Abstract
Background. The generation of heterovariant immunity is a highly desirable feature of influenza vaccines. The goal of this study was to compare the heterovariant B-cell response induced by the monovalent inactivated 2009 pandemic influenza A virus subtype H1N1 (A[H1N1]pdm09) vaccine with that induced by the 2009 seasonal trivalent influenza vaccine (sTIV) containing a seasonal influenza A virus subtype H1N1 (A[H1N1]) component in young and elderly adults.
Methods. Plasmablast-derived polyclonal antibodies (PPAb) from young and elderly recipients of A(H1N1)pdm09 vaccine or sTIV were tested for binding activity to various influenza antigens.
Results. In A(H1N1)pdm09 recipients, the PPAb titers against homotypic A(H1N1)pdm09 vaccine were similar to those against the heterovariant seasonal A(H1N1) vaccine and were similar between young and elderly subjects. The PPAb avidity was higher among elderly individuals, compared with young individuals. In contrast, the young sTIV recipients had 10-fold lower heterovariant PPAb titers against the A(H1N1)pdm09 vaccine than against the homotypic seasonal A(H1N1) vaccine. In binding assays with recombinant head and stalk domains of hemagglutinin, PPAb from the A(H1N1)pdm09 recipients but not PPAb from the sTIV recipients bound to the conserved stalk domain.
Conclusion. The A(H1N1)pdm09 vaccine induced production of PPAb with heterovariant reactivity, including antibodies targeting the conserved hemagglutinin stalk domain.
Keywords: influenza, vaccine, antibody, cross-reactivity
The 2009 pandemic influenza A virus subtype H1N1 (A[H1N1]pdm09) is antigenically distinct from seasonal influenza A virus subtype H1N1 (A[H1N1]) strains that have circulated in recent years [1, 2]. Therefore, a large fraction of the population was immunologically naive to A(H1N1)pdm09 when it emerged in 2009 [3], and the pandemic disproportionally affected the younger population because of their lack of prior exposure to related strains [4].
The antibody response to influenza vaccination, especially inactivated influenza vaccines, is believed to be a critical mediator of the protective immunity [5]. Conventional evaluation of this response measures influenza virus–specific antibody reactivity of serum from the vaccine recipients in a hemagglutination inhibition or a neutralization assay. One limitation of serum-based assays is the intrinsic difficulty of differentiating antibodies induced by recent vaccination from preexisting cross-reactive antibodies resulting from prior exposure. An alternate approach focuses on B cells activated by influenza vaccines, which in adults and older children is largely a memory B-cell response and takes advantage of transiently circulating plasmablasts in the peripheral blood at around day 7 after immunization. These plasmablasts are antibody-secreting cells that are highly enriched with vaccine-activated influenza virus–specific B cells [6]. We and others have generated individual plasmablast-derived recombinant monoclonal antibodies [6] or plasmablast-derived polyclonal antibodies (PPAb) that represent the overall antibody response [7] to examine quantitative and qualitative characteristics of the antibody response to influenza vaccination or influenza virus infection in different age groups [6, 8–11].
Because of the rapid emergence of A(H1N1)pdm09, 2 inactivated influenza vaccines were distributed in 2009, a seasonal trivalent influenza vaccine (sTIV) containing a seasonal A(H1N1) component and a monovalent A(H1N1)pdm09 vaccine. We took advantage of the rare opportunity provided by this dual distribution to directly compare the PPAb responses to the seasonal A(H1N1) and A(H1N1)pdm09 vaccines. In particular, we evaluated the vaccine-specific and heterovariant reactivity of antibodies induced by these 2 H1N1 vaccine strains in young and elderly adults.
MATERIALS AND METHODS
Human Participants, Vaccination Protocols, and Blood Samples
Recipients of A(H1N1)pdm09 vaccine (monovalent inactivated subvirion influenza A/California/2009 without adjuvant; Novartis) were recruited at the University of Rochester during December 2009 and January 2010. Subjects were recruited in 2 age cohorts: 19 young adults aged 18–32 years (mean ± SD, 25.6 ± 4.1 years) and 18 elderly adults aged 70–81 years (mean ± SD, 75.7 ± 3.3 years). None of these subjects received 2009 sTIV within 2 weeks before enrollment. Recipients of the sTIV (Fluzone; Sanofi Pasteur) were enrolled at Stanford University during the 2009–2010 season and included 21 young adults (mean age ± SD, 25.3 ± 3.8 years) and 19 elderly adults (mean age ± SD, 76.7 ± 5.4 years) [9] who had not previously received the A(H1N1)pdm09 vaccine. The 2009 sTIV contained an A/Brisbane/59/2007(H1N1)-like virus, an A/Brisbane/10/2007(H3N2)-like virus, and a B/Brisbane/60/2008-like virus. There was no significant difference in age between the recipients of the A(H1N1)pdm09 vaccine and recipients of the sTIV (P = .749 for young adults and P = .497 for elderly adults, using unpaired t tests). Study protocols were approved by the institutional review boards at the University of Rochester and Stanford University. Informed consent was obtained from all participants. Participants were immunized with 1 dose of either A(H1N1)pdm09 vaccine or sTIV. Blood samples were collected on day 7 or 8 after vaccination. B cells were isolated by negative selection with the RosetteSep Human B Cell Enrichment Cocktail (Stemcell Technologies), following the manufacturer's instructions.
Generation of PPAb
Freshly isolated B cells were cultured in complete medium at a density of 0.5–3 million cells/mL for 7 days to collect PPAb [7]. The concentration of immunoglobulin G (IgG) in PPAb was determined with the Immuno-Tek Quantitative Human IgG enzyme-linked immunosorbent assay (ELISA) kit (Zeptometrix).
