Abstract
Fetal liver epithelial cells (FLEC) are valuable for liver cell therapy and tissue engineering, but methods for culture and characterization of these cells are not well developed. This work explores the influence of multiple soluble factors on FLEC, with the long-term goal of developing an optimal culture system to generate functional liver tissue. Our comparative analysis suggests hepatocyte growth factor (HGF) is required throughout the culture period. In the presence of HGF, addition of oncostatin M (OSM) at culture initiation results in concurrent growth and maturation, while constant presence of protective agents like ascorbic acid enhances cell survival. Study observations led to the development of a culture medium that provided optimal growth and hepatic differentiation conditions. FLEC expansion was observed to be ~2 fold of that under standard conditions, albumin secretion rate was 2 – 3 times greater than maximal values obtained with other media, and the highest level of glycogen accumulation among all conditions was observed with the developed medium. Our findings serve to advance culture methods for liver progenitors in cell therapy and tissue engineering applications.
Keywords: hepatocytes, FLEC, stem cells, hepatocyte growth factor, tissue engineering
INTRODUCTION
A major obstacle to development of liver cell therapy and liver tissue engineering is the difficulty in culturing adult hepatocytes. Despite decades of effort to optimize culture conditions, adult hepatocytes either show limited proliferation or quickly lose their differentiated phenotype in vitro [1]. This hinders the generation of an adequate mass of functional liver tissue for clinical applications [2]. FLEC, which contain a high percentage of liver progenitors, possess high growth potential and readily repopulate diseased livers [3, 4]. The early development stage, pre-immune character, and high telomerase activity of fetal cells make them an attractive alternative source of hepatocytes [5]. To generate a large number of functional hepatocytes from FLEC, however, it is necessary to establish a culture system that supports their optimal proliferation and maturation. Methods established for FLEC would also provide a foundation for developing culture techniques for other liver progenitors, such as oval cells and embryonic stem cells.
Many of the factors that influence adult hepatocytes also exert regulatory effects on FLEC. For example, hepatocyte growth factor (HGF), whose combination with epidermal growth factor (EGF) has optimal mitogenic effect on adult hepatocytes [6], was found to be critical in proliferation of hepatic stem cells and hepatoblasts [7–9]. Dexamethasone, a synthetic glucocorticoid, improves the viability of mature hepatocytes and preserves their function [6, 10]. In the fetal liver, dexamethasone suppresses the expression of α-fetoprotein (AFP) and upregulates albumin synthesis [11]. In culture, it induces E12.5 FLEC to acquire characteristics of perinatal hepatocytes, as demonstrated by the expression of glucose-6-phosphatase (G6Pase) and tyrosine amino transferase (TAT) [12].
Other molecules have been found to affect fetal liver development. Most notably, oncostatin M (OSM), a member of the interleukin-6 (IL-6) family, was shown to be a potent stimulator of the maturation of FLEC along the hepatic lineage [13, 14]. Dexamethasone is absolutely required for the actions of OSM, and augments its effects in a dose-dependent fashion, in the range of 0 to 1 µM [13]. The maturation of FLEC induced by OSM is further enhanced by the presence of DMSO and nicotinamide [15], two pivotal factors that extend the survival, DNA synthesis, and functional preservation of adult hepatocytes in culture [6, 16–19].
In previous studies of FLEC in culture, medium formulations were designed to observations surrounding fetal liver development. Basal media ranged from standard formulations such as DMEM and DMEM/F12, to those specifically designed for long-term culture of adult hepatocytes such as Williams’ E medium [20] and the chemically defined Block’s medium [6]. These basal media were typically supplemented with components commonly required for many cell types, including serum or bovine albumin, L-glutamine, non-essential amino acids, and insulin, in addition to compounds that regulate FLEC, such as dexamethasone and nicotinamide. Depending on the purpose of the experiment, factors that control the growth or differentiation of FLEC were also applied. EGF and HGF were added to stimulate growth, whereas OSM was used to induce hepatic maturation.
Mitogens and differentiation stimulators have also been combined to achieve both maximal proliferation and maturation. Certain studies have applied a two-step approach [21, 22], in which the FLEC were first exposed to a growth-stimulating medium and subsequently, induced to differentiate under conditions that encouraged maturation but might reciprocally repress growth. In other studies, a single medium combining mitogens and differentiation factors were used for the entire culture period [23].
Although a wide variety of FLEC culture media have been described, there has been no direct comparison of their effectiveness nor have the kinetics of cell growth and maturation been delineated under most of these culture conditions. Consequently, it remains unclear how regulatory factors should be utilized to achieve optimal expansion and hepatic differentiation of FLEC. Such studies addressing these issues would provide insight on how to control proliferation and differentiation of FLEC, and would facilitate rational design of culture methods for hepatic progenitors.
In the study described, we directly compared four representative medium conditions. The first medium (denoted as medium G), containing HGF and EGF as the key regulatory factors, has been used previously to support the clonal expansion of liver stem cells isolated from E13.5 mouse livers [7, 8]. The second formulation (denoted as medium D) was designed to induce the morphological and functional maturation of E14.5 FLEC based on use of OSM, DMSO, high concentration of dexamethasone, and basal medium Williams’ E [15]. Media G and D formed the basis for development of a third medium, GD, which contains both growth- and differentiation-stimulating factors. To emulate methods that combined mitogens and differentiation factors, we applied medium GD either throughout the culture period or after one week of culture in medium G. We compared these two conditions with the commonly used media formulations, G and D, to investigate whether the growth and differentiation of FLEC could be enhanced by modulating regulatory factors in the medium and by adjusting the timing of their supplementation.
