Abstract
The present single center, double-blind, delayed start study was conducted to examine possible symptomatic and disease-modifying effects of GM1 ganglioside in Parkinson’s disease (PD). Seventy-seven subjects with PD were randomly assigned to receive GM1 for 120 weeks (early-start group) or placebo for 24 weeks followed by GM1 for 96 weeks (delayed-start group). Washout evaluations occurred at 1 and 2 years after the end of treatment. Seventeen additional subjects who received standard-of-care were followed for comparative information about disease progression. Primary outcome was change from baseline Unified Parkinson’s Disease Rating Scale (UPDRS) motor scores. At week 24, the early-start group had significant improvement in UPDRS motor scores vs. a significant worsening of scores in the delayed-start group. The early-start group also showed a sustained benefit vs. the delayed-start group at week 72 and at week 120. Both groups had significant symptom worsening during washout. This study provides evidence that GM1 use for 24 weeks was superior to placebo for improving motor symptoms and that extended GM1 use (up to 120 weeks) resulted in a lower than expected rate of symptom progression. The data from this small study suggest that GM1 may have symptomatic and potentially disease modifying effects on PD.
Keywords: Parkinson’s disease, GM1, Ganglioside, Treatment, Symptomatic, Disease Modification
INTRODUCTION
Parkinson's disease (PD) is a slowly progressive neurodegenerative disorder characterized by loss of substantia nigra dopamine-producing neurons and loss of forebrain dopamine. Although transient symptomatic improvement can be obtained with currently available pharmacotherapies, functional ability deteriorates over time [1–3]. Development of therapies that can slow progression of the disease would fill a major unmet need in PD.
GM1 ganglioside, a major component of membrane signaling domains, plays important roles in neuronal development and survival and modulates a variety of cell activities. GM1 has been shown in numerous studies to have neurotrophic or neuroprotective effects and influences a variety of cellular activities at the level of the plasma membrane as well as intracellularly [4, 5]. Of potential relevance to the suspected pathophysiology of PD, GM1 modulates intracellular Ca2+ homeostasis [6], promotes lysosomal integrity [7], and may influence mitochondrial function [8, 9]. Preclinical studies show that treatment with GM1 following different types of central and peripheral nervous system lesions [10–15], including those in animal models of PD [14, 16–18], results in significant biochemical and behavioral recovery [19–21]. In particular, GM1 rescues damaged substantia nigra dopamine neurons, increases striatal dopamine levels and enhances dopamine synthetic capacity in residual dopamine neurons in PD animal models [11, 12, 14–18]. In a previous short-term double-blind placebo controlled study of GM1 in PD, GM1-treated subjects showed significant improvement on Unified Parkinson's Disease Rating Scale (UPDRS) motor scores and timed motor task performance (compared to placebo-treated subjects), suggesting a mild symptomatic effect. A follow-up open extension study found long-term use of GM1 to be safe and to result in modest symptom progression over a five year period [22]. A number of subjects had lower UPDRS motor scores after five years of GM1 use than at baseline prior to randomization into the original study.
Due to the complex nature of PD, there are challenges in the clinical assessment of disease progression and the demonstration of disease modification, especially since many drugs with potential disease modifying effects may also exert symptomatic effects. The evaluation of subjects who have already advanced to the point of requiring symptomatic treatment also presents challenges for evaluating potential disease modifying therapies, although it is important to study such subjects rather than confine disease modification trials to de novo PD patients. Since GM1 may potentially have both symptomatic and disease modifying effects on PD, the present trial used a study design previously suggested to possibly differentiate between these aspects of drug response [23–26] and examined subjects with PD evaluated during a practically defined “off” period as well as during a best “on” period.
MATERIALS AND METHODS
Participants
This study (ClinicalTrials.gov NCT00037830) was conducted at a single site in the United States between Nov 1999 and Jun 2010, was approved by the Division of Human Subjects Protection at Thomas Jefferson University, and written informed consent was obtained from all subjects prior to enrollment. Subjects were men or women between 39 and 85 years of age with a diagnosis of idiopathic PD consistent with the UK PD Society brain bank PD diagnostic criteria. Inclusion criteria included “off” UPDRS motor score between 10 and 40, “off” Hoehn and Yahr staging of 1.0 to 3.0, Mini Mental State Exam (MMSE) score of ≥ 25, and Beck Depression Inventory score of ≤ 10. Main exclusion criteria included: atypical/abrupt onset Parkinsonism; lack of response to dopaminergic therapy (if so treated); UPDRS resting tremor total score > 5 or >3 (individual body part) [27, 28]; use of >1000 I.U./day vitamin E and/or >300 mg/day coenzyme Q10; history of Guillain-Barré syndrome; use of anti-coagulant therapy; history of brain surgery for PD. Use of anti-Parkinson medications was allowed (but could not be changed during Phase I). Rasagiline was not available at the start of the study and its use was subsequently not allowed.
A separate group of standard-of-care subjects (“Comparison group”) was recruited according to the criteria described above and was assessed longitudinally to provide descriptive information regarding disease progression in medicated PD patients.
Study design and measurements
In Phase I, subjects were randomly assigned to receive GM1 (early-start, E-S) or placebo (delayed-start, D-S) for 24 weeks. In Phase II, all subjects received GM1 from week 24 - week 120. In Phase III, subjects were evaluated at one and two years after stopping GM1. The Comparison group was followed up to 96 weeks.
UPDRS measures were obtained independently by two raters (to improve test-retest reliability [29]) at each visit and the mean of their scores was used for analysis. Inter-rater reliability was assessed using Pearson linear correlation coefficient for each pair of raters and inter-rater reliability was high (median correlation coefficient was 0.94 for the primary endpoint, UPDRS motor scores, and 0.98 for all other UPDRS sections). Every effort was made to have the same raters assess a given patient throughout the study. All examinations were performed without access to or prior review of any data, records, or notes. An independent safety monitor evaluated and interviewed subjects at each visit, monitored clinical laboratory findings and reported adverse events. Raters had no access to safety or adverse event data.
Primary outcome assessments (UPDRS motor scores) were performed during a practically defined “off” period, before that day’s first dose of anti-Parkinson medication and at least 12 hours after the last dose of levodopa (or 24 hours for dopamine agonists). A secondary assessment was performed at each visit during a best “on” period, typically at least 1 hr. after taking medication. Two baseline assessments were performed one to two weeks apart and clinical stability criteria needed to be met to continue to randomization. The baseline value was the average of these two scores. Phase I evaluations were performed at 6, 12, 18, 23 and 24 weeks (data from weeks 23 and 24 were averaged and analyzed as the end of Phase I). Phase II assessments were performed at weeks 36, 48, 72, 96 and 120; Phase III (washout) assessments were performed yearly. Blood chemistry and urinalysis results were examined regularly to evaluate safety.
