Abstract
Background. A dramatic increase in morbidity and mortality from Clostridium difficile infection (CDI) due to the recent emergence of virulent, antibiotic-resistant strains has led to a search for alternatives to antibiotics, including vaccines and immune-based therapy that target the 2 key toxins—TcdA and TcdB.
Methods. We investigated the efficacy of specific human monoclonal antibodies (HuMab) and alpaca polyclonal antibodies against each toxin separately and in combination in the gnotobiotic piglet model of CDI. Additionally, the HuMab and polyclonal antibodies were exploited to investigate the precise contribution of each toxin to systemic and/or gastrointestinal (GI) tract disease.
Results. Our results indicate that TcdB is an important virulence factor associated with GI and systemic pathology. Administration of anti-TcdB antibody alone or with anti-TcdA protected 100% of piglets from development of systemic CDI and minimized GI lesions. Conversely, 100% of piglets administered only anti-TcdA developed severe GI and systemic disease, with 67%–83% fatality, faring worse than placebo-treated control animals.
Conclusions. These results highlight the importance of TcdB in the pathogenesis of CDI and the effectiveness of TcdB-specific antibody in treating CDI. However, the results raise new questions regarding the nature of TcdA interaction with therapeutic antibodies.
Keywords: Clostridium difficile, anti-toxin antibodies, TcdA, TcdB, systemic disease
Clostridium difficile, an anaerobic, spore-forming, gram-positive bacillus, is the leading cause of antibiotic-associated diarrhea in developing countries. A hypervirulent, epidemic strain, designated NAP1/027/BI, has caused an increase in C. difficile infection (CDI) case and fatality rates since the early 2000s [1, 2]. The symptoms of CDI, ranging from mild diarrhea to severe disease including pseudomembranous colitis, toxic megacolon, sepsis, shock, and even death, are thought to be caused mainly by 2 toxins—toxin A (TcdA) and toxin B (TcdB). Understanding the relative roles of these 2 toxins in disease pathogenesis is of great importance. Although the toxins have been investigated using numerous methods over the past several decades, the results have been conflicting.
In earlier studies using culture supernatants or purified toxins in rodent or rabbit models, TcdA and/or TcdB were administered into the gastrointestinal (GI) tract directly via gavage or intestinal loop. Results indicated that TcdA was the essential virulence factor, with TcdB unable to induce lesions when administered in the absence of TcdA [3, 4]. When highly purified toxins are administered systemically to rodents, either together or separately, both are able to induce disease and death [5]. The finding of cardiotoxicity associated with TcdB in the zebrafish model more recently highlighted the potential for systemic actions of the toxins and the importance of TcdB, in particular [6], although this has yet to apply to mammalian species.
Active and passive immunity have also been used to investigate the roles of the 2 toxins. Using toxoid for immunization in hamsters, 1 study found that immunization with both TcdA and TcdB toxoids was necessary for complete protection [7], but another study found that immunization with only TcdA toxoid was necessary for protection [8]. Investigation of naturally occurring serum antitoxin antibodies in human patients placed an emphasis on the importance of anti-TcdA in preventing recurrence of CDI [9]. Human monoclonal antibodies (HuMab) have been generated against both toxins, and studies in hamsters revealed that HuMab against both toxins prevented CDI in hamsters [10]. Likewise, studies in human patients also highlighted the importance of antibodies against both toxins, not just TcdA, for protection from recurrence [11].
In the past several years, methods for genetic manipulation of C. difficile have allowed for creation of isogenic mutant strains that produce only TcdA or TcdB. One such study showed TcdB to be the essential virulence factor in the hamster model [12], but another study found that both TcdA and TcdB are important for virulence [13]. The results of these numerous studies indicate that neither toxin can be ignored in terms of the importance in disease pathogenesis or for development of novel treatments and therapeutics. However, we are still left with many unanswered questions. In this study we used HuMabs [10] and alpaca polyclonal antibodies against TcdA and/or TcdB in the piglet model of CDI [14] to further investigate the roles of the 2 toxins in pathogenesis and to provide more information on the use of antitoxin antibodies for prevention and treatment of CDI in human patients.
METHODS
Polyclonal Antitoxin Antibody Preparation
Polyclonal antibodies against TcdA and TcdB were generated by immunizing alpacas with recombinant, atoxic TcdA or TcdB (aTcdA or aTcdB), based on the recombinant toxins produced by our laboratory [15]. One animal was immunized with aTcdA and 1 animal was immunized with aTcdB, generating antisera against each toxin separately. Anti-TcdA and anti-TcdB immunoglobulin (IgG) titers were determined using enzyme-linked immunosorbent assay (ELISA) for serum collected from the alpacas. The samples were tested for antibody cross-reactivity to be sure that the separate sera did not have neutralizing ability against the opposite toxin; cross-reactivity was not found to be present for either serum pool. Piglets were dosed with the polyclonal sera based on the neutralizing titer, adjusted to a level that would neutralize previously observed serum toxin concentrations. Alpaca preimmune serum was used as a placebo control.