MATERIALS AND METHODS
Materials
All experiments were approved by the Yale University Institute of Animal Care and Use Committee. C57BL/6 mice were supplied by Jackson Laboratory (Bar Harbor, Maine). Trypsin stock solution, calcium/magnesium-free Dulbecco’s PBS (DPBS), antibiotic-antimycotic, L-glutamine, non-essential amino acids, all basal culture media, human EGF, and Trypsin/EDTA were purchased from Invitrogen Gibco (Grand Island, NY). Fetal bovine serum (FBS) and HEPES buffer solution were procured from Atlanta Biologicals (Lawrenceville, GA), while dexamethasone, nicotinamide, ascorbic acid diphosphate, DMSO, and insulin solutions were from Sigma (St. Louis, MO). Human HGF and mouse OSM were purchased from R&D Systems (Minneapolis, MN) and Pharm Lyse solution was from BD Pharmingen (San Jose, CA). Collagen stock solution was purchased from Angiotech BioMaterials (Palo Alto, CA). Tissue-culture treated polystyrene plates, cell strainers, and collagen-coated chamber slides were obtained from BD Discovery Labware (San Jose, CA), while beta-mercaptoethanol, Tris-HCl, EDTA, and Triton-X100 stock solutions were from American Bioanalytical (Natick, MA). Paraformaldehyde stock solution was acquired from Electron Microscopy Sciences (Hatfield, PA).
Preparation of FLEC
Methods for isolation and processing of FLEC were modified from previously reported protocols [7, 15]. Timed pregnancies were set up using C57BL/6 mice. Noon of the day a vaginal plug was found was considered as embryonic day 0.5 (E0.5). On E14.5, fetal livers were aseptically dissected from the fetuses. Livers were treated with 0.1% Trypsin for 10 minutes at 37°C and triturated to achieve a single cell suspension. The cells were treated with Pharm Lyse solution to break apart erythrocytes, which were then filtered with a cell strainer. Bulk fetal liver cells, depleted of erythrocytes and other non-adherent hematopoietic cells, were used in our experiments based on the fact that this cell population is widely used in mouse FLEC studies [3, 4]
Culture of FLEC
Composition of each culture medium tested in the study is described in Table 1. Incomplete culture media refer to media that were not supplemented with insulin, EGF, HGF, OSM, or DMSO. Complete culture media included these supplemental factors. FLEC were suspended in the incomplete medium of interest and seeded at 5 × 104 cells/cm2 in 12-well plates pre-coated with 0.03% Type-I collagen. After 20 – 24 hours of culture, culture supernatant was removed and culture wells were rinsed with DPBS to remove non-adherent cells. Complete culture medium was used for the remaining culture period and replenished every 2 – 3 days.
Table 1.
Formulation composition of culture media evaluated.
| Components | Medium G | Medium D | Medium GD |
|---|---|---|---|
| Basal medium | DMEM/F-12 | Williams’ E | DMEM/F-12 |
| FBS | 10% | 10% | 10% |
| L-glutamine | 1% | 1% | 1% |
| Non-essential amino acids | 1% | 1% | 1% |
| Antibiotic-antimycotic | 1% | 1% | 1% |
| HEPES | 5 mM | - | 5 mM |
| Dexamethasone | 0.1 µM | 1 µM | 1 µM |
| Nicotinamide | 10 mM | 10 mM | 10 mM |
| Ascorbic acid diphosphate | - | 0.5 mM | 0.5 mM |
| β-mercaptoethanol | 50 µM | - | 50 µM |
| Insulin | 1 µg/ml | 1 µg/ml | 1 µg/ml |
| EGF | 20 ng/ml | 10 ng/ml | 20 ng/ml |
| HGF | 50 ng/ml | - | 50 ng/ml |
| OSM | - | 10 ng/ml | 10 ng/ml |
| DMSO | - | 1% | 1% |
Assessment of cell growth
Cell growth was monitored using the MTS assay and total cellular DNA quantification. At each time point, culture medium was removed and the wells were rinsed with DPBS. The MTS assay was subsequently performed per kit (Promega, Madison, WI) instructions. For DNA quantification, the cells were detached with Trypsin/EDTA, treated with a cell lysis solution (10 mM Tris-HCl + 1 mM EDTA + 0.2% Triton-X100, pH 7.5), and sonicated on ice using a Vibracell sonicator (Sonics & Materials, Newton, CT). The sonicated samples were quantified for DNA using a PicoGreen dsDNA Quantitation kit (Molecular Probes, Eugene, OR).
Analysis of hepatocyte function
Albumin synthesis and glycogen accumulation were analyzed to evaluate the development of hepatocyte function in the FLEC cultures. Mouse albumin secreted into the culture supernatant was measured by ELISA assay according to kit (Bethyl Laboratories Montgomery, TX) instructions. Periodic acid-Schiff (PAS) staining of the cultures was performed to assess glycogen accumulation. Cultures for PAS staining were set up in collagen-coated chamber slides on day 0 in parallel with those in 12-well plates. The number of cells seeded into the chamber wells was scaled according to available culture surface to maintain the plating density at 5 × 104 cells/cm2. At designated time points, spent culture medium was aspirated. The chamber wells were quickly rinsed with DPBS, fixed with 4% paraformaldehyde at room temperature for 10 minutes, and rinsed twice with DPBS. The PAS staining was performed by the Research Histology Laboratory at Yale University. The level of glycogen accumulation was scored in the following manner: “-“ when less than 1% of the cells were stained, “+” for 1 – 25% positive staining, “++” for 25 – 50% staining, “+++” for 50 – 75% staining, and “++++” for > 75% staining.
Statistics
All experiments were performed at a minimum of 3 times using cells from different animal donors, with each growth condition being tested at 3 – 6 replicates. A Student’s t-test was used to evaluate the statistical significance where indicated. Significance level was set at p<0.05.
