Abstract
Background
Loss of fluency is a significant source of functional impairment in many individuals with aphasia. Repetitive transcranial magnetic stimulation (rTMS) administered to the right inferior frontal gyrus (IFG) has been shown to facilitate naming in persons with chronic left hemisphere stroke and non-fluent aphasia. However, changes in fluency in aphasic subjects receiving rTMS have not been adequately explored.
Aims
To determine whether rTMS improves fluency in individuals with chronic nonfluent aphasia, and to identify aspects of fluency that are modulated in persons who respond to rTMS.
Methods & Procedures
Ten individuals with left hemisphere MCA strokes and mild to moderate non-fluent aphasia participated in the study. Before treatment, subjects were asked to describe the Cookie Theft picture in three separate sessions. During treatment, all subjects received 1200 pulses of 1 Hz rTMS daily in 10 sessions over two weeks at a site that had previously been shown to improve naming. Subjects repeated the Cookie Theft description two months after treatment. Five subjects initially received sham stimulation instead of real TMS. Two months after sham treatment, these individuals received real rTMS. Performance both at baseline and after stimulation was coded using Quantitative Production Analysis (Saffran, Berndt & Schwartz, 1989) and Correct Information Unit (Nicholas & Brookshire, 1993) analysis.
Outcomes & Results
Across all subjects (n=10), real rTMS treatment resulted in a significant increase in multiple measures of discourse productivity compared to baseline performance. There was no significant increase in measures of sentence productivity or grammatical accuracy. There was no significant increase from baseline in the sham condition (n=5) on any study measures.
Conclusions
Stimulation of the right IFG in patients with chronic non-fluent aphasia facilitates discourse production. We posit that this effect may be attributable to improved lexical-semantic access.
Keywords: Language, aphasia, TMS, pars triangularis, fluency, neurorehabilitation
Introduction
Failure of spontaneously generated fluent speech is a source of considerable disability for many individuals with aphasia after stroke, particularly those with anterior lesions of the left hemisphere. Nonfluent aphasia is typically characterized by difficulties in speech output, with deficits including interrupted speech, word omission, loss or misuse of inflectional morphology, and utterances with limited syntactic complexity (Goodglass and Kaplan, 1983). However, depending on lesion size and location, different individuals may experience a variety of specific impairments to varying degrees, including but not limited to deficits in speech initiation, poor retrieval of accurate semantic or lexical representations, disrupted sequencing of articulatory movements, or inaccurate grammatical constructions (Gleason et al., 1975; Kolk & Heeschen, 1992)
Recent studies have suggested that exogenous manipulation of cortical activity with repetitive transcranial magnetic stimulation (rTMS) may improve naming in persons with chronic left hemisphere stroke and nonfluent aphasia (e.g. Naeser et al., 2005, Martin et al., 2009; Barwood et al., 2011; Weiduchat et al., 2011, and others). Most investigations in this area have employed low-frequency (1Hz) inhibitory rTMS of the right inferior frontal gyrus (RIFG), and within that region many studies have focused on the right pars triangularis (RPTr). The specific mechanisms by which rTMS administered to this region produces beneficial changes in language ability are debated. Proposed mechanisms have included the dampening of inhibitory transcallosal connections between the right and left hemispheres or modification of intrahemispheric connections within a compensatory network of right hemisphere language areas (see Hamilton et al., 2011 for a review of this topic).
To date, most studies investigating the effects of rTMS on language recovery in patients with chronic nonfluent aphasia have focused on changes in naming ability. Naeser and colleagues (2005) reported improved performance on the Boston Naming Test and naming subtests of the Boston Diagnostic Aphasia Examination in four subjects who received 1Hz rTMS to the RIFG for 10 days (see also Naeser et al., 2009; Barwood et al., 2011; Hamilton et al., 2010). Investigators have used transient changes in naming ability after single sessions of 1Hz rTMS as a strategy for identifying optimal stimulation targets within right inferior frontal gyrus in nonfluent aphasics (Naeser et al., 2011; Hamilton et al., 2010; Turkeltaub et al., 2011a).
Despite the impact of dysfluency on the functional abilities of many individuals with nonfluent aphasia, relatively little has been reported with regard to the effect of right hemisphere rTMS on this aspect of language. To date, only three studies have reported spontaneous speech data in individuals who have undergone right hemisphere rTMS treatment. These studies have reported the greatest number of words in a phrase (Naeser et al., 2005; Martin et al., 2009; Barwood et al., 2011), articulatory agility (Naeser et al., 2005), or picture description complexity index (Barwood et al., 2011) as dependent variables. However, detailed coding schemes, like Quantitative Production Analysis (QPA; Saffran, Berndt & Schwartz, 1989; Rochon et al., 2000), can be used to fully characterize the various dissociable aspects of language production that contribute to fluent spontaneous speech. After coding spontaneous speech, past experimenters have found that specific variables in speech production in aphasics tend to correlate. For example, Rochon and colleagues (2000) found that a three-factor model (sentence structure, unbound morpheme frequency, speech rate) characterized performance in a set of 37 aphasic individuals. Others have grouped measures from QPA into categories based on various aspects of language (Gordon, 2006).
We examined whether right IFG rTMS treatment changed narrative speech production in a group of nonfluent aphasics using QPA and by tallying Correct Information Units (CIUs), a measure of the semantic content of subjects’ utterances (Nicholas & Brookshire, 1993). By identifying the specific aspects of spontaneous speech that are altered as a result of stimulation, we aimed to provide further insight into the specific linguistic processes improved by TMS.
