Abstract
Background
Although maternal infection and inflammation during pregnancy can adversely affect offspring birth weight (BW), whether low grade inflammation in the non-pregnant state predicts BW is unknown.
Aim
Evaluate relationships between offspring BW and pro- and anti-inflammatory factors measured in parous but non-pregnant women.
Subjects and methods
Data come from 234 parous Filipino females (21.5 ± 0.3 yr) in the Cebu Longitudinal Health and Nutrition Survey, a population-based birth cohort in Metropolitan Cebu, Philippines. Pro-inflammatory [Interleukin-6 (IL-6), Interleukin-1 beta (IL-1β), tumor necrosis factor alpha (TNFα), C-reactive protein (CRP)] and anti-inflammatory [Interleukin-10 (IL-10)] factors were measured in fasting plasma when the women were not pregnant, and related to recalled offspring BW.
Results
BW in female offspring was lower only among women with high IL-1β. Although pro-inflammatory cytokines did not predict BW in male offspring, women with higher anti-inflammatory IL-10 gave birth to larger males. Women with a combination of low inflammatory (IL-6) and high anti-inflammatory (IL-10) factors (interaction p<0.104) gave birth to the largest males.
Conclusion
Immune factors measured outside of pregnancy predict offspring BW in these young women. Stable variation in inflammatory phenotype could impact the gestational environment of offspring, thus pointing to potential intergenerational effects of chronic low-grade inflammation.
Keywords: cytokines, C-reactive protein, reproduction, DOHaD, Philippines
INTRODUCTION
Characteristics of the gestational environment have been linked to a wide range of functional and health-related outcomes in offspring, including cardiovascular disease (Barker et al., 1993, Gluckman et al., 2008) (Leeson et al., 2001)), immune phenotype (McDade et al., 2010, McDade et al., 2004), child development (Vohr et al., 2000, Hack et al., 2005), and educational and occupational performance in adulthood (Richards et al., 2001, Strauss, 2000, Black et al., 2007). These findings point to the lingering effects of maternal characteristics on offspring developmental biology (Kuzawa and Sweet, 2009), and are widely probed in humans by documenting relationships between birth weight (BW) and offspring biological and health outcomes (Valdez et al., 1994, Hack et al., 1995, Curhan et al., 1996, Rich-Edwards et al., 1999).
Despite increasing evidence for long-term health impacts of the gestational environment, the biological pathways linking maternal physiology to offspring BW remain poorly understood. Although much work has focused on the role of nutrition and psychosocial stress as influences on birth outcomes (Rini et al., 1999, Fowles, 2004), there is growing evidence that maternal immunity may also be important (Romero et al., 2007). Much of this work has focused on the influence of acute immune activation during pregnancy, which has highlighted several important pathways. First, an excess of pro-inflammatory cytokines during pregnancy can cause vascular damage, compromising placental blood supply and potentially leading to fetal growth restriction (Teran et al., 2001, Greer, 1994, Vince et al., 1995, Stallmach, 1995). The anti-inflammatory cytokine IL-10 generally inhibits the synthesis of pro-inflammatory cytokines (Robertson et al., 2007), and in theory might protect the fetus from these deleterious effects. The second and perhaps better studied pathway involves the role of immunity as a mediator of timing of gestation. Active infection, particularly of the reproductive tract, can influence birth outcomes by intensifying the inflammatory cytokine cascade and leading to preterm delivery (Challis et al., 2009).
Although these acute effects of inflammation during pregnancy on offspring birth outcomes have been widely documented, there is growing evidence and interest in the health impacts of chronic, non-stimulated inflammatory activity in a wide range of health conditions. An extensive literature now indicates that basal variation in pro-inflammatory factors, such as C-reactive protein or IL-6, predict risk for cardiovascular disease (Ridker, 1998), type II diabetes mellitus (Pradhan, 2001), and metabolic syndrome (Ridker et al., 2003). This is thought to reflect the crucial role of inflammatory processes in the pathogenesis of these conditions and underscores a growing awareness of the broad health implications of more subtle variation in non-stimulated immune phenotypes.
Whether basal inflammatory regulation might also impact normal variation in birth outcomes has not been investigated. The potential impact of immune regulation under non-stimulated conditions to birth outcomes is underscored by the crucial role that changes in maternal immunity play in preventing rejection of the fetus during pregnancy. Central to this process are changes in cytokine production, with a characteristic shift from a T-Helper 1 (Th1) cell dominated profile (marked by the production of cytokines such as IL-1, IL-2, IL-6, and IFN-gamma) toward T-Helper 2 (Th2) cell activity (elevated secretion of IL-4, IL-5, and IL-10) (Challis et al., 2009). The predominance of Th2 cytokines during pregnancy is thought to protect the fetus, with experimental work in animal models showing that an increase in Th1 cytokines can have negative effects on the placenta and the fetus (Hahn-Zoric, 2002). Immunological diseases characterized by dysregulation of cytokine production, such as asthma, celiac disease, and systemic lupus (Leung et al., 1995, Forsberg, 2002, Wong et al., 2000) are associated with higher rates of adverse pregnancy outcomes such as spontaneous abortion, intra-uterine growth restriction, and preterm delivery (Clifton and Murphy, 2004, Ludvigsson et al., 2005, Yasmeen, 2001). In light of the key role played by the maternal-fetal immune interface in the maintenance of pregnancy, Goldenberg and Culhane (Goldenberg and Culhane, 2005) hypothesized that women with higher inflammatory status prior to pregnancy would have an attenuated anti-inflammatory response and an increased pro-inflammatory response during pregnancy, which could lead to compromised birth outcomes (Goldenberg and Culhane, 2005). This hypothesis, if correct, suggests that intergenerational impacts on offspring birth outcomes might be added to the growing list of health issues predicted by chronic low grade inflammation.
