Abstract
Importance of the field
The proteasome is responsible for ubiquitin- and ATP-dependent proteolysis of cellular proteins. The latest advances in proteasome studies led to the development of proteasome inhibitors as drugs against human cancer. It has been shown that proteasome inhibitors selectively kill cancer, but not normal cells. However, the exact mechanisms of the anticancer activity of proteasome inhibitors are not well understood. The oncogenic transcription factor Forkhead Box M1 (FOXM1) is overexpressed in a majority of human carcinomas, while its expression is usually low in normal cells. In addition, FOXM1 may also drive tumor invasion, angiogenesis, and metastasis. For these reasons, FOXM1 is an attractive target for anticancer drugs.
Areas covered in this review
My aim is to discuss the recent publications that may point out to novel mechanism of action of proteasome inhibitors. In addition, I will describe the identification of new types of proteasome inhibitors, called thiazole antibiotics. Using a cell–based screening system the thiazole antibiotics Siomycin A and thiostrepton were isolated as inhibitors of FOXM1 transcriptional activity and expression. Paradoxically, it has been showed that these drugs also stabilize the expression of other proteins and act as proteasome inhibitors in vitro. Moreover, it was found that well-known proteasome inhibitors, such as MG115, MG132 and bortezomib inhibit FOXM1 transcriptional activity and FOXM1 expression.
What the reader will gain
It has been shown that proteasome inhibitors suppress FOXM1 expression and simultaneously induce apoptosis in human tumor cell lines. This review describes the correlation between negative regulation of FOXM1 by proteasome inhibitors and apoptosis, and suggests that negative regulation of FOXM1 is a universal feature of these drugs and it may contribute to their anticancer activity.
Take home message
Oncogenic transcription factor FOXM1 is upregulated in a majority of human cancers, suggesting that growth of cancer cells may depend on FOXM1 activity. A short time ago, it has been shown that proteasome inhibitors simultaneously inhibit FOXM1 expression and induce apoptosis in human cancer cells. This effect may explain specificity of proteasome inhibitors to induce apoptosis in cancer, but not in normal cells. Now it is critical to determine the role of suppression of FOXM1 in apoptosis induced by proteasome inhibitors and to establish how significant is the inhibition of FOXM1 for the anticancer activity of proteasome inhibitors.
Keywords: proteasome inhibitors, FOXM1, apoptosis, anticancer drugs, thiazole antibiotics
Proteasome inhibitors are anticancer drugs
The proteasome is a multi-subunit protease complex that degrades proteins that are tagged with ubiquitin chains. Ubiquitin (76 amino-acid protein) is covalently linked by ubiquitinating enzymes to lysine residues of target proteins. The proteasome consists of a cylindrical 20 S catalytic subunit that binds to one or two multi-subunit 19 S regulatory particles, forming 26 S and 30 S proteasomes and recognizes ubiquitinated proteins 1. At the next step ubiquitinated proteins become unfold, translocated into the proteolytic chamber of the 20S proteasome and broken down into small peptides. The 19 S proteasome also has a deubiquitinating activity that removes polyubiquitin tag from the substrate protein.
Since the proteasome target ubiquitin-tagged proteins for degradation, proteasome inhibitors (PI) (Fig 1 C-E) stabilize the expression of the majority of cellular proteins and also induce apoptosis in human cancer cell lines. Six years ago PI, bortezomib (Velcade) (Fig 1E) was the first PI to be approved for the treatment of patients with multiple myeloma, suggesting that PIs could be used for treatment of human cancer. However, at this moment it is not clear how exactly PIs induce programmed cell death in cancer cells and why they selectively kill cancer, but not normal cells. It is very important to establish critical targets for PIs in human cancers of different origin. Several explanations have been presented for the proapoptotic/anticancer abilities of PIs, such as stabilization of IkB and NF-kB inhibition 2, stabilization of p53 3 and Noxa 4, activation of JNK and Fas 5, cleavage of antiapoptotic Mcl-1 6, induction of ROS 7, preventing the destruction of the CDK inhibitor, p27 8, shift in the balance between pro- and antiapoptotic Bcl-2-family proteins 9, 10 and some other possibilities (reviewed in refs. 11, 12. Abnormal NF-kB regulation has been shown in variety of cancers leading to the transcriptional activation of genes responsible for cell proliferation, inhibition of apoptosis, angiogenesis and metastasis 13. It has been suggested that inhibition of NF-kB is one of the major mechanisms of anticancer activity of proteasome inhibitors 13, 14. Proteasome inhibitors hinder NF-kB transcriptional activity via stabilization of IkB and sequestering of NF-kB in the cytoplasm 14. Importance of NF-kB targeting by bortezomib was validated in multiple myeloma cells where an NF-kB signature correlated with their sensitivity to bortezomib 13, 15. In this paper I will describe a novel target for PIs, the oncogenic transcription factor FOXM1 16.