Methods
Subjects
Ten subjects (3 female) ranging in age between 47 and 75 years (mean ± SD, 61.60 ± 8.32) participated. All subjects had sustained a single left hemispheric unilateral ischemic stroke (Figure 1a) and were classified as having mild to moderate nonfluent aphasia by Boston Diagnostic Aphasia Examination (BDAE, Goodglass et al., 2001) tests administered at the initial screening. To be eligible for the study, patients must have been able to produce meaningful words as well as phrases between 2–4 words in length during their baseline language evaluation. To ensure that subjects were able to cooperate during testing, they also must have had relatively intact language comprehension, operationally defined by performance at or above the 25th percentile on the BDAE subtests for word comprehension and commands. Additionally subjects must have been able to name at least 3 items of the first 30 on the Boston Naming Test (Kaplan et al., 2001) and an average of at least 3 pictures out of 20 when presented with 10 sets of picture naming stimuli taken from the Snodgrass and Vanderwart corpus (1980). All subjects were at least 6 months post-stroke, had no other concurrent history of neurological or psychiatric disease or unstable medical conditions and had no contraindications to either MRI or TMS. The study was approved by the Institutional Review Board of the University of Pennsylvania, and all subjects provided informed consent. Additional subject demographic, baseline performance, and lesion data are provided in Table 1.
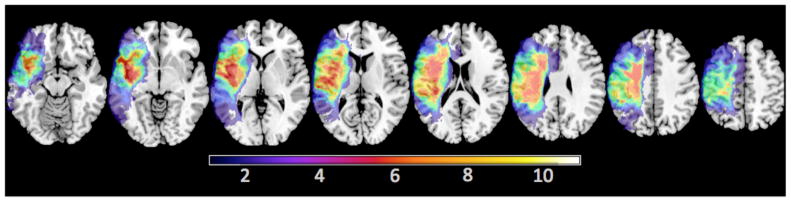
Figure 1.
Map depicts lesion overlap for all 10 left MCA stroke subjects mapped onto the Colin27 MNI template. The location of maximal overlap (bright orange) includes the left Insula, Heschl’s gyrus, and Rolandic operculum.
Table 1.
Demographics, lesion descriptions, and aphasia severity measures for study participants.
| Aphasia Severity | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Subject | Sex | Age, y | Education, y | Time Since Stroke, mo | Lesion Distribution | Lesion Volume, cm3 | Phrase length (BDAE), mean | Naming ability (BNT), (maximum 30) | Auditory Comprehension-Commands (BDAE), (maximum 15) |
| Real TMS | |||||||||
| 1 | M | 60 | 18 | 87 | Large MCA cortical and subcortical, including BA 44, 45, & 47 | 252.11 | 2.5 | 21 | 8 |
| 2 | M | 61 | 18 | 63 | Large MCA cortical and subcortical, including BA 44, 45, & 47 | 130.91 | 2 | 7 | 14 |
| 3 | M | 51 | 14 | 45 | Fronto-parietal cortical and subcortical, including internal capsule, basal ganglia, BA 44, 45, & 47 | 134.03 | 3.5 | 27 | 15 |
| 4 | F | 71 | 18 | 48 | Fronto-temporo-parietal subcortical greater than cortical, including internal capsule, basal ganglia & thalamus; M1 & IFG spared | 91.65 | 5 | 20 | 11 |
| 5 | M | 60 | 24 | 6 | Small fronto-temporo-parietal cortical and subcortical; minor involvement of corona radiata; IFG and insula spared | 36.59 | 3 | 8 | 15 |
| Mean (StdDev) | 60.6 (±7.1) | 18.4 (±3.6) | 49.8 (±29.6) | 129.1 (±79.2) | 3.2 (±1.2) | 16.6 (±8.7) | 12.6 (±3.0) | ||
| Sham TMS | |||||||||
| 1 | M | 65 | 12 | 29 | Subcortical, including corona radiata, internal capsule, basal ganglia, & thalamus; IFG spared | 53.02 | 2.5 | 13 | 11 |
| 2 | F | 65 | 16 | 20 | Large MCA cortical & subcortical, including BA 44, 45, & 47 | 201.62 | 3 | 20 | 8 |
| 3 | M | 47 | 12 | 102 | Cortical & subcortical, including internal capsule, basal ganglia, thalamus, M1, and BA 44; BA 45 & 47 spared | 123.83 | 2.5 | 26 | 11 |
| 4 | F | 61 | 14 | 59 | Large MCA cortical & subcortical, including BA 44, 45, & 47 | 178.99 | 1 | 17 | 14 |
| 5 | M | 75 | 18 | 83 | Fronto-temporo-parietal subcortical, including corona radiata but sparing internal capsule and deep grey structures; IFG spared | 118.49 | 3 | 21 | 11 |
| Mean (StdDev) | 62.6 (±10.1) | 14.4 (±2.6) | 58.6 (±34.8) | 135.2 (±58.1) | 2.4 (±0.8) | 19.4 (±4.8) | 11.0 (±2.1) | ||
Study Overview
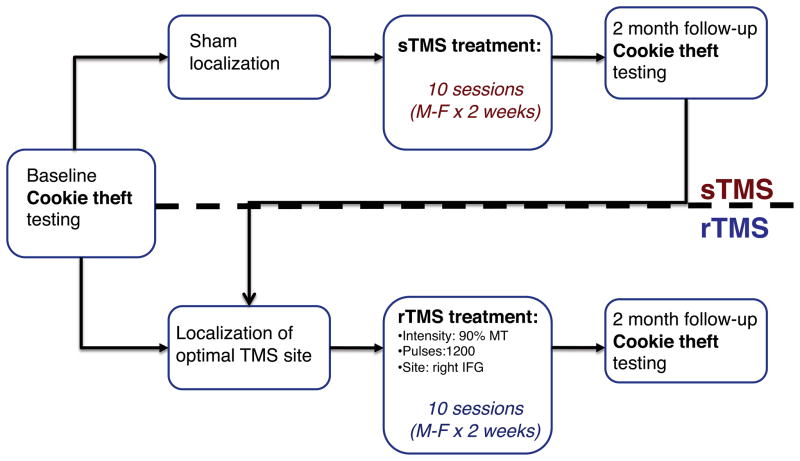
At the start of the study, all subjects underwent baseline language assessment three times on average (range: 1–4). The baseline assessment included testing with the Cookie Theft Picture Description subtest of the BDAE (Goodglass & Kaplan, 1983; Figure 1b), described further below. The average interval between initial and final baseline testing sessions was 33 days (SD = ±34.16 days). Following baseline assessment, each subject was randomized into one of two groups: One group (n=5) received real rTMS while the other group (n=5) initially received sham stimulation (sTMS). These two groups did not differ significantly statistically with respect to age, lesion size, time since stroke onset, or initial measures of aphasia severity. There was a trend toward significance between the two groups with respect to years of education (t(7.3)=2.02; p = 0.081), with a greater number of years of education in the rTMS group (18.4, SD = 3.6) than the sham group (14.4, SD = 2.6). Subjects receiving real rTMS underwent a series of six sessions of rTMS applied to different sites in right IFG in order to identify the optimal target for stimulation. After an optimal site was identified for each individual subject, stimulation was administered to that site in 10 sessions over twelve days (daily sessions occurred Monday through Friday for two weeks with no stimulation on Saturday or Sunday), as described below. Subjects subsequently completed a follow-up language assessment including Cookie Theft Picture Description two months following the completion of stimulation. Testing was not pursued immediately after discontinuation of stimulation because of concerns that repeating language tasks only a short interval after finishing baseline testing might elicit practice effects. Prior evidence (Hamilton et al., 2010) suggested that improvements in spontaneous speech due to rTMS could be observed after two months. Subjects randomized to the sham group received sham rTMS during both the optimal target identification phase of the study as well as during the 10-session treatment phase. These subjects completed a follow-up language assessment including the Cookie Theft Picture Description two months following sTMS, and subsequently crossed over into the real rTMS arm of the study, such that all subjects in the study eventually received real rTMS. Study events are summarized in Figure 2 (See Figure 2).