Here we evaluate relationships between pro- and anti-inflammatory cytokines measured in currently non-pregnant women and BW of their offspring. Data and samples come from a healthy population-based birth cohort study in Metropolitan Cebu, Philippines (Adair et al., 2011). In 2005, we collected anthropometric, dietary, and questionnaire data, and also obtained fasting plasma samples for cytokine analysis. During adult surveys (in 2005, 2007 and 2009), we also obtained retrospective (recalled) reproductive histories for all parous women in the sample, including information on birth outcomes, duration of gestation and other pertinent information for each pregnancy. Our prior analyses in this sample have identified long-term impacts of early life infectious exposure as predictors of adult inflammatory phenotype (McDade et al., 2010, McDade et al., 2004). Here we extend these analyses to evaluate possible intergenerational effects of maternal inflammatory phenotypes. We hypothesized that inflammatory status in the non-pregnant state, reflecting basal immune regulation, would predict offspring birth outcomes. Specifically, we hypothesized that key pro-inflammatory regulators (IL-1β, IL-6, TNFα and CRP) would relate inversely to offspring BW, while anti-inflammatory regulators (IL-10) would relate positively to offspring BW in these women. Because our recent analyses identify stronger relationships between maternal stress hormones and male offspring BW in these women (Thayer et al.), we also evaluated whether relationships between maternal cytokines and offspring BW varied by sex of newborn.
METHODS
Study population
Data come from the Cebu Longitudinal Health and Nutrition Survey (CLHNS), a community-based birth cohort study of mothers and their infants born in 1983-84 (Adair et al., 2011). The women included in the present analyses were the now-adult offspring of the mothers originally enrolled, who were a mean of 21.5 +/− 0.3 years of age at the time of biological sample collection in 2005. Socioeconomic, demographic, health and general behavioral data were collected using questionnaire-based, in-home interviews administered by Cebuano-speaking interviewers (Adair, 1993, Kuzawa and Adair, 2003). Weight (kg) and height (cm) were measured using standard anthropometric techniques (Lohman et al., 1988). This research was conducted under conditions of written informed consent with human subjects oversight and approval from the Institutional Review Boards of the University of North Carolina, Chapel Hill and Northwestern University.
Birth outcomes
Offspring BW was obtained through recall in 2005, 2007, and 2009. Each mother was asked to recall, among other factors, the status of each of her prior pregnancies (twin, singleton, liveborn, stillbirth), birth date, whether the baby’s weight was weighed and if so the BW, gestational age at parturition, and sex of each of her offspring. In addition to birth outcome characteristics, we obtained information on characteristics of each pregnancy, including whether the woman worked while pregnant and whether she obtained prenatal care.
Exclusion criteria
The final analysis sample included the 419 singleton offspring born to the 234 women who were parous by 2009 (the date of the most recent reproductive history) and who had all variables available but who were not pregnant during the 2005 blood draw from which cytokines and CRP were analyzed. Each participant was asked about her current pregnancy status during blood draw in 2005. In addition, because a subset of women who were early in their pregnancies may not have been aware that they were pregnant, we also used birth dates and gestational duration for all pregnancies obtained in 2007 and 2009 reproductive histories, as available, to retrospectively identify women who were likely pregnant in 2005.
Measurement of CRP and cytokines
Venipuncture blood samples were collected using EDTA-coated vacutainer tubes in the participants’ homes in the morning after the overnight fast (~12 hours). Blood samples were kept in coolers on ice packs for no more than 2 h and were then centrifuged to separate plasma prior to freezing at −70°C. Samples were express-shipped in a single batch to Northwestern University on dry ice and stored frozen at −80°C until analysis. CRP concentrations were determined using a high sensitivity immunoturbidimetric method (Synchron LX20, lower detection limit: 0.1 mg/L). Because we were specifically interested in whether inflammatory markers measured in the non-infectious state predicted offspring BW, we excluded women who had CRP >= 10 mg/L (Pearson et al., 2003).
Concentrations of IL-1β, IL-6, IL-10, and TNFα were determined in the Laboratory for Human Biology Research at Northwestern University using a high sensitivity multiplex immunoassay (7-plex) protocol (HSCYTO-60SK, Millipore, Billerica, MA) on the Luminex platform (Luminex Corporation, Austin, TX)(see (McDade et al.) for details). Briefly, samples were incubated with sets of polystyrene microspheres covalently coupled with cytokine-specific capture antibodies, and then incubated with detection antibody labeled with a fluorescent reporter molecule. Concentration of bound analyte was determined by running the samples through a modified flow cytometer. The lower detection limit for the multiplex cytokine assay was 0.08 pg/ml and samples with undetectable concentrations were assigned a value of 0.0001 pg/mL. Percent coefficient of variation (%CV; SD/mean) were as follows (low sample, high sample): IL-6 (14.7, 12.4); IL-10 (15.4, 11.6); IL-1β (15.1, 17.7); TNFα (13.7, 10.1). These levels of analytic variation are comparable to, or lower than, previously reported applications of this method to multiplexed cytokine analysis (Prabhakar et al., 2002).