ELISA for Influenza Virus Antigen–Specific IgG Binding Activity of PPAb
ELISAs were performed as previously described [9]. ELISA plates were coated with inactivated monovalent seasonal A(H1N1) (A/Brisbane/59/2007) vaccine or A(H1N1)pdm09 (A/California/7/2009) vaccine antigen (kindly provided by Dr P. Dormitzer of Novartis) at 0.9 µg/mL in phosphate-buffered saline. Alternatively, plates were coated with recombinant full-length hemagglutinin (HA) proteins derived from A/Brisbane/59/2007(H1N1), A/California/7/2009(H1N1), or A/Vietnam/1203/2004(H5N1) (Immune Technology) or with full-length or headless recombinant HA of A/South Dakota/6/2007 (an A/Brisbane/59/2007-like seasonal A[H1N1] strain) at 5 µg/mL. The full-length and headless HA of A/South Dakota/6/2007 contained the ectodomain of the HA sequence, followed by a thrombin cleavage site, the foldon sequence, and a His-tag [12], and were expressed in 293T cells and purified over a His Talon column (Invitrogen). The HA1 head domain sequence was removed from the headless HA as described elsewhere [13]. HA domain–specific binding activity and avidity were evaluated with and without 7 M urea as previously described [14] using recombinant HA1 or HA2 domains from different influenza strains as the detecting antigen [15, 16].
Statistical Analysis
All data were logarithm transformed for comparison of geometric means. An unpaired 2-tailed t test was used to compare young with elderly individuals and A(H1N1)pdm09 vaccine with sTIV groups.
RESULTS
Homotypic Binding Activity of PPAb Induced by A(H1N1)pdm09 and Seasonal A(H1N1) Vaccines
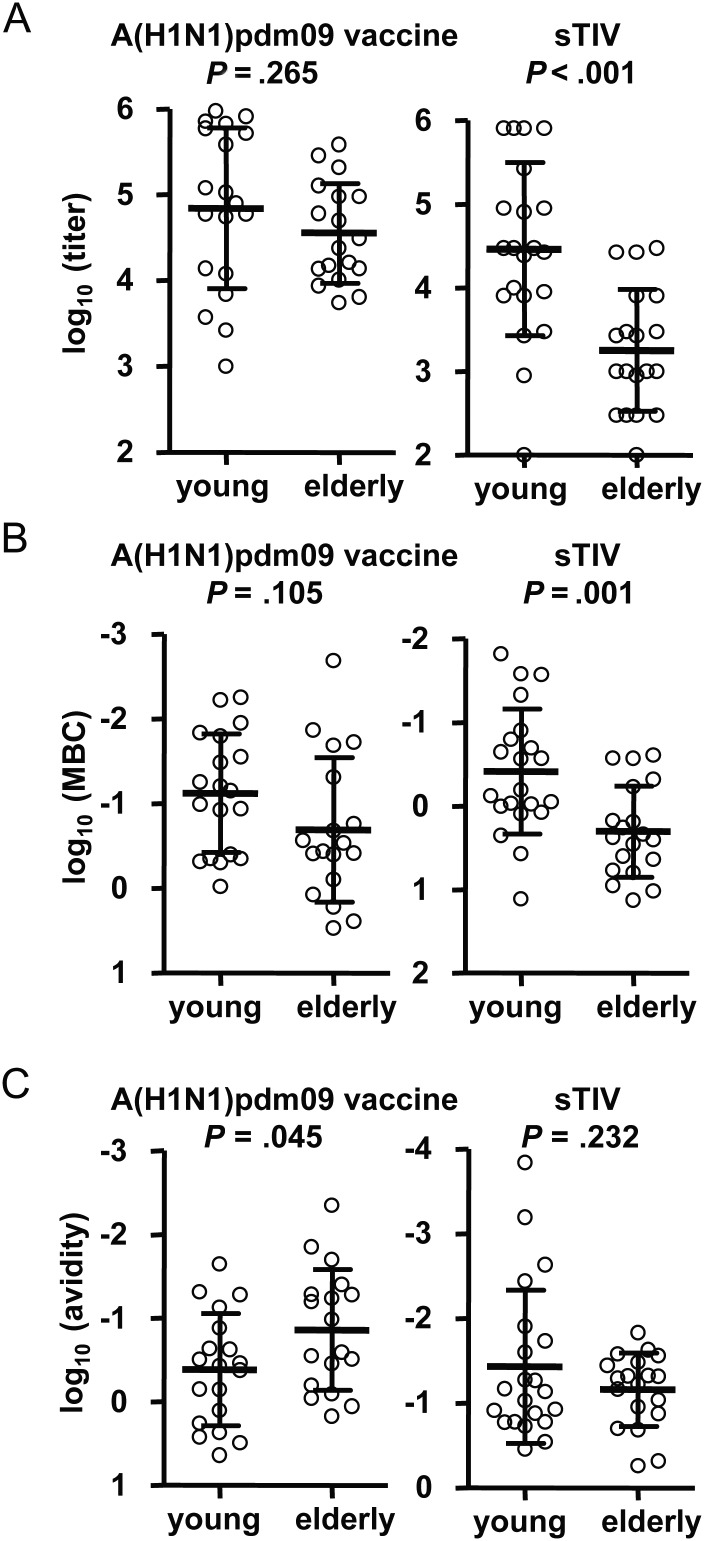
Since the PPAb response to inactivated influenza vaccines is predominantly an IgG response [7, 9], we focused our analysis on the PPAb IgG following immunization of young (age, 18–32 years) and elderly (age, ≥70 years) subjects with either the inactivated A(H1N1)pdm09 monovalent vaccine or the 2009 sTIV containing a seasonal A(H1N1) strain. In the sTIV recipients, as reported previously [9], the seasonal A(H1N1)–specific IgG titer was significantly higher among young individuals; among the A(H1N1)pdm09 recipients, significant differences were not detected between the 2 age groups (Figure 1A).
Figure 1.