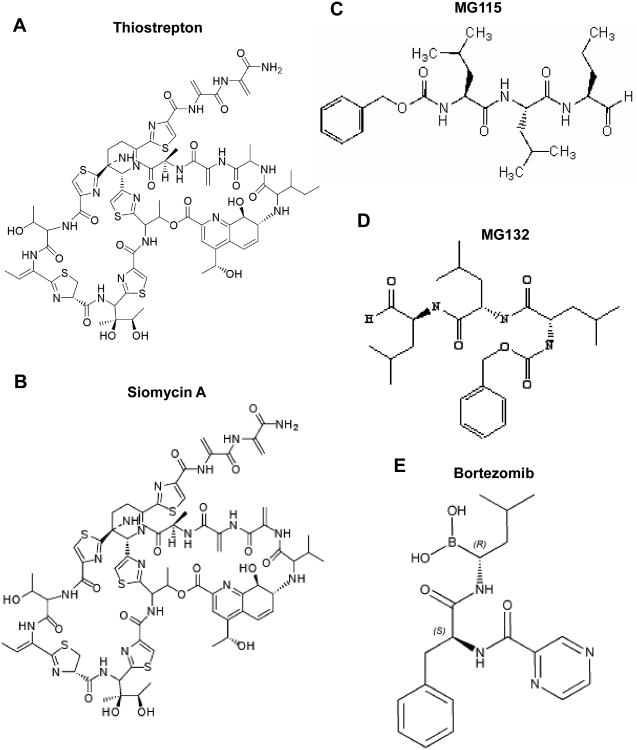
Fig 1. Structure of thiazole antibiotics (A, B) that have activity of proteasome inhibitors and bona-fide proteasome inhibitors (C-E).
The role of FOXM1 in development and cancer
FOXM1 is a transcription factor of the Forkhead family that has a conserved Forkhead/ winged-helix DNA-binding domain (100 amino acids) responsible for binding of Fox proteins the consensus TAAACA site in the promoters of target genes. FOXM1 is expressed in all embryonic tissues and in proliferating cells of epithelial and mesenchymal origin, but its expression is turned off in terminally differentiated, non-dividing cells 17. In addition, it has been shown that FOXM1 plays role in development of nervous system 18 and is required for hepatoblast differentiation 19. Following lung 20 and liver 21 injury FOXM1 expression was induced indicating that FOXM1 is essential for tissue regeneration and repair. Similarly, endothelial cell–restricted FOXM1-deficient mice displayed a significant impairment in endothelial barrier repair and a considerable increase in mortality after acute lung injury 22. Following vascular injury, FOXM1-/- lungs exhibited reduced cell proliferation, diminished expression of cyclin B1 and Cdc25C and increased expression of CDK inhibitor p27 22. These data suggest that FOXM1 plays a essential role in the restoration of endothelial barrier function after vascular injury.
FOXM1 could be activated by oncogenic Ras-MAPK 23, Sonic Hedgehog 24, NF-kB 25 and EGFR 26 pathways, suggesting that it may act as an oncogene. In addition, as a potential oncogene FOXM1 is negatively regulated by tumor suppressor p53 27. When FOXM1 becomes activated, it induces cell cycle progression via transcriptional induction of genes that are involved in mitosis, including Cyclin B, Survivin, Aurora B kinase, Cdc25b phosphatase, Plk1 and some others 28. In addition, FOXM1 transcriptionally activate Skp2 and Cks1 genes (subunits of Skp1-Cullin1-F-box ubiquitin ligase complex), thus targeting CDK/cell cycle inhibitors p21WAF1 and p27KIP1 to degradation 28. These and other data suggest that FOXM1 is required for the execution of the mitotic program 29.