Figure 2.
Outline of study events.
rTMS Methods
Stimulation was administered with a Magstim Rapid transcranial magnetic stimulator, connected to a 70 mm diameter figure-of-eight coil (Magstim, Whitland, UK). A 3.0 Tesla Siemens Trio Scanner was used to collect high-resolution whole-brain T1-weighted images for each subject. The Brainsight system (Rogue Research, Montreal) was used to co-register MRI data with the location of the subject and coil in both phases of the study. The subject’s resting motor threshold (RMT) was established according to published criteria (Rossini et al., 1994). Sham TMS was administered using the same setup, but with the coil perpendicular to head so that only the rim of the coil contacted the head. During real stimulation, the coil was oriented with the handle in a posterior and inferior direction approximately 45 degrees clockwise from the downward position. Real rTMS was delivered at an intensity of 90% RMT and a frequency of 1 Hz. During each of the 10 sessions of the treatment phase of the study subjects in the real rTMS arm of the study received 1200 pulses of 1Hz rTMS at 90% RMT; subjects in the sham arm 1200 pulses of sTMS.
Identification of Optimal Stimulation Site
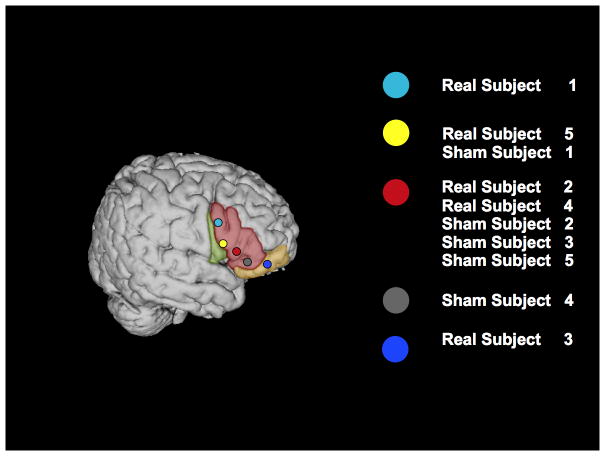
These methods are described in more detail elsewhere (Hamilton et al., 2010; Naeser et al., 2011). In six separate sessions conducted over five days (two sessions were conducted on the last day), different sites in the right inferior frontal lobe were stimulated with rTMS. During these sessions, subjects in the real rTMS arm of the study received 600 pulses of 1Hz rTMS at an intensity of 90% RMT, while subjects in the sham arm received 600 pulses of sTMS. Sites included the region of the motor cortex corresponding to the mouth, a site on pars opercularis (BA 44), three separate sites on the pars triangularis (BA 45; designated in this study as the dorsal posterior, ventral posterior and anterior pars triangularis), and the pars orbitalis (BA 47). Prior to and immediately following rTMS at each candidate site, subjects performed a 40-item picture-naming task. As in previous work, responses that differed from the target by one phoneme were counted as correct (e.g., Naeser et al., 2005). As previously reported, a site was considered to be the optimal target for stimulation if a subject showed the greatest increase in naming accuracy after stimulation of that target and if the change in naming accuracy observed after stimulation of that target was greater than two times the standard deviation of the mean pre-stimulation performance across all sites (Hamilton et al., 2011). Consistent with prior investigations, for 9 out of 10 subjects tested, the optimal site was in the right pars triangularis (RPTr); for one subject the optimal site was found to be in the right pars orbitalis (See Figure 3).
Figure 3.
Right hemisphere overlay of optimal real TMS sites for the 10 subjects in the study mapped onto the Colin27 MNI template. Subjects are labeled as Real or Sham subjects 1–5 (based on which type of treatment they received first, although the sites shown all correspond to their real TMS sites), and correspond with subject labels in Table 1. Shading indicates gyral anatomy: Green=pars opercularis, Red=pars triangularis, Yellow=pars orbitalis.
Stimulus Presentation
Seated comfortably in a quiet testing environment, subjects were presented with the “Cookie Theft” picture from the Boston Diagnostic Aphasia Examination (Goodglass & Kaplan, 1983) by an examiner, and instructed to report everything they saw in the picture. Examiner interruptions were limited to general prompts (e.g. “what’s happening here”); examiners did not point or allude to specific content in the picture. There was no time limit for responses. Subjects performed this task three times at baseline and after 2-month real rTMS follow-up. Subjects randomized to the sham arm performed the task at 2-month sTMS follow-up as well. Speech samples were recorded digitally and coded offline by an experimenter (CN). The order of sessions was randomized during coding and the coder was blinded with respect to subject condition and session order.