Drug administration
GM1 ganglioside (monosialotetrahexosylganglioside GM1 sodium salt, bovine brain derived) was initially supplied as a sterile injectable by Fidia S.p.A. (Abano Terme, Italy) and later, as active pharmaceutical ingredient (manufactured by Fidia S.p.A., provided by TRB Chemedica) formulated into a sterile injectable (Instituto Biologico Contemporaneo (IBC), Buenos Aires, Argentina). Chemistry, manufacturing and controls data for both batches of drug were comparable. Each ampoule contained 100 mg GM1 in 2.0 ml sterile sodium phosphate/sodium chloride. Placebo was supplied in identical ampoules with identical packaging.
A computer-generated randomization list was supplied by Fidia S.p.A.: subjects were assigned treatments sequentially by study staff. After randomization, subjects received a loading dose of GM1 (1000 mg i.v., in 50 ml sterile saline) or placebo, and were instructed to thereafter administer two subcutaneous injections per day. At the end of Phase I, the E-S group blindly received an intravenous placebo infusion and the D-S group received infusion of 1,000 mg of GM1. The route of administration (subcutaneous) and dose were based on pharmacokinetic information obtained from a study of GM1 administration to presenile Alzheimer’s disease patients [30] which showed maximum GM1 blood levels reached 48–72 h after subcutaneous GM1 administration and that administration via the subcutaneous route resulted in at least 50% higher plasma ganglioside levels compared to intramuscular injection. Compliance was assessed at each study visit by accounting for both used and unused ampules of study drug (i.e., recording number of ampules used, missed dose(s) and number of lost or broken ampules).
Statistical analysis
Change from study baseline (CFB), change from week 24 (CFW24) and change from week 120 (CFW120) were calculated for UPDRS part III (primary outcome) and total UPDRS score (sum of Parts I, II, and III) for ‘off’ and ‘on’ period separately for all intent to treat subjects. Sample size was based on effect size estimates from a previous GM1 study in PD [22, 27].
Difference between treatment groups (E-S and D-S) at baseline was assessed using one-way analysis of variance (ANOVA) for continuous variables and Fisher’s exact test for categorical variables. Treatment effects on CFB and CFW24 were evaluated with a mixed effects analysis of covariance (ANCOVA) model for repeated measures. The model included fixed effects for treatment and categorical week in study, interactions between treatment and week, and baseline score (or Week 24 score for CFW24) as covariate; unstructured covariance matrix was used. A separate model was fitted for Phase I CFB, Phase II CFB and Phase II CFW24 ‘Off’ period and ‘On’ period implemented with SAS® procedure PROC MIXED. Subjects who were not on anti-Parkinson’s medications at baseline (5 subjects in the E-S group and 2 subjects in the D-S group) were excluded from the analysis for the ‘On’ period. The primary comparison for treatment effects was a 2-sided t-test for 2 independent groups at the 0.05 significance level based on the adjusted least squares means (LSM) derived from this model at week 24 for Phase I and at week 120 for Phase II. Exploratory paired sample t-test was also performed to examine CFB and CFW24, CFW120 within each group at each time point.
Supportive analyses to examine differences in the rate of symptom progression based on CFB were performed. The model had fixed effects for treatment and continuous week in study, interactions between treatment and week, baseline score as covariate, random intercepts and slopes, and the unstructured covariance matrix between the intercept and slope estimates. Hence, the estimates took into account potential heterogeneity among the different subjects.
A random intercepts and slopes model on CFB with fixed effects of baseline and continuous week in the study and interaction between the baseline and week was used to assess the rate of change in the comparison group subjects.
The impact of missing data in Phase I and Phase II was assessed by using the multiple imputation (MI) method implemented with SAS® procedure PROC MI using the Markov Chain Monte Carlo (MCMC) method. The results from the ANCOVA model based on 100 completed-sets were then combined to derive valid statistical inference through the SAS® procedure PROC MIANALYZE.
The extent to which changes in symptoms were “clinically important” was explored. Calculation of clinical important difference (CID) in UPDRS motor scores was based on distribution-based and anchor-based analyses described by Shulman et al. [31]. Proportion of subjects achieving minimal (≥2.5 point decrease), moderate (≥5.2 point decrease), or large (≥10.8 point decrease) CID [31] was tabulated for each group at each time point without formal inferential statistics.
All analyses were performed using SAS version 9.3; all p-values were reported as is without adjustment for multiplicity due to multiple comparisons and multiple endpoints.
RESULTS
Subject characteristics
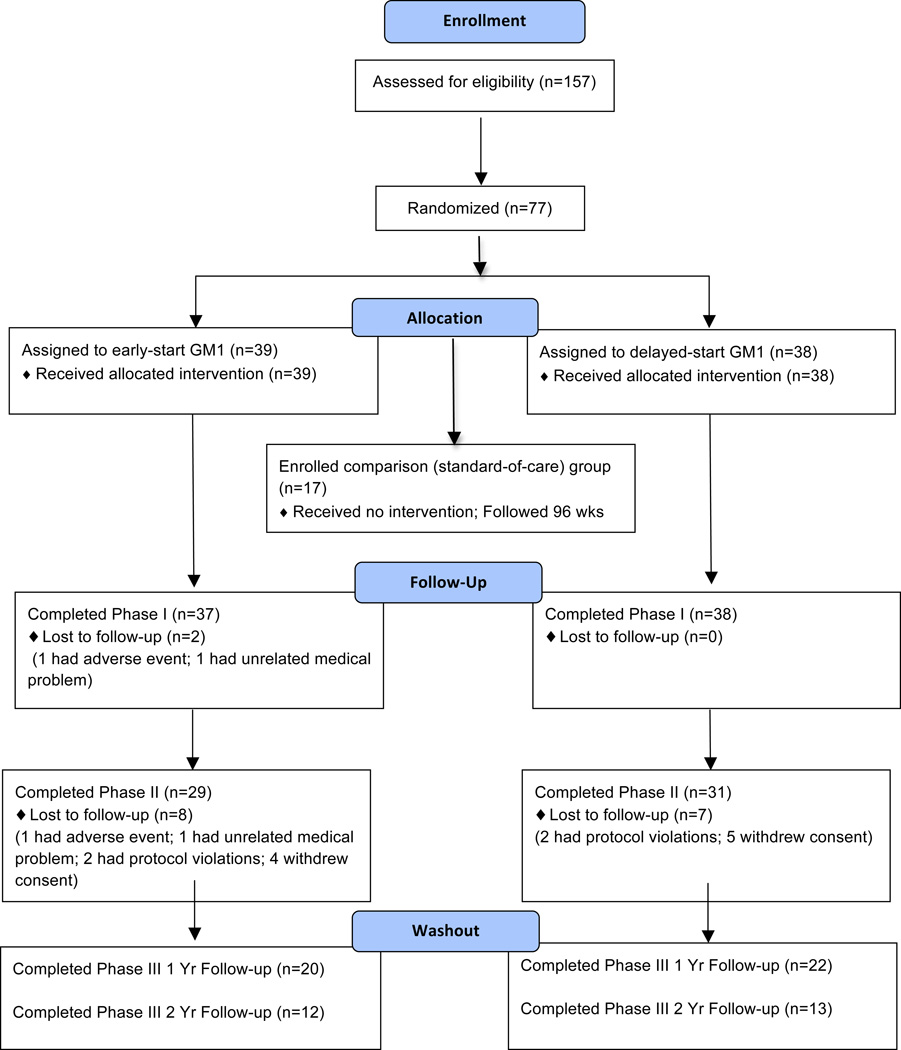
Subject disposition is delineated in Figure 1. There were no statistically significant differences between the groups in baseline demographic and clinical characteristics with exception of time since diagnosis (Table 1). Levodopa dose equivalents were calculated using published guidelines [32]. Comparison group baseline characteristics were comparable to those of the treatment groups.