RESULTS
We compared the culture of E14.5 mouse FLEC under four representative conditions: a growth-stimulating medium (G) based on HGF/EGF as the key mitogens; a differentiation-promoting medium (D) with OSM/DMSO as the key regulating factors; a modified medium (GD) supplemented with HGF, EGF, OSM, and DMSO; and a two-step approach in which media G and GD were applied sequentially. Specifically, FLEC were cultured: 1) for 2 weeks in medium D; 2) for 2 weeks in medium G; 3) in medium G for 1 week followed by another week in medium GD (medium G-GD); or 4) for 2 weeks in medium GD. Similar trends were observed when the experiment was repeated with cells from different animals.
Morphological changes
After the removal of non-adherent cells (predominantly blood cells) at day 1, only about 5% of the seeded cells remained in the culture wells. The attached cells consisted of a few clusters of very small (5 – 10 µm) cells. Under all conditions, these small cells increased in number over time. Consistent with previous reports that hematopoietic cells from fetal livers have poor survival under culture conditions specifically designed for hepatic progenitors [23], less than 1% of the cells in our cultures were macrophages at the 2 week timepoint (based on immunochemical staining, data not shown).
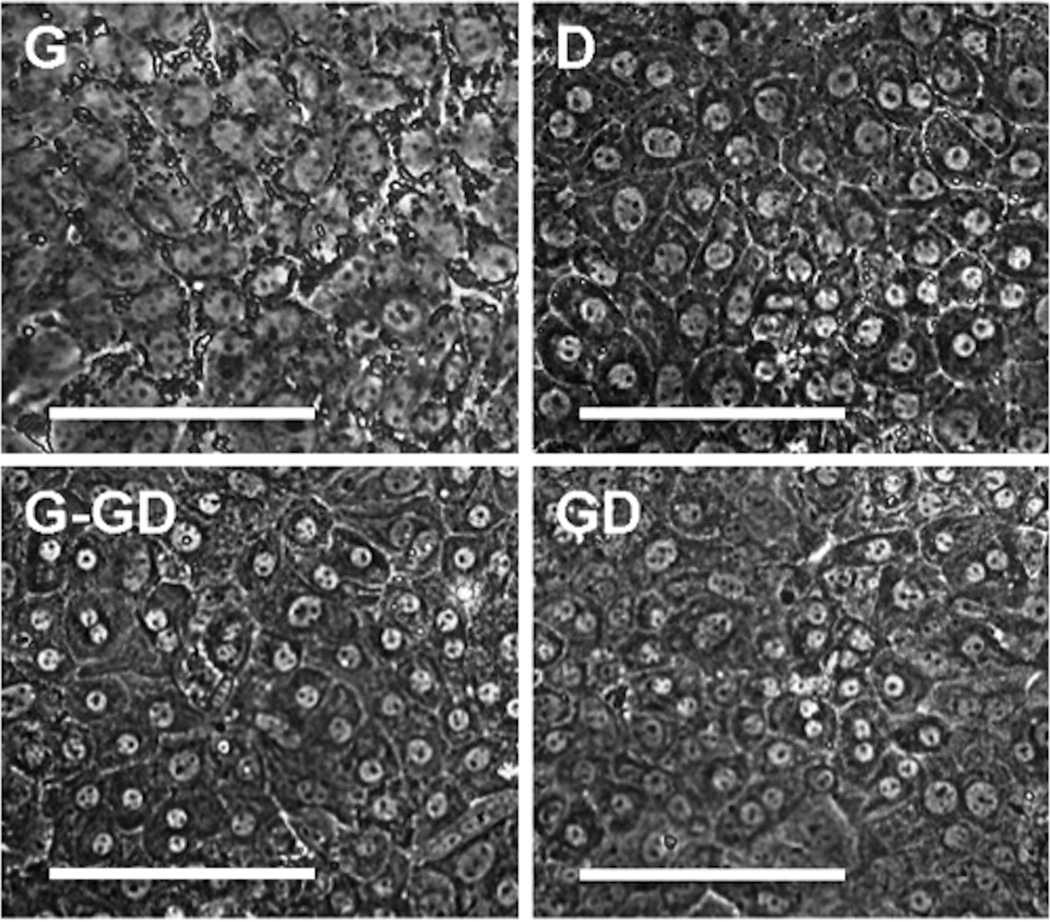
In Group 1, where FLEC were grown in medium D, a cell morphology resembling that of more differentiated hepatocytes was observed, as had been previously reported by other researchers using such media composition [15]. At day 3 – 4, small cells in the culture gradually enlarged in size, reaching 20 – 40µm at day 15, and exhibited the polyhedral shape that is typical of differentiated hepatocytes. These cells also possessed spherical nuclei with one or more prominent nucleoli, which is another signature of hepatocytes. Over time, a portion of the cells became binucleated, similar to what is observed in the adult liver (Figure 1, upper right). Analysis of Group 2, FLEC grown in medium G, indicated that the cells initially proliferate at a much faster rate (as compared to Group 1 samples). However, throughout the 2-week culture period, they remained as small, tightly packed, round cells with a high nucleus-to-cytoplasm ratio. Only a few differentiated hepatocytes were observed at the end of the 2 week period. In Group 3, where the culture medium was switched from G to GD (G-GD) at the one-week timepoint, cells originally cultured in medium G displayed hepatocyte morphology within 2 days: they increased in cell size, became polygonal, and the number of binucleated cells increased over time. Cells belonging to Group 4, in which medium GD was applied throughout the entire 2-week culture period, grew in clusters of small hepatocytes (observed at day 4 – 5) and similar to Group 1 (D medium cultures), these cells enlarged in size and acquired the morphology of mature hepatocytes over time.
