Abstract
Background. We report the first-in-human safety and immunogenicity assessment of a prototype Ad26 vector-based human immunodeficiency virus (HIV) vaccine in humans.
Methods. Sixty Ad26-seronegative, healthy, HIV-uninfected subjects were enrolled in a randomized, double-blinded, placebo-controlled, dose-escalation phase 1 study. Five groups of 12 subjects received 109–1011 vp of the Ad26-EnvA vaccine (N = 10/group) or placebo (N = 2/group) at weeks 0 and 24 or weeks 0, 4, and 24. Safety and immunogenicity were assessed.
Results. Self-limited reactogenicity was observed after the initial immunization at the highest (1011 vp) dose. No product-related SAEs were observed. All subjects who received the Ad26-EnvA vaccine developed Ad26 NAb titers, EnvA-specific enzyme-linked immunosorbent assays (ELISA) titers, and EnvA-specific enzyme-linked immunospot assays (ELISPOT) responses. These responses persisted at week 52. At week 28 in the 109, 1010, 1011 vp 3-dose and the 1010 and 5 × 1010 vp 2-dose groups, geometric mean EnvA ELISA titers were 6113, 12 470, 8545, 3470, and 9655 and mean EnvA ELISPOT responses were 397, 178, 736, 196, and 1311 SFC/106 peripheral blood mononuclear cells, respectively.
Conclusion. This Ad26 vectored vaccine was generally safe and immunogenic at all doses tested. Reactogenicity was minimal with doses of 5 × 1010 vp or less. Ad26 is a promising new vaccine vector for HIV-1.
Clinical Trials Registration. NCT00618605.
Keywords: HIV Vaccine, Adenovirus 26, Safety, Immunogenicity, Dose-escalation
(See the major article by Barouch et al, on pages 248–56.)
Development of a preventive human immunodeficiency virus (HIV)-1 vaccine is a high priority. Three concepts have been evaluated in field trials to date [1–4]. A variety of vaccine approaches to elicit HIV-1–specific immune responses are currently being evaluated in phase I/IIA trials, including plasmid DNA, recombinant viral vectors, and proteins, in addition to a variety of other strategies to augment HIV-1–specific immune responses [5–10]. Adenovirus (Ad) vectors are potent vaccine vectors, and a recombinant adenovirus serotype 5 (Ad5) vector is currently being studied in conjunction with DNA priming in a phase IIB study. Potential limitations of Ad5 vectors include the high level of preexisting antivector immunity in human populations, particularly in the developing world, as well as possibly certain qualitative features of the immune responses elicited [11].
To overcome these potential limitations, alternative Ad vectors have been developed from other serotypes [11, 12]. Alternative serotype Ad vectors have substantial biologic differences from Ad5 in terms of baseline seroepidemiology, receptor usage, tropism, innate immune profile, adaptive immune phenotype, and protective efficacy in the simian immunodeficiency virus (SIV)/macaque model [13–18]. Based on these properties, several alternative serotype Ad vectors, including Ad26 and Ad35, have been advanced into clinical trials as potential vaccine vectors for HIV-1, malaria, tuberculosis, and Ebola vaccines [19, 20]. In this study, we report the first-in-human evaluation of an Ad26 vectored vaccine.
METHODS
Participants and Study Design
This study was a single-center, randomized, double-blind, placebo-controlled, dose-escalation trial to evaluate safety and immunogenicity of a 3-dose regimen (0, 4, and 24 weeks) of Ad26.ENVA.01 (rAd26) HIV-1 vaccine at 109, 1010, and 1011 vp (viral particles) and a 2-dose regimen (0 and 24 weeks) at 1010 and 5 × 1010 vp in HIV-uninfected volunteers. The 2 dose groups allow a comparison of 2 vs 3 vaccinations (at 1 × 1010) and provide data on an intermediate dose (5 × 1010) for product development. Study subjects were healthy, Ad26 seronegative, and at low risk for acquiring HIV as per standard criteria. The protocol was approved by the institutional review board at Brigham and Women's Hospital, and written informed consent was obtained from each subject. The study was registered at ClinicalTrials.gov (NCT00618605).