Statistical analysis
All analyses were conducted with Stata version 10.1. Descriptive statistics were calculated separately for mothers and offspring. Multiple regression was used to determine the relationship between each maternal cytokine and offspring BW adjusting for mother’s age at birth, her height and body mass index at time of blood draw, prenatal care, first born status mother’s education and schooling, household assets, and oral contraceptive use and infectious disease symptoms at the time of cytokine measurement. Because changes in gestational timing may be an important pathway by which inflammation influences birth outcomes, we did not adjust for gestational age at birth in these models. Mean BW stratified on tertiles of each cytokine, adjusting for all variables in the model, were used to generate bar charts. Because most cytokines have highly non-normal distributions, with a fraction of the sample below the detection limit of the assay, we analyzed BW in relation to tertiles of each cytokine (ranges listed in Table I). In Stata, the regress command was used with the cluster option to account for non-independence of BW among multiparous women.
Table I.
Mean (range) of each cytokine stratified by tertile
| Tertile 1 | Tertile 2 | Tertile 3 | |
|---|---|---|---|
| il-1b | 0.06 (0, 0.178) | 0.32 (0.184, 0.470) | 2.53 (0.477, 85.0) |
| n | 79 | 86 | 69 |
| il-6 | 0.13 (0, 0.50) | 1.03 (0.51, 1.96) | 10.91 (2.01, 96.17) |
| n | 92 | 75 | 67 |
| TNFa | 1.91 (0.19, 3.00) | 3.82 (3.06, 4.65) | 7.67 (4.67, 62.2) |
| n | 76 | 77 | 81 |
| CRP | 0 (0, 0) | 0.29 (0.1, 0.6) | 2.50 (0.7, 9.9) |
| n | 77 | 81 | 76 |
| il-10 | 1.46 (0, 4.22) | 7.67 (4.45, 10.56) | 39.1 (10.60, 582.49) |
| n | 85 | 82 | 67 |
RESULTS
Descriptive characteristics of participants at the time of cytokine measurement and of their individual births are reported in Table II. When cytokines were measured, women were an average of 21.5 years of age, and they ranged in age from 15.6 to 25.9 years during individual pregnancies, with roughly half of the births representing first born offspring.
Table II.
Descriptive characteristics of study participants and of individual births1
|
Women at blood draw
(n=234) |
Mean/median | SD/IQR |
|---|---|---|
| Age (years) | 21.5 | 0.3 |
| Height (cm) | 150.9 | 5.3 |
| BMI (kg/m2) | 20.6 | 3.0 |
| IL-1β | 0.30 | (0.12, 0.57) |
| IL-6 | 0.81 | (0.13, 2.23) |
| TNFα | 3.92 | (2.47, 5.47) |
| CRP | 0.2 | (0, 1.0) |
| IL-10 | 7.0 | (2.2, 11.4) |
| Individual births (n=419) | ||
| Birth weight (g) | 3050 | 573 |
| Mother’s age at birth (years) |
21.8 | 2.5 |
| Male offspring | 52.7% | |
| First born | 55.8% | |
| Prenatal care | 99.3% |
all means (SD) other than cytokines and CRP, which are median (interquartile range)
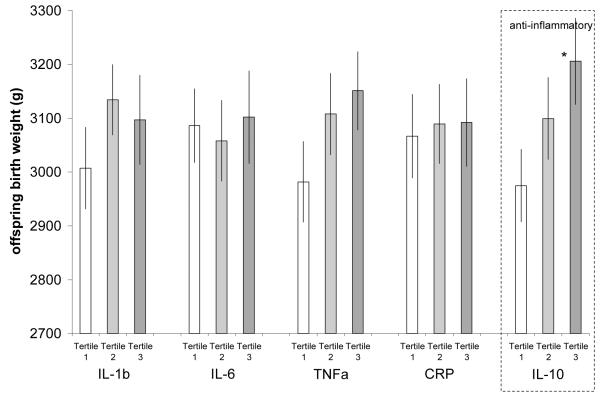
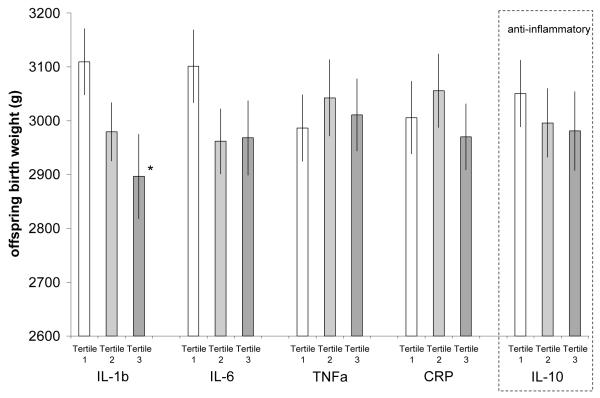
Table III reports regression models predicting offspring BW stratified on sex of offspring and adjusted for mother’s age at birth, prenatal care, recalled gestational age, and first born status. Figures 1 and 2 plot average BW for male and female offspring predicted by these models. In males, offspring BW was unrelated to any pro-inflammatory cytokine. However, women with high levels of anti-inflammatory IL-10 gave birth to larger males than women with lower IL-10. In female offspring, there was a weak negative gradient of BW across levels of IL1-β, IL-6 and CRP, although this only reached statistical significance for offspring born to women with high IL-1β.
