Abstract
Background. Adenovirus serotype 26 (Ad26) has been developed as a novel candidate vaccine vector for human immunodeficiency virus type 1 (HIV-1) and other pathogens. The primary safety and immunogenicity data from the Integrated Preclinical/Clinical AIDS Vaccine Development Program (IPCAVD) 001 trial, the first-in-human evaluation of a prototype Ad26 vector-based vaccine expressing clade A HIV-1 Env (Ad26.ENVA.01), are reported concurrently with this article. Here, we characterize in greater detail the humoral and cellular immune responses elicited by Ad26.ENVA.01 in humans.
Methods. Samples from the IPCAVD 001 trial were used for humoral and cellular immunogenicity assays.
Results. We observed a dose-dependent expansion of the magnitude, breadth, and epitopic diversity of Env-specific binding antibody responses elicited by this vaccine. Antibody-dependent cell-mediated phagocytosis, virus inhibition, and degranulation functional activity were also observed. Env-specific cellular immune responses induced by the vaccine included multiple CD8+ and CD4+ T-lymphocyte memory subpopulations and cytokine secretion phenotypes, although cellular immune breadth was limited. Baseline vector-specific T-lymphocyte responses were common but did not impair Env-specific immune responses in this study.
Conclusion. Ad26.ENVA.01 elicited a broad diversity of humoral and cellular immune responses in humans. These data support the further clinical development of Ad26 as a candidate vaccine vector.
Clinical Trials Registration. NCT00618605.
Keywords: HIV-1, Vaccine, Adenovirus 26, Immunogenicity
(See the major article by Baden et al, on pages 240–7.)
The development of a safe and effective human immunodeficiency virus type (HIV-1) vaccine is an urgent biomedical priority. Only 3 HIV-1 vaccine concepts have completed clinical efficacy testing to date [1–4], suggesting that additional HIV-1 vaccine candidates need to be explored in clinical trials [5–7]. It is important to understand the humoral and cellular immunogenicity profiles of HIV-1 vaccine candidates in humans to inform clinical development of novel vaccine vectors and vaccine strategies.
The failure of the Merck Ad5-gag/pol/nef vaccine in the phase 2b Step Study [3, 8] was a disappointment to the HIV-1 vaccine field but led to the development of alternative serotype adenovirus (Ad) vectors that may offer benefits over Ad5 vectors [9, 10]. Ad serotype 26 (Ad26) is a novel vector that shows substantial differences from Ad5 in several biologic characteristics, including seroepidemiology, cellular receptor use, in vivo tropism, innate immune profiles, adaptive immune phenotypes, and protective efficacy in rhesus monkeys [11–19]. In particular, Ad26 vectors combined with other Ad or poxvirus vectors have afforded partial protective efficacy against both acquisition of infection and virologic control following heterologous SIVmac251 challenges in rhesus monkeys [19].
In an accompanying manuscript [20], we describe the primary safety and immunogenicity results of the Integrated Preclinical/Clinical AIDS Vaccine Development Program (IPCAVD) 001 trial, a randomized, double-blinded, placebo-controlled, first-in-human phase 1 clinical trial of a prototype Ad26 vector–based vaccine expressing clade A HIV-1 Env (Ad26.ENVA.01). Ad26.ENVA.01 proved safe and immunogenic in healthy, Ad26-seronegative, HIV-1–uninfected subjects, and Env-specific humoral and cellular immune responses were assessed by enzyme-linked immunosorbent assays (ELISAs) and interferon γ (IFN-γ) enzyme-linked immunosorbent spot (ELISPOT) assays [20].
Here, we report a detailed characterization of humoral and cellular immune responses elicited by Ad26.ENVA.01 in humans in the IPCAVD 001 trial. Humoral immune responses were assessed by linear peptide arrays to evaluate magnitude, breadth, and epitopic diversity of Env-specific binding antibody responses and by functional nonneutralizing antibody assays. Cellular immune responses were assessed by epitope mapping, multiparameter intracellular cytokine staining (ICS) assays, and cytokine multiplex bead arrays. These data demonstrate the broad diversity of immune responses and the unique immunologic profile induced by this vaccine.
METHODS
Clinical Samples
Serum and peripheral blood mononuclear cell (PBMC) samples from weeks 0, 4, 8, 24, 28, and 52 of the IPCAVD 001 trial [20] were used for immunogenicity studies. All immunologic assays were performed by blinded investigators. The clinical protocol and laboratory studies were approved by the institutional review boards at Brigham and Women's Hospital and Beth Israel Deaconess Medical Center. Written informed consent was obtained from each subject. The clinical study was registered at ClinicalTrials.gov (NCT00618605).