FOXM1 is one of the most overexpressed genes in human solid tumors and it is upregulated in malignant mesothelioma 30, breast cancer 26, 31, non-small cell lung carcinomas 32, anaplastic astrocytomas and glioblastomas 33, 34, basal 24 and squamous 35 cell carcinomas, gastric cancer 36, colorectal cancer 37, hepatocellular carcinomas 38, pancreatic carcinomas 39, intrahepatic cholangiocarcinomas 40 and in some other human cancers. It has been shown that FOXM1 is consistently upregulated in metastatic prostate cancer cells 41 and knockdown of FOXM1 by small interfering RNAs (siRNAs) in several prostate cancer cell lines led to a significant reduction in cell proliferation and anchorage-independent cell growth on soft agar 42, 43. Downregulation of FOXM1 in pancreatic 44 and breast 45 cancer cells by RNA interference led to inhibition of proliferation, migration and invasion of cancer cells. All these data implicate FOXM1 in human tumor growth and metastasis, and suggest that FOXM1 may be the “Achilles' heel” of cancer 46, and an attractive target for drug development 47, 48.
Thiazole antibiotics/proteasome inhibitors target FOXM1
To identify small molecule inhibitors of the transcription factor FOXM1 activity, cell–based screening system was developed and used for screening of NCI libraries of small molecules 49. In the result of the screening thiopeptide, Siomycin A (NSC-285116) was identified as an inhibitor of FOXM1 transcriptional activity 49. Furthermore, another thiazole antibiotic, thiostrepton with small structural differences from Siomycin A was also identified as FOXM1 inhibitor 50-52. Thiazole compounds, Siomycin and thiostrepton are gram-positive bacteria specific, sulphur containing antibiotics 53 (Fig 1 A, B). Genetic and biochemical studies showed that thiazole antibiotics (thiostrepton, siomycin, thiopeptin, sporangiomycin, nosiheptide) have a very similar mode of action. They block the translocation step of translation by binding to the L11 binding domain of the 23S rRNA on the 50S ribosomal subunit 53, but they do not exert any inhibitory effect on eukaryotic protein synthesis (17). In addition, antitumor activities have been reported for both antibiotics. Siomycin A was identified as a strong pro-apoptotic compound in epithelial cells 54 and also as a potential anticancer drug that causes p53-independent apoptosis and induces lysosomal membrane permeabilization 55. Reportedly, thiostrepton also possesses antitumor activity, but a synthesized fragment of thiostrepton showed even more potent antibacterial and anticancer activities than the natural substance 56.
Since FOXM1 is involved in a positive feedback loop and it activates its own transcription (Fig 2) 57, Siomycin A and thiostrepton down-regulate not only the transcriptional activity, but also the protein and mRNA levels of FOXM1 49, 50, 52. To evaluate the anticancer potential of the thiazole antibiotics, their effect on growth and survival of human cancer cell lines of different origin was studied. It has been shown that treatment of human cancer cells with the thiazole antibiotics resulted in downregulation of FOXM1 and potent apoptosis 49-52, suggesting that thiazole antibiotic-induced apoptosis may be linked to the suppression of FOXM1. Surprisingly, it has been discovered later that treatment of cells with thiazole antibiotics leads also to the stabilization of a variety of cellular proteins, including p21, Mcl-1, p53 and others 16. Since these and other cellular proteins are upregulated by PIs 16, 58, it suggested that thiazole antibiotics might also act as PIs. When the thiazole antibiotics were directly tested in vitro and compared with well-known PIs MG132 (Fig 1D) and lactacystin against the 20S proteasome activity, it has been confirmed that they act as PIs in vitro 16. Furthermore, these data implied that bona-fide PIs might also inhibit FOXM1. Additional experiments have shown that PIs strongly inhibit FOXM1 transcriptional activity and protein expression similarly to the thiazole antibiotics 16, 52. These data suggest that down-regulation of FOXM1 may be required for the anticancer activity of PIs. Further experiments are needed to understand the importance of FOXM1 suppression for the activity of PIs as anticancer drugs.
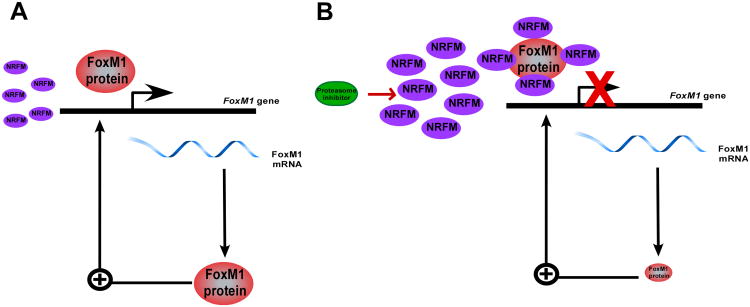
Fig 2. Proteasome inhibitors stabilize hypothetical negative regulator of FOXM1 (NRFM) that inhibits transcriptional activity of FOXM1 and its expression because of positive autoregulation of FOXM1.