Measures of Fluency
A variety of elements were quantified in each speech sample using QPA (Saffran, Berndt & Schwartz, 1989). We categorized these variables based on four aspects of speech fluency: Discourse productivity, sentence productivity, grammatical accuracy, and lexical selection (Gordon 2006).
Discourse Productivity
This category described the production of words related to the picture stimulus. Narrative words (NW) were defined as the total words minus stereotyped utterances, task-related comments, or comments cued by the narrator. The number of narrative words that were verbs and nouns was recorded, as was the number of unique verbs and nouns. Closed-class words (CCW) were defined as the number of narrative words that were determiners, pronouns, conjunctions, and prepositions; other words were counted as open-class words (OCW). The duration and rate (measured in words per minute) of subjects’ speech samples were also recorded.
Sentence Productivity
This category quantified the length and complexity of sentences. Mean sentence length was defined as the average of the number of words per utterance containing a noun and a main verb. Median utterance length referred to the number of narrative words per utterance. The sentence elaboration index is a composite score of the number of narrative words per phrase for both noun and verb phrases. The embedding index is the proportion of sentences that contain an embedded clause.
Grammatical accuracy
This category included the proportion of sentences that were well-formed, in that they contain a subject, predicate, and direct object (where necessary). The auxiliary score was defined as an index of the morphological complexity of the main verb of the sentence. The inflexion index is a measure of verbs that have been changed from their base form.
Lexical Selection
This category measured the usage words relative to other words in an utterance. The proportion of CCWs reflected the number of CCWs relative to the total number of narrative words. Similarly, the proportion of pronouns and the proportion of verbs relative to total narrative words were also measured.
In addition to QPA, speech samples were also scored with respect to Correct Information Units (CIUs), which were defined as the number of words that were intelligible, accurate, informative, and relevant to the eliciting stimulus (see Nicholas & Brookshire, 1993).
Analysis
We pursued a within-subjects comparison contrasting performance two months after real rTMS to baseline performance in all subjects (N=10), using raw performance scores as dependent variables. Because we had strong a priori predictions about the direction of change likely to be induced by rTMS based on prior evidence (e.g. Naeser et al., 2005; Hamilton et al., 2010; Barwood et al., 2011), one-tailed t-tests were employed. We also used within-subjects comparisons to contrast 2-month follow-up performance to baseline performance after sTMS in the subset of subjects receiving sham stimulation (N=5), using raw performance scores as dependent variables. To confirm that significant rTMS vs. baseline effects did not result from practice effects or bias due to unblinding in patients crossed over from the sham arm to the real rTMS arm, we performed a between-subjects comparison contrasting the proportion change from baseline to 2-month follow-up on each study measure in patients who received sTMS (n=5) to those who received rTMS initially (n=5), using independent samples one-tailed t-tests. For all independent samples t-tests (using PASW 18), we used a Levene’s test for equality of variances to test for homogeneity of variance. When assumptions of homogeneity of variance were not met, we adjusted the degrees of freedom using the Welch-Satterthwaite method. We did not make any explicit corrections for multiple comparisons, as many of the variables were complementary, consisting of subdivisions of other variables (e.g. total nouns and verbs as part of narrative words). Furthermore, it has been suggested that in exploratory studies, one should not correct for multiple comparisons but instead present the p-values for all tests for interpretation (see Rothman, 1990). Note that given the number of comparisons we are presenting in the paper (21), we would expect about one comparison to be significant if the data were generated at chance.
Results
Comparison of real rTMS to baseline
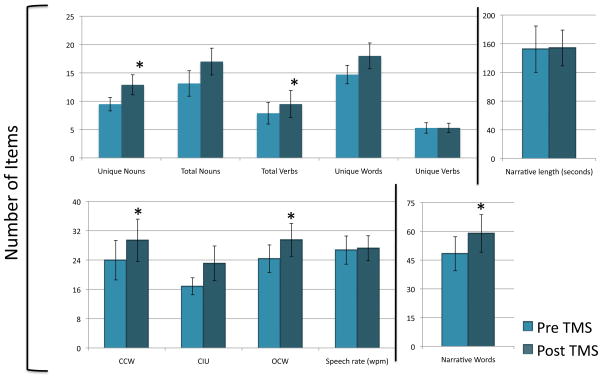
All subjects tolerated stimulation without complaint of physical discomfort or other adverse effects. First, we used t-tests to observe if there were any differences in baseline performance between subjects who received real rTMS initially versus those who received real rTMS after sTMS. For all twenty-one dependent variables, we did not find any significant differences between the two groups in baseline performance (average p-value, .521, range, .131 to .979), performance at 2-month follow-up (average p-value, .494; range, .059 to .984), or proportional change from baseline (average p-value, .468; range, .113 to .879). Therefore, results from these two groups were collapsed for the first analysis. The subjects demonstrated a significant increase in discourse productivity (see Figure 4), as shown by the significant increase in the number of narrative words two-months post-treatment compared to baseline (t(9) = −2.07, p = 0.035). Within narrative words, we found that subjects demonstrated a significant increase in production of open-class words (t(9) = −2.02, p = 0.037), closed-class words (t(9) = −1.95, p = 0.042), total number of verbs (t(9) = −2.28, p = 0.024), unique nouns (t(9) = −2.02, p = 0.037), and marginal effects for total number of nouns (t(9) = −1.82, p = 0.051) and unique words (t(9) = −1.63, p = 0.068). We found no effect for unique verbs (t(9) = −0.015, p = 0.494). Consistent with the notion that subjects were generating more words relevant to the picture stimulus, there was a trend toward an increase in the number of CIUs generated (t(9) = −1.60, p = 0.072). These increases in narrative productivity were not due to subjects speaking for longer periods of time, as there was only a 1.6 second difference in mean passage length (t(9) = −0.13, p = 0.449) between baseline and 2 month follow-up. Nor were subjects simply speaking more rapidly, since there was no significant change in speech rate (t(8) = −2.70, p = 0.397). However, subjects showed a marginally significant increase in the total number of utterances before versus after treatment (t(9) = −1.68, p = 0.064) and also trend toward significance (t(9) = −1.72, p = 0.059) in the percentage of narrative words over total words uttered two-months after treatment (73.6%) versus at baseline (69.5%).