Immunologic Assays
Detailed methods are provided in the Supplementary Materials. Briefly, linear antibody responses were assessed by peptide microarrays (JPT Peptide Technologies) as described elsewhere [21, 22]. Functional nonneutralizing antibody assays were assessed by a THP-1 phagocytosis assay, and antibody-dependent cell-mediated viral inhibition (ADCVI) was assessed as previously described [23, 24]. We also performed a multiplex binding assay that identified HIV-1–specific immunoglobulin G (IgG) to recombinant gp120, gp41, and gp140 to assess the distribution of IgG isotypes (bulk IgG, IgG1, IgG2, IgG3, and IgG4) among the HIV-1–specific antibodies, as previously described [25].
ICS assays were performed as described elsewhere [13, 16, 26]. Cytokine multiplex bead arrays were performed with PBMCs stimulated with the Env peptide pool. Samples were acquired on a Luminex 200 instrument (Millipore), and data analyses were performed using the Rumimex package (http://labs.fhcrc.org/fong/Ruminex/index.html) available on the R statistical programming system [27]. The concentration-response data of the standard samples of each analyte were modeled by a 5-parameter log-logistic curve [28]. B-cell ELISPOT assays were performed with ConS or BaL gp140 antigens.
Vector-specific cellular immune responses were assessed with IFN-γ ELISPOT assays, using Ad26 hexon peptide pools or purified Ad26 virus (103 virus particles [vp] per cell) to stimulate PBMCs [13, 29].
RESULTS
Env-Specific Humoral Immune Responses
As described in the primary manuscript associated with the IPCAVD 001 trial [20], we evaluated the safety and immunogenicity of a novel Ad26.ENVA.01 vaccine in humans at doses of 109 vp, 1010 vp, and 1011 vp (groups 1–3) administered at weeks 0, 4, and 24, as well as at doses of 5 × 1010 vp and 1010 vp (groups 4 and 5) administered at weeks 0 and 24. Vector concentration was determined by spectrophotometry. ELISAs revealed that Ad26.ENVA.01 induced consistent and boostable Env-specific binding antibodies in all groups, although HIV-1–specific neutralizing antibody responses were not detected, [20]. Here, we extend these humoral immune data by assessing the magnitude, breadth, and epitopic diversity of Env-specific binding antibody responses, as well as functional nonneutralizing antibody responses.
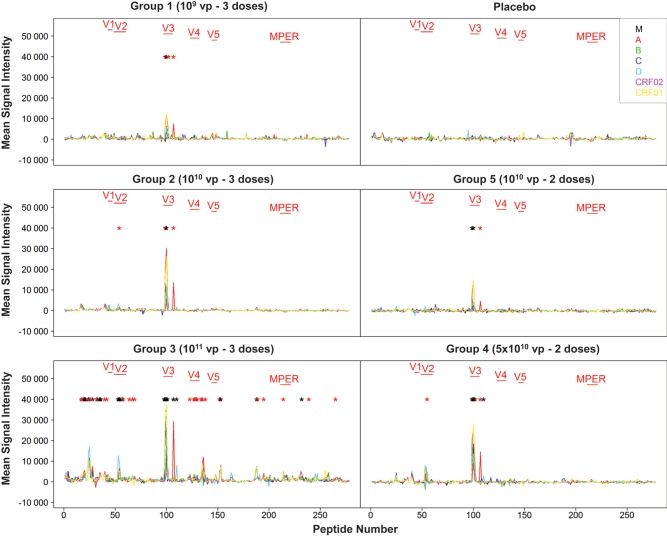
We first used peptide microarrays (JPT Peptide Technologies) to evaluate the capacity of antibodies in serum at peak immunity at week 28 (4 weeks following the final immunization) to bind linear peptides spanning multiple HIV-1 Env sequences from clades M, A, B, C, D, CRF02, and CRF01. This assay detects binding antibody responses against linear epitopes but not conformational epitopes. As shown in Figure 1, we observed robust cross-clade binding antibody responses to peptides in the V3 loop region in all groups. The largest number of peptide responses was observed in subjects who received the 1011 vp dose followed by the 5 × 1010 vp dose, showing that higher vaccine doses resulted in not only higher magnitude responses but also increased breadth and epitopic diversity. For example, responses against the V2 loop [29] were observed with vaccine doses >1010 vp. No responses were detected by these assays in the placebo recipients. Broad binding antibody responses against multiple clades were observed at the highest dose.
Figure 1.
Env-specific binding antibody profiles by peptide arrays. Sera from baseline and week 28 were assessed using peptide arrays (JPT Peptide Technologies) to assess linear antibody responses to peptides spanning Env from clades M, A, B, C, D, CRF02, and CRF01. Mean signal intensity (week 28 with baselines subtracted) from all subjects in each group is depicted. Black asterisks denote peptides with significantly elevated mean signal intensity as compared with placebos (P < .05, by Wilcoxon Mann–Whitney U tests), and red asterisks denote trends (P < .10).