Monoclonal Antitoxin Antibody Preparation
The human monoclonal anti-TcdA (CDA1) and anti-TcdB (CDB1) antibodies used in this study were developed by Massachusetts Biologic Laboratories and Medarex, Inc. [10] and provided for this study and currently licensed by Merck, Inc. These antibodies have already been used in the hamster model [10] and in clinical trials in humans [16, 17]. Both CDA1 and CDB1 are IgG1κ antibodies and bind the receptor-binding domain of TcdA and TcdB, respectively [10]. CDA1 and CDB1 were administered to piglets at a dose of 10 mg/kg, based on the dosing in human studies [16, 17]. As a control, we used the irrelevant human monoclonal anti-shiga toxin 2 (anti-Stx2), developed by our institution [18], at a dose of 10 mg/kg.
Animals and Inoculation
A total of 23 gnotobiotic piglets were used for the polyclonal antibody experiments and 21 were used for the monoclonal antibody experiments. Piglets were derived via Cesarean section and maintained in sterile isolators for the duration of the experiment, as previously described, following approved institutional animal care and use committee guidelines [14]. The piglets for polyclonal antibody experiments were divided into groups as follows: 6 piglets received anti-TcdA antibodies only, 6 piglets received anti-TcdB antibodies only, 6 piglets received anti-TcdA and anti-TcdB antibodies, and 5 piglets received alpaca preimmune serum to serve as controls. The piglets for the monoclonal antibody experiments were divided into groups as follows: 6 piglets received only CDA1, 5 piglets received only CDB1, 6 piglets received CDA1 and CDB1, and 4 piglets received anti-Stx2 to serve as controls. All antitoxin antibodies were administered via intraperitoneal injection when the piglets were aged 4–5 days. The piglets were then orally inoculated with 107 spores of an NAP1/027/BI C. difficile strain, designated strain UK6 [19], approximately 24 hours after administration of the antibodies. Blood was collected every 1–2 days after administration of the antibodies and at the time of euthanasia for evaluation of serum toxin and antibody titers. Feces were collected daily from each piglet for culture, antibody titers, and toxin assays. A necropsy was performed on all animals, and tissues were collected for histopathologic examination. Pleural and abdominal effusions were also collected at the time of necropsy, if present.
Immunocytotoxicity Assay and Cytokine Measurement
We used the ultrasensitive immunocytotoxicity assay developed by our laboratory [20] to measure toxin in serum, body fluids, and fecal samples. Cytokine concentration was determined for interleukin (IL)-1β, IL-4, IL-6, IL-8, IL-10, IL-12, tumor necrosis factor-(TNF)-α, transforming growth factor (TGF)-β, and IFN-γ using porcine cytokine quantification kits (Invitrogen and R&D). All piglet serum samples and large intestinal contents were analyzed. Samples were stored at −20°C until use, and the assays were performed following the manufacturer's directions.
RESULTS
Anti-TcdB Antibodies
In agreement with the hypothesis that TcdB plays an important role in systemic manifestations of CDI, we found that piglets treated with anti-TcdB antibodies, whether alone or in addition to anti-TcdA, were completely protected from the development of systemic signs of CDI (Table 1). None of the 23 piglets in these groups, receiving either the polyclonal or monoclonal antibodies, developed any clinical signs of systemic illness, and they developed only mild GI signs. Administration of both antibodies together did not seem to have any effect, either positive or negative, on the course of clinical disease as compared with administration of only anti-TcdB antibodies (Table 1). Gross GI lesions observed during postmortem examination in polyclonal and monoclonal anti-TcdB only or anti-TcdA + anti-TcdB were mild (Figures 1A and 1D). Figures 2B and 2D show a comparison of segments of the descending colon of piglets in groups treated with only monoclonal anti-TcdB or anti-TcdA + anti-TcdB as compared with other groups. Histopathologic lesions of the large intestine included mild to moderate submucosal edema and minimal neutrophilic infiltration (Figures 3A and 3D). Histopathologic lesions in the large intestine were slightly more severe in piglets treated with monoclonal anti-TcdA + anti-TcdB than in those treated with only monoclonal anti-TcdB. However, this effect was mild and not noted for the polyclonal antibody groups. There were no systemic lesions noted at necropsy or histopathology, and the lungs were normally aerated (Figure 3G).