Homotypic vaccine-specific plasmablast responses to 2009 pandemic influenza A virus subtype H1N1 (A[H1N1]pdm09) vaccine and 2009 seasonal trivalent influenza vaccine (sTIV; containing seasonal influenza A virus subtype H1N1) in young and elderly recipients. The P values in each panel were determined by an unpaired t test for comparison between the young and elderly groups. A, A(H1N1)pdm09-specific and seasonal A(H1N1)-specific plasmablast-derived polyclonal antibody (PPAb) immunoglobulin G (IgG) binding activity in the A(H1N1)pdm09 vaccine and sTIV recipients, respectively. Enzyme-linked immunosorbent assay titers of individual PPAb samples were normalized to a B-cell density of 3 × 106 B cells/mL in PPAb culture. B, Minimum binding concentrations of each PPAb sample were calculated as the IgG concentration (in ng/mL) divided by titer. C, Homotypic vaccine-specific IgG avidity of PPAb. Avidity is defined as the ratio of specific antibody-secreting cell frequency to the normalized PPAb titer (A) (Supplementary Materials). A smaller value indicates higher avidity.
To normalize the PPAb IgG titer to the antibody concentration, we divided the total IgG concentration in each PPAb sample by its homotypic vaccine-specific titer to obtain the minimum binding concentration (Figure 1B), which indicates the lowest IgG concentration of each PPAb sample at which a vaccine-specific binding is detectable by ELISA. A lower minimum binding concentration indicates a higher reactivity. The titers and minimum binding concentrations both reflect the overall antibody response, which is a function of specific antibody quantity and antibody avidity. In both cases, the results indicate that A(H1N1)pdm09 induced similar homotypic vaccine-specific PPAb IgG responses in the 2 age groups, whereas seasonal A(H1N1) vaccine induced a significantly higher homotypic PPAb response in young recipients, compared with elderly recipients (Figure 1A and 1B).
Homotypic Avidity of PPAb Induced by A(H1N1)pdm09 Vaccine
To compare the quality, or avidity, of A(H1N1)pdm09-specific PPAb between young and elderly subjects, we calculated the ratio of the frequency of homotypic vaccine-specific antibody-secreting cells, as determined by ELISPOT assay (Supplementary Materials) over the A(H1N1)pdm09-specific IgG PPAb titers (Figure 1A). This ratio is a measure of the antigen-specific avidity (Supplementary Materials). A lower ratio indicates a higher avidity. The same ratio was also calculated for sTIV recipients. As shown in Figure 1C, the avidity of A(H1N1)pdm09 vaccine–induced PPAb was significantly higher among the elderly recipients, compared with the young recipients; the difference between the age groups was not significant for sTIV recipients.
Heterovariant Reactivity of PPAb Induced by A(H1N1)pdm09 Vaccine
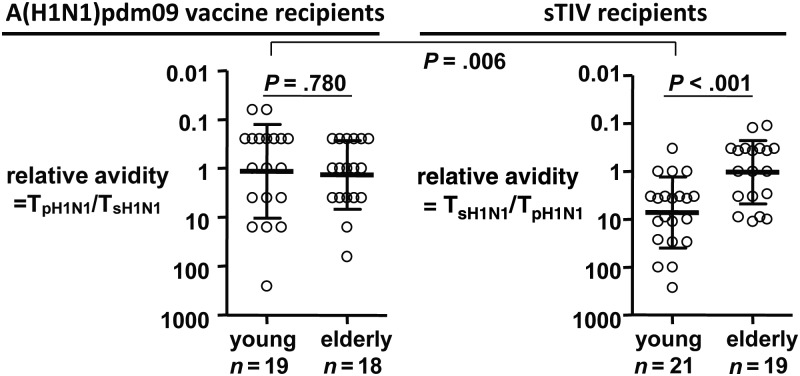
We first determined the heterovariant titer of PPAb from A(H1N1)pdm09 vaccinees, using the seasonal A(H1N1) vaccine as the detecting antigen in ELISA, and then calculated the ratio TA(H1N1)pdm09/TA(H1N1) (shown as TpH1N1/TsH1N1 in Figure 2), where TA(H1N1)pdm09 and TA(H1N1) are the titers against the homotypic A(H1N1)pdm09 antigen and the heterovariant seasonal A(H1N1) antigen, respectively (Figure 2). This ratio is indicative of the heterovariant versus homotypic avidity of A(H1N1)pdm09 vaccine–induced PPAb [9]. A lower value indicates higher PPAb avidity for the heterovariant (ie, seasonal A[H1N1]) strain. The relative avidity of sTIV-induced PPAb against A(H1N1)pdm09 antigen [9] was also shown (Figure 2). sTIV-induced PPAb from elderly recipients had significantly higher heterovariant avidity for A(H1N1)pdm09 antigens than did PPAb from young sTIV recipients; the difference between the age groups was not significant for A(H1N1)pdm09 vaccine recipients. In both young and elderly A(H1N1)pdm09 vaccine recipients, the mean relative avidity was approximately 1, indicating that on average the A(H1N1)pdm09-induced PPAb have similar avidity for A(H1N1)pdm09 and seasonal A(H1N1) antigens. The heterovariant avidity of PPAb from young A(H1N1)pdm09 vaccine recipients was significantly higher than that of PPAb from young sTIV recipients. These results suggest that in young adults the A(H1N1)pdm09 vaccine induced a significant cross-reactive PPAb response to the heterovariant seasonal A(H1N1) strain, which was similar to the level of homotypic reactivity to the A(H1N1)pdm09 strain. In contrast, the seasonal A(H1N1) vaccine did not induce a significant cross-reactive PPAb response to the heterovariant A(H1N1)pdm09 strain.
Figure 2.
Relative heterovariant avidity of PPAb IgG induced by 2009 pandemic inuenza A virus subtype H1N1(A[H1N1]pdm09, or pH1N1) vaccine and 2009 seasonal trivalent inuenza vaccine (sTIV, containing seasonal inuenza A virus subtype H1N1, or sH1N1) vaccine. Relative heterovariant avidity is defined as the ratio of avidity for the heterovariant strain to avidity for the homotypic immunizing strain, which is equal to the ratio of titer, T, against the vaccine strain to the titer against the heterovariant strain. A smaller value indicates higher heterovariant avidity. The P values were determined by an unpaired t test.