(A) Untreated cells where FOXM1 transcriptional activity is required for its expression (B) Cells treated with proteasome inhibitor, which stabilizes NRFM leading to suppression of F OXM1 transcriptional activity and expression.
Conclusion
In summary, recently discovered FOXM1 inhibitors, thiazole antibiotics Siomycin A and thiostrepton were identified also as PIs. In addition, bona-fide PIs such as, MG115, MG132 and bortezomib are FOXM1 inhibitors. Induction of programmed cell death by PIs correlated with the suppression of FOXM1 in human cancer cell lines of different origin. Overall, these data suggest that inhibition of FOXM1 by PIs may partially be responsible for their anticancer activity.
Expert opinion
Oncogenic transcription factor FOXM1 is upregulated in a majority of human cancers 59 and is involved in tumor growth, invasion and metastasis 41, 44. Since cancer, but not normal cells are over-reliant on FOXM1 activity, it was suggested that potential FOXM1 inhibitors would specifically kill cancer cells. Recently, it has been shown that PIs simultaneously inhibit FOXM1 expression and induce apoptosis in human cancer cells. Suppression of FOXM1 by PIs may explain their specificity to kill cancer, but not normal cells, because FOXM1 only overexpressed in cancer cells. It is important to determine what the role FOXM1 inhibition plays in apoptosis induced by PIs in human cancer cells and how significant the inhibition of FOXM1 for the anticancer activity of PIs is. It is also essential to determine the precise mechanism of FOXM1 inhibition by PIs. Since PIs stabilize the majority of cellular proteins, it is plausible to propose that they stabilize a negative regulator of FOXM1 (NRFM) that would inhibit transcriptional activity of FOXM1 and its expression because of FOXM1 auto-regulation loop 57 (Fig 2). Additional experiments are needed to identify NRFM and to establish the mechanism of negative regulation of FOXM1 by PIs. All these data suggest that FOXM1 inhibition by PIs may be important for their anticancer activity.
highlights.
The oncogenic transcription factor FOXM1 is overexpressed in a majority of human cancers
Proteasome inhibitors selectively kill cancer cells, but the exact mechanisms of their anticancer activity are not well understood
Thiazole antibiotics Siomycin A and thiostrepton were isolated as inhibitors of FOXM1 transcriptional activity and expression, and act as proteasome inhibitors
Well-known proteasome inhibitors also inhibit FOXM1 expression and induce apoptosis in human cancer cells
Bibliography
- 1.Rubin DM, Finley D. Proteolysis. The proteasome: a protein-degrading organelle? Curr Biol. 1995 Aug 1;5(8):854–8. doi: 10.1016/s0960-9822(95)00172-2. [DOI] [PubMed] [Google Scholar]
- 2.Mitsiades N, Mitsiades CS, Richardson PG, Poulaki V, Tai YT, Chauhan D, et al. The proteasome inhibitor PS-341 potentiates sensitivity of multiple myeloma cells to conventional chemotherapeutic agents: therapeutic applications. Blood. 2003 Mar 15;101(6):2377–80. doi: 10.1182/blood-2002-06-1768. [DOI] [PubMed] [Google Scholar]
- 3.Chan DW, Yu SY, Chiu PM, Yao KM, Liu VW, Cheung AN, et al. Over-expression of FOXM1 transcription factor is associated with cervical cancer progression and pathogenesis. The Journal of pathology. 2008 Jul;215(3):245–52. doi: 10.1002/path.2355. [DOI] [PubMed] [Google Scholar]
- 4.Fernandez Y, Verhaegen M, Miller TP, Rush JL, Steiner P, Opipari AW, Jr, et al. Differential regulation of noxa in normal melanocytes and melanoma cells by proteasome inhibition: therapeutic implications. Cancer research. 2005 Jul 15;65(14):6294–304. doi: 10.1158/0008-5472.CAN-05-0686. [DOI] [PubMed] [Google Scholar]
