Abstract
Background. Cohort effects, new sex partnerships, and human papillomavirus (HPV) reactivation have been posited as explanations for the bimodal age-specific HPV prevalence observed in some populations; no studies have systematically evaluated the reasons for the lack of a second peak in the United States.
Methods. A cohort of 843 women aged 35–60 years were enrolled into a 2-year, semiannual follow-up study. Age-specific HPV prevalence was estimated in strata defined by a lower risk of prior infection (<5 self-reported lifetime sex partners) and a higher risk of prior infection (≥5 lifetime sex partners). The interaction between age and lifetime sex partners was tested using likelihood ratio statistics. Population attributable risk (PAR) was estimated using Levin's formula.
Results. The age-specific prevalence of 14 high-risk HPV genotypes (HR-HPV) declined with age among women with <5 lifetime sex partners but not among women with ≥5 lifetime sex partners (P = .01 for interaction). The PAR for HR-HPV due to ≥5 lifetime sex partners was higher among older women (87.2%), compared with younger women (28.0%). In contrast, the PAR associated with a new sex partner was 28% among women aged 35–49 years and 7.7% among women aged 50–60 years.
Conclusions. A lower cumulative probability of HPV infection among women with a sexual debut before the sexual revolution may be masking an age-related increase in HPV reactivation in the United States.
Keywords: Human Papillomavirus, menopause, perimenopause, sexual revolution, cervical cancer, reactivation, cohort effect, age
(See the editorial commentary by Brown and Weaver, on pages 211–2.)
Pooled- and meta-analyses of age-specific human papillomavirus (HPV) prevalence have demonstrated considerable variability across geographical regions [1–3]. A recent study updated the age-specific prevalence from a global analysis of 1 million women with normal cervical cytology findings [4]. In all regions, the HPV prevalence was highest among younger women around the age of sexual debut. A second peak in HPV prevalence around the age of menopause was marked in Central and South America and Western Africa but absent in North America, Eastern and Southeast Asia, Eastern Europe, Northern Europe, and Western Europe. An attenuated menopausal peak prevalence was observed in South Asia, Southern Europe, and Southern Africa. Explanations for this variability include differences in relative prevalence of new partnerships at older ages, risk of HPV reactivation at older ages, and cohort effects.
The lack of a second increase in HPV prevalence at menopause in the United States [5, 6] suggests that older US women are not having a significant increase in new sex partnerships at menopause and are not at risk of HPV reactivation, assuming no influence of a cohort effect. To investigate whether these assertions were valid, we compared differences in recent and lifetime sexual behaviors, by age group, to investigate the presence of a cohort effect in an older screening population in Baltimore, Maryland, and its impact on HPV prevalence estimates.
METHODS
Study Population and Data Collection
Women were recruited from outpatient obstetrics-gynecology clinics in and around Baltimore from March 2008 through March 2011; all were attending the clinics for routine gynecological examination. Women were eligible to participate if they were aged 35–60 years, had an intact cervix, and were willing to provide informed consent. Women were not eligible for enrollment if they were pregnant, had plans to become pregnant, had a history of organ transplantation, or were known to be positive for human immunodeficiency virus.
After women provided informed consent, they completed a baseline questionnaire and gynecological examination. Information on sociodemographic characteristics, reproductive and menstrual history, hormonal and nonhormonal medication use, lifetime sexual history, and current sexual behavior were collected using a telephone-administered questionnaire. Data on cervical screening and treatment history were also collected during the interviews. A trained study physician or registered nurse conducted a speculum examination to collect a cervical brush specimen for HPV DNA testing (Digene HPV sampler, Digene, United States) as part of the standardized study protocol. Cervical brushes were placed in standard transport medium, stored at 4°C for <24 hours, and vortexed, and cervical specimens were divided into aliquots and stored at −80°C. All study procedures were approved by the Johns Hopkins Bloomberg School of Public Health Institutional Review Board.
HPV Genotyping
DNA was extracted using the QIAamp DNA Blood Kit (Qiagen, France) and tested for the presence of genotype-specific HPV, using the Roche HPV Linear Array polymerase chain reaction–based assay (Roche Diagnostics, United States) according to a modified version of the manufacturer's instructions, as described elsewhere [7–9].
Current Cervical Cytology Findings
Papanicolaou (Pap) smear cytology findings were not collected as part of the study protocol. Women provided signed consent to allow retrieval of their Pap smear results from the appropriate clinical cytopathology laboratories. The result of the Pap smear that was associated with study enrollment was abstracted from the cytopathology report onto a standardized case report form. Pap smear results were classified according to the most severe diagnosis, and for the purpose of this analysis, a cytology finding of atypical squamous cells of undetermined significance or worse was considered to be abnormal.
Estradiol and Progesterone Measurement
Serum estradiol and progesterone levels in a subset of women who provided serum samples at baseline were measured using a Meso Scale enzyme-linked immunosorbent assay (Meso Scale Discover, Gaithersburg, MD). The assay was validated against standard clinical chemistry measurements in a subset of 60 women, with Spearman ρ coefficients of 0.85 (P < .001) for estradiol level and 0.43 (P = .007) for progesterone level. Results were log-transformed to approximate a normal distribution.
Statistical Analysis
Of 885 women with a baseline interview, 3 had missing or insufficient HPV test results (based on lack of human beta-globin amplification). Other variables of interest that were missing data included marital status (n = 1), lifetime number of sex partners (n = 3), recent sexual behavior (n = 5), history of abnormal Pap smear findings (n = 10), and missing baseline cytology result (n = 21). Women with missing data for any of these variables were excluded from the present analysis, leaving a final analytic sample size of 843.