A(H1N1)pdm09 Vaccine and sTIV-Induced PPAb Binding Activity to Homotypic and Heterovariant HA
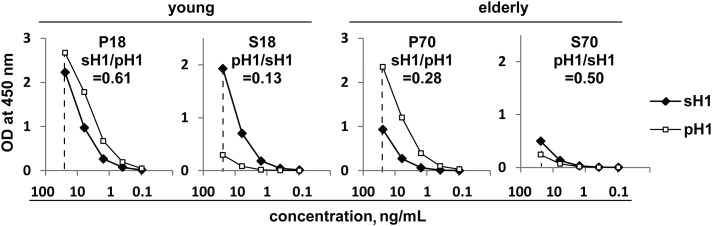
The influenza virus HA is the primary target of neutralizing antibodies following infection or vaccination and is the major component of inactivated influenza vaccines. To characterize the reactivity of vaccine-induced PPAb against the HA of the homotypic vaccine strain versus those of the heterovariant strains, we assembled pools of PPAb by combining equal quantities of IgG from individual PPAb samples for each vaccine and age group. These PPAb pools were designated as P18 (young A[H1N1]pdm09 vaccine recipients), P70 (elderly A[H1N1]pdm09 vaccine recipients), S18 (young sTIV recipients), and S70 (elderly sTIV recipients).
We first measured the binding activity of the PPAb pools to full-length recombinant HA of A(H1N1)pdm09 (pH1) and seasonal A(H1N1) (sH1) by ELISA. Figure 3 shows the titration curves of each PPAb pool and the ratio of the area under the curve (AUC) of the heterovariant HA to that of the homotypic vaccine HA; the latter reflects the relative cross-reactivity of the PPAb pool against the heterovariant HA. Among the 4 PPAb pools, this ratio was greatest for P18 and lowest for S18, supporting our conclusion that the A(H1N1)pdm09 vaccine induced a much greater cross-reactive PPAb response to the heterovariant antigen than the seasonal A(H1N1) vaccine did in the young vaccinees. In the elderly group, the seasonal A(H1N1) vaccine induced greater average cross-reactivity against the heterovariant HA than did the A(H1N1)pdm09 vaccine; however, the overall reactivity to both sH1 and pH1 was lower in the S70 pool (sTIV recipients) than in the P70 pool (A[H1N1]pdm09 vaccine recipients).
Figure 3.
Homotypic and heterovariant binding activity of indicated PPAb pools to recombinant hemagglutinin (HA) protein of seasonal influenza A virus subtype H1N1 (sH1) and 2009 pandemic influenza A virus subtype H1N1 (A[H1N1]pdm09; pH1). A plasmablast-derived polyclonal antibody (PPAb) pool was assembled for each vaccine/age group by combining an equal quantity of immunoglobulin G from individual PPAb samples. All subjects with a sufficient amount of available PPAb samples were included in the pools, without selection on the basis of their response to the vaccination. The pools and the number of subjects included in each pool were P18, young A(H1N1)pdm09 vaccine recipients, n = 15; P70, elderly A(H1N1)pdm09 vaccine recipients, n = 16; S18, young 2009 seasonal trivalent influenza vaccine (sTIV) recipients, n = 20; S70, elderly sTIV recipients, n = 19. The number in each panel indicates the ratio of the area under the curve (AUC) for heterovariant HA to AUC for homotypic vaccine HA. Abbreviation: OD, optical density.
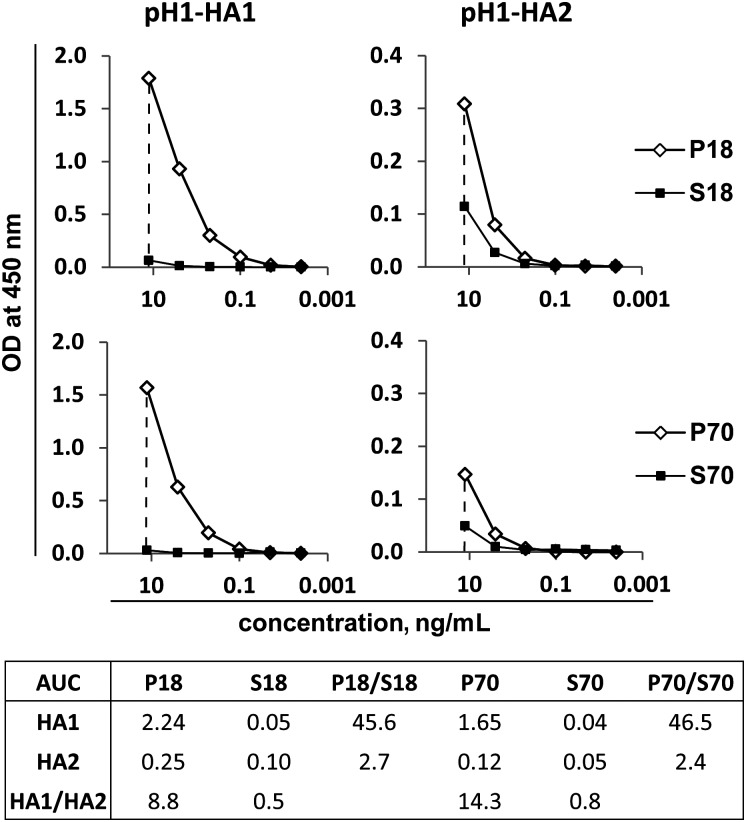
HA Domain–Specific Binding Activity of A(H1N1)pdm09 Vaccine– and sTIV-Induced PPAb
To map the PPAb binding specificity to different domains of HA, we examined binding of the PPAb pools to recombinant head (HA1) and stalk (HA2) domains of A(H1N1)pdm09. HA2 is conserved across group 1 influenza A virus strains, including A/H1N1 strains (seasonal and pandemic) and subtype A/H5N1 strains [17]. The binding titration curves of each PPAb pool for HA1 and for HA2, as well as the ratios of AUCs for the A(H1N1)pdm09 vaccine versus sTIV recipients and the HA1 versus HA2 domains are shown in Figure 4. Strong binding to both HA1 and HA2 was detected in young and elderly A(H1N1)pdm09 vaccine recipients, with the reactivity against HA1 dominant. Cross-reactive binding of the pools from young and elderly sTIV recipients was only detected at the highest IgG concentration tested and was greater for HA2 than for HA1. The difference in the HA1 AUCs between the 2 vaccine groups was >45-fold, whereas the difference in the HA2 AUCs was <3-fold. Together, these results suggest that sTIV induced greater cross-reactivity to pH1-HA2 than to pH1-HA1, although both reactivities were at low levels.