Table III.
Regression models predicting offspring birth weight (grams)1
| Male (n=221) β(SE) |
p-value | Female (n=198) β(SE) |
p-value | ||
|---|---|---|---|---|---|
| IL-1β | tertile 2 | 127.3 (102.3) | 0.215 | −130.3 (82.9) | 0.118 |
| tertile 3 | 89.8 (110.4) | 0.417 | −213.4 (102.3) | 0.039 | |
| model R2 | 0.111 | 0.167 | |||
| IL-6 | tertile 2 | −28.2 (101.4) | 0.781 | −139.4 (93.2) | 0.137 |
| tertile 3 | 15.7 (113.4) | 0.890 | −133.0 (98.7) | 0.179 | |
| model R2 | 0.104 | 0.157 | |||
| TNFα | tertile 2 | 126.1 (106.3) | 0.237 | 55.9 (95.0) | 0.557 |
| tertile 3 | 169.2 (105.0) | 0.109 | 24.4 (93.3) | 0.794 | |
| model R2 | 0.117 | 0.145 | |||
| CRP | tertile 2 | 22.9 (104.2) | 0.826 | 49.8 (96.7) | 0.607 |
| tertile 3 | 25.5 (118.9) | 0.830 | −35.8 (95.5) | 0.708 | |
| model R2 | 0.104 | 0.147 | |||
| IL-10 | tertile 2 | 124.9 (99.4) | 0.210 | −54.5 (89.2) | 0.542 |
| tertile 3 | 231.1 (106.6) | 0.032 | −69.4 (96.2) | 0.472 | |
| model R2 | 0.125 | 0.146 |
All models adjust for mother’s age at birth, stature and body mass index, prenatal care, first born status, mother’s education and schooling, household assets, and oral contraceptive use and infectious disease symptoms at the time of cytokine measurement. Base model R2 male 0.103, female 0.143
Figure 1.
Mean male offspring birth weight stratified on tertile of each maternal cytokine. Adjusted for mother’s age at birth, stature and body mass index, prenatal care, first born status, mother’s education and schooling, household assets, and oral contraceptive use and infectious disease symptoms at the time of cytokine measurement. (*tertile 3 vs. tertile 1 for IL-10 p<0.032)
Figure 2.
Mean female offspring birth weight stratified on tertile of each maternal cytokine. Adjusted for mother’s age at birth, stature and body mass index, prenatal care, first born status, mother’s education and schooling, household assets, and oral contraceptive use and infectious disease symptoms at the time of cytokine measurement (*tertile 3 vs. tertile 1 for IL-1β p<0.039)
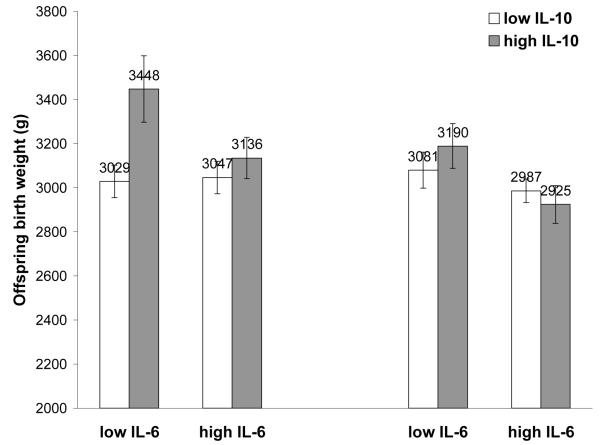
Previously, we have shown that concentrations of IL-6 and IL-10 in the Philippines are positively correlated (McDade et al.), and IL-10 may represent an important anti-inflammatory signal that prevents pro-inflammatory signals from getting out of control. We therefore next tested whether women with an anti-inflammatory phenotype, defined as being in the highest tertile for IL-10 and lowest tertile of IL-6, gave birth to larger babies (Table IV). Women with an anti-inflammatory phenotype tended to give birth to larger male offspring, although this interaction was not significant (p<0.104) (Figure 3). Among females, there was no evidence for an interaction between pro- and anti-inflammatory mediators.
Table IV.