“Any HPV” was defined as positivity for ≥1 HPV type detectable by the Roche Linear Array test (36 individual genotypes and 1 subtype). Because these data are collected from a population participating in routine cervical cancer screening for which screening for high-risk (HR) HPV is recommended, we defined “HR-HPV” according to the standard pool of 14 genotypes (HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68) detectable by the 3 Food and Drug Administration–approved assays used in the routine screening program. The association between HPV prevalence and other categorical variables was estimated using the Wald χ2 test. We used unconditional logistic regression to estimate odds ratios (ORs) as relative effect measures of the associations between HPV prevalence and age, recent sexual behavior, and lifetime number of sex partners. Multivariate models were developed using a combination of biological conceptual models and forward stepwise selection, retaining variables with a Wald P value of <.1. The association between age and presumptive reactivation is difficult to measure by means of cross-sectional observational designs, because there is no molecular marker available to distinguish newly acquired infection from persistently detectable or reactivated latent infection. However, as an indirect measure of this effect, we evaluated the difference in age-specific HPV prevalence in subgroups of women with low prior HPV infection probability and high prior HPV infection probability, defined as <5 and ≥5 lifetime sex partners, respectively. We reasoned that if HPV reactivation is associated with increasing age, as was proposed in previous literature, the risk would be conditioned on prior infection probability (eg, lifetime number of sex partners). Women were divided into age-based subgroups of 35–49 years and 50–60 years for further analysis of the relative impact of recent and lifetime number of sex partners in younger and older perimenopausal women. The biological validity of the bivariate age stratification was examined by comparing the serum estradiol and progesterone levels in a subset of women (607 of 843 [72%]) with an available blood sample at baseline. Supplementary Figure 1 demonstrates that the average levels of estradiol and progesterone begin to decline significantly in the group aged 50–54 years.
Interaction between age and lifetime number of sex partners (using binary categorization) was tested by comparing models with and models without the interaction term, using likelihood ratio statistics. The percentage population attributable risk was estimated as {[pe*(OR-1)]/[pe*(OR-1)+1]}*100, where OR is the unadjusted OR, and pe is the population exposure prevalence [10, 11].
We developed simple models to evaluate the impact of the sexual revolution on age-specific HPV prevalence from 3 hypothetical cross-sectional surveys, conducted in 1998, 2008, and 2018. The details of this model are provided in Supplementary Table 1.
RESULTS
Table 1 represents the basic demographic and behavioral variables of the study population. The age of the study population was normally distributed within the eligible age range of 35–60 years, with a mean age (±SD) of 46.6 ± 6.7 years. The population was 74.3% white and 19.0% black, and the remaining women were Asian or another race. The majority of women in the population had at least a college degree (29.3% had a baccalaureate, and 29.8% had a postgraduate degree) and were married (63.4%). More than 90% of women reported a history of hormonal contraceptive use, and 22.1% were current users of hormonal contraceptives. Few women (8.3%) reported ever using hormone replacement therapy. All women reported a prior history of a Pap smear, 48.9% reported a history of an abnormal Pap smear finding, 21.5% reported a history of colposcopy, and 19.2% reported a history of treatment for cervical intraepithelial neoplasia (CIN). Few women (4.7%) reported a current cytological abnormality [12].
Table 1.
Baseline Characteristics of the HPV in Perimenopause Study Population
| Characteristic | Subjects, No. (%) |
|---|---|
| Age, y | |
| 35–39 | 156 (18.5) |
| 40–44 | 179 (21.2) |
| 45–49 | 208 (24.7) |
| 50–54 | 174 (20.6) |
| 55–60 | 126 (15.0) |
| Race | |
| White | 626 (74.3) |
| Black | 160 (19.0) |
| Other | 57 (6.8) |
| Education | |
| High school | 147 (17.4) |
| Some post-high school | 198 (23.5) |
| College | 247 (29.3) |
| Postgraduate | 251 (29.8) |
| Marital status | |
| Currently married | 534 (63.4) |
| Widowed | 15 (1.8) |
| Separated | 27 (3.2) |
| Divorced | 115 (13.6) |
| Single | 152 (18.0) |
| Hormonal contraceptive use | |
| Never | 76 (9.0) |
| Former | 581 (68.9) |
| Current | 186 (22.1) |
| Hormone replacement therapy | |
| Never | 773 (91.7) |
| Former | 34 (4.0) |
| Current | 36 (4.3) |
| History of abnormal cytology findings | |
| No | 448 (53.1) |
| Yes | 395 (48.9) |
| History of colposcopy | |
| No | 662 (78.5) |
| Yes | 181 (21.5) |
| History of treatmenta | |
| No | 681 (80.8) |
| Yes | 162 (19.2) |
| Current cytology finding | |
| Normal | 803 (95.3) |
| ASC-US+ | 40 (4.7) |
Abbreviation: ASC-US+, atypical squamous cells of undetermined significance or worse.
a Including loop electroexcision procedure, cryotherapy, laser therapy, or cone biopsy.
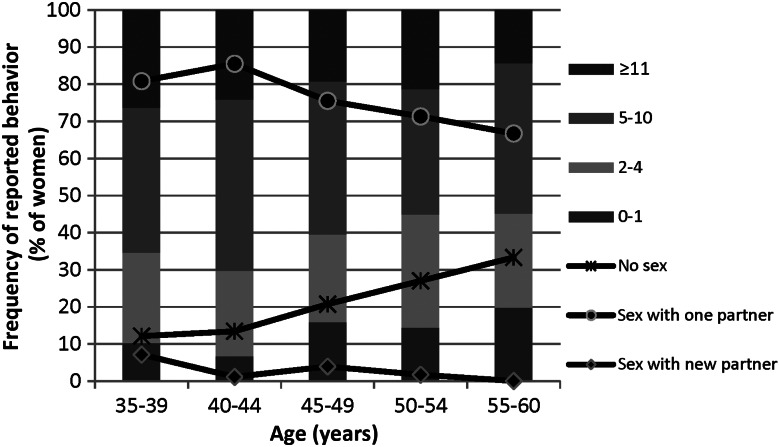
The age-specific patterns of both recent and lifetime sexual behaviors are summarized in Figure 1. A trend was observed of a higher lifetime number of sex partners among younger women (P = .009 for trend). Few women (2.9%) reported having a new sex partner in the past 6 months, and those reporting new partners tended to be younger. Report of no sexual activity in the past 6 months increased with increasing age, from 12.2% in the youngest age group to 33.3% in the oldest age group (P < .001 for trend).