Figure 4.
Binding activity of plasmablast-derived polyclonal antibody (PPAb) pools to recombinant HA1 and HA2 domains of the hemagglutinin (HA) protein of 2009 pandemic influenza A virus subtype H1N1 (pH1). The table lists the areas under the curve (AUCs) for each titration curve and the ratios of AUCs between different PPAb pools (P18/S18 and P70/S70) and between different domains (HA1/HA2) for each pool.
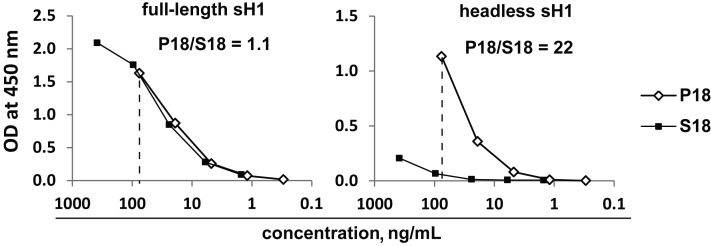
We next examined the binding of P18 and S18 pools to a full-length and a headless recombinant HA of seasonal A(H1N1) (Figure 5). The AUCs for the full-length sH1 were almost identical, whereas the AUC of P18 to the headless sH1 was 22-fold greater than that of S18, indicating that PPAb from young A(H1N1)pdm09 vaccine recipients but not those from young sTIV recipients had substantial binding activity to the conserved stalk domain.
Figure 5.
Binding activity of plasmablast-derived polyclonal antibody pools P18 and S18 to the full-length and headless HA proteins of seasonal influenza A virus subtype H1N1 (sH1). The ratios of areas under the curve (to the right of the dotted lines) are shown in each graph.
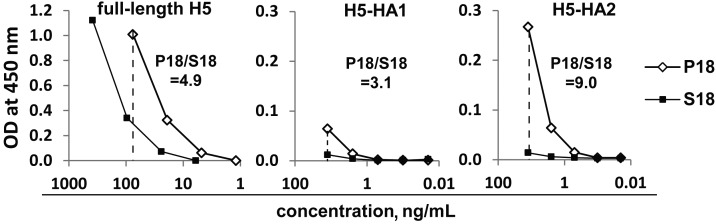
Finally we examined the binding of P18 and S18 pools to the full-length HA of an avian A(H5N1) strain (Figure 6). The AUC of P18 was 4.9-fold greater than that of S18, suggesting that PPAb from the young A(H1N1)pdm09 vaccine recipients but not those from young sTIV recipients recognized the conserved stalk domain of the avian HA (H5). This was confirmed by binding of P18 and S18 to the recombinant H5-HA1 and H5-HA2 domains (Figure 6), which shows substantial binding of P18 to HA2 but not to HA1. Binding of the S18 pool to these H5 domains was only barely detectable at the highest concentration tested.
Figure 6.
Binding activity of plasmablast-derived polyclonal antibody pools P18 and S18 to the full-length hemagglutinin (HA) protein of an avian influenza A virus subtype H5N1 strain (H5) and to the HA1 and HA2 domains of H5.
Taken together, these results (Figures 3–6) indicate that the A(H1N1)pdm09 vaccine induced a substantial PPAb response to the conserved stalk (HA2) domain of HA in addition to the variable head (HA1) domain. In contrast, the seasonal A(H1N1) component of sTIV induced minimal levels of PPAb activity against the stalk domain.
HA Domain–Specific Avidity of A(H1N1)pdm09-Induced PPAb
Vaccine-specific avidity of A(H1N1)pdm09-induced PPAb was greater in elderly recipients, compared with young recipients (Figure 1C). We investigated this finding further by comparing avidity for the recombinant HA of A(H1N1)pdm09 and its HA1 and HA2 domains between the 2 age groups, using an ELISA with and an ELISA without 7 M urea treatment to determine the fraction of high-avidity antibodies in the total antigen-specific binding activity. The avidity of the P70 pool for the full-length HA was higher than that of the P18 pool (Figure 7), in agreement with the avidity for whole A(H1N1)pdm09 vaccine (Figure 1C). Although both PPAb pools had higher avidity for HA2 than for HA1, the difference between the 2 age groups was greater for HA1 (26% vs 57%) than for HA2 (77% vs 83%). These findings suggest that the age difference in A(H1N1)pdm09-induced PPAb avidity is primarily caused by the different avidities to the head domain of HA.
Figure 7.