Figure 4.
Performance on measures of discourse productivity before and 2 months after receiving real rTMS (N=10). Vertical lines represent standard error. Significant improvement (p=<0.05) is indicated by “*.” NW=Narrative Words; CCW= Closed-class Words; OCW= Open-class Words; CIU= Correct Information Units; SL= Sentence Length; UL= Utterance Length; EI = Elaboration Index; WF = Well-formed; VI= Verbs Inflected; Prop= Proportion.
There was no significant improvement among subjects receiving sTMS for any measure of discourse productivity. In contrast to findings within the category of discourse productivity, no significant changes in performance were seen along any variables in the other three fluency categories—sentence productivity, grammatical accuracy, and lexical selection—for either the real rTMS or sTMS conditions (See Figure 5a–c; also see Supplementary Table 1).
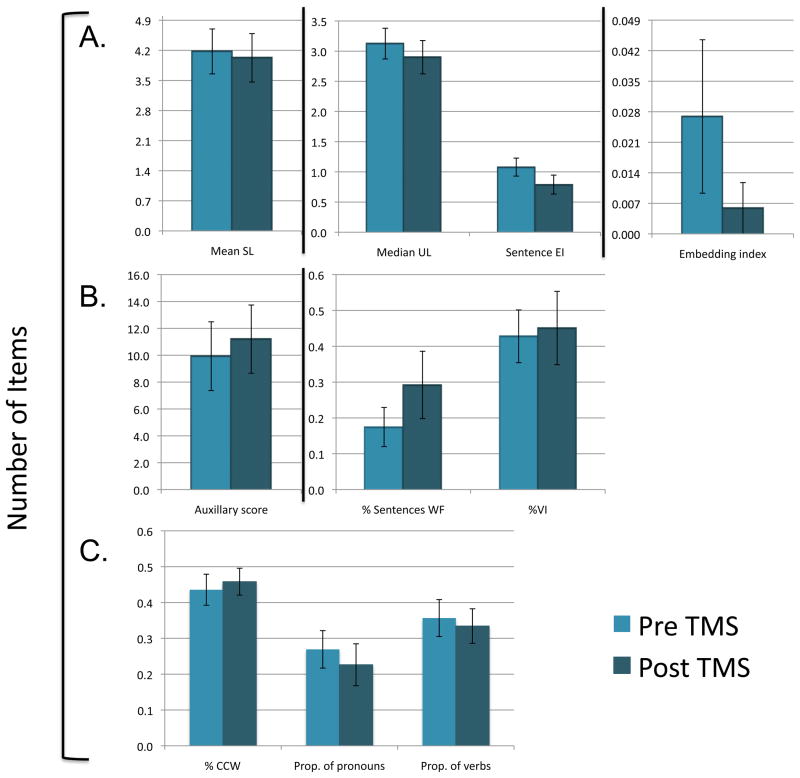
Figure 5.
Performance on measures of (A) sentence productivity, (B) grammatical accuracy, and (C) lexical selection (N=10) before and 2 months after real TMS stimulation (N=10). Vertical lines represent the standard error. NW=Narrative Words; CCW= Closed-class Words; OCW= Open-class Words; CIU= Correct Information Units; SL= Sentence Length; UL= Utterance Length; EI = Elaboration Index; WF = Well-formed; VI= Verbs Inflected; Prop= Proportion.
Comparison of real rTMS to sTMS
Unfortunately, the statistical power to make comparisons between subjects who had initially received rTMS compared to those who had initially received sTMS was limited due to the small sample size. There was a significantly greater increase from baseline in the use of closed-class words for subject receiving rTMS compared to subjects receiving sTMS (t(7.4) = −2.10, p = 0.036). There were also trends toward greater change from baseline in subjects receiving rTMS compared to subjects receiving sTMS for other measures of discourse productivity, including narrative words (t(6.77) = −1.87, p = 0.053), unique words (t(7.73) = −1.65, p = 0.070), unique nouns (t(6.32) = −1.48, p = 0.094), unique verbs (t(7.47) = −1.78, p = 0.058), open-class words (t(7.54) = −1.59, p = 0.076), and CIUs (t(5.3) = 1.7, p=0.072). No significant differences or trends were found for any measures of sentence productivity, grammatical accuracy, or lexical selection (all p values > .1). Finally, there was a very consistent direction of numerical change across all measures of narrative word generation, with subjects receiving rTMS showing a greater improvement from baseline than subjects receiving sTMS. No consistent pattern of performance emerged across measures of sentence productivity, grammatical accuracy, or lexical selection (see Supplementary Table 2).
Discussion
Our data indicate that individuals with chronic nonfluent aphasia who undergo rTMS of the right inferior frontal gyrus experience improvement in fluency two-months after treatment. This improvement in fluency is driven specifically by changes in discourse productivity, as indicated by significant increases in a variety of narrative word types following stimulation. Moreover, the increase in narrative comments exhibited by subjects after receiving real rTMS is not the result of an increase in the speaking speed or duration, but instead reflects a shift in the distribution of utterances away from irrelevant non-narrative statements toward comments that are relevant to the presented picture stimulus. By contrast, our data indicate that subjects with chronic nonfluent aphasia receiving rTMS of the RIFG do not experience a significant benefit with respect to other aspects of fluency, including sentence complexity, grammatical accuracy, or lexical selection.