Figure 1.
Age-specific patterns of recent and lifetime sexual behaviors among study subjects.
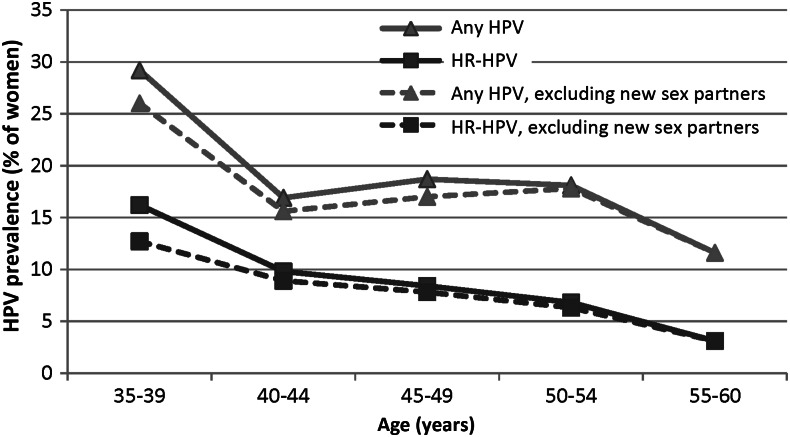
The age-specific HPV prevalence proportions for any HPV and for HR-HPV are summarized in Figure 2. Both any HPV and HR-HPV prevalence proportions decreased with increasing age (P = .002 and P<.001 for trend, respectively). Exclusion of the 24 women with a recent new sex partner did not change these trends.
Figure 2.
Age-specific prevalence proportions of any human papillomavirus (HPV) and high-risk HPV (HR-HPV) among study subjects. See Methods for definitions of any HPV and HR-HPV.
Any HPV and HR-HPV prevalence proportions were higher among women reporting a higher lifetime number of sex partners, with a threshold observed between 4 and 5 lifetime sex partners (Table 2). HPV prevalence was higher among the 24 women reporting a recent new sex partner (OR for any HPV, 11.5 [95% confidence interval {CI}, 4.5–29.5]); OR for HR-HPV, 12.5 [95% CI, 4.7–33.2]) but not among sexually active women with a nonnew partner (OR for any HPV, 1.3 [95% CI, .8–2.0]; OR for HR-HPV, 1.0 [95% CI, .5–1.9]), compared with the prevalence among sexually abstinent women.
Table 2.
Prevalence Proportions of Any Human Papillomavirus (HPV) and High-Risk HPV (HR-HPV), by Age and Sexual Behavior
| Characteristic | Subjects, No. | Any HPV, % of Subjects | OR (95% CI) | HR-HPV, % of Subjects | OR (95% CI) |
|---|---|---|---|---|---|
| Age, y | |||||
| 35–39 | 156 | 28.9 | Reference | 15.4 | Reference |
| 40–44 | 179 | 16.2 | 0.5 (0.3–.08) | 10.1 | 0.6 (0.3–1.2) |
| 45–49 | 208 | 18.3 | 0.6 (0.3–0.9) | 7.7 | 0.5 (0.2–0.9) |
| 50–54 | 174 | 17.8 | 0.5 (0.3–0.9) | 6.9 | 0.4 (0.2–0.8) |
| 55–60 | 126 | 11.9 | 0.3 (0.2–0.6) | 3.2 | 0.2 (0.1–0.5) |
| Lifetime no. of sex partners | |||||
| 0–1 | 111 | 9.9 | Reference | 4.5 | Reference |
| 2 | 57 | 15.8 | 1.7 (0.7–4.4) | 5.3 | 1.2 (0.3–5.1) |
| 3 | 84 | 10.7 | 1.1 (0.4–2.8) | 6 | 1.3 (0.4–4.8) |
| 4 | 72 | 9.7 | 1.0 (0.4–2.7) | 5.6 | 1.2 (0.3–4.8) |
| 5 | 107 | 20.6 | 2.4 (1.1–5.1) | 13.1 | 3.2 (1.1–9.2) |
| 6–10 | 233 | 24 | 2.9 (1.4–5.7) | 9.9 | 2.3 (0.9–6.3) |
| 11–20 | 115 | 23.5 | 2.8 (1.3–5.9) | 10.4 | 2.5 (0.8–7.3) |
| >20 | 64 | 26.6 | 3.3 (1.4–7.6) | 12.5 | 3.0 (0.9–9.7) |
| Sex in past 6 months | |||||
| None | 175 | 14.9 | Reference | 7.4 | Reference |
| With a non-new partner | 644 | 18 | 1.3 (0.8–2.0) | 7.6 | 1.0 (0.5–1.9) |
| With a new partner | 24 | 66.7 | 11.5 (4.5–29.5) | 50 | 12.5 (4.7–33.2) |
Only unadjusted estimates are presented. See Methods for definitions of any HPV and HR-HPV.
Abbreviations: CI, confidence interval; OR, odds ratio.