Table 1.
Clinical Outcome in Anti-TcdA and Anti-TcdB Treated Piglets
| Treatment (number of animals) | Gastrointestinal Disease,a | Systemic Disease,b % | Fatal Diseasec % | TcdA/TcdB in Body Fluids, % | TcdA/TcdB in Feces, % |
|---|---|---|---|---|---|
| Anti-TcdA only | |||||
| Polyclonal (6) | mod-sev | 100 | 83 | 0/50 | 100/100 |
| HuMab (6) | mod-sev | 100 | 67 | 67/67 | 100/100 |
| Anti-TcdB only | |||||
| Polyclonal (6) | mild | 0 | 0 | 0/0 | 100/100 |
| HuMab (5) | mild | 0 | 0* | 0/0 | 100/100 |
| Anti-TcdA and TcdB | |||||
| Polyclonal (6) | mild | 0 | 0 | 0/0 | 100/100 |
| HuMab (6) | mild-mod | 0 | 0* | 0/0 | 100/100 |
| Control | |||||
| Polyclonal (5) | mod-sev | 60 | 20 | 20/20 | 100/100 |
| HuMab (4) | mod-sev | 75 | 50 | 75/75 | 100/100 |
Body fluids included serum, pleural, and/or abdominal effusions collected at the time of necropsy, and percentage reflects the number of piglets in the group with a positive body fluid sample. Serum was evaluated for all piglets. Feces were collected from piglets daily, and percentage reflects the total of samples from all animals in the group beginning at the onset of diarrhea.
Abbreviation: HuMab, human monoclonal antibody.
a Severity of gastrointestinal disease was determined by clinical signs and gross and histopathologic lesions, ranging from mild to severe.
b Systemic disease indicates that piglets developed severe, systemic signs such as lethargy, weakness, anorexia, or dyspnea.
c Fatal disease indicates that piglets died or were euthanized due to the severity of disease.
*P < .05, determined between indicated group and control group using Fisher exact test.
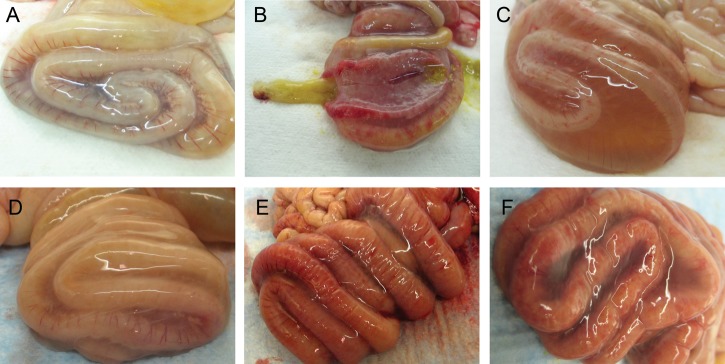
Figure 1.
Necropsy images of the spiral colon from antibody-treated piglets inoculated with Clostridium difficile. A, Mild dilatation with no hyperemia and minimal mesocolonic edema are present in a piglet treated with polyclonal anti-TcdB only. B, Dilatation, hyperemia, and focal mucosal ulceration and hemorrhages are present in a piglet treated with polyclonal anti-TcdA only. The segment of spiral colon in the center has been opened longitudinally to demonstrate the severe thickening of the intestinal wall and the nature of the pseudomembranes found covering the mucosa. C, Severe mesocolonic edema in a control piglet treated with alpaca preimmune sera. D, Mild dilatation and mesocolonic edema in a piglet treated with monoclonal anti-TcdA and anti-TcdB. E, Severe dilatation, moderate mesocolonic edema, hyperemia, and mucosal hemorrhages and ulceration in a piglet treated with monoclonal anti-TcdA only. F, Severe dilatation, moderate mesocolonic edema, and hyperemia in a control piglet treated with monoclonal anti-Stx2.
Figure 2.
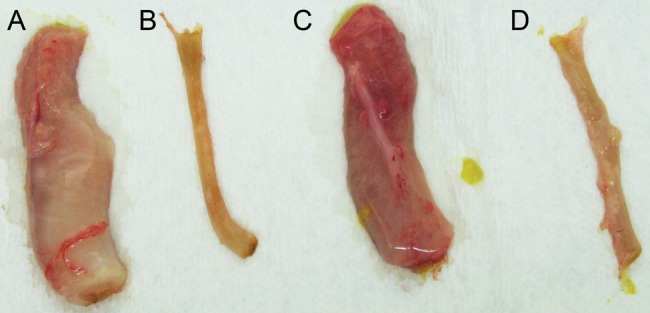
Comparison of the descending colon of monoclonal antibody-treated piglets. Similar sections of approximately 3 cm in length were collected from the distal descending colon at the time of necropsy. A, Section from a control piglet demonstrating severe dilatation, thickening of the intestinal wall, and mesocolonic edema. B, Section from a piglet treated with anti-TcdA and anti-TcdB that is not dilated and has no thickening of the intestinal wall. C, Section from a piglet treated with only anti-TcdA, demonstrating severe dilatation, intestinal thickening, hyperemia, hemorrhages, and mesocolonic edema. D, Section from a piglet treated with anti-TcdB only, with no dilatation, thickening, or hyperemia.