A few prior investigations have suggested that chronic aphasic individuals receiving right hemisphere stimulation experience improvements in fluency. However, one significant limitation of most of these prior studies is the small number of measures used to quantify this aspect of language production. Naeser and colleagues (2005) reported that two out of four subjects receiving rTMS of the IFG experienced an increase in phrase length when describing the Cookie Theft Picture. Martin and colleagues (2009) reported similar results in a single subject. Extending these results to a larger cohort of subjects, Barwood and colleagues (2011) reported that rTMS treatment improved picture description in six subjects receiving real rTMS treatment compared to six other subjects receiving sTMS. However, they only reported two fluency measures: picture description complexity and longest words per phrase. We reported a single case of a chronic aphasic individual who experienced significant and persistent improvement in spontaneous elicited speech on the Cookie Theft Picture Description task and on the Western Aphasia Battery Spontaneous speech subscale (Kertesz, 1982) after receiving 1Hz rTMS of the right IFG (Hamilton et al., 2010). Unlike prior studies, we examined a variety of speech production indices, and found that the subject experienced significant increases in narrative words, different nouns, mean sentence length, and closed class words per sentence. We also recently reported a single case in which a chronically aphasic subject experienced an increase in the number of unique words employed and a marginal increase in the CIUs during a picture description two months after receiving real rTMS (Turkeltaub et al., 2011a). The current study builds upon our prior findings, confirming in one of the largest reported cohorts of chronic aphasic subjects receiving rTMS to date that inhibitory rTMS of the RIFG improves fluency, and clarifying that this effect is related specifically to improved discourse productivity.
The mechanisms by which rTMS of the RIFG improves discourse productivity in individuals with chronic nonfluent aphasia are unclear. However, the performance patterns observed in our subjects militate against certain explanations. One possible account for the increase in narrative words is that TMS facilitates speech via mechanisms that are only indirectly related to language functions, for example by diminishing frontal lobe mediated behavioral inhibition or by modulating arousal. However, this account would predict that subjects would experience an overall increase in speech production, and does not adequately explain why our subjects experience a shift in the distribution of their words toward narrative utterances and away from non-narrative utterances. Neither do our results accord with the notion that rTMS is principally affecting motor aspects of speech production, since this also would not explain a shift toward narrative utterances.
While our findings help to identify which aspects of language production are affected by RIFG rTMS, they do not resolve ongoing debate regarding the neural mechanisms mediating stimulation effects in persons with chronic aphasia. Much of this debate centers on the role of right hemisphere structures in aphasia recovery. One account is that the right hemisphere is deleterious to language recovery, possibly due to the presence of inhibitory interhemispheric connections from the right to the left hemisphere that diminish the recovery of perilesional left hemisphere language areas. A competing account argues that the role of the right hemisphere in aphasia recovery is largely compensatory, and that inhibitory rTMS of specific right hemisphere sites modulates the efficiency of compensatory right hemisphere networks (Hamilton et al. 2011). Based on data from prior imaging studies (Turkeltaub et al., 2011b) and from one specific subject with chronic aphasia who received rTMS and then experienced a second stroke affecting her right hemisphere (Turkeltaub et al., 2011a), we have argued that the role of some right hemisphere regions appears to be compensatory while that of other regions appears to interfere with language processing (Hamilton et al., 2011).
One plausible explanation for the current results is that rTMS of the RIFG selectively improves lexical-semantic access. Lexical-semantic access is often profoundly delayed in subjects with non-fluent aphasia (Edwards, 1995). Subjects who have received inhibitory stimulation may be better able to retrieve the appropriate representations of words and word meanings, and are thus better able to generate more narrative utterances that are relevant to the picture stimulus presented to them. Supporting this notion, all of the study measures in which our subjects showed improvement involved accessing words in various categories, with no improvement in measures of grammatical complexity or sentence construction. Improvement in lexical-semantic processing is also consistent with the already established finding that stimulation of this region improves naming, a process that also relies in large part on the retrieval of accurate lexical-semantic representations (Martin et al., 2004; Naeser et al., 2005b; Winhuisen et al., 2005; Naeser et al., 2010). The fact that the optimal site during TMS site finding in our study is the RPTr in 9 out of 10 subjects further suggests that inhibition of this specific site has a facilitative effect on lexical-semantic selection (see also Naeser et al., 2011). Furthermore, it has also been shown that rTMS presented to the RPTr in patients with chronic nonfluent aphasia induces changes in the N400 signal (Barwood et al., 2011), an ERP marker that has been associated with lexical-semantic aspects of language processing (Kutas et al., 2000; Simos et al., 1997; Brown et al., 1993; Halgren et al., 2002).
The notion that TMS of the right PTr might selectively affect lexical-semantic processing is consistent with converging evidence that suggests that individuals with aphasia engage right hemisphere structures during language processing and that these right hemisphere areas are functionally specific. A recent meta-analysis of neuroimaging studies in aphasia indicates that many right hemisphere perisylvian regions that are activated during language tasks in aphasic individuals are homotopic and functionally homologous with left hemisphere areas that normally subserve language in normal persons (Turkeltaub et al., 2011b). By contrast, the right PTr, while homotopic in location with an area of activation in controls was not homologous with respect to functional activity. Turkeltaub and colleagues suggested that activation observed in the right PTr in aphasics may be dysfunctional, a view further supported by studies that have associated increased right IFG activation with overt naming errors in aphasic subjects (Postman-Caucheteux et al., 2010). A number of studies have demonstrated that, in healthy subjects, more ventral-anterior regions of the left IFG (including PTr) are preferentially involved in lexical-semantic processing, while posterior-dorsal areas (including the pars opercularis; POp) are preferentially involved in phonology (Poldrack et al., 1999; Bookheimer et al., 2002; Hagoort et al., 2005; Gough et al., 2005). Moreover, Hartwigsen and colleagues (2010) found that in healthy individuals disruption of the right POp with TMS resulted in worsened performance on a phonological selection task, while disruption of the right PTr had no effect on a matched semantic task. This finding suggests that, although it is homotopic with a LIFG region that mediates lexical-semantic processing in normal persons, the right PTr is poorly suited to support recovery of lexical-semantic processing after LIFG injury. This is consistent with functional imaging data in aphasia demonstrating that left hemisphere activation was associated with phonemic naming errors, but right hemisphere activity was associated with semantic naming errors (Fridriksson et al., 2009). By this account, inhibition of overactivity in the right PTr might result in suppression of an inefficient node in the remodeled language network that otherwise contributes deleteriously to lexical-semantic processing.