- 5.Mitsiades N, Mitsiades CS, Poulaki V, Chauhan D, Fanourakis G, Gu X, et al. Molecular sequelae of proteasome inhibition in human multiple myeloma cells. Proceedings of the National Academy of Sciences of the United States of America; 2002; Oct 29, pp. 14374–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Podar K, Gouill SL, Zhang J, Opferman JT, Zorn E, Tai YT, et al. A pivotal role for Mcl-1 in Bortezomib-induced apoptosis. Oncogene. 2008 Jan 31;27(6):721–31. doi: 10.1038/sj.onc.1210679. [DOI] [PubMed] [Google Scholar]
- 7.Pei XY, Dai Y, Grant S. The proteasome inhibitor bortezomib promotes mitochondrial injury and apoptosis induced by the small molecule Bcl-2 inhibitor HA14-1 in multiple myeloma cells. Leukemia. 2003 Oct;17(10):2036–45. doi: 10.1038/sj.leu.2403109. [DOI] [PubMed] [Google Scholar]
- 8.Nickeleit I, Zender S, Sasse F, Geffers R, Brandes G, Sorensen I, et al. Argyrin a reveals a critical role for the tumor suppressor protein p27(kip1) in mediating antitumor activities in response to proteasome inhibition. Cancer cell. 2008 Jul 8;14(1):23–35. doi: 10.1016/j.ccr.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 9.Fennell DA, Chacko A, Mutti L. BCL-2 family regulation by the 20S proteasome inhibitor bortezomib. Oncogene. 2008 Feb 21;27(9):1189–97. doi: 10.1038/sj.onc.1210744. [DOI] [PubMed] [Google Scholar]
- 10.Lingbeek ME, Jacobs JJ, van Lohuizen M. The T-box repressors TBX2 and TBX3 specifically regulate the tumor suppressor gene p14ARF via a variant T-site in the initiator. The Journal of biological chemistry. 2002 Jul 19;277(29):26120–7. doi: 10.1074/jbc.M200403200. [DOI] [PubMed] [Google Scholar]
- 11.Nencioni A, Grunebach F, Patrone F, Ballestrero A, Brossart P. Proteasome inhibitors: antitumor effects and beyond. Leukemia. 2007 Jan;21(1):30–6. doi: 10.1038/sj.leu.2404444. [DOI] [PubMed] [Google Scholar]
- 12.Shah JJ, Orlowski RZ. Proteasome inhibitors in the treatment of multiple myeloma. Leukemia. 2009 Sep 10; doi: 10.1038/leu.2009.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baud V, Karin M. Is NF-kappaB a good target for cancer therapy? Hopes and pitfalls. Nat Rev Drug Discov. 2009 Jan;8(1):33–40. doi: 10.1038/nrd2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakanishi C, Toi M. Nuclear factor-kappaB inhibitors as sensitizers to anticancer drugs. Nat Rev Cancer. 2005 Apr;5(4):297–309. doi: 10.1038/nrc1588. [DOI] [PubMed] [Google Scholar]
- 15.Mulligan G, Mitsiades C, Bryant B, Zhan F, Chng WJ, Roels S, et al. Gene expression profiling and correlation with outcome in clinical trials of the proteasome inhibitor bortezomib. Blood. 2007 Apr 15;109(8):3177–88. doi: 10.1182/blood-2006-09-044974. [DOI] [PubMed] [Google Scholar]
- 16.Bhat UG, Halasi M, Gartel AL. FOXM1 is a general target for proteasome inhibitors. PLoS ONE. 2009;4(8):e6593. doi: 10.1371/journal.pone.0006593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laoukili J, Stahl M, Medema RH. FOXM1: At the crossroads of ageing and cancer. Biochim Biophys Acta. 2007 Jan;1775(1):92–102. doi: 10.1016/j.bbcan.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 18.Ueno H, Nakajo N, Watanabe M, Isoda M, Sagata N. Development. 11. Vol. 135. Cambridge; England: 2008. Jun, FOXM1-driven cell division is required for neuronal differentiation in early Xenopus embryos; pp. 2023–30. [DOI] [PubMed] [Google Scholar]
- 19.Krupczak-Hollis K, Wang X, Kalinichenko VV, Gusarova GA, Wang IC, Dennewitz MB, et al. The mouse Forkhead Box m1 transcription factor is essential for hepatoblast mitosis and development of intrahepatic bile ducts and vessels during liver morphogenesis. Developmental biology. 2004 Dec 1;276(1):74–88. doi: 10.1016/j.ydbio.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 20.Kalinichenko VV, Gusarova GA, Tan Y, Wang IC, Major ML, Wang X, et al. Ubiquitous expression of the forkhead box M1B transgene accelerates proliferation of distinct pulmonary cell types following lung injury. The Journal of biological chemistry. 2003 Sep 26;278(39):37888–94. doi: 10.1074/jbc.M305555200. [DOI] [PubMed] [Google Scholar]
