uddin J, Yang R, Gotlinger K, Campbell E, Colgan SP, Petasis NA, Serhan CN. Resolvin D1 and its aspirin-triggered 17R epimer. Stereochemical assignments, anti-inflammatory properties, and enzymatic inactivation. J Biol Chem. 2007;282:9323–9334. doi: 10.1074/jbc.M609212200. [DOI] [PubMed] [Google Scholar]
- 26.Ríos JD, Ghinelli E, Gu J, Hodges RR, Dartt DA. Role of neurotrophins and neurotrophin receptors in rat conjunctival goblet cell secretion and proliferation. Invest Ophthalmol Vis Sci. 2007;48:1543–1551. doi: 10.1167/iovs.06-1226. [DOI] [PubMed] [Google Scholar]
- 27.Labat C, Ortiz JL, Norel X, Gorenne I, Verley J, Abram TS, Cuthbert NJ, Tudhope SR, Norman P, Gardiner P, et al. A second cysteinyl leukotriene receptor in human lung. J Pharmacol Exp Ther. 1992;263:800–805. [PubMed] [Google Scholar]
- 28.Abramovitz M, Wong E, Cox ME, Richardson CD, Li C, Vickers PJ. 5-Lipoxygenase-activating protein stimulates the utilization of arachidonic acid by 5-lipoxygenase. Eur J Biochem. 1993;215:105–111. doi: 10.1111/j.1432-1033.1993.tb18012.x. [DOI] [PubMed] [Google Scholar]
- 29.Krishnamoorthy S, Recchiuti A, Chiang N, Yacoubian S, Lee CH, Yang R, Petasis NA, Serhan CN. Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc Natl Acad Sci U S A. 2010;107:1660–1665. doi: 10.1073/pnas.0907342107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohira T, Arita M, Omori K, Recchiuti A, Van Dyke TE, Serhan CN. Resolvin E1 receptor activation signals phosphorylation and phagocytosis. J Biol Chem. 2010;285:3451–3461. doi: 10.1074/jbc.M109.044131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wittamer V, Grégoire F, Robberecht P, Vassart G, Communi D, Parmentier M. The C-terminal nonapeptide of mature chemerin activates the chemerin receptor with low nanomolar potency. J Biol Chem. 2004;279:9956–9962. doi: 10.1074/jbc.M313016200. [DOI] [PubMed] [Google Scholar]
- 32.Galatowicz G, Ajayi Y, Stern ME, Calder VL. Ocular anti-allergic compounds selectively inhibit human mast cell cytokines in vitro and conjunctival cell infiltration in vivo. Clin Exp Allergy. 2007;37:1648–1656. doi: 10.1111/j.1365-2222.2007.02782.x. [DOI] [PubMed] [Google Scholar]
- 33.Schultz BL. Pharmacology of ocular allergy. Curr Opin Allergy Clin Immunol. 2006;6:383–389. doi: 10.1097/01.all.0000244801.79475.66. [DOI] [PubMed] [Google Scholar]
- 34.Lam H, Bleiden L, de Paiva CS, Farley W, Stern ME, Pflugfelder SC. Tear cytokine profiles in dysfunctional tear syndrome. Am J Ophthalmol. 2009;147:198–205e1. doi: 10.1016/j.ajo.2008.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith J, Albeitz J, Begley C, Caffery B, Nichols K, Schaumberg DA, Schein O. The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5:93–107. doi: 10.1016/s1542-0124(12)70082-4. [DOI] [PubMed] [Google Scholar]
- 36.Ono SJ, Abelson MB. Allergic conjunctivitis: update on patho-physiology and prospects for future treatment. J Allergy Clin Immunol. 2005;115:118–122. doi: 10.1016/j.jaci.2004.10.042. [DOI] [PubMed] [Google Scholar]
- 37.Woodward DF, Spada CS, Nieves AL, Hawley SB, Williams LS. Platelet-activating factor causes goblet cell depletion in the conjunctiva. Eur J Pharmacol. 1989;168:23–30. doi: 10.1016/0014-2999(89)90628-6. [DOI] [PubMed] [Google Scholar]