The current study has limitations. Even though this is one of the largest studies to report on the effects of rTMS on language ability in persons with chronic nonfluent aphasia, the study is still underpowered and additional investigations in larger cohorts of subjects are needed to establish the impact of rTMS on measures of fluency more definitively. Also the number of speech samples gathered from each subject was relatively low compared to the volume of data collected in the original studies validating the QPA (QPA, Berndt et al., 2000) and the use of CIUs (Nicholas & Brookshire, 1993). Baseline testing was not repeated for subjects who initially received sham stimulation prior to receiving real rTMS. While it is therefore possible that introduction of sham rTMS and a two-month time interval could have resulted in a different baseline level of performance for these individuals, the absence of change on any study measure between baseline and 2-month follow-up in subjects who had received sTMS argues against this claim. Finally, owing to the difference in sensory experience between real and sham stimulation, we cannot exclude the possibility that some subjects were not fully blinded to their condition when receiving sTMS. It should be noted, however, that no subject had received TMS prior to the study and would not, therefore, have had expectations regarding the sensory experiences associated with TMS. Despite these caveats, the current data suggest that in addition to improving naming ability rTMS may be a promising technique for remediating dysfluency, one of the most debilitating deficits for many patients with chronic aphasia.
Supplementary Material
Acknowledgments
Sources of Funding
HBC: NIH 2R01 DC05672-04A2
RHH : NIH/NINDS 1K01NS060995-01A1
RHH: Robert Wood Johnson Foundation/Harold Amos Medical Faculty Development Program
PET : American Academy of Neurology Foundation
References
- Barwood CH, Murdoch BE, Whelan BM, Lloyd D, Riek S, O’ Sullivan JD, Coulthard A, Wong A. Improved language performance subsequent to low-frequency rTMS in patients with chronic non-fluent aphasia post-stroke. Eur J Neurol. 2011;18(7):935–943. doi: 10.1111/j.1468-1331.2010.03284.x. [DOI] [PubMed] [Google Scholar]
- Berndt RS, Wayland S, Rochon E, Saffran E, Schwartz M. Quantitative production analysis: A training manual for the analysis of aphasic sentence production. Philadelphia, PA: Psychology Press, Ltd; 2000. [Google Scholar]
- Bookheimer S. Functional MRI of language: new approaches to understanding the cortical organization of semantic processing. Ann Rev Neurosci. 2002;25:151–88. doi: 10.1146/annurev.neuro.25.112701.142946. [DOI] [PubMed] [Google Scholar]
- Brown C, Hargoort P. The processing nature of the N400: evidence from masked priming. J Cogn Neurosci. 1993;5:34–44. doi: 10.1162/jocn.1993.5.1.34. [DOI] [PubMed] [Google Scholar]
- Edwards S. Profiling fluent aphasic spontaneous speech: a comparison of two methodologies. Eur J Disord Commun. 1995;30(3):333–345. doi: 10.3109/13682829509021446. [DOI] [PubMed] [Google Scholar]
- Fridriksson J, Baker JM, Moser D. Cortical mapping of naming errors in aphasia. Hum Brain Mapp. 2009;30(8):2487–2498. doi: 10.1002/hbm.20683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici AD, Ruschemeyer SA, Hahne A, Fiebach CJ. The role of left inferior frontal and superior temporal cortex in sentence comprehension: localizing syntactic and semantic processes. Cereb Cortex. 2003;13(2):170–177. doi: 10.1093/cercor/13.2.170. [DOI] [PubMed] [Google Scholar]
- Gleason JB, Goodglass H, Green E, Ackerman N, Hyde MR. The retrieval of syntax in Broca’s aphasia. Brain Lang. 1975;2(4):451–471. doi: 10.1016/s0093-934x(75)80083-6. [DOI] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E, Barresi B. Boston Diagnostic Aphasia Examination (BDAE) Philadelphia, PA: Lippincott, Williams & Wilkins; 1983. [Google Scholar]
- Goodglass H, Kaplan E, Barresi B. Boston Diagnostic Aphasia Examination (BDAE) Philadelphia, PA: Lippincott, Williams & Wilkins; 2001. [Google Scholar]
- Gordon JK. A quantitative production analysis of picture description. Aphasiology. 2006;20:188–204. [Google Scholar]
- Gough PM, Nobre AC, Devlin JT. Dissociating linguistic processes in the left inferior frontal cortex with transcranial magnetic stimulation. J Neurosci. 2005;25(35):8010–8016. doi: 10.1523/JNEUROSCI.2307-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halgren E, Dhond RP, Christensen N, Van Petten C, Marinkovic K, Lewine JD, Dale AM. N400-like magnetoencephalography responses modulated by semantic context, word frequency, and lexical class in sentences. Neuroimage. 2002;17(3):1101–1116. doi: 10.1006/nimg.2002.1268. [DOI] [PubMed] [Google Scholar]
- Hamilton RH, Chrysikou EG, Coslett B. Mechanisms of aphasia recovery after stroke and the role of noninvasive brain stimulation. Brain Lang. 2011;118(1–2):40–50. doi: 10.1016/j.bandl.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagoort P. On Broca, brain, and binding: a new framework. Trends Cogn Sci. 2005;9(9):416–423. doi: 10.1016/j.tics.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Hamilton RH, Sanders L, Benson J, Faseyitan O, Norise C, Naeser M, Martin P, Coslett HB. Stimulating conversation: enhancement of elicited propositional speech in a patient with chronic non-fluent aphasia following transcranial magnetic stimulation. Brain Lang. 2010;113(1):45–50. doi: 10.1016/j.bandl.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwigsen G, Price CJ, Baumgaertner A, Geiss G, Koehnke M, Ulmer S, Siebner HR. The right posterior inferior frontal gyrus contributes to phonological word decisions in the healthy brain: evidence from dual-site TMS. Neuropsychologia. 2010;48(10):3155–3163. doi: 10.1016/j.neuropsychologia.2010.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. Boston Naming Test (BNT) Austin, TX: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- Kertesz A. Western Aphasia Battery. New York, NY: Grune & Stratton, Inc; 1982. [Google Scholar]