Figure 1.
Subject disposition.
Table 1.
Subject Demographics and Baseline Characteristics
| Early-Start (n=38) |
Delayed-Start (n=38) |
P value1 | Comparison (n=17) |
|
|---|---|---|---|---|
| Age (years) | 59.7 (8.4) | 57.7 (8.7) | 0.3177 | 61.1 (11.8) |
|
Sex: n (%) Male Female |
29 (76.3) 9 (23.7) |
29 (76.3) 9 (23.7) |
1.000 |
14 (82.4) 3 (17.6) |
|
Median time since diagnosis (years) Median, Range (years) |
2.2 (1.5) 1.8, 0.5 – 6.2 |
3.5 (3.1) 2.9, 0.4 – 14.3 |
0.0266 | 3.7 (3.6) 2.7, 0.4 – 13.2 |
| MMSE score | 28.8 (1.1) | 28.4 (1.4) | 0.2296 | 29.2 (1.2) |
| BDI-II score | 5.4 (4.7) | 5.3 (3.5) | 0.8463 | 3.1 (2.6) |
| Total UPDRS score (Off) | 28.0 (10.8) | 29.3 (9.3) | 0.5823 | 32.2 (10.8) |
| UPDRS Motor score (Off) | 19.7 (7.7) | 19.2 (6.2) | 0.7546 | 22.3 (6.5) |
| UPDRS ADL score (Off) | 7.9 (4.2) | 9.4 (4.2) | 0.1148 | 9.3 (4.5) |
| UPDRS Mentation score (Off) | 0.5 (1.0) | 0.7 (1.0) | 0.3254 | 1.6 (1.0) |
| Medication Usage | ||||
| Levodopa (# of subjects (percent)) | 15 (39.5) | 22 (57.8) | 0.1681 | 8 (47.1) |
| Dopamine Agonist* (# of subjects (percent)) | 26 (68.4) | 24 (63.2) | 0.8093 | 14 (82.4) |
| Selegiline (# of subjects (percent)) | 15 (39.5) | 11 (28.9) | 0.4686 | 2 (11.8) |
| Other (# of subjects (percent))^ | 2 (5.2) | 8 (21.1) | 4 (23.5) | |
| Levodopa Equivalent Dose (mg/d) | 384.7 (226.41) | 494.6 (276.41) | 0.0783 | 527.5 (220.81) |
Data presented as mean±SD, unless otherwise noted. One subject from the Early-start group withdrew from the study after randomization but before the first study visit and their data are not included. Levodopa equivalent dose calculations exclude 5 early start subjects and 2 delayed start subjects that were unmedicated at baseline. MMSE: Mini Mental State Exam; BDI-II: Beck Depression Inventory II; UPDRS: Unified Parkinson’s Disease Rating Scale; ADL: Activities of Daily Living.
Dopamine agonists included pramipexole, ropinirole, pergolide and bromocriptine.
Other medications included amantadine, trihexyphenidyl, and benztropine
P value was from one-way ANOVA for continuous variables and 2-sided Fisher’s exact test for categorical variables for testing the differences between Early-Start and Delayed-Start groups.
Phase I treatment effects
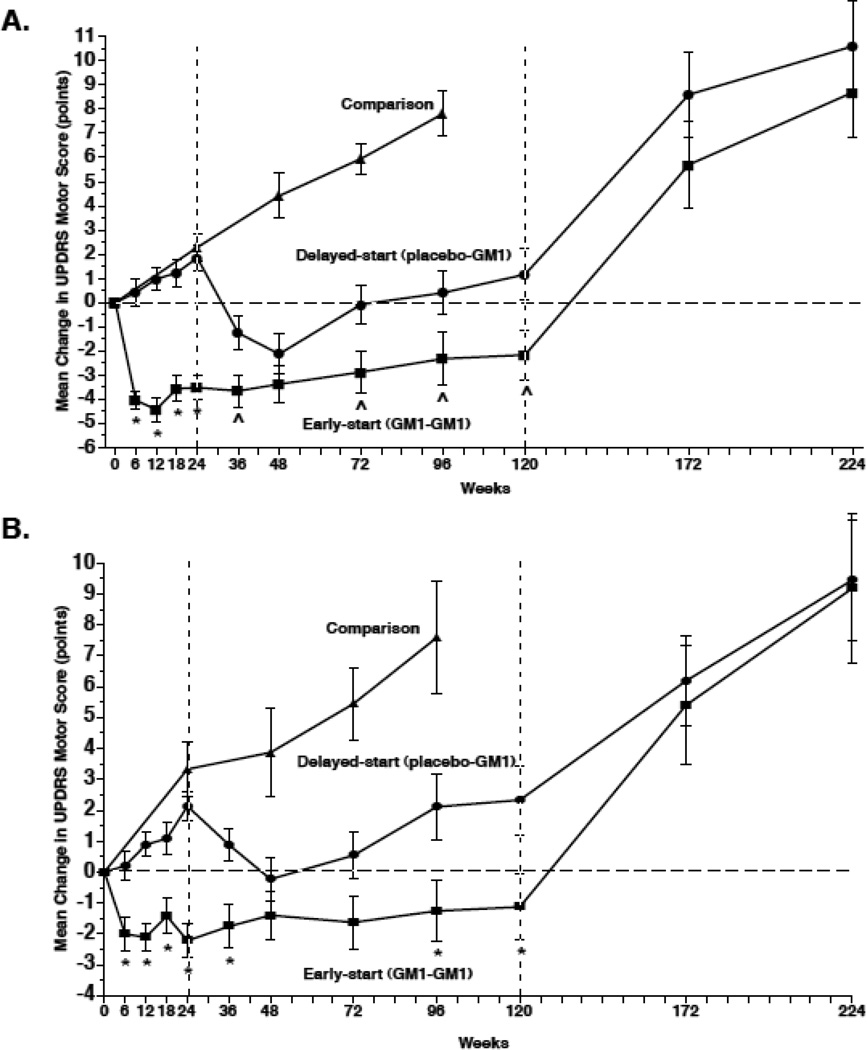
At week 24, E-S subjects had significantly lower mean “off” period UPDRS motor scores (i.e., improvement in motor symptoms) than at baseline while D-S subjects had significantly higher mean motor scores (i.e., worsening of symptoms) than at baseline. The difference between E-S and D-S group motor scores was −5.41 points (p <0.0001) per observed data and - 5.40 points (p <0.0001) after imputing missing data (Table 2, Figure 2A). The estimated rate of change per week during Phase I was 0.04 points (p=0.1903) for the E-S group and 0.08 points (p=0.0063) for the D-S group; the difference, −0.04 points per week, was not statistically significant (Table 2). The rate of change per week was significantly different from zero only for the D-S group, suggesting that the E-S group had minimal symptom worsening compared to baseline whereas the D-S group had significant symptom worsening related to time in the study and similar to the rate of symptom progression of the comparison group (Figure 2A). The observed mean (± SEM) CFB in the Comparison group at week 24 was +2.28 ± 0.866 points (p=0.0181, Table 4).
