Abstract
The processing technology employed in traditional Chinese medicine (TCM) is significant and distinct. Meanwhile, the processed Coptis chinensis Franch. are significant in clinic based on clinical practice and literature. The current study used ultraperformance liquid chromatography method (UPLC) coupled with quadrupole time of flight mass spectrometry (qTOF/MS) and Marklynx software to analyze the chemical profiles of crude and processed C. chinensis Franch. 13 compounds in these samples are identified, including 3 compounds that are detected in C. chinensis Franch. for the first time. Moreover, the results of the experiment show significant chemical differences between crude and processed C. chinensis Franch. with principal component analysis (PCA). The obvious separation in PCA confirms the traditional processing theory in TCM.
1. Introduction
Traditional processing technology (Paozhi technology) is a significant part of traditional Chinese medicine (TCM). Employing the correct processing technology is necessary in manufacturing clinical decoction pieces. According to the restrictions in the science of TCM, any decoction piece must be processed by certain methods before it can be used in clinical practice. Processing technology was developed almost 5000 years ago, along with a number of processing technology theories and methods. Briefly, processing technology plays three main roles in TCM. First, it decreases the toxicity of some TCMs. Some crude TCMs have high toxicity and various side effects; thus, numerous processing technologies have been developed to remove the toxicity and decrease the side effects. Second, processing technology increases the pharmacological effects. Third, processing technology creates new drugs that have new pharmacological effects as a pharmaceutical method. The reason for developing this is when faced with complex human diseases, crude TCMs are sometimes unable to satisfy the need in clinical practice due to the limitations posed by herbal medicine. Thus, the ancient Chinese created processing technology to produce abundant new drugs. In this paper, we investigate the five kinds of processed Coptis chinensis Franch. in chemical profiles.
Processing technology was developed via clinical practice experience and processing technology theories. The processing technology theories were based on the Traditional Five Elements Theory and the Yin-Yang Theory from ancient China. The five kinds of processed C. chinensis Franch. are originally based on the important theories in processing technology: Processing Synergy Theory (PST) or Cong Zhi and Processing Antagonism Theory (PAT) or Fan Zhi. Processed C. chinensis Franch. with rice wine (Jiu HL), with Zingiber officinales Rosc. (Jiang HL), and with Euodia rutaecarpa (Juss.) Benth (Yu HL) belong to PAT, whereas those processed with vinegar (Cu HL) and with salt (Yan HL) belong to PST. Such classification bases on the PST/PAT theory, the contribution of C. chinensis Franch. and their auxiliary materials. These processed products have different pharmacological effect in clinic according to the traditional literatures. The rice wine processed C. chinensis is specially used for the treatment of inflammation in head. The ginger processed C. chinensis is focusing on the treatment in stomach. The Euodia rutaecarpa (Juss.) Benth processed C. chinensis is specially used for the treatment of humidity/heat in liver and gall [1]. The vinegar and salt processed C. chinensis is used for extreme heat symptoms, which is totally different from the earlier three based on the TCM theory. The products of processed C. chinensis Franch. presented unique pharmacological effects in mice experiments [2–6]. In our investigation, ultraperformance liquid chromatography method (UPLC) coupled with quadrupole time of flight mass spectrometry (UPLC-qTOF/MS) is applied to explore the material basis in these processed products with different bioactivities.
The major active constituents in C. chinensis are alkaloids, including berberine, jatrorrhizine, coptisine, and palmatine [7]. The berberine, present at about 10% in C. chinensis Franch., has strong antibacterial bioactivities on Shigella dysenteriae, staphylococci, and streptococci and has been used for of dysentery [8], which is also one of the most popular drugs in academia. Palmatine had extensive pharmacological actions including antibacterial activity such as Escherichia coli, and Staphylococcus aureus [9], anti-inflammation [10], and anticancer effect [11]. Therefore, the analysis on alkaloids in C. chinensis Franch. is significant, which decides the therapeutic effects. Chen et al. has investigated C. chinensis Franch. from different locations based on high-performance liquid chromatography-electrospray ionization-time-off light mass spectrometry (HPLC-ESI-TOF-MS), ultraperformance liquid chromatography/photodiode array detector (UPLC/PDA), and UPLC-MS/MS for authentication and quality evaluation [12]. And these results indicated that both UPLC-PDA and UPLC-MS/MS methods were simple, sensitive, and reliable for the determination of alkaloids in C. chinensis Franch. In this paper, it is the first time to investigate the composition of main compounds in crude and processed C. chinensis Franch. by UPLC-qTOF/MS.