Regression model testing interaction between low IL-6 and high IL-10 as predictors of offspring birth weight, stratified on sex of offspring.1
| Model 1 β(SE) |
p-value | Model 2 β(SE) |
p-value | |
|---|---|---|---|---|
| Males (n=221) | ||||
| Low IL-6 | 49.0 (92.3) | 0.596 | -18.1 (102.9) | 0.861 |
| High IL-10 | 181.4 (101.1) | 0.075 | 88.3 (121.9) | 0.470 |
| IL-6 X IL-10 | - | 330.9 (202.2) | 0.104 | |
| model R2 | 0.119 | 0.130 | ||
| Females (n=198) | ||||
| Low IL-6 | 133.1 (82.1) | 0.107 | 93.5 (101.0) | 0.356 |
| High IL-10 | −20.5 (84.4) | 0.808 | −62.3 (101.7) | 0.541 |
| IL-6 X IL-10 | - | 171.4 (169.4) | 0.313 | |
| model R2 | 0.157 | 0.161 |
All models adjust for mother’s age at birth, stature and body mass index, prenatal care, first born status, mother’s education and schooling, household assets, and oral contraceptive use and infectious disease symptoms at the time of cytokine measurement.
Figure 3.
Adjusted offspring birth weight predicted by combinations of Low IL-6 (lowest tertile) and high anti-inflammatory IL-10 (highest tertile). Male interaction p-value<0.104.
DISCUSSION
We hypothesized that women with higher baseline inflammation outside of pregnancy would give birth to smaller babies. We found modest evidence in support of this hypothesis. In this Filipino population, women who had higher levels of the pro-inflammatory cytokine IL-1β tended to give birth to smaller female offspring, while those with higher levels of the anti-inflammatory cytokine IL-10 gave birth to larger male offspring. Among males, the positive relationship between an anti-inflammatory signal (IL-10) and BW was strongest and only significant when levels of the pro-inflammatory cytokine IL-6 were also low. These findings suggest that individual variation in the regulation ofinflammation among young women, measured outside of their pregnancies, helps explain variation in the birth size of their offspring. By extension, these findings suggest that the gestational environment of the next generation may be among the health outcomes affected by individual variation in inflammatory phenotypes.
In this sample, inflammatory mediators predicted distinct relationships with male and female offspring BW. Differential effects of inflammation and inflammatory conditions on male and female fetal growth have been reported previously. For instance, Murphy and colleagues (Murphy et al., 2003) found that asthmatic women who did not use inhaled steroids during pregnancy had offspring with significantly reduced BW, but only for females (Murphy et al., 2003). A female fetus appears to up-regulate maternal inflammatory pathways associated with asthma that impair placental function, while the male fetus does not (Clifton and Murphy, 2004). These sex-specific fetal effects of maternal asthma, and evidence for involvement of cytokines like IL-1β in the pathogenesis of asthma (Karjalainen et al., 2002, Borish et al., 1992), suggest that our finding of sex-specific relationships between inflammatory cytokines and offspring BW warrant additional attention.
Recent work has highlighted the importance of chronic low grade inflammation as a predictor of many chronic degenerative diseases (Ridker et al., 2000a, Ridker et al., 2000b, Sesso et al., 2007, Pradhan, 2001). Our findings suggest that compromised birth outcomes may be added to the list of adverse health outcomes related to chronic inflammation. Importantly, evidence that a woman’s basal inflammatory profile predicts offspring BW suggests that low grade inflammation could have intergenerational health impacts. There is now extensive evidence that a stressful prenatal environment elevates future risk for a range of risk factors for adult chronic disease, and also predicts mortality (Barker et al., 1993). As an extension of this model, recent work in this and other populations suggests that compromised birth outcomes predict changes in adult immune regulation and a pro-inflammatory phenotype (McDade et al., 2001, McDade et al., 2004). To the extent that a pro-inflammatory phenotype is both a cause and consequence of compromised gestational environments and poor birth outcomes, it could serve as an additional pathway for the intergenerational transmission of health disparities (Drake and Walker, 2004, Kuzawa and Sweet, 2009). This finding underscores the need for additional work aimed at clarifying the links between maternal inflammatory regulation and offspring gestational environment and fetal growth rate.
Several limitations of this study warrant mention. Cytokines and CRP were measured from a single blood sample. It would be preferable to use multiple measurements to define each woman’s stable inflammatory phenotype and then evaluate changes in inflammatory regulation as each woman becomes pregnant. Evidence that pre-pregnancy cytokine concentrations predict elevations in inflammation during pregnancy would significantly strengthen the argument that basal inflammation measured pre-pregnancy influences the intra-uterine environment. In addition, offspring birth information was obtained by maternal recall, which likely reduces measurement reliability and attenuates the power of our analyses to detect significant relationships.
CONCLUSION
In sum, we find evidence that a woman’s inflammatory cytokine profile measured in early adulthood, and in the non-pregnant state, predicts the birth size of her offspring. Female offspring tend to be smaller when born to mothers with higher pro-inflammatory cytokines, with no additional relationship with the anti-inflammatory cytokine considered here. In contrast, male offspring tend to be larger in relation to higher levels of the anti-inflammatory cytokine IL-10. These findings suggest that compromised offspring birth outcomes are an additional adverse health outcome that should be investigated in relation to chronic low-grade inflammation.
ACKNOWLEDGEMENTS
Funding for this study was provided by the National Institutes of Health (RO1 HL085144; 5 RO1 TW05596); biomarker data collection was supported by pilot funds from the Interdisciplinary Obesity Center (RR20649) and the Center for Environmental Health and Susceptibility (ES10126; project 7-2004-E); National Science Foundation (BCS-0746320); PST was supported by an NSF Graduate Research Fellowship while contributing to this analysis; We thank the many scholars and researchers at the Office of Population Studies, University of San Carlos, Cebu, Philippines, for their role in study design and data collection, and the Filipino participants, who generously provided their time for this study.