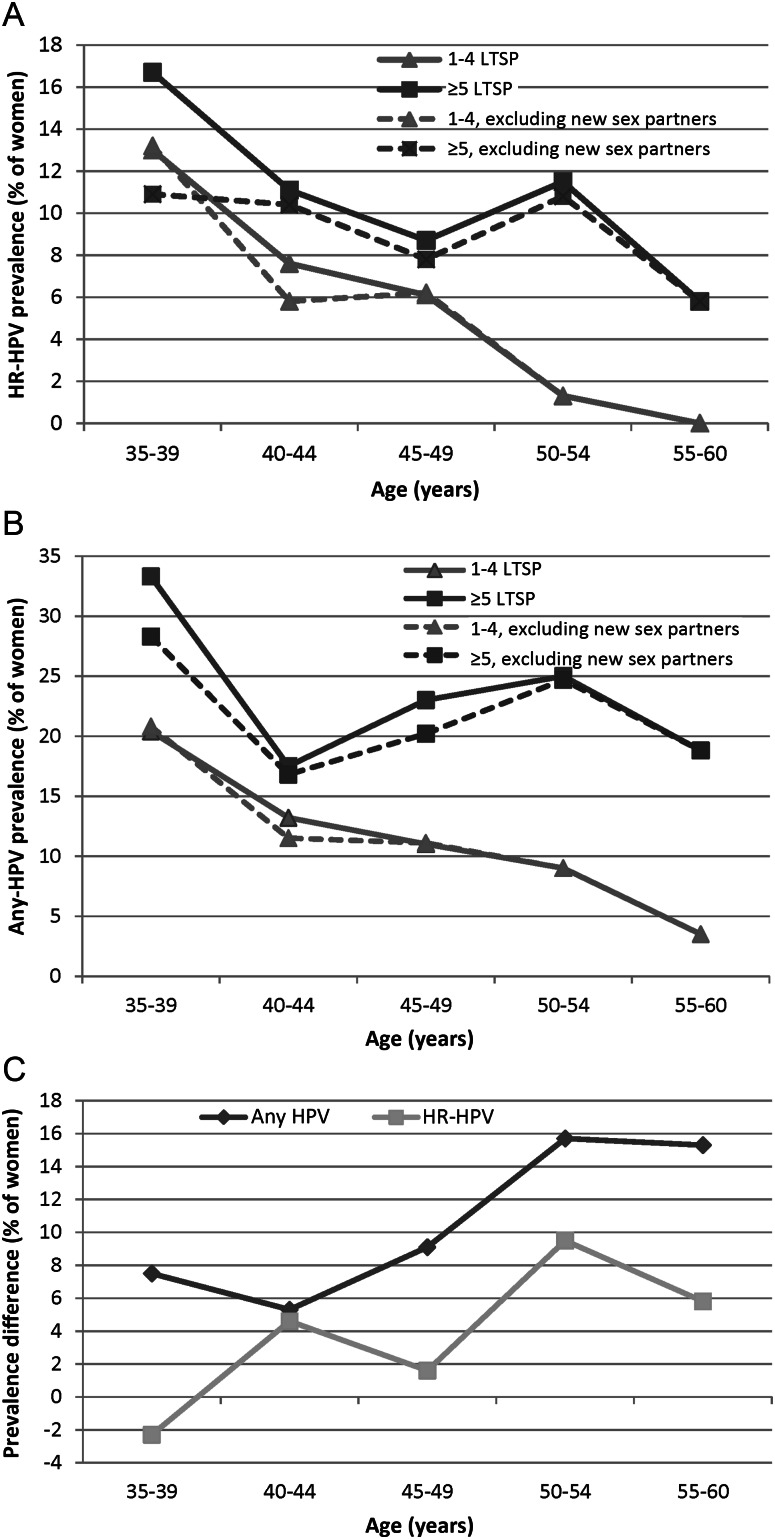
The prevalence proportions of both any HPV and HR-HPV declined with increasing age among women with <5 lifetime sex partners (Figure 3A and 3B, respectively). Among women at presumed higher risk of HPV reactivation (ie, those reporting ≥5 lifetime sex partners), the prevalence proportions of any HPV and HR-HPV declined during ages 35–40 years. The prevalence increased again during ages 40–54 years and decreased among women aged 55–60 years. To control for possible birth cohort differences in past infection probability as a function of the lifetime number of sex partners, we examined the relative difference in HPV prevalence between women with ≥5 lifetime sex partners and women with <5 lifetime sex partners, by age, excluding the 24 women reporting a recent new sex partner. Figure 3C shows that the relative difference in HPV prevalence among the women with a higher lifetime number of sex partners increased with increasing age, compared with women with a lower probability of prior infection. The interaction between age (35–49 years vs 50–60 years) and lifetime number of sex partners (<5 vs ≥5) was modest for any HPV prevalence (likelihood ratio P = .14) and was statistically significant for HR-HPV prevalence (likelihood ratio P = .01), after adjustment for recent sexual behavior, marital status, history of colposcopy, and current cytological abnormality (data not shown).
Figure 3.
A, Age-specific prevalence of high-risk human papillomavirus (HR-HPV), by lifetime number of sex partners (LTSP). B, Age-specific prevalence of any HPV, by LTSP. C, Relative difference in prevalence proportions of any HPV and HR-HPV between subjects with <5 lifetime sex partners and those with ≥5 lifetime sex partners. See Methods for definitions of any HPV and HR-HPV.