2. Materials and Methods
2.1. Chemicals, Reagents, and Materials
Acetonitrile and formic acid were purchased from Fisher Scientific Co. (MA, USA). Ammonium acetate was purchased from Xilong Company (Shanxi, China). All aqueous solutions were prepared with ultrapure water produced by Milli-Q system (18.2 MΩ, Millipore, Ma, USA). Berberine, palmatine, coptisine, epiberberine, and jatrorrhizine standards were purchased from Must Company (Sichuan, China). C. chinensis Franch., Z. officinales Rosc., and E. rutaecarpa (Juss.) Benth were purchased from Sichuan Chinese Herbs Corporation (Sichuan, China). The botanical materials were identified by Professor Chen Shilin, and the voucher specimens were deposited in the Institute of Medicinal Plant Development, Chinese Academy of Medical Sciences, Beijing, China.
2.2. Instrumentation and Chromatographic Conditions
UPLC-qTOF/MS analysis was performed using a qTOF Synapt G2 HDMS system (Waters, Pittsburgh, PA, USA) equipped with an ESI source operated in the positive ion mode. N2 was used as the desolvation gas. The desolvation temperature was set at 450°C with a flow rate of 800 L/h and a source temperature of 120°C. The capillary and cone voltages were set to 2500 and 40 V. Data were collected between 50 Da and 1200 Da, with a scan time of 0.1 s and interscan delay of 0.01 s over an analysis time of 16 min.
2.3. Preparation of the Sample Solution
C. chinensis Franch. samples were cut into 1.5 mm slices and then processed according to the methods described in China pharmacopoeia [1]. Then, the samples were processed at a temperature of 160°C and dried in an oven after mixed with auxiliary material consisting of rice wine (20% w/w), salt (10% w/w), vinegar (20% w/w), E. rutaecarpa (Juss.) Benth (10% w/w), and Z. officinale Rosc. (10% w/w) [13]. All samples were milled into powder and dried at 30°C in oven until they attained constant weight. A total of 0.150 g powder samples were dissolved in a methanol-sulfuric acid (100 : 3) solution. Next, the samples were extracted by ultrasonic cleaner for 30 min before a 15 min water bath (60°C). The sample solutions were subsequently filtered through a 0.22 μm membrane and then injected into the UPLC-qTOF system for analysis.
2.4. Preparation of Standard Solution
The standard stock solutions of berberine (0.12 mg/mL), palmatine (0.11 mg/mL), berberrubine (0.118 mg/mL), coptisine (0.115 mg/mL), epiberberine (0.107 mg/mL), and jatrorrhizine (0.115 mg/mL) were prepared in methanol and stored at −4°C. The solutions were brought to room temperature and filtered through a 0.22 μm membrane filter before injection.
2.5. Data Analysis
The UPLC-qTOF/MS data of crude C. chinensis Franch. and processed C. chinensis Franch. samples were analyzed to identify discriminant variables. The peak finding, peak alignment, and peak filtering of ES+ raw data were carried out with Markerlynx applications manager version 4.1. The parameters used were within the retention time of 0–10 min, and mass range 50–1200 Da, mass tolerance 0.02 Da. Noise elimination level was set at 6.00, and minimum intensity was set to 15% of base peak intensity.
