Abstract
Background. Antiretroviral therapy (ART)–mediated immune reconstitution fails to restore the capacity of the immune system to spontaneously control human immunodeficiency virus (HIV) replication.
Methods. A total of 23 HIV type 1 (HIV-1)–infected, virologically suppressed subjects receiving ART (CD4+ T-cell count, >450 cells/μL) were randomly assigned to have 180 μg/week (for arm A) or 90 μg/week (for arm B) of pegylated (Peg) interferon alfa-2a added to their current ART regimen. After 5 weeks, ART was interrupted, and Peg–interferon alfa-2a was continued for up to 12 weeks (the primary end point), with an option to continue to 24 weeks. End points included virologic failure (viral load, ≥400 copies/mL) and adverse events. Residual viral load and HIV-1 DNA integration were also assessed.
Results. At week 12 of Peg–interferon alfa-2a monotherapy, viral suppression was observed in 9 of 20 subjects (45%), a significantly greater proportion than expected (arm A, P = .0088; arm B, P = .0010; combined arms, P < .0001). Over 24 weeks, both arms had lower proportions of subjects who had viral load, compared with the proportion of subjects in a historical control group (arm A, P = .0046; arm B, P = .0011). Subjects who had a sustained viral load of <400 copies/mL had decreased levels of integrated HIV DNA (P = .0313) but increased residual viral loads (P = .0078), compared with subjects who experienced end-point failure.
Conclusions. Peg–interferon alfa-2a immunotherapy resulted in control of HIV replication and decreased HIV-1 integration, supporting a role for immunomediated approaches in HIV suppression and/or eradication.
Clinical Trials Registration. NCT00594880.
Keywords: HIV-1, interferon-alpha, viral integration, immunotherapy
(See the editorial commentary by McNamara and Collins, on pages 201–3.)
The quest to effect long-term control of human immunodeficiency virus type 1 (HIV-1) in the absence of antiretroviral therapy (ART) has led to numerous therapeutic approaches aimed at increasing host-mediated control of HIV and/or clearance of latent virus reservoirs [1], while maintaining the beneficial effects of immune reconstitution. Pilot strategies currently under investigation include gene therapy [2], therapeutic vaccines [3], cytokines [4], and chemotherapy [5]. Despite intensive investigation, no strategy so far has resulted in sustained control of HIV in the absence of antiretroviral therapy.
Interferon α belongs to a family of type 1 interferons produced by dendritic and others cells as part of the host's Toll-like receptor–mediated antiviral response [6]. While interferon-mediated gene expression is increased in advanced HIV disease [7], the role of this innate response (ie, viral control mechanism vs chronic activation mediator contributing to disease progression) remains under debate [8–10]. The results of several clinical trials support a predominantly antiviral activity (ie, approximately 0.5 log decrease in plasma viral load) when interferon alfa is administered to HIV-1–infected persons in the absence of ART [11–14]. However, in this setting interferon alfa is not completely suppressive, possibly because of the deterioration of immune system effectors due to the ongoing viral replication. The degree to which interferon alfa monotherapy may contribute to virus control (eg, suppression of residual replication) after ART-mediated immune reconstitution has not been tested.
Given the growing interest in identifying novel strategies aimed at controlling HIV in the absence of ART, we sought to establish a proof of concept that interferon alfa can suppress HIV replication in subjects in whom the detrimental effects of uncontrolled HIV replication on immune function have been partially reversed by ART.
METHODS
Study Design
Written informed consent was obtained from patients according to the directives of the institutional review boards at the Wistar Institute, University of Pennsylvania, Philadelphia FIGHT, and Drexel University. All human experimentation was conducted in accordance with the guidelines of the US Department of Health and Human Services and those of the authors’ institutions. The study was registered at ClinicalTrials.gov (http://www.clinicaltrials.gov/; NCT00594880).
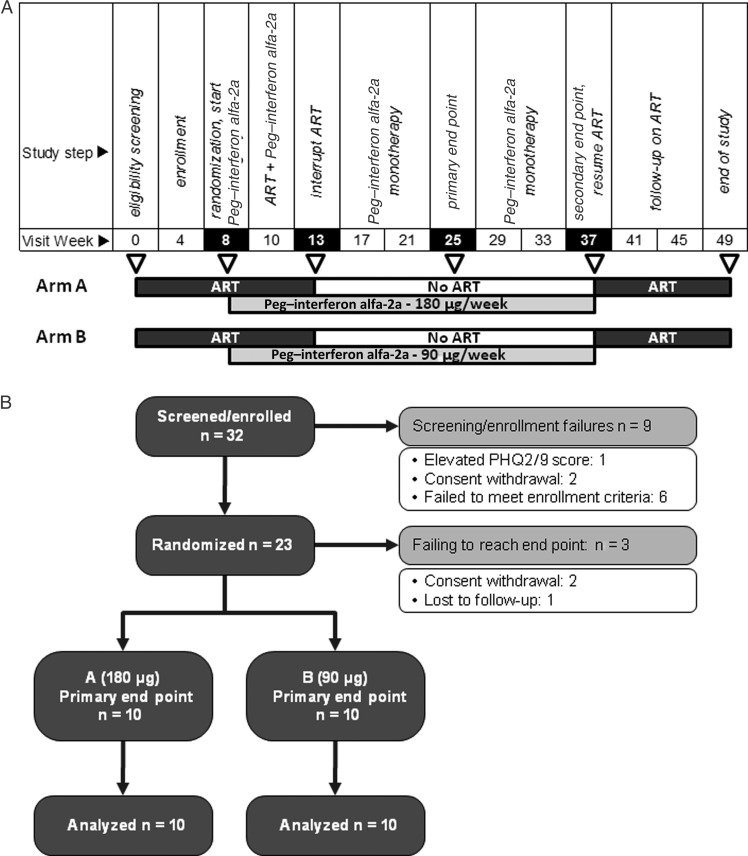
A schematic representation of the study is provided in Figure 1A, together with a CONSORT (Consolidated Standards of Reporting Trials) study subject disposition chart (Figure 1B). A total of 23 eligible subjects (all were in stable health, were receiving ART, had a plasma HIV RNA load of <50 copies/mL, had a current CD4+ T-cell count of >450 cells/μL, and had a nadir CD4+ T-cell count of >200 cells/μL) were randomized. Exclusion criteria included hepatitis C virus coinfection, active hepatitis B virus infection, a history of major depression or autoimmune diseases, a Framingham score of >15% (for men) or >10% (for women) for a 10-year risk for myocardial infarction, and retinal clouds on fundoscopic examination.