Stratification by ages 35–49 years and 50–60 years revealed an increased odds of both any HPV and HR-HPV in both age groups among women reporting a recent new sex partner, compared with women having no new sex partners, although the difference was not statistically significant in the older age group (Table 3). The odds of HPV detection among women reporting >5 lifetime sex partners was modestly elevated in the younger age group (OR for any HPV, 1.9 [95% CI, 1.2–2.0]; OR for HR-HPV, 1.5 [95% CI, .8–2.7]), whereas the odds of HPV detection among women reporting >5 lifetime sex partners was higher in the older age group (OR for any HPV, 4.0 [95% CI, 1.9–8.7]; OR for HR-HPV, 13.4 [95% CI, 1.7–102.8]). Because of the low prevalence of self-report of a new sex partnership in the 6 months prior to study enrollment, the population attributable risk due to new partners was low, with findings of 27.9% for any HPV and 28.0% for HR-HPV among the younger women and only 1.8% and 7.7%, respectively, among the older women. The percentage population attributable risk of HPV from having >5 lifetime sex partners was 37.0% for any HPV and 28.0% for HR-HPV among the younger women and 62.3% and 87.2%, respectively, among the older women.
Table 3.
Unadjusted Odds Ratios and 95% Confidence Intervals for Any and HR-HPV and Lifetime and Recent Sexual Behaviors in Women Aged 35–49 and 50–60 Years
| Any HPV |
HR-HPV |
||||
|---|---|---|---|---|---|
| Characteristic | No. | OR (95% CI) | Percentage PAR | OR (95% CI) | Percentage PAR |
| Age 35–49 y | |||||
| No new sex partner | 522 | Reference | Reference | ||
| New sex partner | 21 | 11 (4.1–29.0) | 27.90 | 11.1 (4.5–27.5) | 28.00 |
| <5 LTSP | 189 | Reference | Reference | ||
| ≥5 LTSP | 354 | 1.9 (1.2–3.0) | 37.00 | 1.5 (.8–2.7) | 28.00 |
| Age 50–60 | |||||
| No new sex partner | 297 | Reference | Reference | ||
| New sex partner | 3 | 2.8 (.2–31.5) | 1.80 | 9.4 (.8–109.6) | 7.70 |
| <5 LTSP | 135 | Reference | Reference | ||
| ≥5 LTSP | 165 | 4 (1.9–8.7) | 62.30 | 13.4 (1.7–102.8) | 87.20 |
Abbreviations: CI, confidence interval; LTSP, lifetime sex partners; OR, odds ratio; PAR, population attributable risk.
In models that assume no risk of reactivation at older ages and decreasing sexual activity with increasing age, the differences in cumulative HPV prevalence by birth cohort would not be predicted to affect the shape of the age-specific HPV prevalence over 3 successive, 10-year HPV prevalence surveys (Figure 4, model A). However, models that include an age-associated increased risk of reactivation would result in marked differences in the shape of the age-specific HPV prevalence curves over the same 20 years (Figure 4, model B), and the increase in HPV prevalence at older ages would not be predicted to be evident in the United States for another 10–15 years because of masking from the cohort effect of the sexual revolution.
Figure 4.
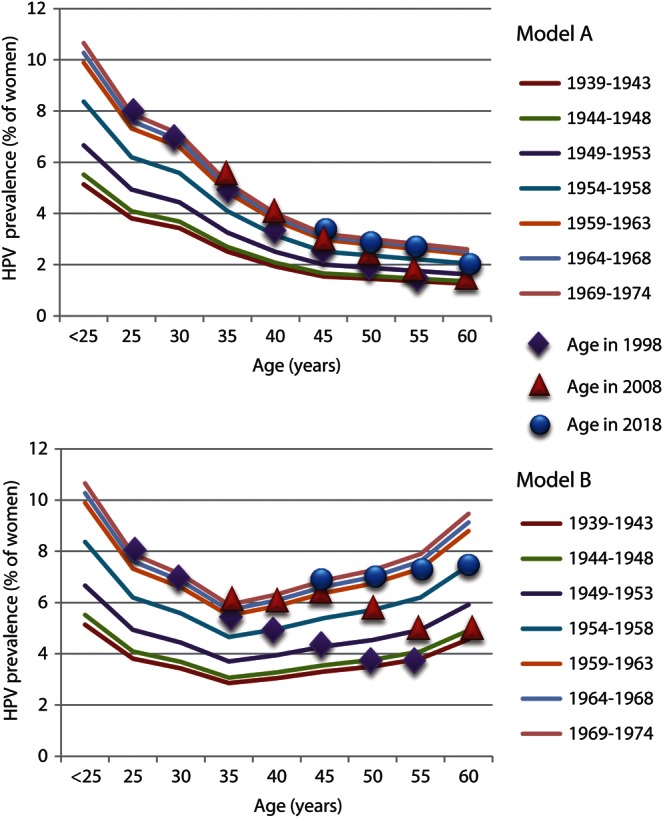
Simple models to evaluate the impact of the cohort effect of the 1965–1975 US sexual revolution on cross-sectional human papillomavirus (HPV) prevalence surveys conducted in 1998, 2008, and 2018 among 5 successive birth cohorts (1939–1974). Model A assumes no risk of reactivation with increasing age, and model B assumes an age-specific increase in reactivation similar to the observed U-shaped HPV prevalence in Morelos, Mexico [38].
DISCUSSION
We show evidence of an interaction between age and lifetime number of sex partners on prevalent HPV detection that is consistent with an age-associated increased risk of HPV reactivation. The older women in our study, who experienced sexual debut at the beginning of the US sexual revolution of the 1960s and 1970s, had a lower lifetime risk of HPV infection, as demonstrated by a lower self-reported lifetime number of sex partners. When we stratified by the lifetime number of sex partners as a crude measure of past probability of HPV infection (and thus risk of reactivation), HPV prevalence declined with age only among the women with <5 lifetime sex partners. The population attributable risk estimates for ≥5 lifetime sex partners were higher among women aged 50–60 years, compared with women aged 35–49 years. The relative prevalence of HPV was high among women with a new sex partner in the past 6 months, compared with the prevalence among women who did not report a new partner, but this exposure was rare, and the population attributable risk was low, especially for the older women. Taken together, our data raise the possibility that reactivation risk may increase around age 50 years and contribute to a larger fraction of HPV detection at older ages, compared with new acquisition.