- 21.Wang X, Hung NJ, Costa RH. Hepatology. 6. Vol. 33. Baltimore, Md: 2001. Jun, Earlier expression of the transcription factor HFH-11B diminishes induction of p21(CIP1/WAF1) levels and accelerates mouse hepatocyte entry into S-phase following carbon tetrachloride liver injury; pp. 1404–14. [DOI] [PubMed] [Google Scholar]
- 22.Zhao YY, Gao XP, Zhao YD, Mirza MK, Frey RS, Kalinichenko VV, et al. Endothelial cell-restricted disruption of FOXM1 impairs endothelial repair following LPS-induced vascular injury. The Journal of clinical investigation. 2006 Sep;116(9):2333–43. doi: 10.1172/JCI27154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Major ML, Lepe R, Costa RH. Forkhead box M1B transcriptional activity requires binding of Cdk-cyclin complexes for phosphorylation-dependent recruitment of p300/CBP coactivators. Molecular and cellular biology. 2004 Apr;24(7):2649–61. doi: 10.1128/MCB.24.7.2649-2661.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teh MT, Wong ST, Neill GW, Ghali LR, Philpott MP, Quinn AG. FOXM1 is a downstream target of Gli1 in basal cell carcinomas. Cancer research. 2002 Aug 15;62(16):4773–80. [PubMed] [Google Scholar]
- 25.Penzo M, Massa PE, Olivotto E, Bianchi F, Borzi RM, Hanidu A, et al. Sustained NF-kappaB activation produces a short-term cell proliferation block in conjunction with repressing effectors of cell cycle progression controlled by E2F or FOXM1. Journal of cellular physiology. 2009 Jan;218(1):215–27. doi: 10.1002/jcp.21596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bektas N, Haaf A, Veeck J, Wild PJ, Luscher-Firzlaff J, Hartmann A, et al. Tight correlation between expression of the Forkhead transcription factor FOXM1 and HER2 in human breast cancer. BMC cancer. 2008;8(1):42. doi: 10.1186/1471-2407-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pandit B, Halasi M, Gartel AL. Cell cycle. 20. Vol. 8. Georgetown, Tex: 2009. Oct 15, p53 negatively regulates expression of FOXM1; pp. 3425–7. [DOI] [PubMed] [Google Scholar]
- 28.Wang IC, Chen YJ, Hughes D, Petrovic V, Major ML, Park HJ, et al. Forkhead box M1 regulates the transcriptional network of genes essential for mitotic progression and genes encoding the SCF (Skp2-Cks1) ubiquitin ligase. Molecular and cellular biology. 2005 Dec;25(24):10875–94. doi: 10.1128/MCB.25.24.10875-10894.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Myatt SS, Lam EW. The emerging roles of forkhead box (Fox) proteins in cancer. Nat Rev Cancer. 2007 Nov;7(11):847–59. doi: 10.1038/nrc2223. [DOI] [PubMed] [Google Scholar]
- 30.Romagnoli S, Fasoli E, Vaira V, Falleni M, Pellegrini C, Catania A, et al. Identification of potential therapeutic targets in malignant mesothelioma using cell-cycle gene expression analysis. The American journal of pathology. 2009 Mar;174(3):762–70. doi: 10.2353/ajpath.2009.080721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wonsey DR, Follettie MT. Loss of the forkhead transcription factor FOXM1 causes centrosome amplification and mitotic catastrophe. Cancer research. 2005 Jun 15;65(12):5181–9. doi: 10.1158/0008-5472.CAN-04-4059. [DOI] [PubMed] [Google Scholar]