Table 2.
Summary and Analysis of GM1 Treatment Effects on “Off” UPDRS Motor Scores: Change from Baseline
| Observed Motor Scores | Observed[1] | With MI for Missing[2] | ||||
|---|---|---|---|---|---|---|
| Time Point | Early-Start (N=38) | Delayed-Start (N=38) | Difference in LSM CFB | Difference in LSM CFB | ||
| N | Mean (SEM)[3] | N | Mean (SEM)[3] | (95% CL) | (95% CL) | |
| Phase I | ||||||
| Week 0 (Baseline) | 38 | 19.65 (1.24) | 38 | 19.15 (0.99) | ||
| Week 6 | 38 | 15.61 (1.24)^ | 38 | 19.57 (1.01) | −4.42 (−5.76, −3.08)* | −4.43 (−5.74, −3.11)* |
| Week 12 | 37 | 14.68 (1.32)^ | 38 | 20.12 (0.96)# | −5.45 (−6.83, −4.06)* | −5.42 (−6.78, −4.05)* |
| Week 18 | 37 | 15.54 (1.26)^ | 38 | 20.38 (1.01)# | −4.85 (−6.42, −3.29)* | −4.87 (−6.42, −3.32)* |
| Week 24 | 37 | 15.59 (1.24)^ | 38 | 21.00 (0.97)$ | −5.41 (−6.82, −4.00)* | −5.40 (−6.79, −4.01)* |
| Slope Estimate (95% CL) | 38 | 0.04 (−0.02, 0.09) | 38 | 0.08 (0.02, 0.13) | −0.04 (−0.12, 0.04) | −0.04 (−0.11, 0.04) |
| P Value[4] | 0.1903 | 0.0063 | 0.3024 | 0.3046 | ||
| Phase II | ||||||
| Week 36 | 35 | 15.34 (1.31)^ | 38 | 17.89 (0.95) | −2.28 (−4.22, −0.34)~ | −2.24 (−4.20, −0.27)~ |
| Week 48 | 36 | 15.60 (1.33)^ | 37 | 16.91 (1.14)# | −1.32 (−3.53, 0.90) | −1.34 (−3.52, 0.83) |
| Week 72 | 32 | 16.64 (1.48)$ | 35 | 18.69 (1.03) | −2.96 (−5.30, −0.61)~ | −2.97 (−5.33, −0.60)~ |
| Week 96 | 33 | 16.74 (1.53) | 32 | 18.83 (1.11) | −3.08 (−5.98, −0.18)~ | −2.97 (−5.90,−0.04)~ |
| Week 120 | 29 | 17.21 (1.42) | 31 | 19.50 (1.29) | −3.33 (−6.45, −0.21)~ | −3.09 (−6.31, 0.12) |
| Slope Estimate (95% CL) | 37 | 0.02 (−0.00, 0.04) | 38 | 0.04 (0.02, 0.06) | −0.02 (−0.05, 0.01) | −0.02 (−0.05, 0.02) |
| P Value[4] | 0.0502 | 0.0003 | 0.2196 | 0.3271 | ||
| Washout | ||||||
| Week 172 | 20 | 24.18 (1.79)$ | 22 | 26.27 (1.91)^ | ||
| Week 224 | 12 | 26.58 (2.62)$ | 13 | 27.12 (2.37)$ | ||
ANCOVA of observed change from baseline; difference was calculated by E-S minus D-S; * p < 0.0001, ~ P<0.05
Combined results of ANCOVA over 100 complete data sets in which missing data were imputed using the Markov Chain Monte Carlo (MCMC) method; * p < 0.0001, ~ p < 0.05
Paired-sample t-test: ^ p < 0.0001; $ p < 0.01; # p < 0.05.
p-value from null hypothesis of the slope or the difference in slope to be zero.
LSM = least square mean; CFB = change from baseline.
Figure 2.
Changes in Unified Parkinson’s Disease Rating Scale (UPDRS) Motor Subsection Scores. A. The mean (±SEM) change from baseline (observed scores) in Early-start and Delayed-start study subjects and in the standard-of-care Comparison group, assessed in the practically defined “off’ condition. The dashed vertical line at week 24 indicates the end of study Phase I. The dashed vertical line at week 120 indicates the end of study Phase II. The horizontal dashed line indicates baseline level. An increase of score indicates symptom worsening; a decrease in score indicates symptom improvement. * = p < 0.0001 Early-start vs. Delayed-start; ^ = p < 0.05 Early-start vs. Delayed-start. B. The mean (±SEM) change from baseline in Early-start and Delayed-start study subjects and in the standard-of-care Comparison group, assessed in the best “on” condition. The dashed vertical line at week 24 indicates the end of study Phase I. The dashed vertical line at week 120 indicates the end of study Phase II. The horizontal dashed line indicates baseline level. * = p < 0.01 Early-start vs. Delayed-start.
Table 4.
Summary and Analysis of “Off” UPDRS Motor Scores in Comparison Group Subjects
| Observed Scores |
Change from Baseline |
Change from Week 24 |
||
|---|---|---|---|---|
| Time Point | N | Mean (SEM) | Mean (SEM)[1] | Mean (SEM)[2] |
| Week 0 (Baseline) | 17 | 22.31 (1.58) | ||
| Week 24 | 17 | 24.59 (1.85) | 2.28 (0.86)# | |
| Week 48 | 16 | 27.00 (1.59) | 4.41 (1.43)$ | 2.13 (1.35) |
| Week 72 | 10 | 29.65 (1.51) | 5.95 (1.22)$ | 5.35 (1.39)$ |
| Week 96 | 8 | 30.50 (1.38) | 7.81 (2.01)$ | 7.13 (1.88)$ |
| Slope Estimate (95% CL) | 17 | 0.24 (0.08, 0.40) | ||
| P Value[3] | 0.0050 |
Paired-sample t-test on change from baseline: $ p<0.01; # p<0.05.
Paired-sample t-test on change from week 24: $ p<0.01.
Model for rate of change included intercept, baseline, continuous weeks in study, and interaction between week and baseline.
Similar analyses were performed for data collected during the “on” period (Supplemental Tables 1a, b). At week 24, E-S subjects had significantly lower mean “on” period UPDRS motor scores (i.e., improvement in motor symptoms) than at baseline while D-S subjects had significantly higher mean motor scores (i.e., worsening of symptoms) than at baseline (Figure 2B). The difference between the E-S and D-S groups was −4.33 points (p <0.0001). The observed CFB in the Comparison group was +3.33 ± 0.87 points, p=0.0018) (Supplemental Table 1c).