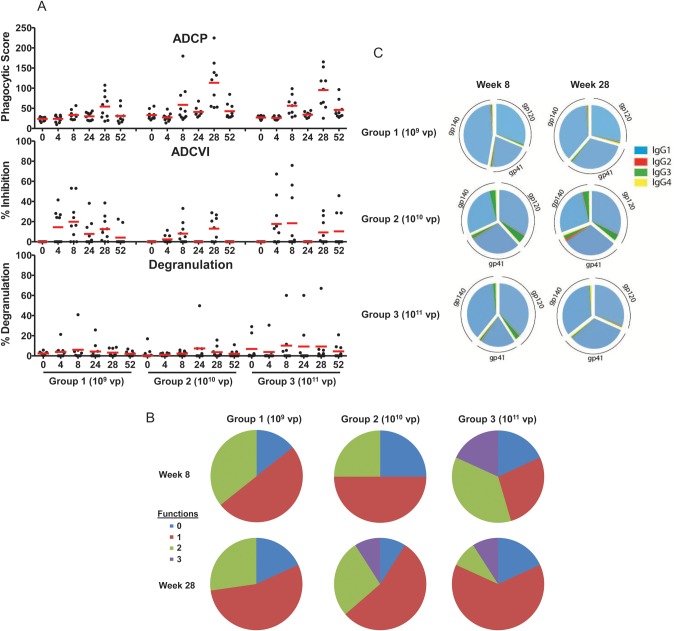
We next assessed functional nonneutralizing antibody responses in individuals who received vaccine doses of 109 vp, 1010 vp, and 1011 vp. As shown in Figure 2A, induction of antibody-dependent cellular phagocytosis (ADCP) was evident in some vaccinees at week 8 and was observed in 90% of vaccinees in the 1010 and 1011 vp groups at peak immunity at week 28. These ADCP responses waned over time but were augmented following a homologous boost immunization. Antibody-dependent cell-mediated virus inhibition (ADCVI) and degranulation responses were also observed in a subset of vaccinees. No responses were detected in placebo recipients (data not shown). As depicted in Figure 2B, serum antibody responses often included multiple functions, with triple function (ADCP/ADCVI/degranulation) responses observed most frequently in subjects who received the higher vaccine doses and following the boost immunization. These data demonstrate that multiple nonneutralizing functional antibody responses were induced by Ad26.ENVA.01, although the protective capacity of these antibody responses remains to be determined.
Figure 2.
Env-specific functional nonneutralizing antibody binding responses and antibody isotypes. A, Serum from multiple time points from subjects who received 109 viral particles (vp), 1010 vp, and 1011 vp of the vaccine were assessed for antibody-dependent cellular phagocytosis (ADCP), cellular inhibition (ADCVI), and macrophage inflammatory protein 1β degranulation. Bars represent mean responses. B, Percentage of vaccinees in these groups at week 8 and week 28 that exhibit multiple antibody functions (ADCP/ADCVI/degranulation). C, Immunoglobulin G (IgG) isotypes of Env gp140-, gp120-, and gp41-specific binding antibodies from week 8 and week 28 from subjects who received 109 vp, 1010 vp, and 1011 vp of the vaccine are shown in pie charts. Mean proportional IgG1, IgG2, IgG3, and IgG4 responses are shown for each group.
We also assessed the isotypes of the antibodies induced by this vaccine. As shown in Figure 2C, Env-specific binding antibodies at week 8 and week 28 were >90% IgG1, with minor contributions of IgG3. Serum Ig A responses were not detected (data not shown). IgG1 and IgG3 antibody isotypes readily bind particular Fc receptors, suggesting that antibodies induced by Ad26.ENVA.01 may be efficient at recruiting innate immune effector functions. Interestingly, the IgG1 and IgG3 profile observed in RV144 parallels the antibody profile observed in this study, whereas antibody responses in VAX003 were characterized by enhanced levels of IgG2 and IgG4 (G. Alter et al, unpublished data).
Env-Specific Cellular Immune Responses
In the primary manuscript associated with the IPCAVD 001 trial, we report that Ad26.ENVA.01 elicited IFN-γ ELISPOT assay responses in all groups, with no clear dose response over the range of doses studied and with durability of responses for 52 weeks [20]. Here, we extend these data with additional studies to define the cellular immune breadth, T-lymphocyte subpopulations, and cytokine secretion phenotypes elicited by this vaccine. For these studies, we selected group 4 for detailed study because the 5 × 1010 vp dose represents the vector dose selected for further clinical development.