Figure 1.
Trial schema and subject disposition. A, Schema of the trial visit and relative activities (top row) during the 14 visits scheduled to occur over 49 weeks. Treatment is represented by dark grey boxes (pegylated [Peg] interferon alfa-2a at 180 or 90 μg/week), light grey boxes (antiretroviral therapy [ART], as previously treated), or white boxes (ART interruption, Peg–interferon alfa-2a monotherapy). B, CONSORT (Consolidated Standards of Reporting Trials) flow diagram of the study subject disposition. PHQ2/9, Patient Health Questionnaire 2/9.
After an 8-week observation period, subjects were randomly assigned to receive either 180 μg/week (for arm A) or 90 μg/week (for arm B) of pegylated (Peg) interferon alfa-2a (Pegasys; Roche) in an open-label manner. After the first 5 weeks of treatment (study week 13), subjects had their ART regiment interrupted and continued to receive Peg–interferon alfa-2a weekly.
The prespecified primary end point was the proportion of subjects who maintained a viral load of <400 copies/mL after 12 weeks of Peg–interferon alfa-2a monotherapy (study week 25). Primary end point failure was defined as 2 consecutive viral load measurements of ≥400 copies/mL, as a decrease in CD4+ T-cell count to <300 cells/μL, or as the presence of significant adverse events, including Patient Health Questionnaire 2/9 depression scores of >10. Subjects with a sustained viral load of <400 copies/mL at the primary end point were allowed to continue Peg–interferon alfa-2a monotherapy for an additional 12 weeks (study week 37). All subjects restarted their prestudy ART regimen at the time of virologic rebound or treatment failure or by study week 37 and were then followed for an additional 12-week period.
Clinical Laboratory Testing and Immunoassays
All clinical laboratory tests were performed by Quest Diagnostics (Madison, NJ). Interferon alfa levels were assessed in cryopreserved plasma, using a cytometric bead array (Invitrogen, Carlsbad, CA) and were analyzed on a Luminex (Austin, TX) platform at the Center for AIDS Research Immunology Core at the University of Pennsylvania.
Ultrasensitive Amplification of HIV RNA in Plasma
Longitudinal plasma HIV RNA levels were measured using the Food and Drug Administration–approved isothermal transcription-mediated amplification assay (Aptima, Gen-Probe, San Diego, CA). This assay has a 50% detection limit of 3.6 RNA copies/mL when performed singly [15]. Data were collected in 5 replicates for each time point (1.5–2.5 mL plasma total), improving the overall limit of detection to <3.5 RNA copies/mL; means are reported. The output for each replicate is a signal/cutoff (S/Co) ratio (range, 0–30), with S/Co of <1.0 considered to be HIV RNA negative. S/Co levels were derived by averaging the S/Co values across replicates [16, 17].
Measures of Integrated HIV DNA
The integration assay was performed on peripheral blood mononuclear cells (PBMCs) as described in detail previously [18]. Briefly, Alu-gag–based polymerase chain reaction with repetitive sampling techniques was used to increase the sensitivity and accuracy of the assay in order to measure low levels of integration in patient samples. Our integration standard accounts for the effect of distance between integration sites and host-Alu sequences. Results are expressed as number of integrated HIV DNA copies per number of CD4+ T cells, as follows: [number of copies of integrated HIV DNA per PBMC] × [(monocyte count + lymphocyte count)/(CD4+ T-cell count)].
Genotype Assessment
A total of 2 × 106 PBMCs were frozen in DNAzol (Molecular Research Center, Cincinnati, OH), and DNA was extracted as per the manufacturer's protocol. HLA class I loci were genotyped by the sequence-based typing method, as recommended by the 13th International Histocompatibility Workshop (http://www.ihwg.org/tmanual/TMcontents.htm). Interleukin 28B (IL-28B) gene alleles were genotyped for the C or T allele at rs12979860, using a custom-designed TaqMan allelic discrimination assay (Applied Biosystems), as described elsewhere [19].
Statistical Analysis
Randomization Procedure
Subjects were randomly allocated 1:1 to receive either 90 or 180 μg/week of Peg–interferon alfa-2a with random block sizes. A Web-based system (http://www.CEDTweb.org) was used to sequentially assign randomization outcomes.
Primary Analysis
We evaluated whether immunotherapy with 180 or 90 μg/week of Peg–interferon alfa-2a maintained control of HIV replication (ie, for 12 weeks) in the absence of antiretroviral therapy to greater levels than observed in the absence of immunomodulatory therapy. On the basis of our previous studies, we expected that, at most, 9% of subjects would maintain a viral load of <400 copies/mL for 12 weeks in the absence of any immunomodulatory therapy [20–22]. A relatively high threshold of ≥400 copies/mL was used to define virologic failure in an effort to avoid classifying patients with transient viral blips as having virologic failure; such viral blips have been described for subjects receiving ART [23]. The primary analysis tested the null hypothesis that the proportion of individuals with a viral load of < 400 copies/mL is equal to 0.09 against the 1-sided alternative that this proportion is >0.09, using a 1-sided exact binomial test with an α level of .05. The analysis was initially stratified by arm and then performed with combined arms. Subjects who withdrew consent or were lost to follow-up (n = 3) were excluded from the primary analysis; a sensitivity analysis including all subjects was also performed.
Secondary Analyses
We also evaluated whether immunotherapy with 180 or 90 μg/week of Peg–interferon alfa-2a maintained control of HIV replication at a viral load of <48 copies/mL (ie, for 12 weeks) in the absence of antiretroviral therapy, using the same approach as with the primary end point analysis described above and with an expected suppression rate of 3% observed in historical control subjects from a structured-treatment-interruption cohort in Philadelphia [20–22].
To determine control over time and on the basis of our previous studies, the proportion of subjects maintaining viral suppression at a viral load of <400 copies/mL over time was illustrated using a Kaplan-Meier plot and differences between study arms and the historical cohort [21] (7% female, 28% African American, and 7% Hispanic; mean [±SD] CD4+ T-cell count before interruption, 677 ± 224 cells/μL) were tested using a log-rank test. To address potential differences in CD4+ T-cell count distributions between cohorts, we fit a Cox proportional hazard model that controlled for CD4+ T-cell count at the time of interruption. Differences in medians between visits for the reported variables were assessed using the Wilcoxon signed rank test or the Fisher exact test. Differences in the levels of HIV integration between visits were tested using Wilcoxon signed rank tests, and differences in end point/baseline ratios between arms were tested using a Wilcoxon rank sum test.