Figure 1.
Morphology of E14.5 mouse FLEC after 2 weeks of culture under medium conditions G, D, G-GD, and GD. Magnification = 40×. Scale bar = 100 µm.
Cell growth
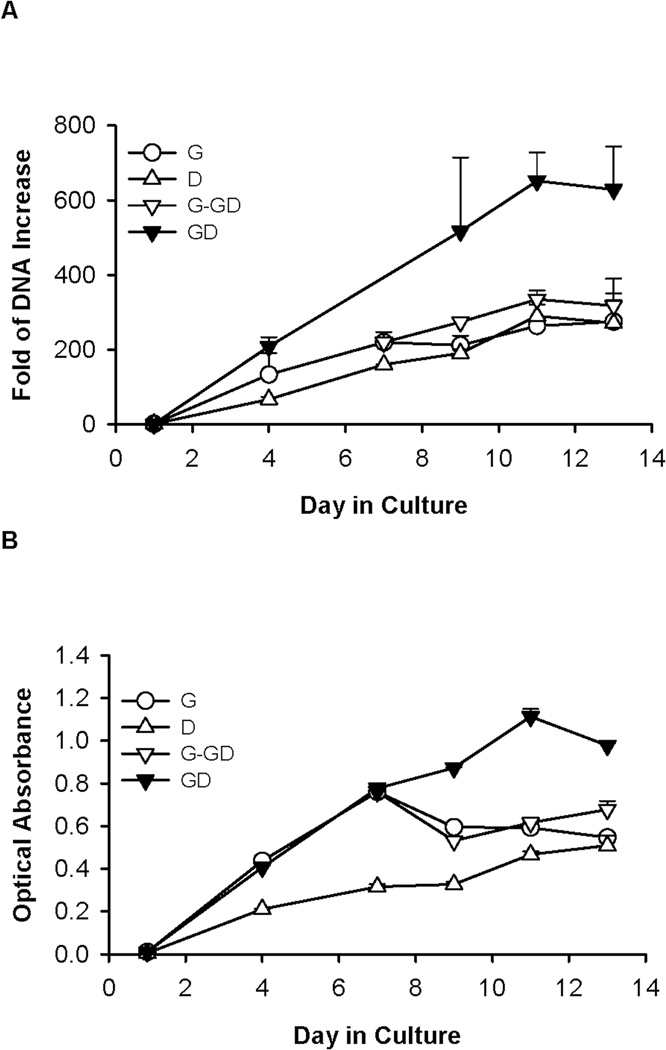
Consistent with visual inspection, results from DNA assay and MTS assay indicated that cells cultured in medium G (Group 2) initially underwent rapid expansion (Figure 2). At day 7, total cellular DNA increased by 219.3 folds compared with day 1. However, the growth declined after this period, as reflected by a plateau in the total DNA and a decrease in the MTS activity after 1 week (Figure 2A). On the other hand, the DNA content and MTS activity of cells cultured in medium D (Group 1) increased slowly but steadily over time, and eventually equaled values of cells grown in medium G. Switching the medium from G to GD at day 7 (Group 3) prolonged the increase of DNA content, which reached a level 16% higher than that in medium G at the end of the culture period (Figure 2A). It appeared that the medium switch also restored the MTS activity to a level higher than that of cultures grown in G medium alone (Figure 2B). When medium GD was used throughout the experiment (Group 4), cellular DNA content and MTS activity continued to increase for almost the entire culture period, reaching levels appreciably higher than those under all other conditions. At the end of a 2-week culture, the increase of DNA content in GD culture was 2.3 folds higher than that in medium G (Figure 2A).
Figure 2.
Proliferation of E14.5 mouse FLEC as measured by (A) fold of DNA increase (compared with day 1 and (B) MTS assay, when cultured under conditions G, D, G-GD, and GD. Data show mean ± SD from one experiment. All experiments were performed a minimum of 3 times using cells from different animal donors, with each growth condition being tested with 3–6 replicates.
Similar trends of cell growth were observed when the experiment was repeated with cells from different animals. In three separate experiments, the GD and G-GD conditions consistently led to higher cell expansion than G or D media alone. On average, cell expansion in medium GD was the highest, at approximately 2 fold-higher than the data from cultures grown under the other conditions (p values for GD versus G, D, and G-GD were 0.04, 0.08, and 0.02, respectively).