The sexual revolution was associated with increased frequency of premarital sex, decreased age of sexual debut [13], and increased rates of several sexually transmitted infections [14]. For example, the number of reported cases of gonorrhea in the United States increased 3.6-fold, from approximately 125 cases/100 000 in 1965 to 450 cases/100 000 in 1975 [15]. HPV seroprevalence in the United States shows an early peak during the second decade (women 20–29 years), with a slow decline among older women [16]. While the decline in HPV seroprevalence at older ages could reflect waning immunity with age, models of a similar age-specific HPV seroprevalence curve in the United Kingdom have demonstrated better fit with cohort-specific changes in cumulative HPV exposure rather than with waning immunity [17]. The increasing incidence trend of non–screen-detectable HPV-associated cancers (ie, vulvar [18], anal [19], and oropharyngeal [20] cancers) among birth cohorts who were likely to have reached sexual debut during or after the sexual revolution further supports the impact of the sexual revolution on the population period prevalence of HPV.
The likelihood that a 2-fold increase in sexually transmitted infections (including HPV infection) in the population between 1965 and 1975 could mask an age-associated increase in HPV reactivation was evaluated using simple models. If a doubling of the cumulative lifetime numbers of HPV infections between 1965 and 1975 is assumed, the age-specific HPV prevalence observed in the US surveys [5, 6, 21, 22] could be observed under assumptions of both (1) a consistent decline in HPV with increasing age and (2) an increase in HPV prevalence due to reactivation at older ages within each birth cohort. We note that our observation of a smooth and continuous decline in HPV prevalence among women at low risk of reactivation because of low prior exposure is consistent with model A, which assumes no reactivation with age. On the other hand, the slight increase in HPV prevalence with age, followed by a sharp decrease at 55 years of age among women at highest risk of reactivation, is consistent with the 2008 prediction in age-specific HPV prevalence in model B, which includes an age-specific increase in reactivation. The residual cohort effects predicted from a 2008–2010 survey in model B, in addition to the imperfection of lifetime number of sex partners as a marker of prior infection probability, likely explains why we do not see an absolute increase in HPV prevalence with age, even after stratification by lifetime number of sex partners. However, the relative increase in HPV prevalence between women with high and those with low numbers of lifetime sex partners increased steadily with age, supporting the possibility that HPV reactivation increases at older ages.
Others have speculated that higher HPV prevalence at older ages represents changes in sexual behaviors in the fifth and sixth decades of life, presumably reflecting an increase in divorce rates and new partnerships [23, 24]. In our population, we find little evidence to support this; in fact, report of a new sex partner decreased with increasing age, and the population attributable risk due to a new sex partner was <10% among women aged >50 years.
The clinical implications of an increase in perimenopausal and postmenopausal HPV prevalence are unclear. Since new sex partnerships explained a minority of prevalent HPV infections among older women, prophylactic vaccination would be unlikely to provide significant benefit, as suggested by others [23, 24]. While some studies suggest that newly detected HPV DNA in older women has no greater risk of CIN2+, compared with newly detected HPV DNA in younger women, the average follow-up time in these studies was short and may not have been sufficient to detect a second peak of disease among older women [25]. An additional concern is whether the early lesions that occur following reactivation in a menopausal woman would have been as readily detected by standard screening algorithms as such lesions in premenopausal women, since the cells at highest risk of transformation—those in the squamocolumnar junction—have receded into the endocervical canal at menopause. This concern is consistent with reports of a higher frequency of CIN2+ diagnosis by endocervical curettage only among older women [26, 27] and with the observation that older women are more likely to have a large, solitary CIN3 lesion detected in tissue obtained by a loop electroexcision procedure [28]. Given the limitations in screening and diagnostic test performance for menopausal women [12, 29, 30], an increase in HPV reactivation at older ages may be predicted to result in an increased proportion of invasive cervical cancer among well-screened women, rather than an increase in CIN2/3. Such a trend has been reported in a large screening population in Hong Kong, where a second peak of HPV prevalence and invasive cervical cancer incidence but not of CIN2/3 incidence was observed at older ages [31].
Our cohort was recruited from a well-screened population of women presenting for routine gynecological care in Baltimore. Although we cannot generalize our findings to the larger US population, we believe that this population is representative of perimenopausal women who are being routinely screened for cervical cancer in the United States. We cannot determine whether HPV detected at baseline in our study was new, reactivated, or persistently detectable prior to enrollment, and thus our inferences support, rather than prove, our hypothesis. We are also unable to determine why HPV reactivation may increase among women aged >50 years, although previous studies have shown lower levels of biomarkers of cellular immune responsiveness among HPV-positive women older than 45 years [32]. In addition, several studies have characterized general systemic changes in immune response to vaccine and infection [33], effects that may be further exacerbated in women because of menopausal hormone changes [34]. Our observation that the relative increase in HPV prevalence among women with a higher past infection probability began at the age in which serum sex hormone levels begin to decline warrants more investigation into the possible role of sex steroid hormones on immunological control of HPV infection.