RESULTS
Participants Characteristics
A total of 23 study participants were randomly assigned to one of the study arms in the 49-week study (Figure 1): 12 were assigned to arm A (180 μg/week), and 11 were assigned to arm B (90 μg/week). The study population included 19 men (83%) and 14 African Americans (61%) and had a median age of 45 years (interquartile range [IQR], 40–49 years). Across both arms, the median baseline CD4+ T-cell count was 840 cells/μL (IQR, 631–1112 cells/μL). Subject baseline characteristics are described in Table 1. No significant difference between arms was observed for baseline variables.
Table 1.
Baseline Characteristics of Subjects With Human Immunodeficiency virus Infection Who Were Randomly Assigned to Receive One of Two Dosages of Pegylated Interferon Alfa-2a
| Study Arm |
Primary Outcome |
|||
|---|---|---|---|---|
| A (180 μg/week) | B (90 μg/week) | Failure | Success | |
| Characteristic | (n = 12) | (n = 11) | (n = 11) | (n = 9) |
| Age (y) | 45.5 (42.8–48.5) | 44 (32–49.5) | 43 (36.5–50) | 43 (39–47) |
| CD4+ T-cell count (cells/μL) | 945 (714.8–1210.8) | 829 (581.5–891.5) | 859 (736.5–1299.5) | 840 (584–1032) |
| Sex | ||||
| Female | 3 (25) | 1 (9.1) | 2 (18.2) | 2 (22.2) |
| Male | 9 (75) | 10 (90.9) | 9 (81.8) | 7 (77.8) |
| Race | ||||
| African American | 9 (75) | 5 (45.5) | 6 (54.5) | 6 (66.7) |
| White | 3 (25) | 6 (54.5) | 5 (45.5) | 3 (33.3) |
Data are median (interquartile range) or no. (%) of subjects.
Polymorphisms in genes encoding for IL-28B and HLA alleles have been associated with the ability to respond to interferon alfa-based therapy during hepatitis C virus infection [19], to control HIV replication in the absence of ART [24], or to control HIV disease progression [25–29]. We did not detect any obvious enrichment in alleles analyzed in the subjects with sustained viral control (Table S1).
Safety Assessments
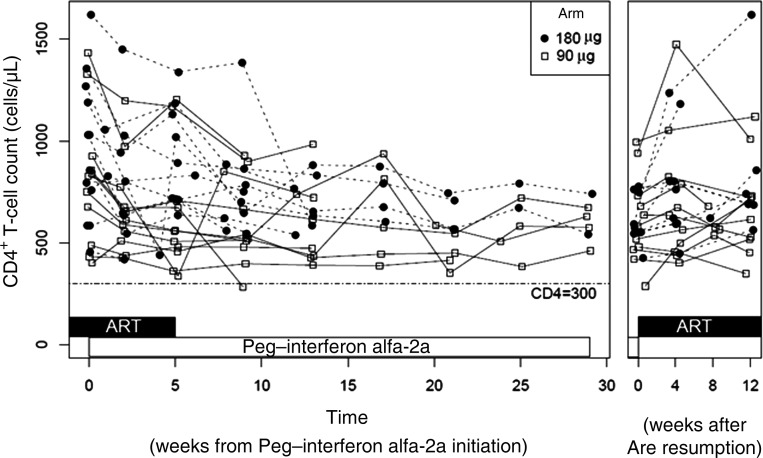
Peg–interferon alfa-2a treatment can result in a number of known adverse events, among which the most prominent are depression, lymphocytopenia, neutropenia and liver toxicities. Three subjects had a Patient Health Questionnaire 2/9 score of >10, indicating moderate depression, and Peg–interferon alfa-2a therapy was discontinued. Although no subject had to discontinue the treatment because of a decrease in CD4+ T-cell count (Figure 2), an initial and expected drop during the first 5 weeks of Peg–interferon alfa-2a treatment was noted (median CD4+ T-cell count at week eight, 840 cells/μL [IQR, 630.5–1112]; median CD4+ T-cell count at week thirteen, 711 cells/μL [IQR, 558–1020]); P = .0004). These levels remained stable over the subsequent monotherapy period. There was no relationship between initial CD4+ T-cell count decline and dose of Peg–interferon alfa-2a (P = .12). One subject experienced grade 3 neutropenia while still receiving ART, resulting in study discontinuation.
Figure 2.
CD4+ T-cell count response to Peg–interferon alfa-2a. CD4+ T cell count was assessed at each study visit for arm A (closed circles) and arm B (open squares). Treatment is represented by black (antiretroviral therapy [ART]) or white horizontal boxes (Peg–interferon alfa-2a). The left panel represents visits up to failure or study end point. The right panel represents visits after ART resumption.
Primary Analysis
Of the 23 subjects enrolled, 2 withdrew from the study before the end point (one was incarcerated, and one withdrew out of concern with protracted grade 2 liver function test results), and 1 was lost to follow-up after week 4, resulting in a sample size of 20 for the primary analysis. During the postrandomization period of Peg–interferon alfa-2a administration, 7 individuals exhibited virologic failure, 3 had moderate depression scores as revealed by the Patient Health Questionnaire 2/9, and 1 had a grade 3 neutropenia; all of these subjects were considered to have achieved end-point failure. Of the 20 subjects with end points by week 12 of Peg–interferon alfa-2a monotherapy (study week 25), 9 subjects (45%) successfully maintained a viral load of <400 copies/mL. The proportion of individuals with a viral load of <400 copies/mL was greater than the anticipated proportion of 9% in either arm or combined (P=.0088 for arm A, P=.0010 for arm B, and P<.0001 for the combined arms, using the exact binomial test). As shown in Table 2, the proportion of subjects who achieved successful outcomes was not significantly different between the 2 arms (P = 1, by the Fisher exact test). We performed a sensitivity analysis that included all 23 randomized subjects, assigning a failure outcome to those who withdrew/were lost to follow-up: the exact binomial test P values were .0180 for arm A, .0017 for arm B, and <.0001 for the combined arms. Secondary analysis at the primary end point, using the <48 copies/mL threshold achieved by 4 of 20 subjects (20%), showed significant differences in both arms, compared with an anticipated rate of 3% based on reports in the literature (P=.03 for arm A, P=.0028 for arm B, and P<.0027 for the combined arms).