The study schema is presented in Table 1. Groups 1–3 received a 10-fold increase in vaccine dose from 109 to 1011 vp. Each dose group had 12 subjects, 10 vaccinees, and 2 placebo recipients, for a total sample size of 60. The placebo recipients in each group were pooled into a 10-subject placebo group for comparison (thus 4 placebo recipients received 2 rather than 3 vaccinations). All vaccines were given by needle and syringe in the deltoid muscle. Systematic safety assessments were conducted (see Supplementary Data). Immunogenicity assessments were performed on samples collected at weeks 0, 2, 4, 6, 8, 24, 26, 28, and 52.
Table 1.
Protocol Schema
| Injection Schedule, weeks |
|||||
|---|---|---|---|---|---|
| Group | Number | Dose | 0 | 4 | 24 |
| 1 | 10 | 109 | rAd26 | rAd26 | rAd26 |
| 2 | FFB | FFB | FFB | ||
| 2 | 10 | 1010 | rAd26 | rAd26 | rAd26 |
| 2 | FFB | FFB | FFB | ||
| 3 | 10 | 1011 | rAd26 | rAd26 | rAd26 |
| 2 | FFB | FFB | FFB | ||
| 4 | 10 | 5 × 1010 | rAd26 | rAd26 | |
| 2 | FFB | FFB | |||
| 5 | 10 | 1010 | rAd26 | rAd26 | |
| 2 | FFB | FFB | |||
| Total | 60 (50/10) | ||||
Abbreviation: FFB, final formulation buffer.
Vaccine
The Ad26.ENVA.01 vaccine product was produced in complementing HER96 cells by Crucell Holland BV (Leiden, Netherlands). A replication-deficient (delta-Early region 1/Early region 3[E1/E3]) adenoviral vector type 26 (Ad26) was constructed to contain an HIV-1 Clade A Env gene encoding a modified envelope gp140 protein (see Supplement Data). The placebo was final formulation buffer.
Immunogenicity Studies
All immunogenicity assays were performed in a blinded fashion under Good Clinical Laboratory Practice (GCLP) conditions. Luciferase-based adenovirus neutralization assays were performed to assess Ad26-specific neutralizing antibodies (NAbs) as previously described [17, 18]. Direct enzyme-linked immunosorbent assays (ELISAs) were performed with sera to assess EnvA-specific binding antibodies against the vaccine immunogen [13]. Interferon (IFN)-γ enzyme-linked immunospot assays (ELISPOTs) were performed to assess Env-specific cellular immune responses using a pool of overlapping EnvA peptides [13]. Criteria for positive Ad26 NAb responses were titer ≥18 and Env ELISA responses were titer ≥100. Criteria for ELISPOT positivity were ≥55 spot-forming cells (SFC) per million peripheral blood mononuclear cell (PBMC) and at least 3× greater than background. The EnvA protein and peptides were kindly provided by the National Institutes of Health Vaccine Research Center (Bethesda, MD).
Statistical Methods
All analyses are based on the intent-to-treat principle including all subjects in the group to which they were randomized. Summaries of response rates are presented with median and geometric mean titers (GMTs) for the Ad NAb and HIV-1 ELISA and means for the HIV-1 ELISPOTs, all with associated 95% confidence intervals (CIs). Analysis of variance (ANOVA) was used to test for differences in means, and the Kruskall-Wallis test was performed as a check on the assumptions in ANOVA.
RESULTS
Of the 60 subjects enrolled, 38 (63%) were female, 40 (67%) were <30 years (range 18–47 years), and 14 (23%) were non-Caucasian/non-Hispanic. Overall retention was 98%, and 148 of 156 (95%) of the planned vaccinations were given (124/130 active vaccine and 24/26 placebo). Of those screened for Ad26 seropositivity, 36 of 365 (9.9%) were positive, all at a relatively low titer. One subject who received the 5 × 1010 vp dose and 1 placebo recipient were lost to follow-up shortly after the first vaccination and were therefore not included in the analyses.