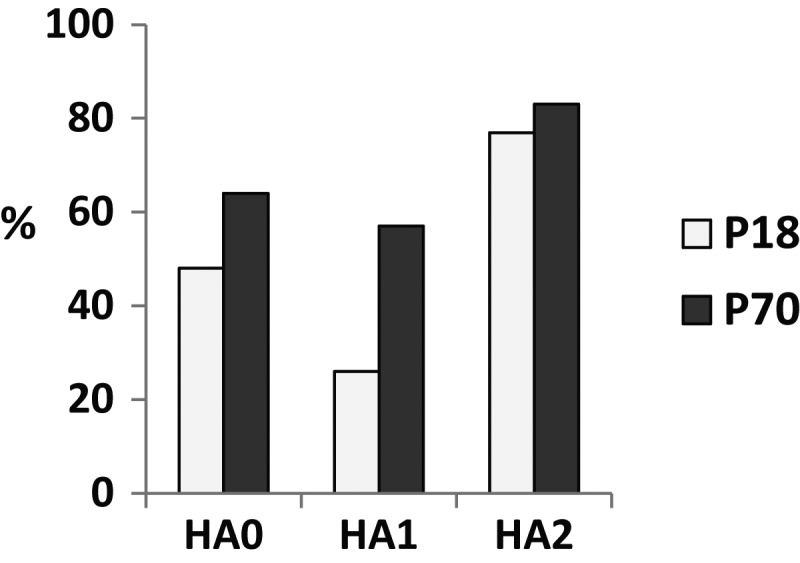
Domain-specific avidity of plasmablast-derived polyclonal antibody pools P18 and P70 for the full-length hemagglutinin (HA; HA0) or HA1 and HA2 domains. The avidity was assessed by enzyme-linked immunosorbent assay, with or without 7 M urea. The bar graph shows the percentage of 7 M urea-resistant high-avidity binding activity.
DISCUSSION
In this study, we showed that the inactivated A(H1N1)pdm09 vaccine induced a similar PPAb response in young and elderly recipients (Figure 1A and 1B). This is in marked contrast to the 2009 sTIV, which induced a significantly higher PPAb response in the young based on binding to sTIV [9], or to the monovalent seasonal A(H1N1) vaccine antigen (Figure 1A and 1B). In the sTIV recipients, the frequency of vaccine-specific antibody-secreting cells was significantly lower in the elderly subjects, compared with the young subjects [9]. A similar difference between the age groups was found in the recipients of A(H1N1)pdm09 vaccine (Supplementary Materials). After immunization with sTIV, the homotypic PPAb avidity was similar in young and elderly recipients ([9] and Figure 1C). In contrast, after immunization with A(H1N1)pdm09 vaccine, the homotypic PPAb avidity was significantly higher in elderly individuals, compared with young individuals (Figure 1C). In other words, the higher avidity of PPAb in the elderly A(H1N1)pdm09 vaccine recipients compensated for the lower frequency of vaccine-specific antibody-secreting cells, resulting in comparable overall PPAb binding activities, or titers, in the 2 age groups. An ELISA that used recombinant HA domains revealed that the difference in avidity for HA between the 2 age groups was primarily due to differences in avidity for the head domain (Figure 7), which is the dominant immunogenic domain of HA and is highly variable between heterovariant influenza virus strains. This result is in agreement with the recent findings of Khurana et al, who demonstrated that after immunization with A(H1N1)pdm09 vaccine the serum antibody avidity for HA1 was significantly higher in older individuals (age, ≥65 years), compared with younger individuals (age < 65 years) [14].
The antibody response after influenza vaccination in virtually all adults is largely an antigen recall response of memory B cells [6]. Generation of high-affinity antibodies against the vaccine antigen depends on the process of affinity maturation, during which somatic hypermutation diversifies the immunoglobulin variable regions of proliferating B cells. This process is mediated by activation-induced cytidine deaminase [18]. The activity of activation-induced cytidine deaminase declines with age [19, 20] and is associated with a reduced serum antibody response to seasonal influenza vaccination in elderly individuals [21]. Despite the potential negative effect of lower activation-induced cytidine deaminase activity on the affinity and avidity of the antibody response, the finding of high-avidity PPAb in the elderly A(H1N1)pdm09 vaccine recipients suggests a role for certain memory B cells present in elderly but not younger adults in the response to this vaccine. Presumably, such B cells were primed by A(H1N1) strains circulating before 1950 that were similar to the A(H1N1)pdm09 strain. This assumption is supported by the more frequent detection of A(H1N1)pdm09-specific antibodies in the prevaccination sera from elderly adults, compared with younger adults [3]. Taken together, these findings suggest that influenza exposure, including natural infection and vaccination, in the distant past can enhance the efficacy of influenza vaccination decades later, even though the overall function of immune cells have declined with age.
A unique challenge in influenza vaccine development is the fact that the population is repeatedly exposed to influenza viruses with new mutations that allow escape from preexisting protective immunity. The primary site of these escape mutations is in the highly variable head domain of the HA that carries the receptor-binding site of the virus, whereas the stalk domain that is involved in virus-host membrane fusion is conserved among group 1 influenza A virus strains, including subtypes H1N1, H2N2, and H5N1 [17]. The current approach for coping with newly emerged influenza virus strains is vaccination with antigenically matched new viral antigens. It is believed that such vaccines induce antibodies that primarily target the variable head domain of the new strain to block its receptor binding, with little or no cross-reactivity to the HA of heterovariant strains. In agreement with this notion, vaccination with recent seasonal influenza vaccines prior to 2010 induced little or no cross-reactive serum antibody response to the novel A(H1N1)pdm09 strain [3]. In fact, vaccination with sTIV has even been associated with increased risk of medically attended A(H1N1)pdm09-associated illness in some epidemiologic studies [22]. In contrast, antibodies specific for the conserved stalk domain had broad cross-reactive neutralizing activity against different group 1 influenza A virus strains and have been proposed as the basis for a universal influenza vaccine [13, 23]. Such antibodies are only detected at very low levels after affinity purification from serum samples of healthy volunteers [24], despite the highly conserved nature of the stalk domain. They have been detected, however, in convalescent-phase sera [25] and in plasmablast-derived recombinant monoclonal antibodies [8, 10] from patients with acute influenza virus infection. Of special interest, it was recently reported that recombinant mAbs targeting the HA stalk domain were isolated frequently from 2 out of the 3 tested recipients of an adjuvanted A(H1N1)pdm09 vaccine [10], or infrequently from recipients of unadjuvanted A(H1N1)pdm09 vaccine [11].