In summary, we propose that the cohort effect of the sexual revolution in the United States is masking an increase in HPV prevalence at older ages, which may be secondary to reactivation of “latent” infection. Further follow-up of the HPV in Perimenopause cohort and national surveillance data will be required to confirm this hypothesis. Although the existence of HPV latency remains contentious, it must be acknowledged that the risk of reactivation would be proportionate to the prevalence of prior HPV infection [35]. Because this risk is unlikely to be uniform across populations (eg, birth cohorts), appropriate measures for risk stratification are needed to evaluate associations between factors such as age and risk of reactivation. Inclusion of biomarkers of depressed or skewed immunity [7, 32] will be important in understanding whether HPV reactivation at older ages is explained by loss of immunologic control of latent HPV infection. The evidence evaluated in recent revisions to screening guidelines [36, 37] is derived from populations of older women with a substantially lower risk of HPV reactivation. Long-term follow-up of previously highly exposed women who will transition through menopause in the next decade is urgently needed to accurately estimate the potential risk of postmenopausal invasive cervical cancer in the US baby boom population and guide prevention strategies.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank Yolanda Eby, Roslyn Howard, and Aleksandra Ogurtsova, for laboratory testing; Lori Hackett, for research administration; Emily Seay, Rebecca Redett, and Jean Murphy, for study recruitment and follow-up; and the clinical providers and support teams at Johns Hopkins Women's Health Center at Green Spring Station, Johns Hopkins Women's Services at the Bayview Medical Offices, and Johns Hopkins Women's Services at White Marsh. Finally, we thank the women who generously volunteered their time as participants in the HIP Cohort, for their invaluable contributions and commitment to this study.
Financial support. This work was supported by the National Cancer Institute of the National Institutes of Health (grant R01 CA123467).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Smith JS, Melendy A, Rana RK, Pimenta JM. Age-Specific Prevalence of Infection with Human Papillomavirus in Females: A Global Review. Journal of Adolescent Health. 2008;43:S5.e1–62. doi: 10.1016/j.jadohealth.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 2.de Sanjose S, Diaz M, Castellsague X, et al. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis. 2007;7:453–9. doi: 10.1016/S1473-3099(07)70158-5. [DOI] [PubMed] [Google Scholar]
- 3.Franceschi S, Herrero R, Clifford GM, et al. Variations in the age-specific curves of human papillomavirus prevalence in women worldwide. Int J Cancer. 2006;119:2677–84. doi: 10.1002/ijc.22241. [DOI] [PubMed] [Google Scholar]
- 4.Bruni L, Diaz M, Castellsagué M, Ferrer E, Bosch FX, de Sanjosé S. Cervical human papillomavirus prevalence in 5 continents: meta-analysis of 1 Million women with normal cytological findings. Journal of Infectious Diseases. 2010;202:1789–99. doi: 10.1086/657321. [DOI] [PubMed] [Google Scholar]
- 5.Castle PE, Fetterman B, Thomas Cox J, et al. The age-specific relationships of abnormal cytology and human papillomavirus DNA results to the risk of cervical precancer and cancer. Obstetrics & Gynecology. 2010;116:76–84. doi: 10.1097/AOG.0b013e3181e3e719. [DOI] [PubMed] [Google Scholar]
- 6.Hariri S, Unger ER, Sternberg M, et al. Prevalence of genital human Papillomavirus among females in the United States, the National Health and Nutrition Examination Survey, 2003–2006. Journal of Infectious Diseases. 2011;204:566–73. doi: 10.1093/infdis/jir341. [DOI] [PubMed] [Google Scholar]
- 7.Marks MA, Viscidi RP, Chang K, et al. Differences in the concentration and correlation of cervical immune markers among HPV positive and negative perimenopausal women. Cytokine. 2011;56(3):798–803. doi: 10.1016/j.cyto.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gravitt PE, Peyton CL, Alessi TQ, et al. Improved amplification of genital human papillomaviruses. Journal of Clinical Microbiology. 2000;38:357–61. doi: 10.1128/jcm.38.1.357-361.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castle PE, Gravitt PE, Solomon D, Wheeler CM, Schiffman M. Comparison of linear array and line blot assay for detection of human papillomavirus and diagnosis of cervical precancer and cancer in the atypical squamous cell of undetermined significance and low-grade squamous intraepithelial lesion triage study. Journal of Clinical Microbiology. 2008;46:109–17. doi: 10.1128/JCM.01667-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walter SD. The distribution of Levin's measure of attributable risk. Biometrika. 1975;62:371–2. [Google Scholar]
- 11.Walter SD. The estimation and interpretation of attributable risk in health research. Biometrics. 1976;32:829–49. [PubMed] [Google Scholar]
- 12.Rositch AF, Silver MI, Burke A, et al. The correlation between HPV positivity and abnormal cervical cytology differs by age among perimenopausal women. Journal of Lower Genital Tract Diseases. 2012 doi: 10.1097/LGT.0b013e3182503402. Aug 9 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aral S, Johnson R, Zaidi A, Fichtner R, Reynolds G. Demographic effects on sexually transmitted diseases in the 1970s: the problem could be worse. Sex Trans Dis. 1983;10:100–1. doi: 10.1097/00007435-198304000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Zaidi A, Aral S, Reynolds G, Blount J, Jones O, Fichtner R. Gonorrhea in the United States: 1967–79. Sex Trans Dis. 1983;10:72–6. doi: 10.1097/00007435-198304000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. 2009 sexually transmitted disease surveillance. Figure 14: gonorrhea—rates, United States, 1941–2009. http://www.cdc.gov/std/stats09/figures/14.htm. 22 November 2010. Accessed 4 November 2012. [Google Scholar]