Phase II treatment effects
At the first assessment of Phase II (week 36), E-S group motor scores did not differ significantly from those at week 24 and remained significantly decreased from baseline. In contrast, D-S group motor scores significantly improved compared to week 24 but not compared to baseline. The difference between the groups at week 36 remained statistically significant: difference in CFB was −2.28 points (p=0.0222), (Table 2); difference in CFW24 was 2.70 points (p=0.0003) (Tables 2, 3). At approximately one year into Phase II (week 72), the E-S group showed no significant symptom worsening from week 24 and remained significantly improved from baseline; the D-S group also remained significantly improved compared to week 24 (Tables 2, 3). At week 120, E-S group motor scores were not significantly changed from week 24 and still lower than at baseline (−2.29 ± 1.22 points, p = 0.0706). The D-S group had motor scores slightly lower than at week 24 and slightly higher than at baseline (+1.16 ± 1.20 points, p=0.3396). The difference in CFB between the groups at week 120 was −3.33 (p=0.0368, observed data) and −3.09 points (p=0.0594, imputed data). Estimated rate of change per week (Phase II) was 0.02 points (p=0.0502) for the E-S group and 0.04 points (p=0.0003) for the D-S group; the difference was not statistically significant (Table 2). Comparison group subjects showed a significant increase in motor scores at week 48 (+4.41 ± 1.43 points, p=0.0077), week 72 (+5.95 ± 1.22 points, p = 0.0009) and week 96 (their last observation, +7.81 ± 2.01 points, p=0.0061), compared to baseline (Table 4).
Table 3.
Summary and Analysis of GM1 Treatment Effects on “Off” UPDRS Motor Scores: Change from Week 24 During Phase II.
| Change in Motor Scores from Week 24 | |||||
|---|---|---|---|---|---|
| Time Point | Early-Start (N=38) | Delayed-Start (N=38) | Observed Difference in CFW24[1] |
||
| N | Mean (SEM)[2] | N | Mean (SEM)[2] | (95% CL) | |
| Week 36 | 35 | −0.07 (0.51) | 38 | −3.11 (0.45)^ | 2.70 (1.30, 4.11)~ |
| Week 48 | 36 | 0.10 (0.49) | 37 | −4.03 (0.56)^ | 3.70 (2.11, 5.29)* |
| Week 72 | 32 | 0.63 (0.79) | 35 | −1.86 (0.53)$ | 2.03 (0.14, 3.91)~ |
| Week 96 | 33 | 1.14 (1.01) | 32 | −1.36 (0.75) | 1.87 (−0.64, 4.39) |
| Week 120 | 29 | 1.11 (1.05) | 31 | −0.63 (0.95) | 1.64 (−1.15, 4.43) |
2-sample t-test for difference in change from baseline between Early-Start and Delayed-Start groups at given time point derived from mixed effects ANCOVA: * p <0.0001; ~ p <0.05
Paired-sample t-test: ^ p<0.0001; $ p<0.01.
CFW24 = change from week 24.
When assessed during the “on” period, changes in motor scores of the E-S group broadly followed the changes observed during “off” period evaluations: scores at week 36 were not significantly different form those at week 24 (p=0.5443) but significantly decreased from baseline (p=0.0185) (Supplemental Tables 1a, b; Figure 2B). At week 72, the E-S group showed no significant change from week 24; scores were still slightly decreased compared to baseline (−1.63 ± 0.865, points, p=0.0721) and the D-S group remained significantly improved compared to week 24 (−1.48 ± 0.652, points, p=0.0309) (Figure 2B). At week 120, E-S group motor scores were not significantly changed from week 24 (+1.01 ± 0.976, p = 0.3114) and lower than at baseline, but not significantly so (−1.13 ± 1.08 points, p = 0.3086). D-S group motor scores were slightly higher than at week 24 (0.17 ± 0.815 points, p = 0.8395) and significantly higher than at baseline (+2.33 ± 1.124 points, p=0.0480). The difference in CFB between E-S and D-S groups at week 120 was statistically significant (−3.94 points, p=0.0111, observed data and −3.89 points, p=0.0094, imputed data). The Comparison group had significantly higher “on” motor scores at week 48 (+3.87 ± 1.48 points, p=0.0182), week 72 (+5.45 ± 1.18 points, p = 0.0012) and at week 96 (+7.59 ± 1.81 points, p=0.0040), compared to baseline (Supplemental Table 1c).
Additional treatment effects
“Off” UPDRS motor scores significantly increased in both E-S and D-S subjects at one year of washout (+7.30 ± 0.96 points (p< 0.0001) and 6.52 ± 0.97 points (p<0.0001), respectively) and at two years of washout (+10.33 ± 1.46 (p< 0.0001) and 10.69 ± 1.88 points (p = 0.0001), respectively), compared to scores at week 120 (Figure 2, Table 2).
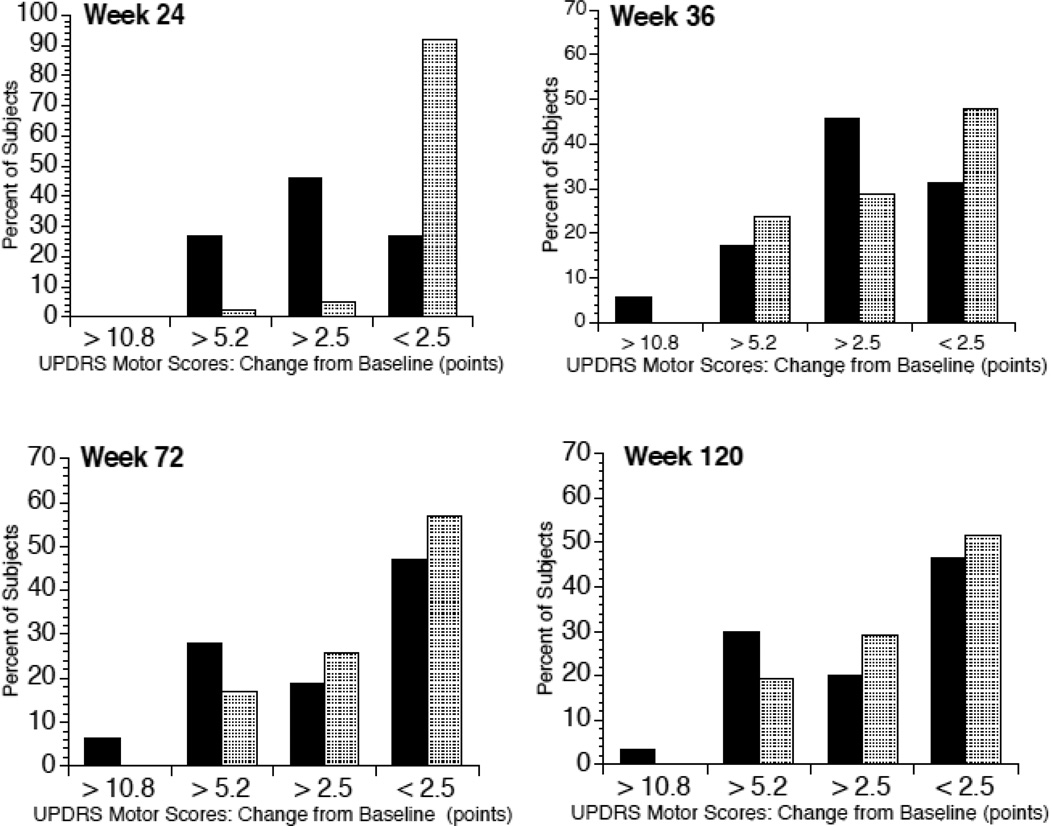
Assessment of clinically important differences (CID) in the UPDRS motor score [22] showed 73% of E-S subjects exceeded thresholds for minimum or moderate CID compared to 8% of D-S subjects at week 24 (Figure 3). By week 120, 53% of E-S subjects continued to have at least minimum CID vs. 48% of D-S subjects.