Safety and Tolerability
The Ad26.ENVA.01 vaccine was safe and generally well tolerated at all 4 doses (see Supplementary Data for further safety information, including Supplementary Figures 1A and 1B). Minimal reactogenicity was observed in the 109 or 1010 vp dose groups. Overall, no significant differences in local or systemic reactogenicity were observed between groups (Supplementary Figures 1A and 1B). Some reactogenicity was observed after the initial immunization of the 1011 vp dose. This reactogenicity after the first dose of Ad26 at 1011 vp is similar to that reported with Ad5 at this dose [21–23]. No vaccine-related serious adverse events (SAEs) occurred, and no pattern of clinical or laboratory adverse events was identified.
Immunogenicity
Ad26 Neutralizing Antibody Responses
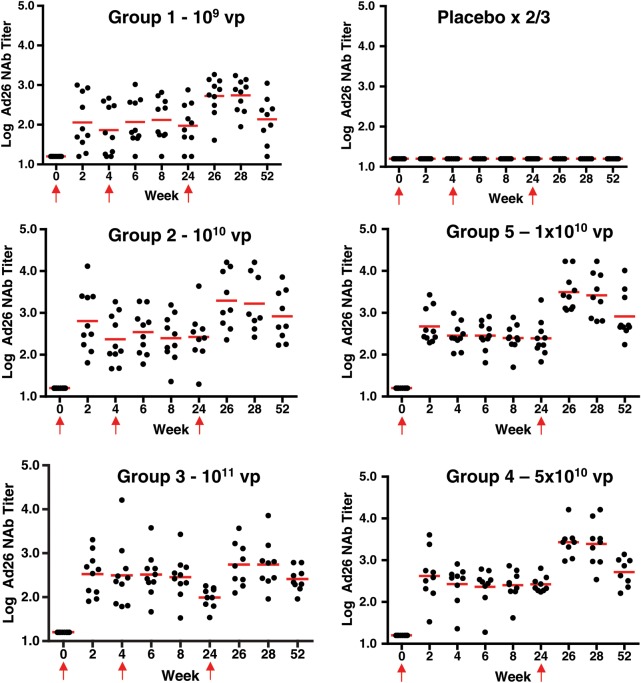
All subjects were seronegative for Ad26 at baseline. Figure 1 shows the kinetics of the response by dose group. All placebo recipients had negative Ad26 NAb titers throughout the course of the study. Ninety-eight percent of vaccine recipients had detectable Ad26 NAb titers by day 14 after first vaccination (only 1 subject had a negative titer in the 109 vp dose group; this subject developed a NAb titer by day 42). The Ad26 NAb titers persisted in all but 1 subject (a 109 vp recipient) at week 52.
Figure 1.
Ad26 neutralizing antibody responses by group. Individual Ad26 neutralizing antibody responses from subjects by week and vaccine group are shown. Dots show individual responses at a given time point. Red horizontal lines show the median values at a given time point for the group. Red arrows show times when vaccine or placebo was administered (the week 4 placebo injection was not given in groups 4 and 5).
A dose response was observed in the Ad26 NAb GMTs at week 4 after the initial immunization: 73 (group 1–109; 95% CI, 26, 204), 233 (group 2–1010; 95% CI, 89, 606), 313 (group 3–1011; 95% CI, 95, 1035), 268 (group 4–5 × 1010; 95% CI, 118, 611), and 284 (group 5–1010; 95% CI, 173, 468; P < .01 for all groups compared to the placebo group).
Week 24 Ad26 NAb titers (prior to the second/third boost immunization) were similar to week 4 titers but were substantially increased by week 28 (4 weeks following the boost immunization) as follows: 551 (group 1–109; 95% CI, 279, 1087), 1678 (group 2–1010; 95% CI, 442, 6367), 549 (group 3–1011; 95% CI, 209, 1445), 2559 (group 4–5 × 1010; 95% CI, 987, 6638), and 2616 (group 5–1010; 95% CI, 1019, 6718). In subjects who received a dose of 1010 or greater, no clear dose or schedule effects were observed. No interference was identified between the induction of antivector immune responses and the subsequent elicitation of HIV-specific immune responses. These data show that Ad26.ENVA.01 consistently elicited vector-specific NAbs that increased with subsequent boost immunizations.