Table 2.
Primary End Point Results for Subjects With Human Immunodeficiency virus Infection Who Were Randomly Assigned to Receive One of Two Dosages of Pegylated Interferon Alfa-2a
| Study Arm, No. (%) |
|||
|---|---|---|---|
| Outcome | A (180 μg/week) | B (90 μg/week) | Overall, No. (%) |
| Failure | 6 (60) | 5 (50) | 11 (55) |
| Success | 4 (40) | 5 (50) | 9 (45) |
| Overall | 10 (100) | 10 (100) | 20 (100) |
P = 1, by the Fisher exact test, for differences in failure and success rates between arms A and B.
Plasma interferon alfa-2a concentrations were higher than baseline values in most subjects, with levels at week 5 slightly higher in arm A (P = .0782; data not shown) and no significant difference between subjects with treatment success or failure outcomes, suggesting that either dose tested can achieve drug levels associated with suppression.
Assessment of Plasma HIV Levels During 24-Week Peg–Interferon Alfa-2a Monotherapy
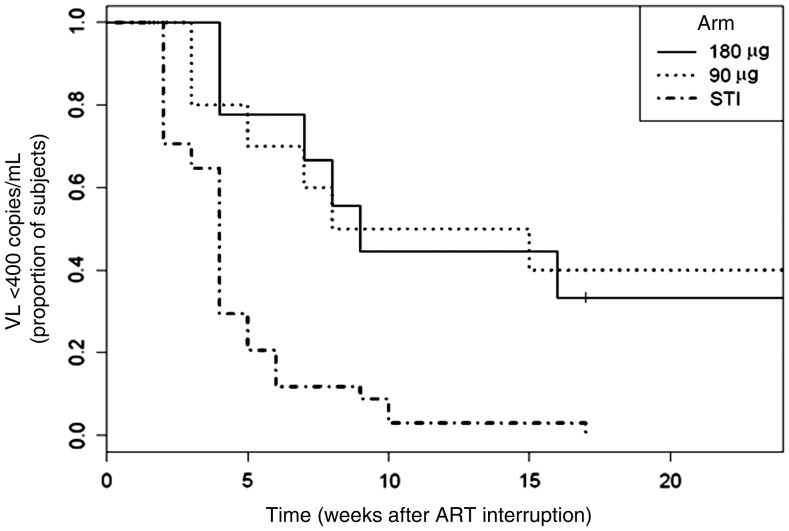
The 9 subjects with HIV levels controlled to <400 copies/mL at 12 weeks on Peg–interferon alfa-2a monotherapy (4 of 9 had a sustained viral load of <48 copies/mL) were allowed to continue to receive monotherapy for another 12 weeks. One subject withdrew consent after week 12, resulting in 8 subjects followed for 24 weeks. As illustrated in Figure 3, 6 of these 8 subjects maintained a viral load of <400 copies/mL for the entire period (3 of 6 with a viral load of <48 copies/mL). A log-rank test performed using data over the 24-week period revealed that the rate in each arm of maintaining viral suppression was significantly different than that of historical controls (P = .0046 for arm A and P = .0011 for arm B, compared with an ART-interruption arm of historical control subjects [21]); accounting for baseline CD4+ T-cell count did not alter these findings.
Figure 3.
Viral load (VL) response to Peg–interferon alfa-2a. The VL was assessed at each visit and is represented as the Kaplan–Meier plot of the viral response to Peg–interferon alfa-2a treatment. The vertical axis represents the fraction of subjects in arm A (solid line) and arm B (dotted line) with a VL of <400 RNA copies/mL. The dashed line represent the comparator historical structured treatment interruption (STI) group [21]. Abbreviation: ART, antiretroviral therapy.
Detection of Residual HIV-1 RNA
We measured plasma samples from subjects with a viral load of <400 copies/mL, using an ultrasensitive polymerase chain reaction technique able to detect 1–100 HIV RNA copies/mL [16, 30] (Table 3). First, we determined the effects of 5 weeks of Peg–interferon alfa-2a added to a suppressive ART regimen (viral load, <400 copies/mL at both time points) from all available subjects (n = 20).
Table 3.
Transcription-Mediated Amplification (TMA)–Based Assessment of Viral Replication During the Study Period Among Subjects With Human Immunodeficiency virus Infection, by Primary Outcome
| Week |
|||||
|---|---|---|---|---|---|
| Outcome | 4 | 8 | 13 | 25 | 37 |
| Success | |||||
| TMA (S/Co) | 4.6 (1.5–7.4) | 5.3 (0.1–6.1) | 2.6 (1.4–8) | 20.9 (8.9–30.2) | 22.4 (12–30.4) |
| TMA | 9 | 9 | 9 | 8 | 5 |
| PCR viral load (RNA copies/mL) | 48 (48–48) | 48 (48–48) | 48 (48–48) | 77 (48–230) | 167 (48–416) |
| PCR |
9 |
9 |
9 |
9 |
8 |
| Week |
|||||
| 4 | 8 | 13 | Failure visit | ||
| Failure | |||||
| TMA (S/Co) | 3.85 (2.5–7.6) | 3 (1.9–9.8) | 0.6 (0.1–4.3) | Not donea | |
| TMA | 10 | 10 | 10 | 0 | |
| PCR viral load (RNA copies/mL) | 48 (48–126.5) | 48 (48–48) | 48 (48–48) | 16 370 (5556–28 586) | |
| PCR | 11b | 10 | 10 | 10 | |
Data are median (interquartile range) or no. of subjects.
Abbreviations: PCR, polymerase chain reaction; S/Co, signal/cutoff ratio.
aTMA was not done for individuals with a viral load of >400 copies/mL.
dOne individual had a viral load of 486 copies/mL, but this was not confirmed because the individual withdrew from the study immediately after week 4.
Residual HIV plasma levels during ART (median S/Co value at week eight, 3.3 [IQR, 0.15–7.9]) were not significantly different after 5 weeks of Peg–interferon alfa-2a (median S/Co value at week thirteen, 2.4 [IQR, 0.1–4.95]; P = .5619).