Albumin synthesis
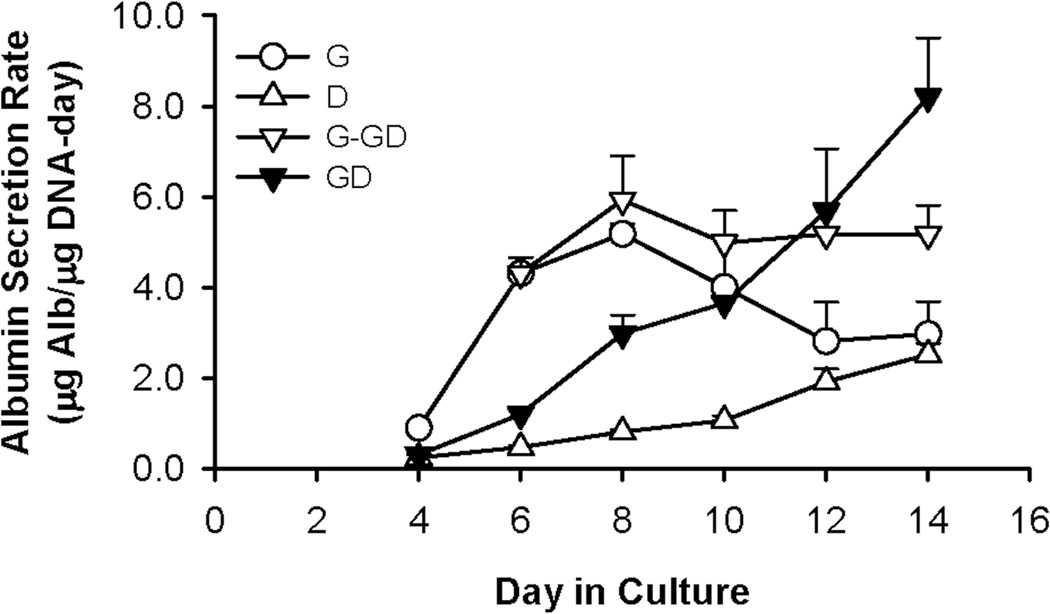
The level of albumin synthesis in medium G was much higher than that in medium D for the first 10 – 11 days (Figure 3). The albumin secretion rate in medium G climbed rapidly during the first week, reaching 6.4 fold higher than that of medium D on day 8. However, albumin synthesis decreased steadily thereafter, eventually to a level similar to that of medium D at the end of a 2-week culture. Switching the G cultures to medium GD at day 7 (Group 3), when albumin secretion was at its peak, stabilized the secretion at this level. Interestingly, although albumin secretion in the GD medium (Group 4) was initially low, it increased with time in a nearly linear fashion and eventually surpassed the secretion level in G-GD. At the end of a 2-week culture, the albumin secretion rate in medium GD was 59% higher than that in G-GD.
Figure 3.
Albumin secretion of E14.5 mouse FLEC when cultured in media G, D, G-GD, and GD. Data show mean ± SD from one experiment. All experiments were performed a minimum of 3 times using cells from different animal donors, with each growth condition being tested with 3 – 6 replicates.
As with cell growth and morphology, similar trends of albumin synthesis were observed when this experiment was repeated with cells from different animals. On average, at the end of a 2-week culture, the albumin secretion rate in medium GD was 2 – 3 times higher than the maximal values under the other conditions (p values for GD versus G, D, and G-GD were 0.006, 0.00005, and 0.008, respectively).
Glycogen accumulation
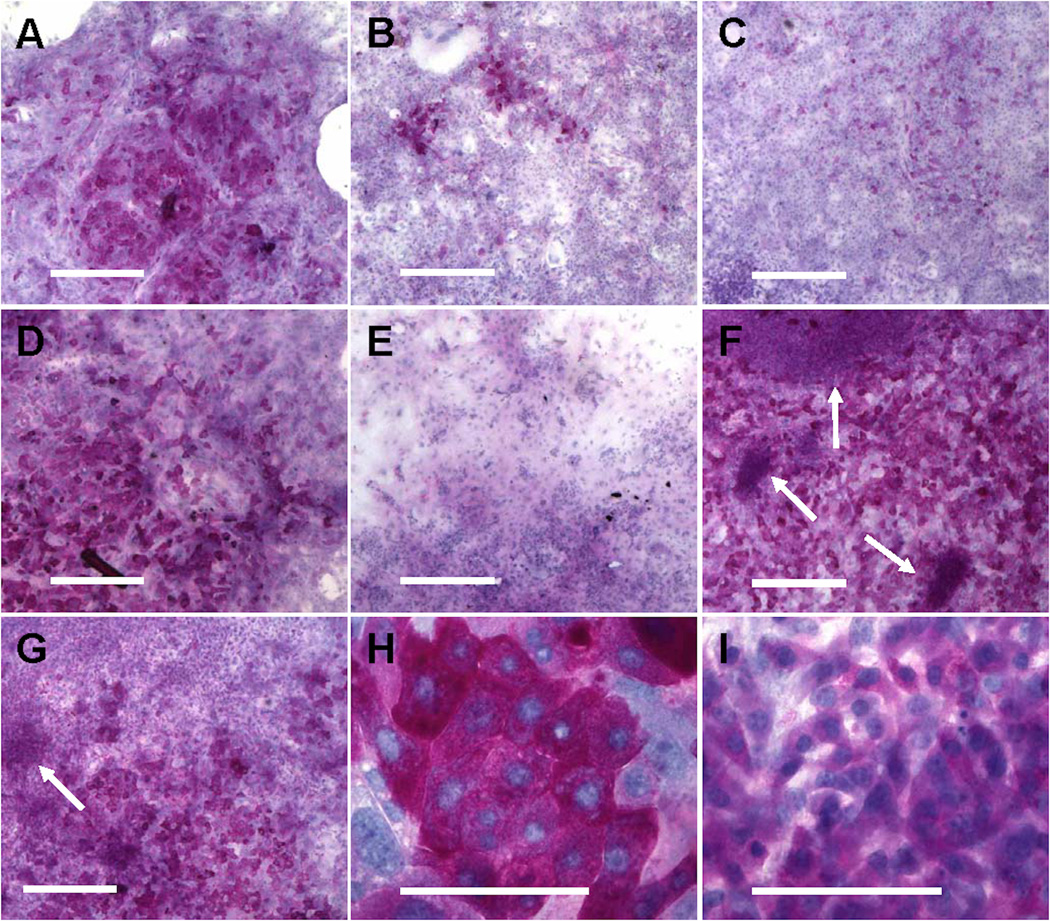
The ability of the cultured cells to produce and store glycogen was also examined under the four different growth conditions. Glycogen accumulation was evaluated at day 7 and day 15 for each of the groups. After one week of culture, many cells in medium D (Group 1) showed an intense staining that indicated high levels of glycogen accumulation (Figure 4A) and all of the stained cells had the appearance of more differentiated hepatocytes. The staining pattern of the medium D cultures remained consistent until the end of the 2-week culture (Figure 4D). In contrast, the cultures grown in medium G (Group 2) contained only a small percentage of intensely stained hepatocytes at day 7 (Figure 4B) and the staining was largely diminished at day 15 (Figure 4E). This was overcome by switching the medium to GD at day 7 (Group 3): a large number of intensely stained cells were observed at day 15, all of which exhibited the typical hepatocyte morphology (Figure 4G). The staining of GD cultures (Group 4) was unremarkable at day 7 (Figure 4C). However by day 15, the staining level in GD was the highest among all culture conditions (Figure 4F). Interestingly, the staining in GD cultures was contributed by two groups of cells with distinct morphologies: large hepatocytes with intense staining (Figure 4H), and intermediately stained clusters of tightly packed, small cells that did not resemble mature hepatocytes (Figure 4I). The latter was also observed in the G-GD cultures (Group 3), but the number of clusters was lower than in GD (Group 4).