HIV-1 Env-Specific Antibody Responses
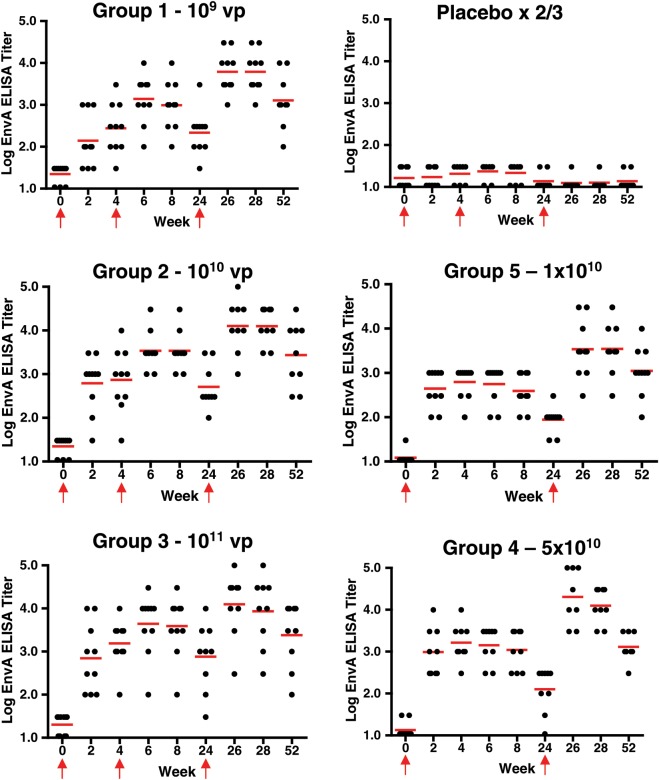
All subjects had negative EnvA-specific ELISA binding antibody titers at baseline, and all subjects in the placebo group remained negative throughout the course of the study. Figure 2 shows the kinetics of the response by dose group. Following 1 vaccination at week 4, 47/49 (96%) of vaccine recipients developed an EnvA-specific ELISA titer. The 2 subjects who did not develop a response were in the 109 and 1010 groups and developed a response at week 6, 2 weeks following the second injection. All vaccinees had persistent antibody responses at week 52.
Figure 2.
EnvA enzyme-linked immunosorbent assays (ELISA) responses by group. Individual EnvA ELISA responses from subjects by week and vaccine group are shown. Dots show individual responses at a given time point. Red horizontal lines show the median values at a given time point for the group. Red arrows show times when vaccine or placebo was administered (the week 4 placebo injection was not given in groups 4 and 5).
A dose response was observed with the ELISA GMTs at week 4: 275 (group 1–109; 95% CI, 103, 735), 739 (group 2–1010; 95% CI, 227, 2411), 1552 (group 3–1011; 95% CI, 646, 3727), 1630 (group 4–5 × 1010; 95% CI, 748, 3553), and 624 (group 5–1010; 95% CI, 348, 1119). All active vaccination groups had responses significantly greater than placebo (P < .0001). Significant differences were seen between group 3 and group 1 (P = .002) and between group 4 and group 1 (P = .002).
Despite the induction of vector-specific NAbs, Env-specific ELISA responses were augmented at week 8 (4 weeks after the second immunization), again in a dose-dependent fashion: (group 1–109: 979–95% CI, 378, 2535; group 2–1010: 3420–95% CI, 1673, 6991; and group 3–1011: 3898–95% CI, 1236, 12 290). ELISA responses were further increased at week 28 (4 weeks after the third vaccination): (group 1–109: 6113–95% CI, 2763, 13 526; group 2–1010: 12 470–95% CI, 5987, 25 971; and group 3–1011: 8545–95% CI, 2041, 35 764) and persisted at week 52.