In this study, we observed that immunization with unadjuvanted inactivated A(H1N1)pdm09 vaccine resulted in plasmablasts that secreted IgG with similar activity to A(H1N1)pdm09 and seasonal A(H1N1) in both young and elderly recipients (Figure 2). In contrast, such cross-reactive heterovariant reactivity was not seen in young recipients of sTIV ([9] and Figure 2). Of note, PPAb from elderly sTIV recipients also had similar activity for A(H1N1)pdm09 and seasonal A(H1N1), although at significantly lower levels than the homotypic binding activity of PPAb from the young adults [9]. By using recombinant HA proteins to test for domain-specific binding, we found that in addition to the head-specific reactivity, the A(H1N1)pdm09 vaccine induced stalk-specific antibodies more efficiently than the seasonal A(H1N1) vaccine. This raises the question of why the highly conserved HA2 peptide is more immunogenic in the context of pH1 than in sH1. We postulate that in truly immune-naive individuals, such as very young children, both HA1 and HA2 are immunogenic, with HA1 being dominant. Thus primary exposure to influenza virus results in memory B cells specific for both HA1 and HA2, with the former in the majority. Subsequent exposure to new seasonal influenza virus strains with relatively small changes in HA1 sequence (antigenic drift) will activate both HA1- and HA2-specific memory B cells. The diversity of HA1-specific B cells will be increased through somatic hypermutation, resulting in a further expanded memory B-cell pool that is primed by the variant HA1 epitopes; there will be fewer HA2-specific memory B cells. Repeated exposure to seasonal influenza virus strains, altered through antigenic drift, will enhance the immunodominance of HA1 over time, to the extent that eventually the antibody response to a new seasonal influenza virus vaccine will be almost entirely targeted at the head domain. Only when the immune system is challenged with an antigenically shifted strain, such as A(H1N1)pdm09, to which the younger population has not been exposed, will the B-cell response consist of a primary response of naive B cells to the new HA1 and a memory response to HA2. In this case, the immunodominance of HA1 will no longer play the major role, and antibodies specific for both HA1 and HA2 will be generated. This hypothetical scenario is in agreement with the following observations: (1) HA2-specific memory B cells were detected from peripheral blood, albeit at very low frequency, in those vaccinated with sTIV [26]; (2) broadly cross-reactive monoclonal antibodies from individuals infected or vaccinated with A(H1N1)pdm09 had high frequency of immunoglobulin-variable gene mutations, whereas highly A(H1N1)pdm09-specific monoclonal antibodies had the lowest number of somatic mutations [8, 11]; (3) after A(H1N1)pdm09 vaccination, the PPAb avidity for HA2 was higher than that for HA1 (Figure 7), suggesting that HA2-specific memory B cells have undergone more extensive affinity maturation during repeated past exposure; (4) sequential immunization of mice with influenza virus HA that carries highly diverse HA1 peptides resulted in antibody response to the conserved HA2 [27, 28]; and (5) from a recipient of A(H1N1)pdm09 vaccine who was >60 years old and hence was likely to have been exposed to an A(H1N1)pdm09-related strain in the distant past, recombinant monoclonal antibodies dominantly targeted the HA head domain without any stalk-specific ones [10].
Our results suggest that prior exposure to influenza virus infection and/or vaccination has profound effects on the vaccine-specific and heterovariant reactivity of PPAb. Studies should be performed to determine whether the observed heterovariant PPAb reactivity induced by the A(H1N1)pdm09 vaccine is associated with broad protective efficacy.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank our study subjects, for their participation; P. Dormitzer, for providing critical reagents; C. Zhang, for technical assistance; W. Wang, A. Hussain, R. Vepachedu, and A. Suguitan Jr, for producing the headless HA; S. Mackey, for coordinating the clinical study; S. Swope, S. Cathey, C. Walsh, S. French, and M. Ugur (Stanford University School of Medicine) and the Vaccine Research Unit in the University of Rochester, for enrolling subjects, administering vaccine, and collecting samples and clinical data; T. Quan, K. Spann, S. Batra, and B. Tse, for scheduling subjects and providing clinical data management; and VA Palo Alto Health Care System, for supporting this study.
Financial support. This work was supported by the National Institutes of Health (AI090019, AI057229, and UL1 RR025744), the New York Influenza Center of Excellence (HHSN266200700008C), the National Center for Immunization and Respiratory Diseases (5U18IP000172-03), the intramural research program of the National Institute of Allergy and Infectious Diseases, the Department of Health and Human Services (HHSN272200900026C), and the Medical Countermeasures Initiative and the Pandemic Influenza Funding of the Food and Drug Administration.