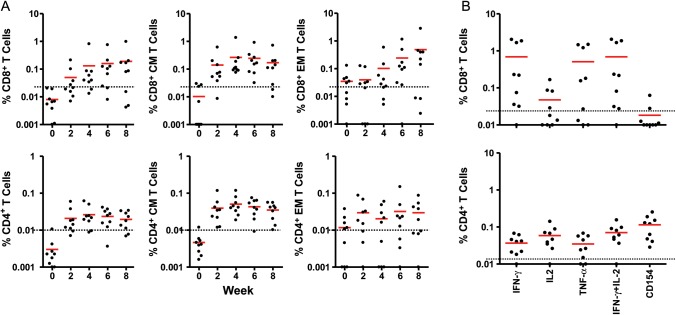
Epitope mapping studies revealed the induction of a median of 1 epitope (range, 0–3 epitopes) per individual (data not shown), suggesting limited cellular immune breadth to this prototype clade A Env immunogen. Multiparameter ICS assays demonstrated that the vaccine elicited both central memory (CD27+CD45RO+) and effector memory (CD27–CD45RO+) CD8+ and CD4+ T-lymphocyte responses, as shown in Figure 3A. Positive total and central memory CD8+ ICS responses were detected in 67% and 100% of subjects, respectively, at week 4. CD4+ T-lymphocyte responses were approximately 4-fold lower than CD8+ T-lymphocyte responses. Moreover, Env-specific CD8+ and CD4+ T lymphocytes at peak immunity at week 28 secreted multiple cytokines, including IFN-γ, interleukin 2 (IL-2), and tumor necrosis factor α (TNF-α), and upregulated CD40L (CD154), as depicted in Figure 3B. A broad range of response magnitudes was observed (0.03%–2.04% of CD8+ T lymphocytes and 0.01%–0.07% of CD4+ T lymphocytes for IFN-γ secretion).
Figure 3.
Env-specific CD8+ and CD4+ T-lymphocyte responses by intracellular cytokine staining assays. A, Peripheral blood mononuclear cells (PBMCs) from multiple time points from subjects who received 5 × 1010 viral particles (vp) of the vaccine were assessed for Env-specific interferon-γ (IFN-γ)–secreting CD8+ and CD4+ total, central memory (CM), and effector memory (EM) T-lymphocyte responses. Responses are depicted as percentage of IFN-γ–secreting cells divided by total, CM, or EM CD8+ or CD4+ T lymphocytes. B, PBMCs from these subjects at week 28 were assessed for secretion of multiple cytokines (IFN-γ, interleukin 2 [IL-2], and tumor necrosis factor-α [TNF-α]) and expression of CD154 from Env-specific CD8+ and CD4+ T-lymphocytes. T-lymphocyte responses were assessed following stimulation with pooled clade A Env peptides. Bars reflect mean responses. Average placebo assay backgrounds are shown as dotted lines.
Additional cytokine secretion phenotype data were generated by stimulation of PBMCs from week 28 with pooled clade A Env peptides and analysis of cytokines in culture supernatants by cytokine multiplex bead arrays. As shown in Figure 4A, multiple cytokines were induced, in particular granulocyte-macrophage colony-stimulating factor, IFN-γ, IL-2, interleukin 10, interleukin 13, macrophage inflammatory protein 1β, and TNF-α. Taken together, these data demonstrate that Ad26.ENVA.01 elicited limited cellular immune breadth but induced a diversity of T-lymphocyte subpopulations and cytokine secretion phenotypes.
Figure 4.
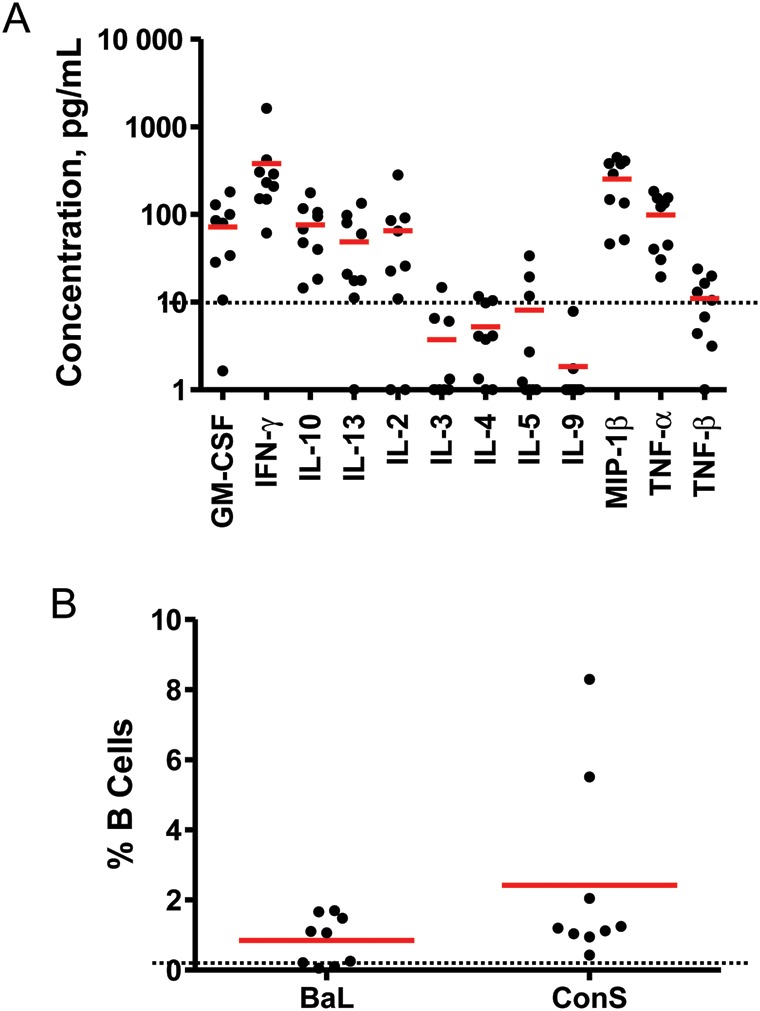
Env-specific cytokine secretion and B-lymphocyte enzyme-linked immuno spot (ELISPOT) assay responses. A, Peripheral blood mononuclear cells (PBMCs) from week 28 from subjects who received 5 × 1010 viral particles of the vaccine were assessed for secretion of multiple cytokines following stimulation with pooled clade A Env peptides by cytokine multiplex bead arrays. Concentrations of each cytokine are shown. Bars reflect mean responses. GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN-γ, interferon γ; IL-2, interleukin 2; IL-3, interleukin 3; IL-4, interleukin 4; IL-5, interleukin 5; IL-9, interleukin 9; IL-10, interleukin 10; IL-13, interleukin 13; MIP-1β, macrophage inflammatory protein 1β; TNF-α, tumor necrosis factor α; TNF-β, tumor necrosis factor β. B, PBMCs from week 28 from subjects who received 5 × 1010 vp of the vaccine were assessed for memory B-lymphocyte responses following stimulation with purified BaL or ConS gp140 in B-lymphocyte ELISPOT assays. Bars reflect mean responses. Average placebo assay backgrounds are shown as dotted lines.