The impact of vaccination schedule was also assessed by comparing group 2 (3 doses) and group 5 (2 doses). Similar ELISA titers were observed at week 4 after the first vaccination. As expected, higher titers were observed at week 8 after 2 doses (group 2: 3419) vs 1 dose (group 5: 390; P < .0001). This difference persisted at week 24 (506 vs 88), week 28 (12 470 vs 3470), and week 52 (2718 vs 1104). These data show that Ad26.ENVA.01 consistently induced EnvA-specific binding antibody responses and that these responses could be augmented by boosting with a homologous vector. No significant HIV-specific neutralizing antibody responses were detected by TZM-bl assays.
HIV-1 Env-Specific Cellular Immune Responses
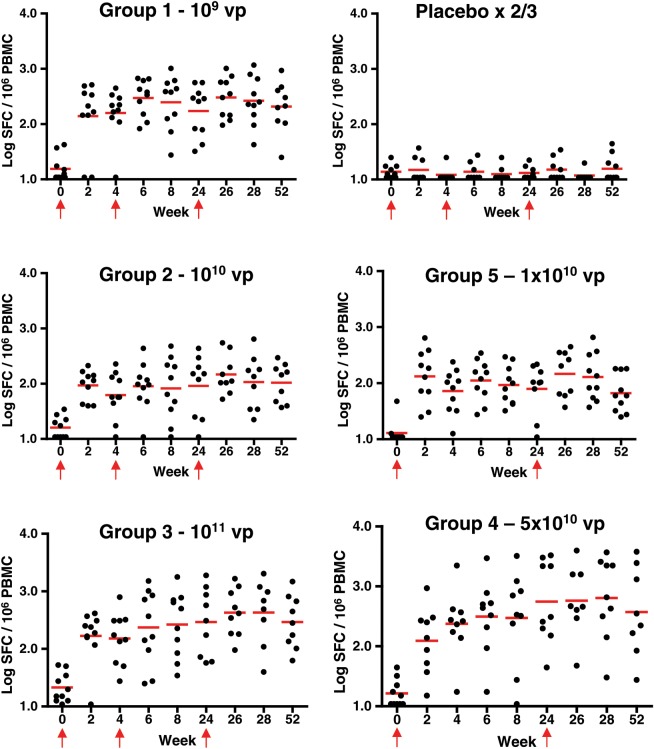
No Env-specific cellular immune responses above background were detected by IFN-γ ELISPOT assays at baseline, and none of the placebo subjects developed Env-specific ELISPOT responses at any time point in the study. Figure 3 shows the kinetics of the response by dose group. At week 4, 38/49 (78%) of vaccinees had detectable ELISPOT responses. Of the 11 without a detectable response at week 4, 9 had a response at week 2 and the other 2 (1 each in groups 2 and 5) developed a response subsequently. By week 52, responses were still observed in 80% of vaccinees. Of note, 7 of 8 (88%) volunteers in group 4 and 6 of 10 (60%) in group 5 had responses at week 52.
Figure 3.
EnvA enzyme-linked immunospot assays (ELISPOT) responses by group. Individual EnvA ELISPOT responses from subjects by week and vaccine group are shown. Dots show individual responses at a given time point. Red horizontal lines show the median values at a given time point for the group. Red arrows show times when vaccine or placebo was administered (the week 4 placebo injection was not given in groups 4 and 5). Abbreviations: PBMC, peripheral blood mononuclear cell; SFC, spot-forming cell.
Mean ELISPOT responses at weeks 4 and 28 were as follows: 212 and 397 SFC per million PBMC (group 1–109; 95% CI, 121, 302 and 137, 658), 88 and 178 (group 2–1010; 95% CI, 37, 138 and 27, 328), 232 and 736 (group 3–1011; 95% CI, 71, 394 and 176, 1297), 458 and 1312 (group 4–5 × 1010; 95% CI, 0, 975 and 314 and 2309), and 98 and 196 (group 5–1010; 95% CI, 47, 148 and 52 and 340; all significantly greater than the placebo group). These responses were similar in all groups at weeks 8 and 52 as well. No clear dose or schedule effects were observed. These data show that Ad26.ENVA.01 consistently induced EnvA-specific cellular immune responses.