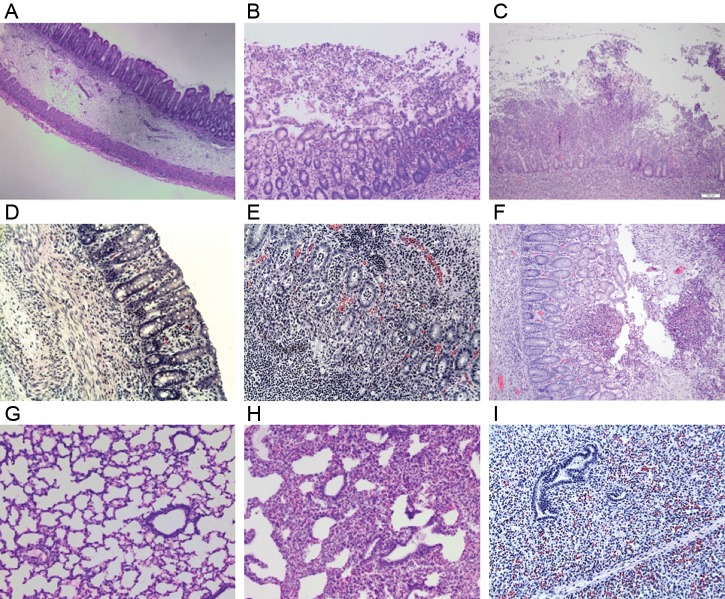
Figure 3.
Histopathology images from antibody-treated piglets inoculated with Clostridium difficile. A, Submucosal edema with intact mucosa, minimal neutrophilic infiltration, and no formation of pseudomembranes in the spiral colon of a piglet treated with polyclonal anti-TcdB. B, Extensive neutrophilic infiltration of the mucosa with pseudomembrane formation in the spiral colon of a piglet treated with polyclonal anti-TcdA. C, Neutrophilic inflammation, pseudomembrane formation, and a complete ulceration of the mucosa in the spiral colon of a control piglet treated with alpaca preimmune serum. D, Mucosal and submucosal edema with intact mucosa and minimal neutrophilic inflammation in the spiral colon of a piglet treated with monoclonal anti-TcdA and anti-TcdB. E, Severe neutrophilic infiltration in the mucosa of the spiral colon in a piglet treated with monoclonal anti-TcdA. F, An area of mucosal ulceration, neutrophilic infiltration, and pseudomembrane formation in the spiral colon of a control piglet treated with monoclonal anti-Stx2. G, Lung from a piglet treated with polyclonal anti-TcdB only illustrating normal histology. H, Lung from a piglet treated with polyclonal anti-TcdA demonstrating regional atelectasis without inflammatory or bacterial infiltration. I, Lung from a piglet treated with monoclonal anti-TcdA demonstrating an area of complete atelectasis from a consolidated area of the lung noted at necropsy.
Anti-TcdA Antibodies
Interestingly, administration of only anti-TcdA antibodies did not provide any level of protection against either localized GI disease or the development of systemic CDI. In fact, piglets in the groups receiving only polyclonal or monoclonal anti-TcdA antibodies developed more severe disease than controls; 5 of 6 piglets given polyclonal anti-TcdA and 4 of 6 piglets given monoclonal anti-TcdA developed fatal disease with severe GI disease as well as systemic disease (Table 1). Pleural effusion, cranial–ventral lung consolidation, and ascites were notable systemic lesions present at the time of necropsy. Additionally, these piglets had severe lesions of the large intestine, including dilatation, hyperemia, focal hemorrhages, and mesocolonic edema extending from the cecum to the rectum, with the most severe lesions and pseudomembranes present in the spiral and descending colon (Figures 1B and 1E). Figure 2C shows a segment of the descending colon of a piglet treated with monoclonal anti-TcdA as compared with other groups. Histopathologic examination of the large intestine revealed severe neutrophilic infiltration of the mucosa, with focal mucosal erosions and ulcerations and formation of pseudomembranes (Figures 3B and 3E). Histopathologic lesions of the lungs included regional atelectasis with scattered neutrophil and macrophage infiltration, but no bacterial infiltration (Figures 3H and 3I). One piglet in the group treated with only monoclonal anti-TcdA had particularly severe lung lesions, with extensive consolidation noted in the cranial–ventral regions at necropsy (Figure 3I). The gross and microscopic lesions of the anti-TcdA–treated piglets were as severe or more severe than those of control piglets (Figure 1C and 1F; Figure 2A; Figures 3C and 3F).