Figure 4.
Glycogenesis of E14.5 mouse FLEC under the four culture conditions. PAS staining was performed on culture D at day 7 (A) and day 15 (D), culture G at day 7 (B) and day 15 (E), culture G-GD at day 15 (G), and culture GD at day 7 (C) and day 15 (F). Arrows in (F, G, I) indicate intermediately stained clusters of small cells. Scale bar = 500 µm (A-G) or 100 µm (H-I).
DISCUSSION
The purpose of our study was to investigate how specific changes in culture conditions affect the proliferation and hepatic differentiation of mouse FLEC, and to establish culture medium conditions that would promote both optimal growth and hepatic differentiation. Table 2 summarizes observations from our comparative study.
Table 2.
Proliferation and hepatic differentiation of E14.5 mouse FLEC cultured under the medium conditions G, D, G-GD, and GD.
| Medium | Cell Growth | Morphological Maturation |
Albumin Synthesis |
Glycogen Accumulation |
|---|---|---|---|---|
| G | Week 1: high Week 2: declined |
No | Week 1: high Week 2: declined |
- |
| D | Slow | Yes | Low | +++ (Large hepatocytes) |
| G-GD | Improved compared with G |
Yes | Stabilized at week 1 level |
+++ (Large and small hepatocytes) |
| GD | Highest | Yes | Highest Continued increase |
+++ (Large and small hepatocytes) |
Evaluation of cells grown in media G and D, representing published methods that promote mouse FLEC expansion and hepatic maturation, respectively, yielded unexpected results. Rapid cell proliferation and albumin synthesis were promoted in medium G but could not be sustained beyond one week of culture (Figure 2 and Figure 3). Furthermore, albumin secretion in medium D was much lower than in medium G for most of the culture period (Figure 3), suggesting that the cells cultured in medium D either synthesized less albumin on a per-cell basis or contained a lower percentage of albumin-secreting cells. This was especially surprising because only a few cells in medium G possessed the mature hepatocyte morphology and glycogen storage ability that was prevalent in cultures maintained in medium D (Figure 1 and Figure 4). Therefore, the published methods of culture were not as effective in our study as previously reported [7, 8, 15].
Based on formulations G and D, we designed a modified medium, GD, which contains both growth- and differentiation-stimulating factors (Table 1). Similar to a 2-step approach used by Tanimizu et al. [22], switching from medium G to GD induced the hepatic maturation of FLEC, as evidenced by the extensive mature hepatocyte morphology and glycogen accumulation that were absent in medium G alone (Figure 1 and 4). When used alone, medium GD promoted continuous, vigorous cell growth and led to the highest cell expansion among all conditions tested (Figure 2). Hepatic maturation, demonstrated by morphological maturation (Figure 1) and glycogenesis (Figure 4), was also strongly induced. Furthermore, albumin secretion in medium GD increased continuously over time and became significantly higher than in all other cultures (Figure 3). These results are consistent with the report by Minguet et al. in that compared with HGF or OSM alone, the HGF/OSM combination induced a higher level of proliferation, albumin gene expression, and glycogen synthesis of putative hepatic progenitors [23]. Furthermore, by directly comparing the kinetics of cell proliferation and differentiation under all four conditions, we showed that while the G-GD approach ameliorated the decline of cell growth and albumin synthesis observed in G cultures, culturing FLEC in GD alone promoted the DNA content and albumin secretion rate to increase continuously and reach the highest levels among all conditions. Our findings suggest that the growth and hepatic differentiation of FLEC are not only affected by the type of soluble factors present in the medium, but also by the timing of their supplementation.
Compared with the two-step approach G-GD, cell growth, albumin synthesis, and glycogenesis were further enhanced when medium GD was used throughout the culture period. The improvement may be due to the presence of ascorbic acid in medium GD, which is known to protect both adult and fetal hepatocytes against apoptosis [24, 25]. After the medium switch, total DNA content and albumin secretion increased at a much slower rate in G-GD than in GD. This is further illustrated by the pattern of PAS staining, wherein more extensive staining and more intermediately stained small cells were observed in GD than in G-GD. Therefore, supplying protective reagents from the very beginning of the culture period may augment the growth and hepatic differentiation of FLEC. In future studies, it would be interesting to analyze the apoptosis and cell proliferation events in order to understand the relative contribution of protective agents and mitogenic factors in enhancing cell growth in medium GD. In addition, studies that characterize phenotypes within mouse FLEC cultures—as was done by Suzuki et al. [26]—may be helpful in determining the mechanisms underlying enhanced growth and differentiation of the cell population.