Second, we sought to determine whether a change in residual viral load occurred in responders (<400 copies/mL) from the time of ART interruption to 12 or 24 weeks of Peg–interferon alfa-2a monotherapy. The 9 responder subjects showed an increase in residual HIV RNA levels, from a median S/Co value of 2.6 (IQR, 1.4–8) before ART interruption (week 13) to a median S/Co value of 20.9 (IQR, 8.9–30.2) at the primary end point (week 25; P = .0078, by the Wilcoxon signed rank test). No change was detected between 12 weeks (S/Co value at week twenty-five, 9.2 [IQR, 4.9–26.5]) and 24 weeks (S/Co value at week thirty-seven, 22.4 [IQR, 6.1–31.5]; P = .625) after ART interruption among the 6 subjects whose viral load remained at <400 copies/mL for 24 weeks despite not receiving ART.
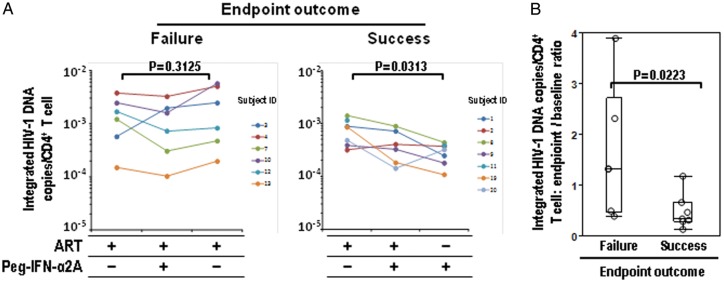
Evaluation of Integrated HIV DNA Copies Per CD4+ T Cell in Association With Treatment Success
HIV persists during ART as integrated DNA in memory T cells and perhaps other cells. We compared the amount of integrated viral DNA present in PBMCs from 6 subjects who exhibited virologic failure and 7 who maintained viral suppression at week 12 (representing those subjects with primary end point success and with a sufficient PBMC yield to complete the analysis; Figure 4). Samples were studied at weeks 8, 13, and 25. The number of integrated HIV DNA copies per CD4+ T cell (Figure 4A) did not change significantly between baseline and the time of virologic failure in the 6 subjects who had virologic failure. In contrast, the number of integrated HIV DNA copies per CD4+ T cell between baseline and the primary end point declined significantly in the 7 subjects who exhibited durable viral control (mean baseline value [±SD], 7.83 × 10−4 ± 4.08 × 10−4 copies/CD4+ T cell; mean end point value [±SD], 2.92 × 10−4 ± 1.19 × 10−4copies/CD4+ T cell; P = .0313). The ratio of integrated HIV DNA copies/CD4+ T cell between end point and baseline was also significantly lower in subjects sustaining viral control (Figure 4B; P = .0223).
Figure 4.
Assessment of integrated HIV DNA levels per circulating CD4+ T cell. A, Integrated HIV DNA was measured by Alu–polymerase chain reaction, as described in the Methods section. The number of integrated HIV DNA copies/CD4+ T cell are represented. The left panel shows 6 subjects experiencing protocol failure (viral load, ≥400 HIV RNA copies/mL) before the 12-week end point. Assessed are protocol week 8 (antiretroviral therapy [ART]), week 13 (ART + Peg–interferon alfa-2a), and after failure (after ART resumption). The right panel shows 7 subjects experiencing protocol-defined success (a sustained viral load of <400 copies/mL until the 12-week end point). Assessed are protocol week 8 (ART), week 13 (ART + Peg–interferon alfa-2a; subject 1 = not done), and week 25 (Peg–interferon alfa-2a monotherapy), as indicated. P values calculated by the Wilcoxon signed rank test are indicated. B, Boxes represent the median, quartiles, and extremes of the distribution of end point:baseline ratios of integrated DNA copies/CD4+ T cell in subjects with virological failure or success. Individual values are superimposed as open circles. A P value calculated by the Wilcoxon rank sum test is indicated.
DISCUSSION
We report that administration of Peg–interferon alfa-2a (90 or 180 μg/week) to individuals with an ART-suppressed HIV RNA load results in a sustained control of viral replication in 45% of subjects when ART is interrupted. The observed response rate was significantly greater than that reported in trials evaluating ART interruption alone and remained significant when including all randomized subjects (39% suppression).
Prior approaches based on interferon alfa administration without ART (ie, in treatment-naive subjects or subjects with ART interruption) have failed to achieve similar suppression rates [12, 14]. Differences in study design might have contributed to this disparity. We started Peg–interferon alfa-2a treatment in subjects receiving suppressive ART (viral load, <50 copies/mL) and with high degree of immune reconstitution (CD4+ T-cell count >450 cells/μL), which likely resulted in restored immune subsets (eg, natural killer [NK] cells and CD8+ T cells) and activation of antiviral host factors (eg, apolipoprotein-B messenger RNA editing enzyme [APOBEC]), mediating some of the antiviral effects of type 1 interferons. Moreover, unlike prior studies [12, 13], Peg–interferon alfa-2a was coadministered with ART for 5 weeks, potentially allowing for steady-state drug levels and immunomodulation to be achieved before ART was interrupted.
The mechanisms by which Peg–interferon alfa-2a restricted viral replication in our subjects remain to be elucidated. Type 1 interferons control viremia by reducing viral replication in infected cells and preventing infection of new targets. The mechanisms of type 1 interferon–dependent viral control fall into 3 areas: direct interferon α anti-HIV activity, mediated by host proteins (eg, tetherin, APOBEC 1, and protein kinase R activation [31, 32]); enhancement of adaptive effector function (eg, HIV-specific CD8+ T cells and ADCC); and enhancement of innate immune effector function (eg, NK cells).
A role for interferon-mediated immune clearance of HIV-infected cells is supported by clinical studies in which the administration of interferon alfa to patients with HIV infection or melanoma was associated with increased perforin and granzyme expression in NK and CD8+ T cells, respectively [33, 34]. While several studies suggest that the majority of integrated HIV DNA is defective [1, 35, 36], latently infected cells may still express HIV protein and be subject to immune clearance.