Systemic and Fecal Toxin
Serum and body fluid samples collected from piglets were assessed for the presence of toxin using the ultrasensitive immunocytotoxicity assay. Piglets that develop systemic CDI often have detectable TcdA and TcdB in their serum, but piglets with localized GI disease do not [21]. Because none of the piglets in the groups receiving anti-TcdB developed signs of systemic illness, we would not expect to find either toxin in the serum, which was confirmed (Table 1). In the control piglet groups, that is, 20% of the polyclonal group and 75% of the monoclonal group, both TcdA and TcdB were detectable in the serum or body fluids (Table 1). In the group that received only polyclonal anti-TcdA, we found that TcdA was not detectable in any of the serum or body fluid samples, but TcdB was detectable in the serum or body fluids of 50% of the group (Table 1). Because we know that both toxins are taken up into the circulatory system in severe CDI, these findings indicate that the dose of the polyclonal antibodies given in this study fully neutralized the TcdA present in the circulation of these piglets and that the systemic pathology observed was likely due to the action of TcdB. In the group that received only monoclonal anti-TcdA, 67% of the piglets developed systemic disease and had both toxins present in the serum or body fluids, indicating that the dose of monoclonal antibodies given did not fully neutralize TcdA in vivo. The concentration of TcdA was reduced, as compared with that of controls, from approximately 10 ng/mL to 10 pg/mL.
Fecal alpaca and human antitoxin antibodies were measured using ELISA, and although the average concentration was quite variable in the feces, these results indicate that parenterally administered antitoxin antibodies are able to reach the gut lumen (data not shown). In fecal cytotoxicity assays for all polyclonal– and monoclonal antibody–treated groups, both TcdA and TcdB were detectable in 100% of samples collected from the onset of diarrhea, beginning 24–48 hours after inoculation with C. difficile, and still had cytotoxic activity, indicating that the concentration of antibodies in the gut lumen was not great enough to fully neutralize toxin (Table 1).
Serum and Intestinal Cytokines
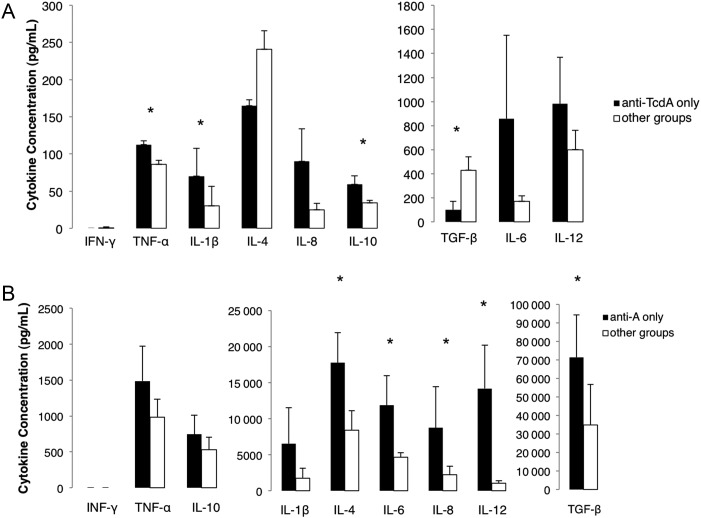
For initial analysis, mean cytokine concentrations between all 4 groups were compared using the Kruskall-Wallis test, with post-hoc comparisons within groups. No significant differences were present between all groups for serum cytokines, but IL-4, IL-8, IL-10, and IL-12 significantly differed for large intestinal cytokines. Within-group comparisons revealed that the group treated with only anti-TcdA differed most from other groups for several of the cytokines measured. So, a second analysis to compare the anti-TcdA–treated group with all other groups combined was performed for serum and intestinal cytokines using the Mann-Whitney U test. In this case, significant differences in serum cytokine concentrations were present for IL-1β, IL-10, TNF-α, and TGF-β (Figure 4A), and significant differences in large intestinal cytokine concentrations were present for IL-4, IL-6, IL-8, IL-12, and TGF-β (Figure 4B).
Figure 4.