We also extended the cellular immune analysis to evaluate memory B-lymphocyte responses in PBMCs obtained during week 28 from this group, using a B-cell ELISPOT assay. As depicted in Figure 4B, we observed that 100% of vaccinees demonstrated B-lymphocyte responses to the clade B BaL and consensus ConS gp140 antigens, with 0.1%–1.7% of B lymphocytes responding to BaL and 0.4%–8.3% of B lymphocytes responding to ConS. These data are consistent with the binding antibody data (Figure 1) and show that Ad26.ENVA.01 induced robust cross-clade, Env-specific memory B-lymphocyte responses.
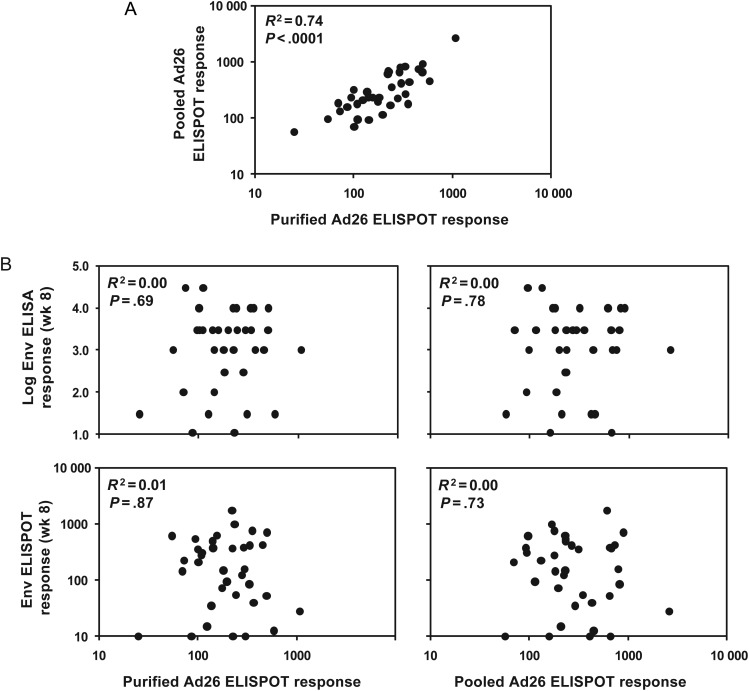
Vector-Specific Cellular Immune Responses
All individuals in the IPCAVD 001 trial were selected to be Ad26 seronegative at study entry, and thus this study does not address whether baseline Ad26 neutralizing antibodies may impact vaccine immunogenicity. However, as shown in Figure 5A, we observed that nearly all (>95%) subjects who received the 109 vp, 1010 vp, and 1011 vp doses of the vaccine had detectable Ad26-specific cellular immune responses at baseline, as measured by vector-specific ELISPOT assays that used either purified Ad26 virus or overlapping Ad26 hexon peptides for stimulation. These responses likely reflect Ad-specific T lymphocytes that are cross-reactive across multiple Ad serotypes, consistent with previous reports from our laboratories and others [29, 31–33]. As shown in Figure 5B, baseline Ad26-specific cellular immune responses at week 0 were not correlated with Env-specific ELISA or ELISPOT assay responses following vaccination at week 8 (R2 = 0.00–0.01; P = .69–.87). Similarly, baseline Ad26-specific cellular immune responses were not correlated with Env-specific ELISA or ELISPOT assay responses at week 28 (data not shown). These data show that baseline Ad26-specific T-lymphocyte responses did not have a detectable impact on vaccine immunogenicity in this study.