We report a significant reduction of CD4+ T cell–integrated HIV DNA in subjects with viral control. Our therapeutic approach (ie, ART + Peg–interferon alfa-2a therapy, followed by Peg–interferon alfa-2a alone) may have contributed to this decrease by allowing residual HIV replication to trigger immune responses that are restored by long-term ART and that are capable of clearing infected cells expressing HIV proteins. The implications of this observation remains unclear, but one hypothesis is that Peg–interferon alfa-2a resulted in an immunomediated reduction in the size of the cellular latent reservoir (as defined by the level of integrated HIV DNA). As indicated by the transcription-mediated amplification-based assessment, residual viremia or enhanced viral release occurred when ART was stopped. This may have contributed to the amplification of cytotoxic T-lymphocyte responses, which are reduced in frequency in ART-treated subjects as compared to long-term nonprogressors [37]. Regardless of the mechanism, our data suggest that detection of residual HIV in plasma can be disassociated from changes in HIV integration levels within peripheral cells. Further studies in larger populations will be required to establish whether ART interruption and residual viremia are necessary to produce the observed reduction in viral integration.
It will be important for future studies to determine whether Peg–interferon alfa-2a treatment can stabilize the viral set point to these low levels, sustaining viral control over time. Of the 8 subjects who elected to continue monotherapy past the primary end point to week 24 (9 subjects were eligible), 6 had a viral load of <400 copies/mL at week 48 (3 of 8 had a viral load of <48 copies/mL), supporting the view that in some individuals interferon alfa-mediated immune control may be extended beyond 24 weeks. Interestingly, 2 of the 6 subjects with a viral load of <400 copies had a stable set point between 50 and 400 copies/mL throughout the 24 weeks of monotherapy.
We did not observe any rise in integrated HIV DNA levels in patients in whom the viral load was not controlled to <400 copies/mL. This was unexpected and may indicate delayed kinetics of reservoir change after ART interruption and viremic rebound; importantly, in our study the viremic episodes were of short duration, since our patients restarted ART as soon as their viral load was confirmed to be <400 copies/mL. Our data suggest that future studies aimed at determining the kinetics of CD4+ T cell–integrated HIV DNA rebound may require longer periods of ART interruption with sustained viremia.
Overall, our observations are consistent with interferon alfa contributing to long-term control despite residual viral replication, as observed in “elite” suppressors (ie, individuals sustaining low-level viremia without ART [16, 17]). Notably, the latter individuals also preserve functional circulating plasmacytoid dendritic cells better than chronic progressors do [38, 39], and they have low levels of viral integration [18], supporting the hypothesis that type 1 interferon–mediated mechanisms contribute to HIV control in vivo.
Our study has some limitations. First, the original design (including a period of ART interruption without Peg–interferon alfa-2a for each subject, providing for the determination of individual viral set points) was modified at the request of the Food and Drug Administration in the course of the Investigational New Drug submission, on the basis of the results of the SMART study [20], which showed that CD4+ T cell–guided sexually transmitted infections are associated with disease progression, and on the basis of extensive literature reporting viral rebound following ART interruption in >95% of subjects. To address this limitation, we compared the proportion of failures in each arm with an expected rate obtained from prior studies [20–22, 40–43], and we elected to use an estimated proportion of subjects with sustained viral suppression (9%) that was higher than the proportions reported in similar populations. In a secondary analysis, we also compared (using a log-rank test) each study arm to historical cohort of control subjects for whom ART was interrupted, confirming that the 2 arms had significantly higher rates of suppression than the ART-interruption group, even after adjustment for initial CD4+ T-cell levels. A second limitation is the unavailability of many pre-ART viral loads (most subjects received long-time ART from multiple providers), which prevented us from directly assessing the relationship between viral set points before ART and those during Peg–interferon alfa-2a monotherapy. However, we did establish that our study group did not include an overrepresentation of individuals with HLA and KIR alleles associated with HIV control. Finally, because our study cohort represents a proof-of-concept study with a limited sample size, it will be important to confirm our results in future, larger longitudinal studies.
In conclusion, we report that treatment with 90 or 180 μg/week of Peg–interferon α2a can support viral control and reduction of peripheral HIV integration levels in subjects for whom ART has been interrupted. Our study provides a proof of concept that immunotherapy can be pursued in HIV-infected, ART-dependent subjects to reach a status of viral control beyond ART and could complement current research approaches to current “functional cure” and eradication.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We are grateful for the altruism and determination of study participants and to their providers, who contributed to overall study oversight. We acknowledge clinical recruitment support provided by Jane Shull and the Philadelphia FIGHT staff (A. Kapalko, K. Richards, S. Smith), Drexel University (S. Lewis, D. Downie, C. M. Randazzo, A. Johnson, S. Skinner), and the University of Pennsylvania (C. Carty, K. Maffei, J. Quinn, J. Gilmore, J. Hines, Z. Dorey-Stein). We are grateful to Dr J. Chehimi, for initial study support, and to Drs K. Brady, J. Hines, E. Hollen, S. Hansen-Flaschen, and R. Maniglia, for patient referrals. Pharmacy support was provided by the University of Pennsylvania (K. Rockwell, D. Kim) and Drexel University (K. George). We thank the data safety monitoring board members K. Squires, MD; J. Merz, PhD; G. Bisson, MD; A. Troxel, PhD; S. Bellamy, PhD; R. Goldfein, Esq; and G. Brake. We acknowledge statistical analysis support (X. Yin); Web development support for data collection (M. Picone); ultrasensitive amplification of HIV RNA (V. Winkelman at Creative Testing Solutions, Temple AZ); National Institutes of Health program support (D. Livnat, M. Dehlinger, E. Pouilot); genotyping support (F.-M. Dui, M. P. Martin), administrative support for institutional review board, data safety monitoring board, National Institutes of Health, and Food and Drug Administration reporting (B. O'Brien, M. O'Neill, J. Dubin); sample/subject transport support (J. Starnes, B. Fisher); and laboratory support (G. Reynolds, B. Ross, A. Mackiewicz, N. Opsitnick, C. Calloway, M. Cummins, L. Hubbard, A. Quartlebaum).
Author contributions are as follows: Livio Azzoni, study design, data management, analysis, and manuscript preparation; Andrea S. Foulkes, study design, analysis, and manuscript preparation; Emmanouil Papasavvas, study management, analysis, and manuscript preparation; Angela M. Mexas, integrated DNA assays, data analysis, and manuscript preparation; Kenneth M. Lynn, clinical care, study coordination, analysis, and manuscript preparation; Michael Busch, transcription-mediated amplification assays, analysis, and manuscript preparation; Steven Deeks, transcription-mediated amplification assays, analysis, and manuscript preparation; Mary Carrington, HLA haplotypes, analysis, and manuscript preparation; Karam Mounzer, clinical care, study coordination, analysis, and manuscript preparation; Pablo Tebas, clinical care, study coordination, analysis, and manuscript preparation; Ian Frank, clinical care, study coordination, analysis, and manuscript preparation; Una O'Doherty, integrated DNA assays, data analysis, and manuscript preparation; Jeffrey M. Jacobson, clinical care, study coordination, analysis, and manuscript preparation; Jay Kostman, clinical principal investigator, study coordination, analysis, and manuscript preparation; and Luis J. Montaner, principal investigator, study design and management, data collection, analysis, and manuscript preparation.