Serum and intestinal cytokines in anti-TcdA–treated piglets. The mean cytokine concentration in piglets treated with only polyclonal anti-TcdA is compared with other groups. A, Serum cytokines. B, Large intestinal cytokines. *P < .05
CONCLUSIONS
Specific HuMabs against TcdA and TcdB are currently being seriously considered for therapeutic use in patients with CDI [16], making our investigation very relevant. Our observations in the piglet model indicate that the presence of antibodies against TcdB in the blood stream is highly effective in preventing systemic and GI tract disease due to CDI. Conversely, the administration of an antibody against TcdA alone not only failed to protect piglets, it appears to have exacerbated the outcome of the disease, leading to more serious consequences, as compared with control animals. The fact that almost identical results were obtained using 2 distinctly different sets of antibodies, including monoclonal and polyclonal and human and alpaca, adds considerable strength to these observations. We chose to evaluate the antibodies for prevention of disease based on the fact that this may be an effective way to reduce disease rates in high-risk patients and because treatment trials in human patients showed no significant difference during acute disease in hospitalized patients.
Distinguishing the relative roles of the 2 main virulence factors of C. difficile, TcdA and TcdB, has received considerable attention in recent years. However, the picture is still not entirely clear due to conflicting evidence that apparently depends on the animal model, bacterial strains, or antibodies used. These studies indicate that neither toxin can be ignored and that many questions concerning the roles of the 2 toxins remain unanswered. In this study we used neutralizing polyclonal and monoclonal antitoxin antibodies to compare the relative contributions of TcdA and TcdB in the pathogenesis of CDI using the gnotobiotic piglet model we previously characterized [14].
Our results support the current notion that TcdB is important in the pathogenesis of systemic complications of CDI and showed that administration of anti-TcdB alone was sufficient to completely prevent the development of severe disease and death in piglets. Anti-TcdB also substantially reduced the severity of the signs and gross and microscopic lesions of GI disease. These results are somewhat unexpected because previous studies have demonstrated that although it is cytotoxic, TcdB may not be an important virulence factor causing intestinal mucosal damage in neonatal pigs [22]. Yet, here we have evidence that neutralization of TcdB with antibodies provides protection from systemic as well as intestinal lesions of CDI.
In contrast to anti-TcdB, administration of only anti-TcdA, whether polyclonal or monoclonal, provided no protection from disease and, in fact, appeared to worsen prognosis after inoculation with C. difficile. Recent experiments in which the monoclonal anti-TcdA was administered to uninfected piglets have shown that the antibodies cause no negative effects when administered alone (unpublished data). The experiments involving the polyclonal antibodies were performed first; when we observed this effect, a hypothesis was that the existence of toxin-enhancing antibodies in the polyclonal antisera could be enhancing TcdA and causing the worsened clinical signs and lesions. The ultrasensitive toxin detection assay developed by our laboratory, for example, takes advantage of one such enhancing antibody, A1H3 [20, 23]. For this reason, the experiments with monoclonal antibodies were undertaken. We used monoclonal antibodies that had already been exhaustively examined for their toxin-neutralizing activity in vitro and in vivo in animals and humans, and these antibodies were not previously shown to have any toxin-enhancing effect [10, 16, 17]. In vitro cytotoxicity assay results from our laboratory have not shown either the polyclonal or monoclonal antibodies to have any toxin-enhancing capability at any concentration, and both types of antibodies are able to fully neutralize TcdA and TcdB in vitro.
Another hypothesis is that anti-TcdA antibodies could form complexes with TcdA, which could cause acute illness. This seems unlikely, given that the clinical signs and lesions of disease in anti-TcdA–treated piglets are those of CDI, not of immune-complex disease. These animals have CDI lesions as severe as or more severe than those in control piglets, without lesions such as vasculitis or glomerulonephritis that would accompany severe immune-complex deposition. Although immune-complex deposition was evident, it may be possible that the anti-TcdA antibodies, possibly in complex with TcdA, caused hyperstimulation of the immune system. The toxins themselves are known to be pro-inflammatory [24], but the immune response in terms of cytokine production in the anti-TcdA–treated group appears to be over and above that observed when no antibodies or only anti-TcdB are present. Given the results from the cytokine profiles in these piglets, it appears that multiple cytokines with diverse functions are elevated in those treated with anti-TcdA as compared with other groups, including controls. Some of the cytokines are pro-inflammatory, such as IL-6, IL-8, IL-1β, and TNF-α, but others are anti-inflammatory or immune regulatory, such as IL-4, IL-10, IL-12, and TGF-β. The pro-inflammatory mediators could worsen disease if some aspect of the toxin–antibody interaction induces their overproduction. The increased production of the anti-inflammatory and regulatory cytokines in these piglets may be in response to an imbalance in the pro-inflammatory mediators or could be the result of a more generalized immune stimulation.