DISCUSSION
The novel Ad26.ENVA.01 HIV-1 vaccine candidate was generally safe and well tolerated and induced HIV-specific humoral and cellular immune responses. These responses were induced after a single immunization in nearly all subjects, including those who received the lowest dose (109 vp). All subjects who received the vaccine developed both Ad26 vector-specific and EnvA insert-specific immune responses that persisted for at least 1 year. These findings are consistent with the data in the nonhuman primate model where Ad26 vectors have been shown to raise consistent humoral and cellular responses and provide some protection against SIV [13, 16, 24, 25]. Because Ad26 has many biologic properties that are different from those of Ad5 and as seen in the accompanying paper by Barouch et al, [28] its potential as a vaccine platform warrants further study.
Reactogenicity was observed only at the highest dose (1011 vp) administered, was self-limited in nature, and was observed only with the initial vaccination. This is similar to reactogenicity reported for Ad5 vectors at this same dose [21–23] and possibly less than the reactogenicty observed for Ad5 at doses less than 1011 vp. No vaccine-associated SAEs were identified, and the overall safety findings were similar in placebo and vaccine recipients.
Env-specific T-cell responses were elicited at all 3 doses, including the lowest dose (109 vp). Because lower doses were not studied, the threshold for the induction of T-cell responses in humans with this vector is unknown. T-cell responses were apparently not boosted by dose (more than a 100-fold range) or increased number (2 vs 3) of immunizations. Further characterization of the immune responses elicited by these vaccine regimens are presented in the accompanying paper by Barouch et al [28].
Env-specific binding antibody responses detected by ELISA were elicited in nearly all subjects after the first immunization, including those who received the lowest dose. Responses were boosted substantially in all groups. A dose-response effect was observed with significantly higher titers in the 1011 vp dose group compared with the 109 vp group. In the 1010 vp dose group, a direct comparison of the effect of the number of immunizations can be made. Here the 3-dose regimen elicited higher ELISA titers compared with the 2-dose regimen by week 6 (2 weeks after the second vaccination), and this difference was maintained for up to 1 year. Further research is warranted to determine if more vaccinations will further augment immune responses over time.
Ad26-specific NAb responses were also elicited at all vaccine doses, were evident after the first dose and were boosted substantially in all groups. Despite the induction of robust vector-specific NAb responses, EnvA insert-specific antibody responses were increased following boosts with the homologous vectors.
A phase 2b study of the Merck Ad5-gag/pol/nef vaccine showed no protective efficacy and possible enhanced HIV-1 acquisition in certain subgroups [1]. Our vaccine candidate is different from this prior vaccine in important ways. First, we utilize Ad26 instead of Ad5 as a vaccine vector, which has major biological differences, including different receptor usage, innate immune profiles, and adaptive immune phenotypes [13–18]. Second, our vector expresses HIV-1 Env, which is potentially relevant since Env-specific antibodies appear to be important in blocking acquisition of infection in both human and nonhuman primate studies [13, 26].
These data demonstrate the safety and immunogenicity of a novel Ad26 vaccine vector in humans for the first time. As no safety concerns were identified, future studies of the 5 × 1010 vp dose, given the lower reactogenicity and comparable immunogenicity, are warranted. Importantly, Env-specific humoral and cellular immune responses were consistently elicited over a 100-fold dose range with only minimal reactogenicity at the highest dose studied. Further evaluation is needed to determine if pre-existing Ad26 immunity will alter the safety or immunogenicity profile observed. Ad26 vectors therefore warrant additional clinical investigation as vaccine vectors for both HIV and other pathogens [27].