Disclaimer. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
Financial support. This work was supported by NIH/NIAID grant U01AI065279 to LJM, K08AI073102 to AM, K02 AI078766 and R21 AI087461 to UO. Additional support was provided by Genentech/Roche (drug support under protocol PEG224), The Philadelphia Foundation (Robert I. Jacobs Fund), Henry S. Miller, Jr. and J. Kenneth Nimblett, AIDS funds from the Commonwealth of Pennsylvania and from the Commonwealth Universal Research Enhancement Program, Pennsylvania Department of Health, the Penn Center for AIDS Research (P30 AI 045008), and Cancer Center Grant (P30 CA10815), the American Foundation for AIDS Research, the National Cancer Institute, National Institutes of Health (Contract No. HHSN261200800001E), and the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Chun TW, Carruth L, Finzi D, et al. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature. 1997;387:183–8. doi: 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- 2.Wilen CB, Wang J, Tilton JC, et al. Engineering HIV-resistant human CD4+ T cells with CXCR4-specific zinc-finger nucleases. PLoS Pathog. 2011;7:e1002020. doi: 10.1371/journal.ppat.1002020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angel JB, Routy JP, Tremblay C, et al. A randomized controlled trial of HIV therapeutic vaccination using ALVAC with or without Remune. AIDS. 2011;25:731–9. doi: 10.1097/QAD.0b013e328344cea5. [DOI] [PubMed] [Google Scholar]
- 4.Henry K, Katzenstein D, Cherng DW, et al. A pilot study evaluating time to CD4 T-cell count <350 cells/mm(3) after treatment interruption following antiretroviral therapy +/- interleukin 2: results of ACTG A5102. J Acquir Immune Defic Syndr. 2006;42:140–8. doi: 10.1097/01.qai.0000225319.59652.1e. [DOI] [PubMed] [Google Scholar]
- 5.Matalon S, Rasmussen TA, Dinarello CA. Histone deacetylase inhibitors for purging HIV-1 from the latent reservoir. Mol Med. 2011;17:466–72. doi: 10.2119/molmed.2011.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang JJ, Altfeld M. Innate immune activation in primary HIV-1 infection. J Infect Dis. 2011;202(Suppl. 2):S297–301. doi: 10.1086/655657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rotger M, Dang KK, Fellay J, et al. Genome-wide mRNA expression correlates of viral control in CD4+ T-cells from HIV-1-infected individuals. PLoS Pathog. 2010;6:e1000781. doi: 10.1371/journal.ppat.1000781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chehimi J, Papasavvas E, Tomescu C, et al. Inability of plasmacytoid dendritic cells to directly lyse HIV-infected autologous CD4+ T cells despite induction of tumor necrosis factor-related apoptosis-inducing ligand. J Virol. 2010;84:2762–73. doi: 10.1128/JVI.01350-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tomescu C, Chehimi J, Maino VC, Montaner LJ. NK cell lysis of HIV-1-infected autologous CD4 primary T cells: requirement for IFN-mediated NK activation by plasmacytoid dendritic cells. J Immunol. 2007;179:2097–104. doi: 10.4049/jimmunol.179.4.2097. [DOI] [PubMed] [Google Scholar]
- 10.Boasso A, Shearer GM. Chronic innate immune activation as a cause of HIV-1 immunopathogenesis. Clin Immunol. 2008;126:235–42. doi: 10.1016/j.clim.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lane HC, Kovacs JA, Feinberg J, et al. Anti-retroviral effects of interferon-alpha in AIDS-associated Kaposi's sarcoma. Lancet. 1988;2:1218–22. doi: 10.1016/s0140-6736(88)90811-2. [DOI] [PubMed] [Google Scholar]
- 12.Boue F, Reynes J, Rouzioux C, et al. Alpha interferon administration during structured interruptions of combination antiretroviral therapy in patients with chronic HIV-1 infection: INTERVAC ANRS 105 trial. AIDS. 2011;25:115–8. doi: 10.1097/QAD.0b013e328340a1e7. [DOI] [PubMed] [Google Scholar]
- 13.Dianzani F, Rozera G, Abbate I, et al. Interferon may prevent HIV viral rebound after HAART interruption in HIV patients. J Interferon Cytokine Res. 2008;28:1–3. doi: 10.1089/jir.2007.0076. [DOI] [PubMed] [Google Scholar]
- 14.Asmuth DM, Murphy RL, Rosenkranz SL, et al. Safety, tolerability, and mechanisms of antiretroviral activity of pegylated interferon alfa-2a in HIV-1-monoinfected participants: a phase II clinical trial. J Infect Dis. 2010;201:1686–96. doi: 10.1086/652420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Linnen JM, Gilker JM, Menez A, et al. Sensitive detection of genetic variants of HIV-1 and HCV with an HIV-1/HCV assay based on transcription-mediated amplification. J Virol Methods. 2002;102:139–55. doi: 10.1016/s0166-0934(02)00012-5. [DOI] [PubMed] [Google Scholar]
- 16.Hatano H, Delwart EL, Norris PJ, et al. Evidence for persistent low-level viremia in individuals who control human immunodeficiency virus in the absence of antiretroviral therapy. J Virol. 2009;83:329–35. doi: 10.1128/JVI.01763-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hatano H, Delwart EL, Norris PJ, et al. Evidence of persistent low-level viremia in long-term HAART-suppressed, HIV-infected individuals. AIDS. 2010;24:2535–9. doi: 10.1097/QAD.0b013e32833dba03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graf EH, Mexas AM, Yu JJ, et al. Elite suppressors harbor low levels of integrated HIV DNA and high levels of 2-LTR circular HIV DNA compared to HIV+ patients on and off HAART. PLoS Pathog. 2011;7:e1001300. doi: 10.1371/journal.ppat.1001300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ge D, Fellay J, Thompson AJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399–401. doi: 10.1038/nature08309. [DOI] [PubMed] [Google Scholar]