Figure 5.
Adenovirus serotype 26 (Ad26)–specific T-lymphocyte responses by enzyme-linked immuno spot (ELISPOT) assays. A, Baseline Ad26-specific cellular immune responses were assessed by ELISPOT assays following stimulation with purified Ad26 virus or pooled Ad26 hexon peptides. B, Lack of correlation between baseline Ad26-specific cellular immune responses at week 0 and Env-specific humoral ELISA (top) and cellular ELISPOT assay (bottom) responses at week 8 from subjects who received 109 viral particles (vp), 1010 vp, and 1011 vp of the vaccine (Spearman rank-correlation tests).
DISCUSSION
In this article, we describe detailed humoral and cellular immune responses elicited by Ad26.ENVA.01 in the IPCAVD 001 trial, the first-in-human evaluation of an Ad26 vaccine vector. We used state-of-the-art immunologic assays to complement the primary immunogenicity data, which are reported in an accompanying manuscript [20]. The magnitude, breadth, and epitopic diversity of binding antibody responses appeared to be dose dependent, and functional nonneutralizing antibody responses were observed. The vaccine elicited both B- and T-lymphocyte responses, and multiple T-lymphocyte subpopulations and cytokine secretion phenotypes were induced. These data demonstrate that Ad26.ENVA.01 elicited a broad diversity of humoral and cellular immune responses that help define the unique immunologic profiles elicited by this vaccine vector.
Ad26 is biologically substantially different from the well-characterized Ad5 vector by lower titers of vector-specific neutralizing antibodies worldwide [12], the use of CD46 rather than the coxsackievirus and Ad receptor (CAR) as its primary cellular receptor [11], and the induction of different immune phenotypes in preclinical studies [13, 16]. Moreover, Ad26 vectors combined with either MVA vectors or Ad35 vectors afforded partial protection against both acquisition of infection and virologic control following heterologous SIVmac251 challenges in rhesus monkeys [19]. Ad vectors from other species, such as chimpanzees, are also being explored in clinical trials [34–36]. Moreover, in the present study, we evaluated Ad26 vectors expressing HIV-1 Env, which represents an important antigen for protection [19, 30]. Thus, Ad26.ENVA.01 has key differences from the Ad5-Gag/Pol/Nef vaccine that was used in the Step study [3].
The humoral immune responses elicited by Ad26.ENVA.01 demonstrated broad and potent binding antibody responses against multiple clades (Figure 1), as well as nonneutralizing functional activity (Figure 2). The lack of neutralizing activity induced by Ad26.ENVA.01, however, suggests that the use of improved or engineered Env antigen sequences and/or more potent heterologous prime-boost regimens may be desirable for future studies. Moreover, the induction of V2-specific antibody responses with vaccine doses of >1010 vp (Figure 1) may be relevant in light of the RV144 correlates analysis that raised the hypothesis that V2-specific antibodies elicited by the ALVAC/gp120 vaccine may have reduced HIV-1 acquisition risk [30].
Ad26.ENVA.01 induced both CD8+ and CD4+ T-lymphocyte responses with a diversity of central and effector memory subpopulations and cytokine secretion phenotypes (Figures 3–4). Cellular immune breadth, however, remained limited. Future studies are therefore planned to evaluate the immunogenicity of Ad26 vectors expressing bioinformatically optimized mosaic HIV-1 Gag/Pol/Env immunogens [9, 37], which expanded CD8+ and CD4+ T-lymphocyte breadth without compromising Env-specific antibody responses in rhesus monkeys [38]. A limitation of the present studies is that we were only able to assess peripheral immune responses in the IPCAVD 001 trial. Preclinical studies in both mice and rhesus monkeys have shown that Ad26 vectors delivered by the intramuscular route can also elicit robust mucosal immune responses [39–41]. Future studies are therefore warranted to assess antigen- and vector-specific mucosal immune responses and mucosal inflammatory responses elicited by Ad26 vectors in humans.
A previous study reported that baseline Ad5-specific T-lymphocyte responses inhibited the induction of antigen-specific cellular immune responses by Ad5-gag/pol/nef vaccine vectors in humans [31]. In contrast to these data, we did not observe an impact of baseline Ad26-specific T-lymphocyte responses on the subsequent development of Env-specific humoral or cellular immune responses in this study (Figure 5). We suspect that these differences may relate to biological differences between these Ad serotypes, differences in the characteristics of vector-specific cellular immune responses, and technical differences in the assays that were used. We cannot exclude the possibility that we may have missed a small effect, given the limited sample size of the present study.
In summary, we have shown that Ad26.ENVA.01 elicits a broad diversity of humoral and cellular immune responses in humans. However, the immune correlates of protection against HIV-1 infection in humans are not yet fully defined. Nevertheless, these data characterize the detailed immunologic profiles elicited by a prototype Ad26 vector in humans and suggest that further clinical development of Ad26 vaccine vectors is warranted.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank M. Pensiero, A. Fix, K. Brandariz, D. Carter, O. Defawe, L. Digilio, R. Dilan, D. Friedrich, S. Kik, A. LaPorte, M. Lifton, P. Meyer, D. Morris, J. Sadoff, and H. Schuitemaker, for generous advice and assistance; and the National Institutes of Health Vaccine Research Center for the clade A Env antigen and immunologic reagents.