- Kolk H, Heeschen C. Adaptation symptoms and impairment symptoms in Broca’s aphasia. Aphasiology. 1990;4:221–231. [Google Scholar]
- Kutas M, Federmeier KD. Electrophysiology reveals semantic memory use in language comprehension. Trends Cogn Sci. 2000;4(12):463–470. doi: 10.1016/s1364-6613(00)01560-6. [DOI] [PubMed] [Google Scholar]
- Martin PI, Naeser MA, Ho M, Doron KW, Kurland J, Kaplan J, Wang Y, Nicholas M, Baker EH, Alonso M, Fregni F, Pascual-Leone A. Overt naming fMRI pre- and post-TMS: Two nonfluent aphasia patients, with and without improved naming post-TMS. Brain Lang. 2009;111(1):20–35. doi: 10.1016/j.bandl.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PI, Naeser MA, Ho M, Treglia E, Kaplan E, Baker EH, Pascual-Leone A. Research with transcranial magnetic stimulation in the treatment of aphasia. Curr Neurol Neurosci Rep. 2009;9(6):451–458. doi: 10.1007/s11910-009-0067-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PI, Naeser MA, Theoret H, Tormos JM, Nicholas M, Kurland J, Fregni F, Seekins H, Doron K, Pascual-Leone A. Transcranial magnetic stimulation as a complementary treatment for aphasia. Semin Speech Lang. 2004;25(2):181–191. doi: 10.1055/s-2004-825654. [DOI] [PubMed] [Google Scholar]
- Naeser MA, Martin PI, Nicholas M, Baker EH, Seekins H, Kobayashi M, Theoret H, Fregni F, Maria-Tormos J, Kurland J, Doron KW, Pascual-Leone A. Improved picture naming in chronic aphasia after TMS to part of right Broca’s area: an open-protocol study. Brain Lang. 2005;93:95–105. doi: 10.1016/j.bandl.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Naeser MA, Martin PI, Theoret H, Kobayashi M, Fregni F, Nicholas M, Tormos JM, Steven MS, Baker EH, Pascual-Leone A. TMS suppression of right pars triangularis, but not pars opercularis, improves naming in aphasia. Brain Lang. 2011;119(3):206–213. doi: 10.1016/j.bandl.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeser MA, Martin PI, Treglia E, Ho M, Kaplan E, Bashir S, Hamilton R, Coslett HB, Pascual-Leone A. Research with rTMS in the treatment of aphasia. Restor Neurol Neurosci. 2010;28:511–529. doi: 10.3233/RNN-2010-0559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas LE, Brookshire RH. A system for quantifying the informativeness and efficiency of the connected speech of adults with aphasia. J Speech Hear Res. 1993;36(2):338–350. doi: 10.1044/jshr.3602.338. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JD. Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. Neuroimage. 1999;10(1):15–35. doi: 10.1006/nimg.1999.0441. [DOI] [PubMed] [Google Scholar]
- Postman-Caucheteux WA, Birn RM, Pursley RH, Butman JA, Solomon JM, Picchioni D, McArdle J, Braun AR. Single-trial fMRI shows contralesional activity linked to overt naming errors in chronic aphasic patients. J Cogn Neurosci. 2010;22:1299–1318. doi: 10.1162/jocn.2009.21261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochon E, Saffran EM, Berndt RS, Schwartz MF. Quantitative analysis of aphasic sentence production: further development and new data. Brain Lang. 2000;72(3):193–218. doi: 10.1006/brln.1999.2285. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, Dimitrijevic MR, Hallett M, Katayama Y, Lucking CH, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol. 1994;91(2):79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–46. [PubMed] [Google Scholar]
- Saffran EM, Berndt RS, Schwartz MF. The quantitative analysis of agrammatic production: procedure and data. Brain Lang. 1989;37(3):440–479. doi: 10.1016/0093-934x(89)90030-8. [DOI] [PubMed] [Google Scholar]
- Simos PG, Basile LF, Papanicolaou AC. Source localization of the N400 response in a sentence-reading paradigm using evoked magnetic fields and magnetic resonance imaging. Brain Res. 1997;762(1–2):29–39. doi: 10.1016/s0006-8993(97)00349-1. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Vanderwart M. A standardized set of 260 pictures: norms for name agreement, image agreement, familiarity, and visual complexity. J Exp Psychol Hum Learn. 1980;6(2):174–215. doi: 10.1037//0278-7393.6.2.174. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Coslett HB, Thomas AL, Faseyitan O, Benson J, Norise C, Hamilton RH. The right hemisphere is not unitary in its role in aphasia recovery. Cortex. 2011a doi: 10.1016/j.cortex.2011.06.010. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub PE, Messing S, Norise C, Hamilton RH. Are networks for residual language function and recovery consistent across aphasic patients? Neurology. 2011b;76(20):1726–1734. doi: 10.1212/WNL.0b013e31821a44c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiduschat N, Thiel A, Rubi-Fessen I, Hartmann A, Kessler J, Merl P, Kracht L, Rommel T, Heiss WD. Effects of repetitive transcranial magnetic stimulation in aphasic stroke: a randomized controlled pilot study. Stroke. 2011;42(2):409–415. doi: 10.1161/STROKEAHA.110.597864. [DOI] [PubMed] [Google Scholar]
- Winhuisen L, Thiel A, Schumacher B, Kessler J, Rudolf J, Haupt WF, Heiss WD. Role of the contralateral inferior frontal gyrus in recovery of language function in poststroke aphasia: a combined repetitive transcranial magnetic stimulation and positron emission tomography study. Stroke. 2005;36(8):1759–1763. doi: 10.1161/01.STR.0000174487.81126.ef. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.