- 16.Dunne EF, Sternberg M, Markowitz LE, et al. Human papillomavirus (HPV) 6, 11, 16, and 18 prevalence among females in the United States—National Health and Nutrition Examination Survey, 2003–2006: opportunity to measure HPV vaccine impact? J Infect Dis. 2011;204:562–5. doi: 10.1093/infdis/jir342. [DOI] [PubMed] [Google Scholar]
- 17.Desai S, Chapman R, Jit M, et al. Prevalence of Human Papillomavirus Antibodies in Males and Females in England. Sexually Transmitted Diseases. 2011;38:622–9. doi: 10.1097/OLQ.0b013e31820bc880. [DOI] [PubMed] [Google Scholar]
- 18.Saraiya M, Watson M, Wu X, et al. Incidence of in situ and invasive vulvar cancer in the US, 1998–2003. Cancer. 2008;113:2865–72. doi: 10.1002/cncr.23759. [DOI] [PubMed] [Google Scholar]
- 19.Joseph DA, Miller JW, Wu X, et al. Understanding the burden of human papillomavirus-associated anal cancers in the US. Cancer. 2008;113:2892–2900. doi: 10.1002/cncr.23744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human Papillomavirus and Rising Oropharyngeal Cancer Incidence in the United States. Journal of Clinical Oncology. 2011;29:4294–301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sherman ME, Lorincz AT, Scott DR, et al. Baseline cytology, human papillomavirus testing, and risk for cervical neoplasia: a 10-year cohort analysis. J Natl Cancer Inst. 2003;95:46–52. doi: 10.1093/jnci/95.1.46. [DOI] [PubMed] [Google Scholar]
- 22.Datta SD, Koutsky LA, Ratelle S, et al. Human Papillomavirus infection and cervical cytology in women screened for cervical cancer in the United States, 2003-2005. Ann Intern Med. 2008;148:493–500. doi: 10.7326/0003-4819-148-7-200804010-00004. [DOI] [PubMed] [Google Scholar]
- 23.Bosch FX, Burchell AN, Schiffman M, et al. Epidemiology and natural history of human papillomavirus infections and type-specific implications in cervical neoplasia. Vaccine. 2008;26S:K1–16. doi: 10.1016/j.vaccine.2008.05.064. [DOI] [PubMed] [Google Scholar]
- 24.Trottier H, Ferreira S, Thomann P, et al. Human Papillomavirus Infection and Reinfection in Adult Women: the Role of Sexual Activity and Natural Immunity. Cancer Research. 2010;70:8569–77. doi: 10.1158/0008-5472.CAN-10-0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodríguez AC, Schiffman M, Herrero R, et al. Longitudinal Study of Human Papillomavirus Persistence and Cervical Intraepithelial Neoplasia Grade 2/3: Critical Role of Duration of Infection. Journal of the National Cancer Institute. 2010;102:315–24. doi: 10.1093/jnci/djq001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gage JC, Duggan MA, Nation JG, Gao S, Castle PE. Detection of cervical cancer and its precursors by endocervical curettage in 13,115 colposcopically guided biopsy examinations. American Journal of Obstetrics and Gynecology. 2010;203:481.e1–9. doi: 10.1016/j.ajog.2010.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Solomon D, Stoler M, Jeronimo J, Khan M, Castle P, Schiffman M. Diagnostic utility of endocervical curettage in women undergoing colposcopy for equivocal or low-grade cytologic abnormalities. Obstetrics and Gynecology. 2007;110:288–95. doi: 10.1097/01.AOG.0000270154.69879.09. [DOI] [PubMed] [Google Scholar]
- 28.Yang HP, Zuna RE, Schiffman M, et al. Clinical and Pathological Heterogeneity of Cervical Intraepithelial Neoplasia Grade 3. PLoS ONE. 2012;7:e29051. doi: 10.1371/journal.pone.0029051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stoler MH, Wright TC, Jr., Sharma A, et al. The interplay of age stratification and HPV testing on the predictive value of ASC-US cytology. Results from the ATHENA HPV study. American Journal of Clinical Pathology. 2012;137:295–303. doi: 10.1309/AJCPGW1V2BBWMOCX. [DOI] [PubMed] [Google Scholar]
- 30.Wright TC, Jr., Stoler MH, Behrens CM, Apple R, Derion T, Wright TL. The ATHENA human papillomavirus study: design, methods, and baseline results. American Journal of Obstetrics and Gynecology. 2012;206:46 e1–11. doi: 10.1016/j.ajog.2011.07.024. [DOI] [PubMed] [Google Scholar]
- 31.Chan PKS, Chang AR, Yu MY, et al. Age distribution of human papillomavirus infection and cervical neoplasia reflects caveats of cervical screening policies. International Journal of Cancer. 2010;126:297–301. doi: 10.1002/ijc.24731. [DOI] [PubMed] [Google Scholar]
- 32.González P, Hildesheim A, Rodríguez AC, et al. Behavioral/lifestyle and immunologic factors associated with HPV Infection among women older than 45 years. Cancer Epidemiology Biomarkers & Prevention. 2010;19:3044–54. doi: 10.1158/1055-9965.EPI-10-0645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reber AJ, Chirkova T, Kim JH, et al. Immunosenescence and challenges of vaccination against influenza in the aging population. Aging and Disease. 2012;3:68–90. [PMC free article] [PubMed] [Google Scholar]
- 34.Gameiro CM, Romao F, Castelo-Branco C. Menopause and aging: changes in the immune system—a review. Maturitas. 2010;67:316–20. doi: 10.1016/j.maturitas.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 35.Gravitt PE. The Known Unknowns of HPV Natural History. Journal of Clinical Investigation. 2011;121:4593–9. doi: 10.1172/JCI57149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saslow D, Solomon D, Lawson HW, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology Screening Guidelines for the Prevention and Early Detection of Cervical Cancer. American Journal of Clinical Pathology. 2012;137(4):516–42. doi: 10.1309/AJCPTGD94EVRSJCG. [DOI] [PubMed] [Google Scholar]
- 37.Moyer VA. Screening for Cervical Cancer: U.S. Preventive Services Task Force Recommendation Statement. Annals of Internal Medicine. 2012;137:516–42. doi: 10.7326/0003-4819-156-12-201206190-00424. [DOI] [PubMed] [Google Scholar]
- 38.Flores Y, Bishai D, Lazcano E, et al. Improving cervical cancer screening in Mexico: results from the Morelos HPV study. Salud Publica Mex. 2003;45(Suppl 3):S388–98. doi: 10.1590/s0036-36342003000900013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.