ACKNOWLEDGMENTS
Michael L. Shuler is a world leader in biotechnology and biochemical engineering, and it is our honor to dedicate this paper in recognition of his contributions to research and education in biotechnology. Here, we present an original study of the effects of culture conditions on the growth and differentiation of fetal liver epithelial cells (FLEC), which represent a relevant source of progenitor cells for liver cell culture and liver tissue engineering. This work was inspired by our association of Mike Shuler: one of us (W.M.S.) was a faculty colleague in the School of Chemical Engineering at Cornell during the period 1996–2002 and another of us (L.Q.) was a doctoral student, who had Dr. Shuler on her thesis committee. Dr. Shuler has long experience with optimization of cell cultures, including substantial work on liver cells, which began with his work on effects of shear on expression of hepatic detoxification enzymes in microcarrier-attached liver cells [27] and his pioneering early work to use cultured cells (including heptocytes) in complex bioreactors that mimicked human physiology [28]. This later work evolved into a major effort for his laboratory, leading most recently to the demonstration that micro cell culture analogs can be used for pharmacokinetic-based screening of drug compounds [29]. These devices incorporate multiple technologies, including microfluidics [30] and mathematical models [31], but an essential element is identifying ways to incorporate cultured liver cells in devices so that they mimic the physiological functions of whole liver in drug metabolism [32]. This body of work by Mike Shuler and his students has informed the present work on the optimization of culture conditions for new therapies based on hepatocytes.
The described experiments were supported by grants from the NIH (HL085416, DE14097, DK61846, and HL073742) and the Human Frontier Science Program (RGP5/2006). We thank Stephanie Donaldson for her assistance in mouse mating, Dr. Scott Swenson for helpful discussion, and Margaret Cartiera for editorial assistance.
ABBREVIATIONS
- FLEC
Fetal liver epithelial cells
- HGF
hepatocyte growth factor
- OSM
oncostatin M
- EGF
epidermal growth factor
- AFP
α-fetoprotein
- G6Pase
glucose-6-phosphatase
- TAT
tyrosine amino transferase
- IL-6
interleukin-6
- DPBS
Dulbecco’s PBS
- FBS
fetal bovine serum
- PAS
periodic acid-Schiff
Footnotes
DISCLOSURE STATEMENT
The authors declare no financial or commercial conflict of interest.
REFERENCES
- 1.Allen JW, Bhatia SN. Engineering liver therapies for the future. Tissue Engineering. 2002;8:725–737. doi: 10.1089/10763270260424097. [DOI] [PubMed] [Google Scholar]
- 2.Strom SC, Bruzzone P, Cai H, Ellis E, et al. Hepatocyte transplantation: clinical experience and potential for future Use. Cell Transplantation. 2006;15:105–110. doi: 10.3727/000000006783982395. [DOI] [PubMed] [Google Scholar]
- 3.Sandhu JS, Petkov PM, Dabeva MD, Shafritz DA. Stem cell properties and repopulation of the rat liver by fetal liver epithelial progenitor cells. Am. J. Pathol. 2001;159:1323–1334. doi: 10.1016/S0002-9440(10)62519-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng JF, Liang LJ, Wu CX, Chen JS, Zhang ZS. Transplantation of fetal liver epithelial progenitor cells ameliorates experimental liver fibrosis in mice. World J. Gastroenterol. 2006;12:7292–7298. doi: 10.3748/wjg.v12.i45.7292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu Y, Shatapathy CC, Minger SL. Isolation, in vitro cultivation and characterisation of foetal liver cells. Methods Mol Biol. 2009;481:181–192. doi: 10.1007/978-1-59745-201-4_15. [DOI] [PubMed] [Google Scholar]
- 6.Block G, Locker J, Bowen W, Petersen B, et al. Population expansion, clonal growth, and specific differentiation patterns in primary cultures of hepatocytes induced by HGF/SF, EGF and TGF alpha in a chemically defined (HGM) medium. J. Cell Biol. 1996;132:1133–1149. doi: 10.1083/jcb.132.6.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suzuki A, Zheng Y, Kondo R, Kusakabe M, et al. Flow-cytometric separation and enrichment of hepatic progenitor cells in the developing mouse liver. Hepatology. 2000;32:1230–1239. doi: 10.1053/jhep.2000.20349. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki A, Zheng Y, Kaneko S, Onodera M, et al. Clonal identification and characterization of self-renewing pluripotent stem cells in the developing liver. J. Cell Biol. 2002;156:173–184. doi: 10.1083/jcb.200108066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanimizu N, Nishikawa M, Saito H, Tsujimura T, Miyajima A. Isolation of hepatoblasts based on the expression of Dlk/Pref-1. J. Cell Sci. 2003;116:1775–1786. doi: 10.1242/jcs.00388. [DOI] [PubMed] [Google Scholar]
- 10.Nawa K, Nakamura T, Kumatori A, Noda C, Ichihara A. Glucocorticoid-dependent expression of the albumin gene in adult rat hepatocytes. J. Biol. Chem. 1986;261:16883–16888. [PubMed] [Google Scholar]
- 11.Belanger L, Frain M, Baril P, Gingras MC, et al. Glucocorticosteroid suppression of alpha1-fetoprotein synthesis in developing rat liver. Evidence for selective gene repression at the transcriptional level. Biochemistry. 1981;20:6665–6672. doi: 10.1021/bi00526a022. [DOI] [PubMed] [Google Scholar]