Supplementary Data
Supplementary materials are available at The Journal of Ifectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank the Safety Monitoring Committee members Drs Peter Wright (Chair), Michael Keefer, and Paul Goepfert; the NIH Vaccine Research Center, Bethesda, MD, for the EnvA vaccine antigen, EnvA protein, and peptides; the Investigational Drug Service at Brigham and Women's Hospital; the clinical research staff at Brigham and Women's Hospital (Rozalia Kocjan, Patrick Falahee, Christine Matera, Brian Engelson, John Gothing, Daniel Worrall, Marissa Wilck, Nicolas Issa, Francisco Marty); research staff at Beth Israel Deaconess Medical Center (Kara Brandariz, Annalena LaPorte, Rebecca Dilan, Giannina Santos, Elizabeth Christian, Alexis Burbank, Avinash Oza, Caroline Miller, Justin Dalrymple, Shirin Bajimaya, Brittany Carey, David Dominguez, and Lauren E. Grandpre); Crucell Holland BV staff (Laura Digilio, Sandra Kik, Jerry Sadoff, Hanneke Schuitemaker); and National Institute of Allergy and Infectious Diseases/NIH staff (Mike Pensiero, Alan Fix, Elizabeth Adams, and MaryAnne Luzar).
Financial support. The project was supported in part by the following NIH grants: AI066305; AI078526; AI069412; AI096040; AI060354; RR025758; and the Ragon Institute of MGH, MIT, and Harvard.
Potential conflicts of interest. P.M.G., W.M., and G.J. are employees of Crucell Holland, B. V. Patents for the Ad26 construct are held in part by Crucell and BIDMC Beth Israel Deaconess Medical Center. No licensing agreements, royalties, or income are associated with these patents. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Buchbinder SP, Mehrotra DV, Duerr A, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372:1881–93. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McElrath MJ, De Rosa SC, Moodie Z, et al. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet. 2008;372:1894–905. doi: 10.1016/S0140-6736(08)61592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–20. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 4.Flynn NM, Forthal DN, Harro CD, Judson FN, Mayer KH, Para MF. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J Infect Dis. 2005;191:654–65. doi: 10.1086/428404. [DOI] [PubMed] [Google Scholar]
- 5.Baden LR, Blattner WA, Morgan C, et al. Timing of plasmid cytokine (IL-2/Ig) administration affects HIV-1 vaccine immunogenicity in HIV-seronegative subjects. J Infect Dis. 2011;204:1541–9. doi: 10.1093/infdis/jir615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bart PA, Goodall R, Barber T, et al. EV01: a phase I trial in healthy HIV negative volunteers to evaluate a clade C HIV vaccine, NYVAC-C undertaken by the EuroVacc Consortium. Vaccine. 2008;26:3153–61. doi: 10.1016/j.vaccine.2008.03.083. [DOI] [PubMed] [Google Scholar]
- 7.Churchyard GJ, Morgan C, Adams E, et al. A phase IIA randomized clinical trial of a multiclade HIV-1 DNA prime followed by a multiclade rAd5 HIV-1 vaccine boost in healthy adults (HVTN204) PLoS One. 2011;6:e21225. [Google Scholar]
- 8.Goepfert PA, Elizaga ML, Sato A, et al. Phase 1 safety and immunogenicity testing of DNA and recombinant modified vaccinia Ankara vaccines expressing HIV-1 virus-like particles. J Infect Dis. 2011;203:610–9. doi: 10.1093/infdis/jiq105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koblin BA, Casapia M, Morgan C, et al. Safety and immunogenicity of an HIV adenoviral vector boost after DNA plasmid vaccine prime by route of administration: a randomized clinical trial. PLoS One. 2011;6:e24517. doi: 10.1371/journal.pone.0024517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalams SA, Parker S, Jin X, et al. Safety and immunogenicity of an HIV-1 gag DNA vaccine with or without IL-12 and/or IL-15 plasmid cytokine adjuvant in healthy, HIV-1 uninfected adults. PLoS One. 2012;7:e29231. doi: 10.1371/journal.pone.0029231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barouch DH, Kik SV, Weverling GJ, et al. International seroepidemiology of adenovirus serotypes 5, 26, 35, and 48 in pediatric and adult populations. Vaccine. 2011;29:5203–9. doi: 10.1016/j.vaccine.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mast TC, Kierstead L, Gupta SB, et al. International epidemiology of human pre-existing adenovirus (Ad) type-5, type-6, type-26 and type-36 neutralizing antibodies: correlates of high Ad5 titers and implications for potential HIV vaccine trials. Vaccine. 2010;28:950–7. doi: 10.1016/j.vaccine.2009.10.145. [DOI] [PubMed] [Google Scholar]