Footnotes
DECLARATION OF INTERESTS
The authors report no declarations of interest.
REFERENCES
- Adair L, Popkin Bm, Vanderslice J, Akin J, Guilkey D, Black R, Briscoe J, Flieger W. Growth dynamics during the first two years of life: a prospective study in the Philippines. Eur J Clin Nutr. 1993;47:42–51. [PubMed] [Google Scholar]
- Adair LS, Popkin BM, Akin JS, Guilkey DK, Gultiano S, Borja J, Perez L, Kuzawa CW, McDade T, Hindin MJ. Cohort Profile: The Cebu Longitudinal Health and Nutrition Survey. International Journal of Epidemiology. 2011;40:619–625. doi: 10.1093/ije/dyq085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJP, Godfrey KM, Gluckman PD, Harding JE, Owens JA, Robinson JS. Fetal nutrition and cardiovascular disease in adult life. The Lancet. 1993;341:938–941. doi: 10.1016/0140-6736(93)91224-a. [DOI] [PubMed] [Google Scholar]
- Black SE, Devereux PJ, Salvanes KG. From the Cradle to the Labor Market? The Effect of Birth Weight on Adult Outcomes. The Quarterly Journal of Economics. 2007;122:409–439. [Google Scholar]
- Borish L, Mascali J, Dishuck J, Beam W, Martin R, Rosenwasser L. Detection of alveolar macrophage-derived IL-1 beta in asthma. Inhibition with corticosteroids. The Journal of Immunology. 1992;149:3078–3082. [PubMed] [Google Scholar]
- Challis JR, Lockwood CJ, Myatt L, Norman JE, Strauss JF, Petraglia F. Inflammation and Pregnancy. Reproductive Sciences. 2009;16:206–215. doi: 10.1177/1933719108329095. [DOI] [PubMed] [Google Scholar]
- Clifton VL, Murphy VE. Maternal Asthma as a Model for Examining Fetal Sex-specific Effects on Maternal Physiology and Placental Mechanisms that Regulate Human Fetal Growth. Placenta. 2004;25:S45–S52. doi: 10.1016/j.placenta.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Curhan GC, Willett WC, Rimm EB, Spiegelman D, Ascherio AL, Stampfer MJ. Birth Weight and Adult Hypertension, Diabetes Mellitus, and Obesity in US Men. Circulation. 1996;94:3246–3250. doi: 10.1161/01.cir.94.12.3246. [DOI] [PubMed] [Google Scholar]
- Drake A, Walker B. The intergenerational effects of fetal programming: non-genomic mechanisms for the inheritance of low birth weight and cardiovascular risk. Journal of Endocrinology. 2004;180:1–16. doi: 10.1677/joe.0.1800001. [DOI] [PubMed] [Google Scholar]
- Forsberg G, Hernell Olle, Melgar Silvia, Israelsson Anne, Hammarström Sten, Hammarström Marie-Louise. Paradoxical coexpression of proinflammatory and down-regulatory cytokines in intestinal T cells in childhood celiac disease. Gastroenterology. 2002;123:667–678. doi: 10.1053/gast.2002.35355. [DOI] [PubMed] [Google Scholar]
- Fowles ER. Prenatal Nutrition and Birth Outcomes. Journal of Obstetric, Gynecologic, & Neonatal Nursing. 2004;33:809–822. doi: 10.1177/0884217504270599. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of In Utero and Early-Life Conditions on Adult Health and Disease. New England Journal of Medicine. 2008;359:61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg RL, Culhane JF. Prepregnancy Health Status and the Risk of Preterm Delivery. Arch Pediatr Adolesc Med. 2005;159:89–90. doi: 10.1001/archpedi.159.1.89. [DOI] [PubMed] [Google Scholar]
- Greer I, Lyall F, Perera T, Boswell F, Macara Lm. Increased Concentrations of Cytokines Interleukin-6 and Interleukin-1 Receptor Antagonist in Plasma of Women With Preeclampsia: A Mechanism for Endothelial Dysfunction? Obstetrics & Gynecology. 1994;84:937–940. [PubMed] [Google Scholar]
- Hack M, Klein NK, Taylor HG. Long-Term Developmental Outcomes of Low Birth Weight Infants. The Future of Children. 1995;5:176–196. [PubMed] [Google Scholar]
- Hack M, Taylor HG, Drotar D, Schluchter M, Cartar L, Andreias L, Wilson-Costello D, Klein N. Chronic Conditions, Functional Limitations, and Special Health Care Needs of School-aged Children Born With Extremely Low-Birth-Weight in the 1990s. JAMA: The Journal of the American Medical Association. 2005;294:318–325. doi: 10.1001/jama.294.3.318. [DOI] [PubMed] [Google Scholar]
- Hahn-Zoric M, Hagberg H, Kjellmer I, Ellis J, Wennergren M, Hanson La. Aberrations in Placental Cytokine mRNA Related to Intrauterine Growth Retardation. Pediatric Research. 2002;51:201–206. doi: 10.1203/00006450-200202000-00013. [DOI] [PubMed] [Google Scholar]
- Karjalainen J, Nieminen MM, Aromaa A, Klaukka T, Hurme M. The IL-1[beta] genotype carries asthma susceptibility only in men. Journal of Allergy and Clinical Immunology. 2002;109:514–516. doi: 10.1067/mai.2002.121948. [DOI] [PubMed] [Google Scholar]