- 12.Nierhoff D, Ogawa A, Oertel M, Chen Y-Q, Shafritz DA. Purification and characterization of mouse fetal liver epithelial cells with high in vivo repopulation capacity. Hepatology. 2005;42:130–139. doi: 10.1002/hep.20735. [DOI] [PubMed] [Google Scholar]
- 13.Kamiya A, Kinoshita T, Ito Y, Matsui T, et al. Fetal liver development requires a paracrine action of oncostatin M through the gp130 signal transducer. EMBO J. 1999;18:2127–2136. doi: 10.1093/emboj/18.8.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lazaro C, Croager E, Mitchell C, Campbell J, et al. Establishment, characterization, and long-term maintenance of cultures of human fetal hepatocytes. Hepatology. 2003;38:1095–2106. doi: 10.1053/jhep.2003.50448. [DOI] [PubMed] [Google Scholar]
- 15.Sakai Y, Jiang J, Kojima N, Kinoshita T, Miyajima A. Enhanced in vitro maturation of fetal mouse liver cells with oncostatin M, nicotinamide, and dimethyl sulfoxide. Cell Transplant. 2002;11:435–441. [PubMed] [Google Scholar]
- 16.Isom I, Georgoff I, Salditt-Georgieff M, Darnell J., Jr. Persistence of liver-specific messenger RNA in cultured hepatocytes: different regulatory events for different genes. J. Cell Biol. 1987;105:2877–2885. doi: 10.1083/jcb.105.6.2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De La Vega FM, Mendoza-Figueroa T. Dimethyl sulfoxide enhances lipid synthesis and secretion by long-term cultures of adult rat hepatocytes. Biochimie. 1991;73:621–624. doi: 10.1016/0300-9084(91)90033-w. [DOI] [PubMed] [Google Scholar]
- 18.Mitaka T. The current status of primary hepatocyte culture. International Journal of Experimental Pathology. 1998;79:393–409. doi: 10.1046/j.1365-2613.1998.00083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sato F, Mitaka T, Mizuguchi T, Mochizuki Y, Hirata K. Effects of nicotinamide-related agents on the growth of primary rat hepatocytes and formation of small hepatocyte colonies. Liver. 1999;19:481–488. doi: 10.1111/j.1478-3231.1999.tb00080.x. [DOI] [PubMed] [Google Scholar]
- 20.Williams GM, Gunn JM. Long-term cell culture of adult rat liver epithelial cells. Experimental Cell Research. 1974;89:139–142. doi: 10.1016/0014-4827(74)90196-7. [DOI] [PubMed] [Google Scholar]
- 21.Miyoshi H, Ehashi T, Ema H, Hsu HC, et al. Long-term culture of fetal liver cells using a three-dimensional porous polymer substrate. ASAIO Journal. 2000;46:397–402. doi: 10.1097/00002480-200007000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Tanimizu N, Saito H, Mostov K, Miyajima A. Long-term culture of hepatic progenitors derived from mouse Dlk+ hepatoblasts. J. Cell Sci. 2004;117:6425–6434. doi: 10.1242/jcs.01572. [DOI] [PubMed] [Google Scholar]
- 23.Minguet S, Cortegano I, Gonzalo P, Martinez-Marin J, et al. A population of c-Kit(low)(CD45/TER119)-hepatic cell progenitors of 11-day postcoitus mouse embryo liver reconstitutes cell-depleted liver organoids. J. Clin. Invest. 2003;112:1152–1163. doi: 10.1172/JCI17409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sánchez A, Alvarez AM, Benito M, Fabregat I. Apoptosis induced by transforming growth factor-beta in fetal hepatocyte primary cultures. J. Biol. Chem. 1996;271:7416–7422. doi: 10.1074/jbc.271.13.7416. [DOI] [PubMed] [Google Scholar]
- 25.Zhou C, Zhang C. Protective effects of antioxidant vitamins on Aroclor 1254-induced toxicity in cultured chicken embryo hepatocytes. Toxicol. In Vitro. 2005;19:665–673. doi: 10.1016/j.tiv.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki A, Iwama A, Miyashita H, Nakauchi H, Taniguchi H. Role for growth factors and extracellular matrix in controlling differentiation of prospectively isolated hepatic stem cells. Development. 2003;130:2513–2524. doi: 10.1242/dev.00459. [DOI] [PubMed] [Google Scholar]
- 27.Mufti NA, Bleckwenn NA, Babish JG, Shuler ML. Possible involvement of the AH receptor in the induction of cytochrome P-450IA1 under conditions of hydrodynamic shear in microcarrier-attached hepatoma cell lines. Biochemical and Biophysical Research Communications. 1995;208:144–152. doi: 10.1006/bbrc.1995.1316. [DOI] [PubMed] [Google Scholar]
- 28.Sweeney LM, Shuler ML, Babish JG, Ghanem A. A cell culture analogue of rodent physiology: application to naphthalene toxicology. Toxic. in Vitro. 1995;9:307–316. doi: 10.1016/0887-2333(95)00007-u. [DOI] [PubMed] [Google Scholar]
- 29.Sung JH, Shuler ML. A micro cell culture analog with 3-D hydrogel culture of multiple cell lines to assess metabolism-dependent cytotoxicity of anti-cancer drugs. Lab on a Chip. 2009;9:1385–1394. doi: 10.1039/b901377f. [DOI] [PubMed] [Google Scholar]
- 30.Shuler ML, Esch MB. Body-on-a chip: using microfluidic systems to predict human responses to drugs. Pure Appl. Chem. 2010;82:1635–1645. [Google Scholar]
- 31.Sung JH, Esch MB, Shuler ML. Integration of in silico and in vitro platforms for pharmacokinetic-pharmacodynamic modeling. Expert Opin. Drug Metab. Toxicol. 2010;6:1063–1081. doi: 10.1517/17425255.2010.496251. [DOI] [PubMed] [Google Scholar]
- 32.Viravaidya K, Sin A, Shuler ML. Develoment of a microscale cell culture analog to probe naphthalene toxicity. Biotechnol. Prog. 2004;20:316–323. doi: 10.1021/bp0341996. [DOI] [PubMed] [Google Scholar]