- 13.Barouch DH, Liu J, Li H, et al. Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature. 2012;482:89–93. doi: 10.1038/nature10766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barouch DH. Novel adenovirus vector-based vaccines for HIV-1. Curr Opin HIV AIDS. 2010;5:386–90. doi: 10.1097/COH.0b013e32833cfe4c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu J, Ewald BA, Lynch DM, et al. Magnitude and phenotype of cellular immune responses elicited by recombinant adenovirus vectors and heterologous prime-boost regimens in rhesus monkeys. J Virol. 2008;82:4844–52. doi: 10.1128/JVI.02616-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu J, O'Brien KL, Lynch DM, et al. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature. 2009;457:87–91. doi: 10.1038/nature07469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lore K, Adams WC, Havenga MJ, et al. Myeloid and plasmacytoid dendritic cells are susceptible to recombinant adenovirus vectors and stimulate polyfunctional memory T cell responses. J Immunol. 2007;179:1721–9. doi: 10.4049/jimmunol.179.3.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li H, Rhee EG, Masek-Hammerman K, Teigler JE, Abbink P, Barouch DH. Adenovirus serotype 26 utilizes CD46 as a primary cellular receptor and only transiently activates T lymphocytes following vaccination of rhesus monkeys. J Virol. 2012;86:10862–5. doi: 10.1128/JVI.00928-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Radosevic K, Rodriguez A, Lemckert AA, et al. The Th1 immune response to Plasmodium falciparum circumsporozoite protein is boosted by adenovirus vectors 35 and 26 with a homologous insert. Clin Vaccine Immunol. 2010;17:1687–94. doi: 10.1128/CVI.00311-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geisbert TW, Bailey M, Hensley L, et al. Recombinant adenovirus serotype 26 (Ad26) and Ad35 vaccine vectors bypass immunity to Ad5 and protect nonhuman primates against ebolavirus challenge. J Virol. 2011;85:4222–33. doi: 10.1128/JVI.02407-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Catanzaro AT, Koup RA, Roederer M, et al. Phase 1 safety and immunogenicity evaluation of a multiclade HIV-1 candidate vaccine delivered by a replication-defective recombinant adenovirus vector. J Infect Dis. 2006;194:1638–49. doi: 10.1086/509258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Priddy FH, Brown D, Kublin J, et al. Safety and immunogenicity of a replication-incompetent adenovirus type 5 HIV-1 clade B gag/pol/nef vaccine in healthy adults. Clin Infect Dis. 2008;46:1769–81. doi: 10.1086/587993. [DOI] [PubMed] [Google Scholar]
- 23.Harro CD, Robertson MN, Lally MA, et al. Safety and immunogenicity of adenovirus-vectored near-consensus HIV type 1 clade B gag vaccines in healthy adults. AIDS Res Hum Retroviruses. 2009;25:103–14. doi: 10.1089/aid.2008.0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abbink P, Lemckert AA, Ewald BA, et al. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J Virol. 2007;81:4654–63. doi: 10.1128/JVI.02696-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li H, Liu J, Carville A, Mansfield KG, Lynch D, Barouch DH. Durable mucosal simian immunodeficiency virus-specific effector memory T lymphocyte responses elicited by recombinant adenovirus vectors in rhesus monkeys. J Virol. 2011;85:11007–15. doi: 10.1128/JVI.05346-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haynes BF, Gilbert PB, McElrath MJ, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med. 2012;366:1275–86. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barouch DH, O'Brien KL, Simmons NL, et al. Mosaic HIV-1 vaccines expand the breadth and depth of cellular immune responses in rhesus monkeys. Nat Med. 2010;16:319–23. doi: 10.1038/nm.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barouch DH, Liu J, Peter L, et al. Detailed Characterization of Humoral and Cellular Immune Responses Elicited by a Recombinant Adenovirus Serotype 26 HIV-1 Env Vaccine in Healthy Adults (IPCAVD 001). J Infect Dis. 2013 doi: 10.1093/infdis/jis671. 207:248-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.