We also consider that differences in the toxins produced by different strains of C. difficile could play a role in some of our findings regarding lack of protection or disease-worsening effects noted with anti-TcdA. The alpaca polyclonal antibodies and HuMabs were generated against atoxic toxins or toxoid based on TcdA and TcdB of strain VPI 10463, whereas strain UK6 was used for challenge of piglets in these studies. The antibodies directed against the toxins produced by VPI 10463 and the toxins produced by strain UK6 during the course of infection in the piglets may not interact as antibodies directed against UK6 would due to genetic differences in the toxin genes of laboratory and hypervirulent epidemic strains. Further studies with antitoxin antibodies generated against the toxins of various strains would be needed to determine whether this difference is important for consideration of antibody products aimed at treatment of patients.
We know from this study that although antitoxin antibodies administered systemically did reach the gut lumen, the antibodies were not present in great enough concentrations to fully neutralize either TcdA or TcdB in the gut. Both toxins were present at similar concentrations in the feces of all piglet groups, yet in groups treated with only anti-TcdB or anti-TcdA + anti-TcdB, GI lesions were mild as compared with the severe lesions developed by controls and animals only treated with anti-TcdA. Based on antibody titers and toxin detection in the serum and body fluids of inoculated piglets, we know that anti-TcdA was present in the serum at expected levels and was able to partially (in the case of monoclonal antibodies) or fully (in the case of polyclonal antibodies) neutralize TcdA. Only TcdB was detectable in the serum of the polyclonal anti-TcdA treated piglets, indicating that it alone was likely responsible for the systemic manifestations of disease observed in these animals.
Although the exact cause for the disease-worsening effect observed in piglets treated with only anti-TcdA in these experiments cannot yet be explained, it certainly warrants close investigation because these products are intended for use in human patients. The rodent models that have been used in previous investigations of antibodies for treatment or prevention of CDI have not indicated the same outcome, but rather show that anti-TcdA is protective. Lesions most similar to human CDI are reproducible in the piglet model. Whether or not piglets also offer a closer representation of therapeutic outcomes than rodent models is yet to be determined. Our results indicate the importance of TcdB in both GI and systemic CDI and that the potential for adverse effects due to anti-TcdA either given directly in the form of therapeutic antibodies or generated by the host in response to natural infection or immunization is clearly a subject that needs careful investigation.
Notes
Acknowledgments. We give special thanks to the members of Dr Jean Mukherjee's laboratory, who were not only involved with the alpaca immunization and blood collection but also helped greatly with the preparation of the sera for these experiments. Our animal care technicians, Patricia Boucher and Rachel Nieminen, cared for all the piglets used in these experiments. We thank Merck, Inc. for providing the monoclonal antibodies used in these experiments via a grant, which covers a portion of this work.
Financial support. This work was supported by Merck, Inc. grant LKR8118; by National Institutes of Health grants N01AI30050, R01AI088748, and F32AI081497; and New England Regional Center of Excellence grant U54 AI057159.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Gerding DN. Global epidemiology of Clostridium difficile infection in 2010. Infect Control Hosp Epidemiol. 2010;31(Suppl 1):S32–4. doi: 10.1086/655998. [DOI] [PubMed] [Google Scholar]
- 2.Rupnik M, Wilcox MH, Gerding DN. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat Rev Microbiol. 2009;7:526–36. doi: 10.1038/nrmicro2164. [DOI] [PubMed] [Google Scholar]
- 3.Lyerly DM, Saum KE, MacDonald DK, Wilkins TD. Effects of Clostridium difficile toxins given intragastrically to animals. Infect Immun. 1985;47:349–52. doi: 10.1128/iai.47.2.349-352.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Triadafilopoulos G, Pothoulakis C, O'Brien MJ, LaMont JT. Differential effects of Clostridium difficile toxins A and B on rabbit ileum. Gastroenterology. 1987;93:273–9. doi: 10.1016/0016-5085(87)91014-6. [DOI] [PubMed] [Google Scholar]
- 5.Ehrich M. Interaction of Clostridium difficile toxins and mouse hepatic microsomes. Toxicon. 1983;21:903–7. doi: 10.1016/0041-0101(83)90083-1. [DOI] [PubMed] [Google Scholar]