- Kuzawa CW, Adair LS. Lipid profiles in adolescent Filipinos: relation to birth weight and maternal energy status during pregnancy. The American Journal of Clinical Nutrition. 2003;77:960–966. doi: 10.1093/ajcn/77.4.960. [DOI] [PubMed] [Google Scholar]
- Kuzawa CW, Sweet E. Epigenetics and the embodiment of race: Developmental origins of US racial disparities in cardiovascular health. American Journal of Human Biology. 2009;21:2–15. doi: 10.1002/ajhb.20822. [DOI] [PubMed] [Google Scholar]
- Leeson CPM, Kattenhorn M, Morley R, Lucas A, Deanfield JE. Impact of Low Birth Weight and Cardiovascular Risk Factors on Endothelial Function in Early Adult Life. Circulation. 2001;103:1264–1268. doi: 10.1161/01.cir.103.9.1264. [DOI] [PubMed] [Google Scholar]
- Leung DY, Martin RJ, Szefler SJ, Sher ER, Ying S, Kay AB, Hamid Q. Dysregulation of interleukin 4, interleukin 5, and interferon gamma gene expression in steroid-resistant asthma. The Journal of Experimental Medicine. 1995;181:33–40. doi: 10.1084/jem.181.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohman TG, Roche AF, Martorell R. Anthropometric Standardization Reference Manual. Human Kinetics Books; Champaign, IL: 1988. [Google Scholar]
- Ludvigsson JF, Montgomery SM, Ekbom A. Celiac Disease and Risk of Adverse Fetal Outcome: A Population-Based Cohort Study. Gastroenterology. 2005;129:454–463. doi: 10.1016/j.gastro.2005.05.065. [DOI] [PubMed] [Google Scholar]
- McDade TW, Beck MA, Kuzawa C, Adair LS. Prenatal undernutrition, postnatal environments, and antibody response to vaccination in adolescence. Am J Clin Nutr. 2001;74:543–548. doi: 10.1093/ajcn/74.4.543. [DOI] [PubMed] [Google Scholar]
- McDade TW, Kuzawa CW, Adair LS, Beck MA. Prenatal and early postnatal environments are significant predictors of total immunoglobulin E concentration in Filipino adolescents. Clinical & Experimental Allergy. 2004;34:44–50. doi: 10.1111/j.1365-2222.2004.01834.x. [DOI] [PubMed] [Google Scholar]
- McDade TW, Rutherford J, Adair L, Kuzawa CW. Early origins of inflammation: microbial exposures in infancy predict lower levels of C-reactive protein in adulthood. Proceedings of the Royal Society B-Biological Sciences. 2010;277:1129–1137. doi: 10.1098/rspb.2009.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDade TW, Tallman PS, Adair LS, Borja J, Kuzawa CW. Comparative insights into the regulation of inflammation: Levels and predictors of interleukin 6 and inteleukin 10 in young adults in the Philippines. Am J Phys Anthropol. doi: 10.1002/ajpa.21586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy VE, Gibson PG, Giles WB, Zakar T, Smith R, Bisits AM, Kessell CG, Clifton VL. Maternal Asthma Is Associated with Reduced Female Fetal Growth. Am. J. Respir. Crit. Care Med. 2003;168:1317–1323. doi: 10.1164/rccm.200303-374OC. [DOI] [PubMed] [Google Scholar]
- Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, Iii, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC, Jr, Taubert K, Tracy RP, Vinicor F. Markers of Inflammation and Cardiovascular Disease: Application to Clinical and Public Health Practice: A Statement for Healthcare Professionals From the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- Prabhakar U, Eirikis E, Davis HM. Simultaneous quantification of proinflammatory cytokines in human plasma using the LabMAP(TM) assay. Journal of Immunological Methods. 2002;260:207–218. doi: 10.1016/s0022-1759(01)00543-9. [DOI] [PubMed] [Google Scholar]
- Pradhan AD, Manson Joann E., Rifai Nader, Buring Julie E., Ridker Paul M. C-Reactive Protein, Interleukin 6, and Risk of Developing Type 2 Diabetes Mellitus. JAMA: The Journal of the American Medical Association. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- Rich-Edwards JW, Colditz GA, Stampfer MJ, Willett WC, Gillman MW, Hennekens CH, Speizer FE, Manson JE. Birthweight and the Risk for Type 2 Diabetes Mellitus in Adult Women. Annals of Internal Medicine. 1999;130:278–284. doi: 10.7326/0003-4819-130-4_part_1-199902160-00005. [DOI] [PubMed] [Google Scholar]