- 38.Horikawa Y, Shatos MA, Hodges RR, Zoukhri D, Rios JD, Chang EL, Bernardino CR, Rubin PA, Dartt DA. Activation of mitogen-activated protein kinase by cholinergic agonists and EGF in human compared with rat cultured conjunctival goblet cells. Invest Ophthalmol Vis Sci. 2003;44:2535–2544. doi: 10.1167/iovs.02-1117. [DOI] [PubMed] [Google Scholar]
- 39.Lambiase A, Bonini S, Rasi G, Coassin M, Bruscolini A, Bonini S. Montelukast, a leukotriene receptor antagonist, in vernal keratoconjunctivitis associated with asthma. Arch Ophthalmol. 2003;121:615–620. doi: 10.1001/archopht.121.5.615. [DOI] [PubMed] [Google Scholar]
- 40.Sousa AR, Parikh A, Scadding G, Corrigan CJ, Lee TH. Leukotriene-receptor expression on nasal mucosal inflammatory cells in aspirin-sensitive rhinosinusitis. N Engl J Med. 2002;347:1493–1499. doi: 10.1056/NEJMoa013508. [DOI] [PubMed] [Google Scholar]
- 41.Capra V. Molecular and functional aspects of human cysteinyl leukotriene receptors. Pharmacol Res. 2004;50:1–11. doi: 10.1016/j.phrs.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 42.Paruchuri S, Tashimo H, Feng C, Maekawa A, Xing W, Jiang Y, Kanaoka Y, Conley P, Boyce JA. Leukotriene E4-induced pulmonary inflammation is mediated by the P2Y12 receptor. J Exp Med. 2009;206:2543–2555. doi: 10.1084/jem.20091240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aoki H, Hisada T, Ishizuka T, Utsugi M, Kawata T, Shimizu Y, Okajima F, Dobashi K, Mori M. Resolvin E1 dampens airway inflammation and hyperresponsiveness in a murine model of asthma. Biochem Biophys Res Commun. 2008;367:509–515. doi: 10.1016/j.bbrc.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 44.Nieto N, Torres MI, Ríos A, Gil A. Dietary polyunsaturated fatty acids improve histological and biochemical alterations in rats with experimental ulcerative colitis. J Nutr. 2002;132:11–19. doi: 10.1093/jn/132.1.11. [DOI] [PubMed] [Google Scholar]
- 45.Jin Y, Arita M, Zhang Q, Saban DR, Chauhan SK, Chiang N, Serhan CN, Dana R. Anti-angiogenesis effect of the novel anti-inflammatory and pro-resolving lipid mediators. Invest Ophthalmol Vis Sci. 2009;50:4743–4752. doi: 10.1167/iovs.08-2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Herrera BS, Ohira T, Gao L, Omori K, Yang R, Zhu M, Muscara MN, Serhan CN, Van Dyke TE, Gyurko R. An endogenous regulator of inflammation, resolvin E1, modulates osteoclast differentiation and bone resorption. Br J Pharmacol. 2008;155:1214–1223. doi: 10.1038/bjp.2008.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arita M, Yoshida M, Hong S, Tjonahen E, Glickman JN, Petasis NA, Blumberg RS, Serhan CN. Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proc Natl Acad Sci USA. 2005;102:7671–7676. doi: 10.1073/pnas.0409271102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hasturk H, Kantarci A, Ohira T, Arita M, Ebrahimi N, Chiang N, Petasis NA, Levy BD, Serhan CN, Van Dyke TE. RvE1 protects from local inflammation and osteoclast-mediated bone destruction in periodontitis. FASEB J. 2006;20:401–403. doi: 10.1096/fj.05-4724fje. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.