- 32.Garber ME, Troyanskaya OG, Schluens K, Petersen S, Thaesler Z, Pacyna-Gengelbach M, et al. Diversity of gene expression in adenocarcinoma of the lung. Proceedings of the National Academy of Sciences of the United States of America; 2001; Nov 20, pp. 13784–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van den Boom J, Wolter M, Kuick R, Misek DE, Youkilis AS, Wechsler DS, et al. Characterization of gene expression profiles associated with glioma progression using oligonucleotide-based microarray analysis and real-time reverse transcription-polymerase chain reaction. The American journal of pathology. 2003 Sep;163(3):1033–43. doi: 10.1016/S0002-9440(10)63463-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu M, Dai B, Kang SH, Ban K, Huang FJ, Lang FF, et al. FOXM1B is overexpressed in human glioblastomas and critically regulates the tumorigenicity of glioma cells. Cancer research. 2006 Apr 1;66(7):3593–602. doi: 10.1158/0008-5472.CAN-05-2912. [DOI] [PubMed] [Google Scholar]
- 35.Gemenetzidis E, Bose A, Riaz AM, Chaplin T, Young BD, Ali M, et al. FOXM1 upregulation is an early event in human squamous cell carcinoma and it is enhanced by nicotine during malignant transformation. PLoS ONE. 2009;4(3):e4849. doi: 10.1371/journal.pone.0004849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Q, Zhang N, Jia Z, Le X, Dai B, Wei D, et al. Critical role and regulation of transcription factor FOXM1 in human gastric cancer angiogenesis and progression. Cancer research. 2009 Apr 15;69(8):3501–9. doi: 10.1158/0008-5472.CAN-08-3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Douard R, Moutereau S, Pernet P, Chimingqi M, Allory Y, Manivet P, et al. Sonic Hedgehog-dependent proliferation in a series of patients with colorectal cancer. Surgery. 2006 May;139(5):665–70. doi: 10.1016/j.surg.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 38.Okabe H, Satoh S, Kato T, Kitahara O, Yanagawa R, Yamaoka Y, et al. Genome-wide analysis of gene expression in human hepatocellular carcinomas using cDNA microarray: identification of genes involved in viral carcinogenesis and tumor progression. Cancer research. 2001 Mar 1;61(5):2129–37. [PubMed] [Google Scholar]
- 39.Nakamura T, Furukawa Y, Nakagawa H, Tsunoda T, Ohigashi H, Murata K, et al. Genome-wide cDNA microarray analysis of gene expression profiles in pancreatic cancers using populations of tumor cells and normal ductal epithelial cells selected for purity by laser microdissection. Oncogene. 2004 Mar 25;23(13):2385–400. doi: 10.1038/sj.onc.1207392. [DOI] [PubMed] [Google Scholar]
- 40.Obama K, Ura K, Li M, Katagiri T, Tsunoda T, Nomura A, et al. Hepatology. 6. Vol. 41. Baltimore, Md: 2005. Jun, Genome-wide analysis of gene expression in human intrahepatic cholangiocarcinoma; pp. 1339–48. [DOI] [PubMed] [Google Scholar]
- 41.Chandran UR, Ma C, Dhir R, Bisceglia M, Lyons-Weiler M, Liang W, et al. Gene expression profiles of prostate cancer reveal involvement of multiple molecular pathways in the metastatic process. BMC cancer. 2007;7:64. doi: 10.1186/1471-2407-7-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kalin TV, Wang IC, Ackerson TJ, Major ML, Detrisac CJ, Kalinichenko VV, et al. Increased Levels of the FOXM1 Transcription Factor Accelerate Development and Progression of Prostate Carcinomas in both TRAMP and LADY Transgenic Mice. Cancer research. 2006 Feb 1;66(3):1712–20. doi: 10.1158/0008-5472.CAN-05-3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim IM, Ackerson T, Ramakrishna S, Tretiakova M, Wang IC, Kalin TV, et al. The Forkhead Box m1 transcription factor stimulates the proliferation of tumor cells during development of lung cancer. Cancer research. 2006 Feb 15;66(4):2153–61. doi: 10.1158/0008-5472.CAN-05-3003. [DOI] [PubMed] [Google Scholar]
- 44.Wang Z, Banerjee S, Kong D, Li Y, Sarkar FH. Down-regulation of Forkhead Box M1 Transcription Factor Leads to the Inhibition of Invasion and Angiogenesis of Pancreatic Cancer Cells. Cancer research. 2007 Sep 1;67(17):8293–300. doi: 10.1158/0008-5472.CAN-07-1265. [DOI] [PubMed] [Google Scholar]