Figure 3.
Percent of study subjects showing clinically important differences in UPDRS motor subsection scores. Graphs indicate the percent of subjects judged to have minimal (≥2.5 points), moderate (≥5.2 points), large (≥10.8 points) or no (<2.5 points) clinically important change in UPDRS motor scores compared to baseline. Solid bars indicate early-start subjects; shaded bars indicate delayed-start subjects. Week 24 = end of the placebo-controlled phase of the study; Week 36 = 12 weeks after delayed-start group began GM1 use; Week 72 = approximately 1 year after start of Phase II of the study; Week 120 = approximately 2 years after start of Phase II of the study.
Results similar to those already reported were obtained from analysis of total UPDRS scores (Supplemental Tables 2a, 2b, 2c).
Adverse events and safety
The most prevalent adverse events were injection site reactions including injection site pain, erythema, pruritis, swelling, nodule, hemorrhage/hematoma, urticaria, and induration. These occurred more frequently in the E-S group during phase 1 of the study and increased in the D-S group in Phase II (Supplemental Table 3). Pain and erythema were the most common injection site reactions in the E-S group in Phase I: pain and hemorrhage were most frequently reported in the D-S group in Phase I. Pain and erythema were the most common injection site reactions in the both groups in Phase II. Three subjects reported serious adverse events (i.e., asthenia, worsening of Parkinson’s symptoms, anastomotic ulcer/stomach cancer). There were no consistent clinically significant clinical chemistry changes in either treatment group.
DISCUSSION
In Phase I, there was a significant improvement in “off” and “on” UPDRS motor (and total UPDRS) scores in GM1-treated subjects and a small (but significant) symptom worsening in placebo-treated subjects that mirrored the change observed in the standard-of-care Comparison group. The symptomatic effect from GM1 may relate to functional improvement in residual dopaminergic neurons, a conclusion supported by preclinical data showing enhanced dopamine synthesis in residual neurons in a mouse model of Parkinsonism following GM1 treatment [18]. Interestingly, bradykinesia, a clinical sign thought to best reflect a nigrostriatal dopaminergic deficit [33] appeared to be most responsive to GM1 treatment, while tremor and posture/balance/gait were less responsive.
In Phase II, E-S subjects maintained much of the initial benefit of GM1 treatment and by the end of the study, their scores remained below those recorded at study baseline. The D-S subjects showed symptomatic benefit after switching to GM1 use. By the end of the study, their scores were below those observed at the end of the placebo period (during which time there was some symptom worsening). These data suggest a sustained benefit for the E-S group and show that both treatment groups fared better than the Comparison subjects. These findings are unlikely related to a symptomatic effect alone since the symptom progression trajectory for the two groups was divergent at the end of the study, a result suggestive of a potential disease-modifying effect [25].
During the extended washout period, symptoms significantly worsened in all subjects. The increase in UPDRS scores seen in the first washout year could be related to loss of a GM1-induced effect on dopamine synthesis and release in residual dopamine neurons combined with a loss of any compensatory responses and/or withdrawal of trophic support stimulated by GM1.
The mechanisms through which potential neuroprotective/neurorestorative effects from GM1 may be exerted are uncertain but may involve modulation of lipid raft structure/function. Lipid rafts, modulatory platforms for a variety of signaling pathways [34, 35], are influenced by the GM1 content. Several PD-relevant proteins specifically associate with lipid rafts and co-localize with GM1. Alpha-synuclein fibrillization may contribute to PD pathophysiology [36] and binding of α-synuclein to GM1 inhibits fibril formation, dependent upon the amount of GM1 present [37]. LRRK2, parkin, and PINK1 also associate with lipid rafts and co-localize with GM1, potentially influencing neurodegeneration in PD [38, 39].
Long-term use of GM1 was not associated with any consistent non-injection site related adverse events. Although Guillain–Barré Syndrome (GBS), an acute inflammatory polyneuropathy, was previously suggested to develop in a small number of patients using ganglioside preparations [40–43], there have been no such reports in patients receiving purified GM1 in clinical studies [44] and no increase in anti-GM1 antibody titres in patients previously followed by us [28]. Further arguing against a GM1-GBS connection, there has been no decrease in the GBS incidence after the withdrawal of gangliosides from the market in Italy [45].
A limitation of this study is that it was performed at a single center and studied a relatively small number of subjects. Additionally, this study utilized subjects already receiving anti-PD medications, including levodopa. Although results obtained during a practically defined “off” period may be potentially complicated by the any “long duration response” to levodopa, it is possible, but unlikely, that this effect substantially contributed to the current findings as it would have been expected to influence the groups similarly. To further account for potential influences in medication status on the study outcome, subjects were also examined during a best “on” period. Data derived during the “on” period followed the same general pattern seen in the “off” condition. Even when assessed in the “on” condition, E-S subjects improved during Phase I while D-S subjects worsened somewhat as did Comparison group subjects. E-S subjects maintained an improved “on” response throughout the study and remained divergent from the D-S group. Comparison group subjects continued to deteriorate over time, despite being “optimally medicated”.
The groups were well matched for demographics and symptom severity although there were non-significant differences in anti-PD drug use at baseline. The D-S group (which included 2 subjects with the highest LEDs) had a higher mean LED at baseline than the E-S group (which included 5 unmedicated subjects compared to 2 unmedicated subject in the D-S group). While these differences in medication status may suggest that the E-S group contained less advanced subjects, the UPDRS data does not support that assertion. In addition, there was a small but statistically significant difference in median time since diagnosis, with a slightly shorter time since diagnosis in the E-S group compared to the D-S group. Although this could suggest that the E-S group had milder disease at the outset of the study, the measure of time since diagnosis is not a reliable measure of disease progression or symptom status. A better index of disease severity is the UPDRS motor scores, which were not significantly different between the groups at baseline.
There are potential problems and limitations to all PD trials that attempt to assess a potential disease modifying therapy that might also have a symptomatic effect, as is the case here as well as in prior studies using a similar design [26]. However, the current data do not support the existence of a potential confound from “unequally-distributed placebo responses”, as suggested by de la Fuente-Fernandez et al. in their interpretation of the ADAGIO Study results [46]. Even though a GM1-related improvement in UPDRS score during the first 12 weeks of treatment was slightly smaller in the D-S group (weeks 24–36) than in the E-S group (weeks 0–12), there was little or no placebo response in the E-S group at the start of Phase II. Also, since raters saw subjects at 12–24 week intervals during Phase II and two independent raters examined each subject, it is unlikely that rater bias played a significant role in the study outcome.