- Richards M, Hardy R, Kuh D, Wadsworth MEJ. Birth weight and cognitive function in the British 1946 birth cohort: longitudinal population based study. BMJ. 2001;322:199–203. doi: 10.1136/bmj.322.7280.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker PM, Buring JE, Cook NR, Rifai N. C-Reactive Protein, the Metabolic Syndrome, and Risk of Incident Cardiovascular Events. Circulation. 2003;107:391–397. doi: 10.1161/01.cir.0000055014.62083.05. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Buring Julie E., Shih Jessie, Matias Mathew, Hennekens Charles H. Prospective Study of C-Reactive Protein and the Risk of Future Cardiovascular Events Among Apparently Healthy Women. Circulation. 1998;98:731–733. doi: 10.1161/01.cir.98.8.731. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Hennekens CH, Buring JE, Rifai N. C-Reactive Protein and Other Markers of Inflammation in the Prediction of Cardiovascular Disease in Women. New England Journal of Medicine. 2000a;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma Concentration of Interleukin-6 and the Risk of Future Myocardial Infarction Among Apparently Healthy Men. Circulation. 2000b;101:1767–1772. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- Rini CK, Dunkel-Schetter C, Wadhwa PD, Sandman CA. Psychological Adaptation and Birth Outcomes: The Role of Personal Resources, Stress, and Sociocultural Context in Pregnancy. Health Psychology. 1999;18:333–345. doi: 10.1037//0278-6133.18.4.333. [DOI] [PubMed] [Google Scholar]
- Robertson SA, Care AS, Skinner RJ. Interleukin 10 Regulates Inflammatory Cytokine Synthesis to Protect Against Lipopolysaccharide-Induced Abortion and Fetal Growth Restriction in Mice. Biology of Reproduction. 2007;76:738–748. doi: 10.1095/biolreprod.106.056143. [DOI] [PubMed] [Google Scholar]
- Romero R, Espinoza J, Gonçalves LF, Kusanovic JP, Friel L, Hassan S. The Role of Inflammation and Infection in Preterm Birth. Semin Reprod Med. 2007;25:021, 039. doi: 10.1055/s-2006-956773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesso HD, Wang L, Buring JE, Ridker PM, Gaziano JM. Comparison of Interleukin-6 and C-Reactive Protein for the Risk of Developing Hypertension in Women. Hypertension. 2007;49:304–310. doi: 10.1161/01.HYP.0000252664.24294.ff. [DOI] [PubMed] [Google Scholar]
- Stallmach T, Hebisch G, Joller H, Kolditz P, Engelmann M. Expression pattern of cytokines in the different compartments of the feto-maternal unit under various conditions. Reprod Fertil Dev. 1995;7:1573–80. doi: 10.1071/rd9951573. [DOI] [PubMed] [Google Scholar]
- Strauss RS. Adult Functional Outcome of Those Born Small for Gestational Age. JAMA: The Journal of the American Medical Association. 2000;283:625–632. doi: 10.1001/jama.283.5.625. [DOI] [PubMed] [Google Scholar]
- Teran E, Escudero C, Moya W, Flores M, Vallance P, Lopez-Jaramillo P. Elevated C-reactive protein and pro-inflammatory cytokines in Andean women with pre-eclampsia. International Journal of Gynecology & Obstetrics. 2001;75:243–249. doi: 10.1016/s0020-7292(01)00499-4. [DOI] [PubMed] [Google Scholar]
- Thayer ZM, Feranil AB, Kuzawa CW. Maternal cortisol disproportionately impacts fetal growth in male offspring: Evidence from the Philippines. Am J Hum Biol. doi: 10.1002/ajhb.21226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez R, Athens M, Thompson G, Bradshaw B, Stern M. Birthweight and adult health outcomes in a biethnic population in the USA. Diabetologia. 1994;37:624–631. doi: 10.1007/BF00403383. [DOI] [PubMed] [Google Scholar]
- Vince GS, Starkey PM, Austgulen R, Kwiatkowski D, Redman CWG. Interleukin-6, turnour necrosis factor and soluble turnour necrosis factor receptors in women with pre-eclampsia. BJOG: An International Journal of Obstetrics & Gynaecology. 1995;102:20–25. doi: 10.1111/j.1471-0528.1995.tb09020.x. [DOI] [PubMed] [Google Scholar]
- Vohr BR, Wright LL, Dusick AM, Mele L, Verter J, Steichen JJ, Simon NP, Wilson DC, Broyles S, Bauer CR, Delaney-Black V, Yolton KA, Fleisher BE, Papile L-A, Kaplan MD. Neurodevelopmental and Functional Outcomes of Extremely Low Birth Weight Infants in the National Institute of Child Health and Human Development Neonatal Research Network, 1993-1994. Pediatrics. 2000;105:1216–1226. doi: 10.1542/peds.105.6.1216. [DOI] [PubMed] [Google Scholar]
- Wong CK, Ho CY, Li E, Lam C. Elevation of proinflammatory cytokine (IL-18, IL-17, IL-12) and Th2 cytokine (IL-4) concentrations in patients with systemic lupus erythematosus. Lupus. 2000;9:589–593. doi: 10.1191/096120300678828703. [DOI] [PubMed] [Google Scholar]
- Yasmeen S, Wilkins Ee, Field Nt, Sheikh Ra, Gilbert Wm. Pregnancy outcomes in women with systemic lupus erythematosus. Journal of Maternal-Fetal Medicine. 2001;10:91–96. doi: 10.1080/714904302. [DOI] [PubMed] [Google Scholar]