Financial support. This work was supported by the National Institutes of Health (grants AI060354, AI066305, AI066924, AI069412, AI078526, and AI096040), the Bill & Melinda Gates Foundation, and the Ragon Institute of MGH, MIT, and Harvard.
Potential conflicts of interest. M. G. P, M. W., and J. G. are employees of Crucell. Patents for the Ad26 vector are held in part by Crucell and BIDMC. No licensing agreements, royalties, or income is associated with these patents. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Flynn NM, Forthal DN, Harro CD, Judson FN, Mayer KH, Para MF. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J Infect Dis. 2005;191:654–65. doi: 10.1086/428404. [DOI] [PubMed] [Google Scholar]
- 2.Pitisuttithum P, Gilbert P, Gurwith M, et al. Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J Infect Dis. 2006;194:1661–71. doi: 10.1086/508748. [DOI] [PubMed] [Google Scholar]
- 3.Buchbinder SP, Mehrotra DV, Duerr A, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372:1881–93. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–20. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 5.Barouch DH. Challenges in the development of an HIV-1 vaccine. Nature. 2008;455:613–9. doi: 10.1038/nature07352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Picker LJ, Hansen SG, Lifson JD. New paradigms for HIV/AIDS vaccine development. Annu Rev Med. 2012;63:95–111. doi: 10.1146/annurev-med-042010-085643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fauci AS, Johnston MI, Dieffenbach CW, et al. HIV vaccine research: the way forward. Science. 2008;321:530–2. doi: 10.1126/science.1161000. [DOI] [PubMed] [Google Scholar]
- 8.McElrath MJ, De Rosa SC, Moodie Z, et al. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet. 2008;372:1894–905. doi: 10.1016/S0140-6736(08)61592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barouch DH, Korber B. HIV-1 vaccine development after STEP. Annu Rev Med. 2010;61:153–67. doi: 10.1146/annurev.med.042508.093728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barouch DH. Novel adenovirus vector-based vaccines for HIV-1. Curr Opin HIV AIDS. 2010;5:386–90. doi: 10.1097/COH.0b013e32833cfe4c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abbink P, Lemckert AA, Ewald BA, et al. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J Virol. 2007;81:4654–63. doi: 10.1128/JVI.02696-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barouch DH, Kik SV, Weverling GJ, et al. International seroepidemiology of adenovirus serotypes 5, 26, 35, and 48 in pediatric and adult populations. Vaccine. 2011;29:5203–9. doi: 10.1016/j.vaccine.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu J, O'Brien KL, Lynch DM, et al. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature. 2009;457:87–91. doi: 10.1038/nature07469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waddington SN, McVey JH, Bhella D, et al. Adenovirus serotype 5 hexon mediates liver gene transfer. Cell. 2008;132:397–409. doi: 10.1016/j.cell.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 15.Vogels R, Zuijdgeest D, van Rijnsoever R, et al. Replication-deficient human adenovirus type 35 vectors for gene transfer and vaccination: efficient human cell infection and bypass of preexisting adenovirus immunity. J Virol. 2003;77:8263–71. doi: 10.1128/JVI.77.15.8263-8271.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu J, Ewald BA, Lynch DM, et al. Magnitude and phenotype of cellular immune responses elicited by recombinant adenovirus vectors and heterologous prime-boost regimens in rhesus monkeys. J Virol. 2008;82:4844–52. doi: 10.1128/JVI.02616-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lore K, Adams WC, Havenga MJ, et al. Myeloid and plasmacytoid dendritic cells are susceptible to recombinant adenovirus vectors and stimulate polyfunctional memory T cell responses. J Immunol. 2007;179:1721–9. doi: 10.4049/jimmunol.179.3.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mast TC, Kierstead L, Gupta SB, et al. International epidemiology of human pre-existing adenovirus (Ad) type-5, type-6, type-26 and type-36 neutralizing antibodies: correlates of high Ad5 titers and implications for potential HIV vaccine trials. Vaccine. 2010;28:950–7. doi: 10.1016/j.vaccine.2009.10.145. [DOI] [PubMed] [Google Scholar]
- 19.Barouch DH, Liu J, Li H, et al. Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature. 2012;482:89–93. doi: 10.1038/nature10766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baden LR, Walsh SR, Seaman MS, et al. First-in-human evaluation of the safety and immunogenicity of a recombinant adenovirus serotype 26 HIV-1 Env vaccine (IPCAVD 001) J Infect Dis. 2012 doi: 10.1093/infdis/jis670. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomaras GD, Binley JM, Gray ES, et al. Polyclonal B cell responses to conserved neutralization epitopes in a subset of HIV-1-infected individuals. J Virol. 2011;85:11502–19. doi: 10.1128/JVI.05363-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Masch A, Zerweck J, Reimer U, Wenschuh H, Schutkowski M. Antibody signatures defined by high-content peptide microarray analysis. Methods Mol Biol. 2010;669:161–72. doi: 10.1007/978-1-60761-845-4_13. [DOI] [PubMed] [Google Scholar]