- 20.El-Sadr WM, Lundgren JD, Neaton JD, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–96. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 21.Papasavvas E, Kostman JR, Mounzer K, et al. Randomized, controlled trial of therapy interruption in chronic HIV-1 infection. PLoS Med. 2004;1:e64. doi: 10.1371/journal.pmed.0010064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davey RT, Jr, Bhat N, Yoder C, et al. HIV-1 and T cell dynamics after interruption of highly active antiretroviral therapy (HAART) in patients with a history of sustained viral suppression. Proc Natl Acad Sci U S A. 1999;96:15109–14. doi: 10.1073/pnas.96.26.15109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nettles RE, Kieffer TL, Kwon P, et al. Intermittent HIV-1 viremia (blips) and drug resistance in patients receiving HAART. JAMA. 2005;293:817–29. doi: 10.1001/jama.293.7.817. [DOI] [PubMed] [Google Scholar]
- 24.Bashirova AA, Thomas R, Carrington M. HLA/KIR restraint of HIV: surviving the fittest. Annu Rev Immunol. 2011;29:295–317. doi: 10.1146/annurev-immunol-031210-101332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McMichael AJ, Jones EY. First-class control of HIV-1. Science. 2010;330:1488–90. doi: 10.1126/science.1200035. [DOI] [PubMed] [Google Scholar]
- 26.Thomas R, Apps R, Qi Y, et al. HLA-C cell surface expression and control of HIV/AIDS correlate with a variant upstream of HLA-C. Nat Genet. 2009;41:1290–4. doi: 10.1038/ng.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poropatich K, Sullivan DJ., Jr Human immunodeficiency virus type 1 long-term non-progressors: the viral, genetic and immunological basis for disease non-progression. The Journal of General Virology. 2011;92:247–68. doi: 10.1099/vir.0.027102-0. [DOI] [PubMed] [Google Scholar]
- 28.Kaur G, Mehra N. Genetic determinants of HIV-1 infection and progression to AIDS: immune response genes. Tissue Antigens. 2009;74:373–85. doi: 10.1111/j.1399-0039.2009.01337.x. [DOI] [PubMed] [Google Scholar]
- 29.Leslie A, Matthews PC, Listgarten J, et al. Additive contribution of HLA class I alleles in the immune control of HIV-1 infection. J Virol. 2010;84:9879–88. doi: 10.1128/JVI.00320-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roland ME, Elbeik TA, Kahn JO, et al. HIV RNA testing in the context of nonoccupational postexposure prophylaxis. J Infect Dis. 2004;190:598–604. doi: 10.1086/421278. [DOI] [PubMed] [Google Scholar]
- 31.Pillai SK, Abdel-Mohsen M, Guatelli J, et al. Role of retroviral restriction factors in the interferon-alpha-mediated suppression of HIV-1 in vivo. Proc Natl Acad Sci U S A. 2012;109:3035–40. doi: 10.1073/pnas.1111573109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goujon C, Malim MH. Characterization of the alpha interferon-induced postentry block to HIV-1 infection in primary human macrophages and T cells. J Virol. 2010;84:9254–66. doi: 10.1128/JVI.00854-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guillot B, Portales P, Thanh AD, et al. The expression of cytotoxic mediators is altered in mononuclear cells of patients with melanoma and increased by interferon-alpha treatment. Br J Dermatol. 2005;152:690–6. doi: 10.1111/j.1365-2133.2005.06512.x. [DOI] [PubMed] [Google Scholar]
- 34.Portales P, Reynes J, Pinet V, et al. Interferon-alpha restores HIV-induced alteration of natural killer cell perforin expression in vivo. Aids. 2003;17:495–504. doi: 10.1097/00002030-200303070-00004. [DOI] [PubMed] [Google Scholar]
- 35.Chun TW, Stuyver L, Mizell SB, et al. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci U S A. 1997;94:13193–7. doi: 10.1073/pnas.94.24.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chun TW, Engel D, Berrey MM, Shea T, Corey L, Fauci AS. Early establishment of a pool of latently infected, resting CD4(+) T cells during primary HIV-1 infection. Proc Natl Acad Sci U S A. 1998;95:8869–73. doi: 10.1073/pnas.95.15.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Migueles SA, Osborne CM, Royce C, et al. Lytic granule loading of CD8+ T cells is required for HIV-infected cell elimination associated with immune control. Immunity. 2008;29:1009–21. doi: 10.1016/j.immuni.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soumelis V, Scott I, Gheyas F, et al. Depletion of circulating natural type 1 interferon-producing cells in HIV-infected AIDS patients. Blood. 2001;98:906–12. doi: 10.1182/blood.v98.4.906. [DOI] [PubMed] [Google Scholar]
- 39.Machmach K, Leal M, Gras C, et al. Plasmacytoid dendritic cells reduce HIV production in Elite controllers. J Virol. 2012;86:4245–52. doi: 10.1128/JVI.07114-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Autran B, Murphy RL, Costagliola D, et al. Greater viral rebound and reduced time to resume antiretroviral therapy after therapeutic immunization with the ALVAC-HIV vaccine (vCP1452) AIDS. 2008;22:1313–22. doi: 10.1097/QAD.0b013e3282fdce94. [DOI] [PubMed] [Google Scholar]
- 41.Kilby JM, Bucy RP, Mildvan D, et al. A randomized, partially blinded phase 2 trial of antiretroviral therapy, HIV-specific immunizations, and interleukin-2 cycles to promote efficient control of viral replication (ACTG A5024) J Infect Dis. 2006;194:1672–6. doi: 10.1086/509508. [DOI] [PubMed] [Google Scholar]
- 42.Neumann AU, Tubiana R, Calvez V, et al. HIV-1 rebound during interruption of highly active antiretroviral therapy has no deleterious effect on reinitiated treatment. Comet Study Group. AIDS. 1999;13:677–83. doi: 10.1097/00002030-199904160-00008. [DOI] [PubMed] [Google Scholar]
- 43.Steingrover R, Pogany K, Fernandez Garcia E, et al. HIV-1 viral rebound dynamics after a single treatment interruption depends on time of initiation of highly active antiretroviral therapy. AIDS. 2008;22:1583–8. doi: 10.1097/QAD.0b013e328305bd77. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.