Potential conflicts of interest. H. J. is an employee of MedImmune, the producer of the live attenuated influenza vaccine. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Brockwell-Staats C, Webster RG, Webby RJ. Diversity of influenza viruses in swine and the emergence of a novel human pandemic influenza A (H1N1) Influenza Other Respi Viruses. 2009;3:207–13. doi: 10.1111/j.1750-2659.2009.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu R, Ekiert DC, Krause JC, Hai R, Crowe JE, Jr, Wilson IA. Structural basis of preexisting immunity to the 2009 H1N1 pandemic influenza virus. Science. 2010;328:357–60. doi: 10.1126/science.1186430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hancock K, Veguilla V, Lu X, et al. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N Engl J Med. 2009;361:1945–52. doi: 10.1056/NEJMoa0906453. [DOI] [PubMed] [Google Scholar]
- 4.Dawood FS, Jain S, Finelli L, et al. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;360:2605–15. doi: 10.1056/NEJMoa0903810. [DOI] [PubMed] [Google Scholar]
- 5.Gerhard W. The role of the antibody response in influenza virus infection. Curr Top Microbiol Immunol. 2001;260:171–90. doi: 10.1007/978-3-662-05783-4_9. [DOI] [PubMed] [Google Scholar]
- 6.Wrammert J, Smith K, Miller J, et al. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature. 2008;453:667–71. doi: 10.1038/nature06890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He XS, Sasaki S, Narvaez CF, et al. Plasmablast-derived polyclonal antibody response after influenza vaccination. J Immunol Methods. 2011;365:67–75. doi: 10.1016/j.jim.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wrammert J, Koutsonanos D, Li GM, et al. Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. J Exp Med. 2011;208:181–93. doi: 10.1084/jem.20101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sasaki S, Sullivan M, Narvaez CF, et al. Limited efficacy of inactivated influenza vaccine in elderly individuals is associated with decreased production of vaccine-specific antibodies. J Clin Invest. 2011;121:3109–19. doi: 10.1172/JCI57834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomson CA, Wang Y, Jackson LM, et al. Pandemic H1N1 influenza infection and vaccination in humans induces cross-protective antibodies that target the hemagglutinin stem. Front Immun. 2012;3 doi: 10.3389/fimmu.2012.00087. 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li GM, Chiu C, Wrammert J, et al. Pandemic H1N1 influenza vaccine induces a recall response in humans that favors broadly cross-reactive memory B cells. Proc Natl Acad Sci U S A. 2012;109:9047–52. doi: 10.1073/pnas.1118979109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stevens J, Blixt O, Tumpey TM, Taubenberger JK, Paulson JC, Wilson IA. Structure and receptor specificity of the hemagglutinin from an H5N1 influenza virus. Science. 2006;312:404–10. doi: 10.1126/science.1124513. [DOI] [PubMed] [Google Scholar]
- 13.Steel J, Lowen AC, Wang TT, et al. Influenza virus vaccine based on the conserved hemagglutinin stalk domain. MBio. 2010;1:e00018–10. doi: 10.1128/mBio.00018-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khurana S, Verma N, Talaat KR, Karron RA, Golding H. Immune response following H1N1pdm09 vaccination: differences in antibody repertoire and avidity in young adults and elderly populations stratified by age and gender. J Infect Dis. 2012;205:610–20. doi: 10.1093/infdis/jir791. [DOI] [PubMed] [Google Scholar]
- 15.Khurana S, Verma S, Verma N, et al. Properly folded bacterially expressed H1N1 hemagglutinin globular head and ectodomain vaccines protect ferrets against H1N1 pandemic influenza virus. PLoS One. 2010;5:e11548. doi: 10.1371/journal.pone.0011548. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Khurana S, Verma N, Yewdell JW, et al. MF59 adjuvant enhances diversity and affinity of antibody-mediated immune response to pandemic influenza vaccines. Sci Transl Med. 2011;3:85ra48. doi: 10.1126/scitranslmed.3002336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sui J, Hwang WC, Perez S, et al. Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat Struct Mol Biol. 2009;16:265–73. doi: 10.1038/nsmb.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nussenzweig MC, Alt FW. Antibody diversity: one enzyme to rule them all. Nat Med. 2004;10:1304–5. doi: 10.1038/nm1204-1304. [DOI] [PubMed] [Google Scholar]
- 19.Frasca D, Landin AM, Lechner SC, et al. Aging down-regulates the transcription factor E2A, activation-induced cytidine deaminase, and Ig class switch in human B cells. J Immunol. 2008;180:5283–90. doi: 10.4049/jimmunol.180.8.5283. [DOI] [PubMed] [Google Scholar]
- 20.Frasca D, Landin AM, Riley RL, Blomberg BB. Mechanisms for decreased function of B cells in aged mice and humans. J Immunol. 2008;180:2741–6. doi: 10.4049/jimmunol.180.5.2741. [DOI] [PubMed] [Google Scholar]
- 21.Frasca D, Diaz A, Romero M, et al. Intrinsic defects in B cell response to seasonal influenza vaccination in elderly humans. Vaccine. 2010;28:8077–84. doi: 10.1016/j.vaccine.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skowronski DM, De Serres G, Crowcroft NS, et al. Association between the 2008–09 seasonal influenza vaccine and pandemic H1N1 illness during Spring-Summer 2009: four observational studies from Canada. PLoS Med. 2010;7:e1000258. doi: 10.1371/journal.pmed.1000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang TT, Tan GS, Hai R, et al. Vaccination with a synthetic peptide from the influenza virus hemagglutinin provides protection against distinct viral subtypes. Proc Natl Acad Sci U S A. 2010;107:18979–84. doi: 10.1073/pnas.1013387107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sui J, Sheehan J, Hwang WC, et al. Wide prevalence of heterosubtypic broadly neutralizing human anti-influenza A antibodies. Clin Infect Dis. 2011;52:1003–9. doi: 10.1093/cid/cir121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stanekova Z, Mucha V, Sladkova T, Blaskovicova H, Kostolansky F, Vareckova E. Epitope specificity of anti-HA2 antibodies induced in humans during influenza infection. Influenza Other Respi Viruses. 2012;6:389–95. doi: 10.1111/j.1750-2659.2011.00328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corti D, Suguitan AL, Jr, Pinna D, et al. Heterosubtypic neutralizing antibodies are produced by individuals immunized with a seasonal influenza vaccine. J Clin Invest. 2010;120:1663–73. doi: 10.1172/JCI41902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang TT, Tan GS, Hai R, et al. Broadly protective monoclonal antibodies against H3 influenza viruses following sequential immunization with different hemagglutinins. PLoS Pathog. 2010;6:e1000796. doi: 10.1371/journal.ppat.1000796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei CJ, Boyington JC, McTamney PM, et al. Induction of broadly neutralizing H1N1 influenza antibodies by vaccination. Science. 2010;329:1060–4. doi: 10.1126/science.1192517. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.