- 45.Ahmad A, Wang Z, Kong D, Ali S, Li Y, Banerjee S, et al. FOXM1 down-regulation leads to inhibition of proliferation, migration and invasion of breast cancer cells through the modulation of extra-cellular matrix degrading factors. Breast cancer research and treatment. 2009 Oct 8; doi: 10.1007/s10549-009-0572-1. [DOI] [PubMed] [Google Scholar]
- 46.Radhakrishnan SK, Gartel AL. FOXM1: the Achilles' heel of cancer? Nat Rev Cancer. 2008 Mar;8(3):c1. doi: 10.1038/nrc2223-c1. author reply c2. [DOI] [PubMed] [Google Scholar]
- 47.Adami GR, Ye H. Future roles for FOXM1 inhibitors in cancer treatments. Future Oncol. 2007 Feb;3(1):1–3. doi: 10.2217/14796694.3.1.1. [DOI] [PubMed] [Google Scholar]
- 48.Gartel AL. FOXM1 inhibitors as potential anticancer drugs. Expert Opin Ther Targets. 2008 Jun;12(6):663–5. doi: 10.1517/14728222.12.6.663. [DOI] [PubMed] [Google Scholar]
- 49.Radhakrishnan SK, Bhat UG, Hughes DE, Wang IC, Costa RH, Gartel AL. Identification of a Chemical Inhibitor of the Oncogenic Transcription Factor Forkhead Box M1. Cancer research. 2006;66(19):9731–35. doi: 10.1158/0008-5472.CAN-06-1576. [DOI] [PubMed] [Google Scholar]
- 50.Bhat UG, Zipfel PA, Tyler DS, Gartel AL. Cell cycle. 12. Vol. 7. Georgetown, Tex: 2008. Jun 15, Novel anticancer compounds induce apoptosis in melanoma cells; pp. 1851–5. [DOI] [PubMed] [Google Scholar]
- 51.Kwok JM, Myatt SS, Marson CM, Coombes RC, Constantinidou D, Lam EW. Thiostrepton selectively targets breast cancer cells through inhibition of forkhead box M1 expression. Molecular cancer therapeutics. 2008 Jul;7(7):2022–32. doi: 10.1158/1535-7163.MCT-08-0188. [DOI] [PubMed] [Google Scholar]
- 52.Bhat UG, Halasi M, Gartel AL. Thiazole antibiotics target FOXM1 and induce apoptosis in human cancer cells. PLoS ONE. 2009;4(5):e5592. doi: 10.1371/journal.pone.0005592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lentzen G, Klinck R, Matassova N, Aboul-ela F, Murchie AI. Structural basis for contrasting activities of ribosome binding thiazole antibiotics. Chem Biol. 2003 Aug;10(8):769–78. doi: 10.1016/s1074-5521(03)00173-x. [DOI] [PubMed] [Google Scholar]
- 54.Hagg M, Biven K, Ueno T, Rydlander L, Bjorklund P, Wiman KG, et al. A novel high-through-put assay for screening of pro-apoptotic drugs. Investigational new drugs. 2002 Aug;20(3):253–9. doi: 10.1023/a:1016249728664. [DOI] [PubMed] [Google Scholar]
- 55.Erdal H, Berndtsson M, Castro J, Brunk U, Shoshan MC, Linder S. Induction of lysosomal membrane permeabilization by compounds that activate p53-independent apoptosis. Proceedings of the National Academy of Sciences of the United States of America; 2005; Jan 4, pp. 192–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nicolaou KC, Zak M, Rahimipour S, Estrada AA, Lee SH, O'Brate A, et al. Discovery of a biologically active thiostrepton fragment. Journal of the American Chemical Society. 2005 Nov 2;127(43):15042–4. doi: 10.1021/ja0552803. [DOI] [PubMed] [Google Scholar]
- 57.Halasi M, Gartel AL. Cell cycle. 12. Vol. 8. Georgetown, Tex: 2009. Jun 15, A novel mode of FOXM1 regulation: positive auto-regulatory loop; pp. 1966–7. [DOI] [PubMed] [Google Scholar]
- 58.Matta H, Chaudhary PM. The proteasome inhibitor bortezomib (PS-341) inhibits growth and induces apoptosis in primary effusion lymphoma cells. Cancer biology & therapy. 2005 Jan;4(1):77–82. doi: 10.4161/cbt.4.1.1379. [DOI] [PubMed] [Google Scholar]
- 59.Wierstra I, Alves J. FOXM1, a typical proliferation-associated transcription factor. Biol Chem. 2007 Dec;388(12):1257–74. doi: 10.1515/BC.2007.159. [DOI] [PubMed] [Google Scholar]