In conclusion, the present results from this small proof of concept study suggest that extended use of GM1 ganglioside is safe in PD patients, has an early symptomatic effect and may slow symptom progression over a 2 year period. Specifically, the latter point needs to be confirmed in a larger, multi-center study. In addition, the effects of GM1 ganglioside treatment in earlier, unmedicated patients should also be assessed.
Supplementary Material
Acknowledgements
We thank Drs. David P. Roeltgen and Sandra Weibel for serving as independent medical monitors for this study. We also thank Dr. Roberto Fiorentini for providing helpful comments and advice throughout the project.
Funding: This study was funded by NIH grant NS038681.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
J.S. originally designed the study and S.G. subsequently provided input on study design. J.S., S.G., S.S., A.C. and F.C. collected data. W.D. conducted the statistical analysis of the data. J.S. drafted the manuscript. S.G., S.S., A.C., F.C. and W.D. revised the manuscript.
References
- 1.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 2.Uitti RJ, Ahlskog JE, Maraganore DM, et al. Levodopa therapy and survival in idiopathic Parkinson's disease: Olmstead County project. Neurology. 1993;43:1918–1926. doi: 10.1212/wnl.43.10.1918. [DOI] [PubMed] [Google Scholar]
- 3.Olanow CW, Schapira AH, Agid Y. Neuroprotection for Parkinson's disease: prospects and promises. Ann Neurol. 2003;53(Suppl 3):S1–S2. doi: 10.1002/ana.10566. [DOI] [PubMed] [Google Scholar]
- 4.Allende ML, Proia RL. Lubricating cell signaling pathways with gangliosides. Curr Opin Struct Biol. 2002;12:587–592. doi: 10.1016/s0959-440x(02)00376-7. [DOI] [PubMed] [Google Scholar]
- 5.Hakomori S, Igarashi Y. Gangliosides and glycosphingolipids as modulators of cell growth, adhesion, and transmembrane signaling. Adv Lipid Res. 1993;25:147–162. [PubMed] [Google Scholar]
- 6.Ledeen RW, Wu G. Ganglioside function in calcium homeostatsis and signaling. Neurochem Res. 2002;27:637–647. doi: 10.1023/a:1020224016830. [DOI] [PubMed] [Google Scholar]
- 7.Wei J, Fujita M, Nakai M, et al. Protective role of endogenous gangliosides for lysosomal pathology in a cellular model of synucleinopathies. Am J Pathol. 2009;174:1891–1909. doi: 10.2353/ajpath.2009.080680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shield AJ, Murray TP, Board PG. Functional characterisation of ganglioside-induced differentiation-associated protein 1 as a glutathione transferase. Biochem Biophys Res Commun. 2006;347:859–866. doi: 10.1016/j.bbrc.2006.06.189. [DOI] [PubMed] [Google Scholar]
- 9.Bianchi R, Janigro D, Milan F, Giudici G, Gorio A. In vivo treatment with GMI prevents the rapid decay of ATPase activity and mitochondria1damage in hippocampal slices. Brain Res. 1986;364:400–404. doi: 10.1016/0006-8993(86)90856-5. [DOI] [PubMed] [Google Scholar]
- 10.Jonsson G, Gorio A, Hallman H, et al. Effects of GM1 ganglioside on developing and mature serotonin and noradrenaline neurons after lesion by selective neurotoxins. J Neurosci Res. 1984;12:459–475. doi: 10.1002/jnr.490120229. [DOI] [PubMed] [Google Scholar]
- 11.Hadjiconstantino M, Rosetti ZL, Paxton RC, Neff NH. Administration of GM1 ganglioside restores the dopamine content in striatum after chronic treatment with MPTP. Neuropharmacology. 1986;25:1075–1077. doi: 10.1016/0028-3908(86)90206-6. [DOI] [PubMed] [Google Scholar]
- 12.Tilson HA, Harry GJ, Nanry K, Hudson PM, Hong JS. Ganglioside interactions with the dopaminergic system of rats. J Neurosci Res. 1988;19:88–93. doi: 10.1002/jnr.490190112. [DOI] [PubMed] [Google Scholar]
- 13.Oderfeld-Nowak B, Skup M, Ulas J, Jezierska M, Gradkowska M, Zarenba M. Effect of ganglioside GM1 treatment on post-lesion responses of cholinergic enzymes in rat hippocampus after various partial deafferentations. J Neurosci Res. 1984;12:409–420. doi: 10.1002/jnr.490120225. [DOI] [PubMed] [Google Scholar]
- 14.Schneider JS, Pope A, Simpson K, Taggart J, Smith MG, DiStefano L. Recovery from experimental parkinsonism in primates with GM1 ganglioside treatment. Science. 1992;256:843–846. doi: 10.1126/science.1350379. [DOI] [PubMed] [Google Scholar]
- 15.Stull ND, Schneider JS, Iacovitti L. GM1 ganglioside partially rescues cultured dopaminergic neurons from MPP+ induced damage: dependence on initial damage and time of treatment. Brain Res. 1994;640:308–315. doi: 10.1016/0006-8993(94)91886-4. [DOI] [PubMed] [Google Scholar]
- 16.Pope-Coleman A, Tinker JP, Schneider JS. Effects of GM1 ganglioside treatment on pre-and postsynaptic dopaminergic markers in the striatum of parkinsonian monkeys. Synapse. 2000;36:120–128. doi: 10.1002/(SICI)1098-2396(200005)36:2<120::AID-SYN5>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 17.Herrero MT, Perez-Otano I, Oset C, et al. GM1 ganglioside promotes the recovery of surviving midbrain dopaminergic neurons in MPTP-treated monkeys. Neuroscience. 1993;56:965–972. doi: 10.1016/0306-4522(93)90142-3. [DOI] [PubMed] [Google Scholar]
- 18.Schneider JS, Kean A, DiStefano L. GM1 ganglioside rescues substantia nigra pars compacta neurons and increases dopamine synthesis in residual nigrostriatal dopaminergic neurons in MPTP-treated mice. J Neurosci Res. 1995;42:117–123. doi: 10.1002/jnr.490420113. [DOI] [PubMed] [Google Scholar]
- 19.Tseng EE, Brock MV, Lange MS, et al. Monosialoganglioside GM1 inhibits neurotoxicity after hypothermic circulatory arrest. Surgery. 1998;124:298–306. [PubMed] [Google Scholar]
- 20.Kojima H, Gorio A, Janigro D, Jonsson G. GM1 ganglioside enhances regrowth of noradrenaline nerve terminals in rat cerebral cortex lesioned by the neurotoxin 6-hydroxydopamine. Neuroscience. 1984;13:1011–1022. doi: 10.1016/0306-4522(84)90285-9. [DOI] [PubMed] [Google Scholar]