- 23.Ackerman ME, Moldt B, Wyatt RT, et al. A robust, high-throughput assay to determine the phagocytic activity of clinical antibody samples. J Immunol Methods. 2011;366:8–19. doi: 10.1016/j.jim.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gomez-Roman VR, Florese RH, Patterson LJ, et al. A simplified method for the rapid fluorometric assessment of antibody-dependent cell-mediated cytotoxicity. J Immunol Methods. 2006;308:53–67. doi: 10.1016/j.jim.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 25.Tomaras GD, Yates NL, Liu P, et al. Initial B-cell responses to transmitted human immunodeficiency virus type 1: virion-binding immunoglobulin M (IgM) and IgG antibodies followed by plasma anti-gp41 antibodies with ineffective control of initial viremia. J Virol. 2008;82:12449–63. doi: 10.1128/JVI.01708-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horton H, Thomas EP, Stucky JA, et al. Optimization and validation of an 8-color intracellular cytokine staining (ICS) assay to quantify antigen-specific T cells induced by vaccination. J Immunol Methods. 2007;323:39–54. doi: 10.1016/j.jim.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ritz C, Streibig JC. Bioassay analysis using R. J Stat Software. 2005;12:1–22. [Google Scholar]
- 28.Finney DJ. Bioassay and the practice of statistical inference. Int Stat Rev. 1979;47:1–12. [Google Scholar]
- 29.O'Brien KL, Liu J, King SL, et al. Adenovirus-specific immunity after immunization with an Ad5 HIV-1 vaccine candidate in humans. Nat Med. 2009;15:873–5. doi: 10.1038/nm.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haynes BF, Gilbert PB, McElrath MJ, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med. 2012;366:1275–86. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frahm N, DeCamp AC, Friedrich DP, et al. Human adenovirus-specific T cells modulate HIV-specific T cell responses to an Ad5-vectored HIV-1 vaccine. J Clin Invest. 2012;122:359–67. doi: 10.1172/JCI60202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hutnick NA, Carnathan D, Demers K, Makedonas G, Ertl HC, Betts MR. Adenovirus-specific human T cells are pervasive, polyfunctional, and cross-reactive. Vaccine. 2010;28:1932–41. doi: 10.1016/j.vaccine.2009.10.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hutnick NA, Carnathan DG, Dubey SA, et al. Baseline Ad5 serostatus does not predict Ad5 HIV vaccine-induced expansion of adenovirus-specific CD4+ T cells. Nat Med. 2009;15:876–8. doi: 10.1038/nm.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Colloca S, Barnes E, Folgori A, et al. Vaccine vectors derived from a large collection of simian adenoviruses induce potent cellular immunity across multiple species. Sci Transl Med. 2012;4 doi: 10.1126/scitranslmed.3002925. 115ra2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barnes E, Folgori A, Capone S, et al. Novel adenovirus-based vaccines induce broad and sustained T cell responses to HCV in man. Sci Transl Med. 2012;4 doi: 10.1126/scitranslmed.3003155. 115ra1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Hara GA, Duncan CJ, Ewer KJ, et al. Clinical assessment of a recombinant simian adenovirus ChAd63: a potent new vaccine vector. J Infect Dis. 2012;205:772–81. doi: 10.1093/infdis/jir850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fischer W, Perkins S, Theiler J, et al. Polyvalent vaccines for optimal coverage of potential T-cell epitopes in global HIV-1 variants. Nat Med. 2007;13:100–6. doi: 10.1038/nm1461. [DOI] [PubMed] [Google Scholar]
- 38.Barouch DH, O'Brien KL, Simmons NL, et al. Mosaic HIV-1 vaccines expand the breadth and depth of cellular immune responses in rhesus monkeys. Nat Med. 2010;16:319–23. doi: 10.1038/nm.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li H, Liu J, Carville A, Mansfield KG, Lynch D, Barouch DH. Durable mucosal simian immunodeficiency virus-specific effector memory T lymphocyte responses elicited by recombinant adenovirus vectors in rhesus monkeys. J Virol. 2011;85:11007–15. doi: 10.1128/JVI.05346-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaufman DR, De Calisto J, Simmons NL, et al. Vitamin A deficiency impairs vaccine-elicited gastrointestinal immunity. J Immunol. 2011;187:1877–83. doi: 10.4049/jimmunol.1101248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaufman DR, Liu J, Carville A, et al. Trafficking of antigen-specific CD8+ T lymphocytes to mucosal surfaces following intramuscular vaccination. J Immunol. 2008;181:4188–98. doi: 10.4049/jimmunol.181.6.4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.