- 6.Hamm EE. Identification of Clostridium difficile toxin B cardiotoxicity using a zebrafish embryo model of intoxication. Proc Natl Acad Sci U S A. 2006;103:14176–81. doi: 10.1073/pnas.0604725103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Libby JM, Jortner BS, Wilkins TD. Effects of the two toxins of Clostridium difficile in antibiotic-associated cecitis in hamsters. Infect Immun. 1982;36:822–9. doi: 10.1128/iai.36.2.822-829.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim PH, Iaconis JP, Rolfe RD. Immunization of adult hamsters against Clostridium difficile-associated ileocecitis and transfer of protection to infant hamsters. Infect Immun. 1987;55:2984–92. doi: 10.1128/iai.55.12.2984-2992.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kyne L, Warny M, Qamar A, Kelly CP. Association between antibody response to toxin A and protection against recurrent Clostridium difficile diarrhoea. Lancet. 2001;357:189–93. doi: 10.1016/S0140-6736(00)03592-3. [DOI] [PubMed] [Google Scholar]
- 10.Babcock GJ, Broering TJ, Hernandez HJ, et al. Human monoclonal antibodies directed against toxins A and B prevent Clostridium difficile-induced mortality in hamsters. Infect Immun. 2006;74:6339–47. doi: 10.1128/IAI.00982-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leav BA, Blair B, Leney M, et al. Serum anti-toxin B antibody correlates with protection from recurrent Clostridium difficile infection (CDI) Vaccine. 2010;28:965–9. doi: 10.1016/j.vaccine.2009.10.144. [DOI] [PubMed] [Google Scholar]
- 12.Lyras D, O'Connor JR, Howarth PM, et al. Toxin B is essential for virulence of Clostridium difficile. Nature. 2009;458:1176–9. doi: 10.1038/nature07822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuehne SA, Cartman ST, Heap JT, Kelly ML, Cockayne A, Minton NP. The role of toxin A and toxin B in Clostridium difficile infection. Nature. 2010;467:711–3. doi: 10.1038/nature09397. [DOI] [PubMed] [Google Scholar]
- 14.Steele J, Feng H, Parry N, Tzipori S. Piglet models of acute or chronic Clostridium difficile illness. J Infect Dis. 2010;201:428–34. doi: 10.1086/649799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang G, Zhou B, Wang J, et al. Expression of recombinant Clostridium difficile toxin A and B in Bacillus megaterium. BMC Microbiol. 2008;8:192. doi: 10.1186/1471-2180-8-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lowy I, Molrine DC, Leav BA, et al. Treatment with monoclonal antibodies against Clostridium difficile toxins. N Engl J Med. 2010;362:197–205. doi: 10.1056/NEJMoa0907635. [DOI] [PubMed] [Google Scholar]
- 17.Taylor CP, Tummala S, Molrine D, et al. Open-label, dose escalation phase I study in healthy volunteers to evaluate the safety and pharmacokinetics of a human monoclonal antibody to Clostridium difficile toxin A. Vaccine. 2008;26:3404–9. doi: 10.1016/j.vaccine.2008.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akiyoshi DE, Rich CM, O'Sullivan-Murphy S, et al. Characterization of a human monoclonal antibody against Shiga toxin 2 expressed in Chinese hamster ovary cells. Infect Immun. 2005;73:4054–61. doi: 10.1128/IAI.73.7.4054-4061.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Killgore G, Thompson A, Johnson S, et al. Comparison of seven techniques for typing international epidemic strains of Clostridium difficile: restriction endonuclease analysis, pulsed-field gel electrophoresis, PCR-ribotyping, multilocus sequence typing, multilocus variable-number tandem-repeat analysis, amplified fragment length polymorphism, and surface layer protein A gene sequence typing. J Clin Microbiol. 2008;46:431–7. doi: 10.1128/JCM.01484-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He X, Wang J, Steele J, et al. An ultrasensitive rapid immunocytotoxicity assay for detecting Clostridium difficile toxins. J Microbiol Methods. 2009;78:97–100. doi: 10.1016/j.mimet.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steele J, Chen K, Sun X, et al. Systemic dissemination of Clostridium difficile toxins A and B is associated with severe, fatal disease in animal models. J Infect Dis. 2012;205:384–91. doi: 10.1093/infdis/jir748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keel MK, Songer JG. The distribution and density of Clostridium difficile toxin receptors on the intestinal mucosa of neonatal pigs. Vet Pathol. 2007;44:814–22. doi: 10.1354/vp.44-6-814. [DOI] [PubMed] [Google Scholar]
- 23.He X, Sun X, Wang J, et al. Antibody-enhanced, Fcγ R-mediated endocytosis of Clostridium difficile toxin A. Infect Immun. 2009;77:2294–303. doi: 10.1128/IAI.01577-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carter GP, Rood JI, Lyras D. The role of toxin A and toxin B in Clostridium difficile-associated disease: Past and present perspectives. Gut Microbes. 2010;1:58–64. doi: 10.4161/gmic.1.1.10768. [DOI] [PMC free article] [PubMed] [Google Scholar]