- 21.Ballough GPH, Cann FJ, Smith CD, Forster JS, Kling CE, Filbert MG. GM1 monosialoganglioside pretreatment protects against soman-induced seizure related brain damage. Mol Chem Neuropathol. 1998;34:1–23. doi: 10.1007/BF02815133. [DOI] [PubMed] [Google Scholar]
- 22.Schneider JS, Sendek S, Daskalakis C, Cambi F. GM1 ganglioside in Parkinson’s disease: Results of a five year open study. J Neurol Sci. 2010;292:45–51. doi: 10.1016/j.jns.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 23.Parkinson Study Group. A controlled, randomized, delayed-start study of rasagiline in early Parkinson’s disease. Arch Neurol. 2004;61:561–566. doi: 10.1001/archneur.61.4.561. [DOI] [PubMed] [Google Scholar]
- 24.Olanow CW, Hauser RA, Jankovic J, et al. A randomized, double-blind, placebo-controlled, delayed start study to assess rasagiline as a disease modifying therapy in Parkinson’s disease (The ADAGIO Study): rationale, design, and baseline characteristics. Mov Disord. 2008;23:2194–2201. doi: 10.1002/mds.22218. [DOI] [PubMed] [Google Scholar]
- 25.D’Agostino RB., Sr The delayed-start study design. N Engl J Med. 2009;361:1304–1306. doi: 10.1056/NEJMsm0904209. [DOI] [PubMed] [Google Scholar]
- 26.Olanow CW, Rascol O, Hauser R, et al. A double-blind, delayed-start trial of rasagiline in Parkinson’s disease. N Engl J Med. 2009;361:1268–1278. doi: 10.1056/NEJMoa0809335. [DOI] [PubMed] [Google Scholar]
- 27.Schneider JS, Roeltgen DP, Mancall EL, et al. Parkinson's disease: Improved function with GM1 ganglioside treatment in a randomized placebo-controlled study. Neurology. 1998;50:1630–1636. doi: 10.1212/wnl.50.6.1630. [DOI] [PubMed] [Google Scholar]
- 28.Schneider JS, Roeltgen DP, Rothblat DS, Chapas-Crilly J, Seraydarian L, Rao J. GM1 ganglioside treatment of Parkinson's disease: an open pilot study of safety and efficacy. Neurology. 1995;45:1149–1154. doi: 10.1212/wnl.45.6.1149. [DOI] [PubMed] [Google Scholar]
- 29.Kim M, Gollomp SM, Schneider JS. Does a two rater system improve reliability of UPDRS motor scoring of mild Parkinson’s disease patients? Mov Dis. 2006;21(Suppl.13):S26. [Google Scholar]
- 30.Svennerholm L, Gottfries CG, Blennow K, et al. Parenteral administration of GM1 ganglioside to presenile Alzheimer patients. Acta Neurol Scand. 1990;81:48–53. doi: 10.1111/j.1600-0404.1990.tb00930.x. [DOI] [PubMed] [Google Scholar]
- 31.Shulman LM, Gruber-Baldini AL, Anderson KE, et al. The clinically important difference on the Unified Parkinson’s Disease Rating Scale. Arch Neurol. 2010;67:64–70. doi: 10.1001/archneurol.2009.295. [DOI] [PubMed] [Google Scholar]
- 32.Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Dis. 2010;25:2649–2685. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- 33.Vingerhoets FJG, Schulzer M, Calne DB, Snow BJ. Which clinical signs of Parkinson’s disease best reflect the nigrostriatal lesion? Ann Neurol. 1997;41:58–64. doi: 10.1002/ana.410410111. [DOI] [PubMed] [Google Scholar]
- 34.Zajchowski LD, Robbins SM. Lipid rafts and little caves. Compartmentalized signaling in membrane microdomains. Eur J Biochem. 2002;269:737–752. doi: 10.1046/j.0014-2956.2001.02715.x. [DOI] [PubMed] [Google Scholar]
- 35.Michel V, Bakovic M. Lipid rafts in health and disease. Biol Cell. 2007;99:129–140. doi: 10.1042/BC20060051. [DOI] [PubMed] [Google Scholar]
- 36.Goldberg MS, Lansbury PT., Jr Is there a cause-and-effect relationship between α-synuclein fibrillization and Parkinson’s disease? Nature Cell Biol. 2000;2:E115–E119. doi: 10.1038/35017124. [DOI] [PubMed] [Google Scholar]
- 37.Martinez Z, Zhu M, Han S, Fink AL. GM1 specifically interacts with α-synuclein and inhibits fibrillation. Biochemistry. 2007;46:1868–1877. doi: 10.1021/bi061749a. [DOI] [PubMed] [Google Scholar]
- 38.Fallon L, Moreau F, Croft BG, et al. Parkin and CASK/LIN-2 associate via a PDZ-mediated interaction and are co-localized in lipid rafts and postsynaptic densities in brain. J Biol Chem. 2002;277:486–491. doi: 10.1074/jbc.M109806200. [DOI] [PubMed] [Google Scholar]
- 39.Hatano T, Kubo S-I, Imai S, et al. Leucine-rich repeat kinase 2 associates with lipid rafts. Human Mol Genetics. 2007;16:678–690. doi: 10.1093/hmg/ddm013. [DOI] [PubMed] [Google Scholar]
- 40.Figueras A, Morales-Olivas FJ, Capella D, Palop V, Laporte JR. Bovine gangliosides in acute polyneuropathy. BMJ. 1992;305:1330–1331. doi: 10.1136/bmj.305.6865.1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Landi G, D'Alessandro R, Dossi BC. Guillain–Barré syndrome after exposure to gangliosides. BMJ. 1993;307:1463–1464. doi: 10.1136/bmj.307.6917.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raschetti R, Maggini M, Popoli P, et al. Gangliosides and Guillain–Barré syndrome. J Clin Epidemiol. 1995;48:1399–1405. doi: 10.1016/0895-4356(95)00557-9. [DOI] [PubMed] [Google Scholar]
- 43.Illa I, Ortiz N, Gallard E, Juarez C, Grau JM, Dalakas MC. Acute inflammatory demyelinating axonal Guillain–Barré syndrome with IgG antibodies against motor polyradiculoneuropathy. Ann Neurol. 1995;38:218–224. doi: 10.1002/ana.410380214. [DOI] [PubMed] [Google Scholar]
- 44.Yu RK, Saito RK, Zhang Y, Fiorentini R, Khin-Maung-Gyi F, Friday G. Analysis of serum anti-GM1 antibody titres in patients receiving GM1 therapy. Soc Neurosci Abstr. 1992;18:1472. [Google Scholar]
- 45.Govoni V, Granieri E, Manconi M, Capone J, Casetta I. Is there a decrease in Guillain–Barré syndrome incidence after bovine ganglioside withdrawal in Italy? A population-based study in the Local Health District of Ferrara, Italy. J Neurol Sci. 2003;216:99–103. doi: 10.1016/s0022-510x(03)00215-6. [DOI] [PubMed] [Google Scholar]
- 46.de la Fuente-Fernandez R, Schulzer M, Mak E, Sossi V. Trials of neuroprotective therapies for Parkinson’s disease: Problems and limitations. Parkinsonism and Related Disorders. 2010;16:365–369. doi: 10.1016/j.parkreldis.2010.04.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.