3. Results and Discussion
3.1. UPLC Method Development
To produce a better chromatogram in UPLC-qTOF, the UPLC method was developed with consideration for such factors as mobile phases, modifiers, and flow rates. Methanol and acetonitrile were tested with different ratios, linear gradients, and flow rates of the mobile phase (0.1, 0.2, and 0.25 mL/min). The modifiers, such as formic acid, ammonium acetate, sodium dodecyl sulfate (SDS), phosphoric acid, and diethylamine, were all detected in the present experiment. As a result, water containing 1% formic acid and 1% ammonium acetate (A)—acetonitrile (B) with a flow rate of 0.25 mL/min was chosen as the optimum chromatographic condition with a linear gradient (0–10 min, 80%–70% A) at room temperature.
3.2. UPLC-qTOF/MS Chemical Analysis
Figure 1(a) presents the representative chromatogram of C. chinensis Franch. by UPLC-qTOF/MS. Figure 1(b) shows the five standard compounds chromatograms. Table 1 shows 13 compounds in the chromatograms that have been identified based on [M+H]+ m/z and retention time analysis by the database and references. In the experiments, the chromatograms of the samples were analyzed using the Marklynx software. More than 3000 markers (differences) were detected to be present among the crude and processed C. chinensis Franch.
Figure 1.
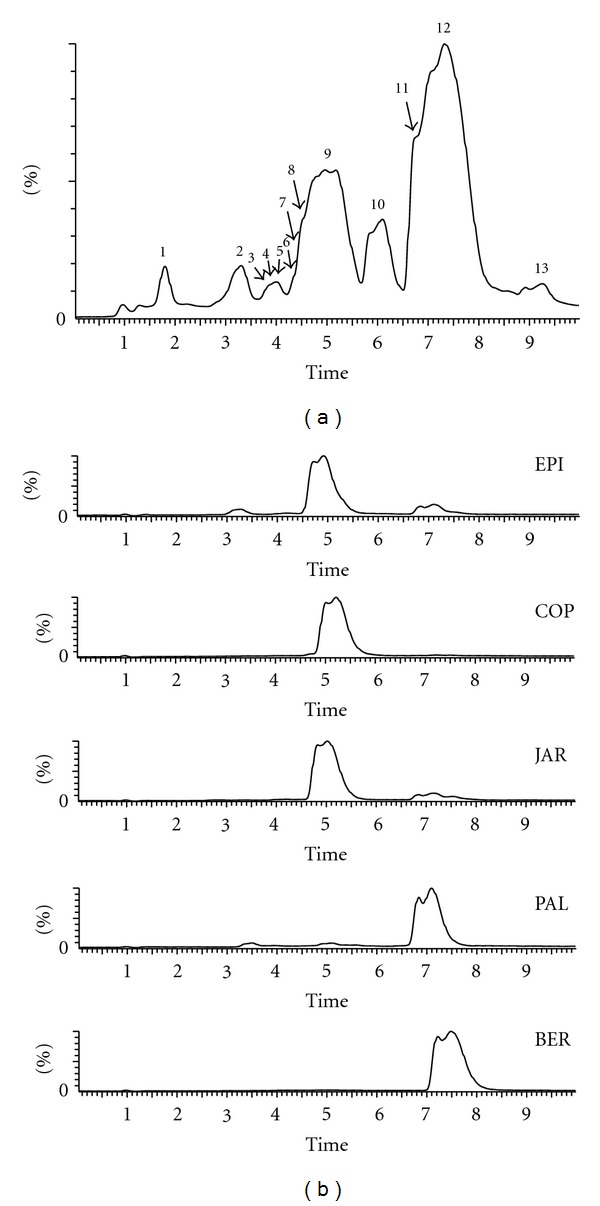
(a) The representative chromatogram of C. chinensis Franch. by UPLC-qTOF/MS (the numbers of peaks are same with the identification in Table 1). (b) The chromatograms of standard compounds (EPI: epiberberine; COP: coptisine; JAR: jatrorrhizine; PAL: palmatine; BER: berberine).
Table 1.
Identified compounds in C. chinensis Franch. by UPLC-QTOF/MS.
| Peak no. | t R (min) | Identified compounds | Molecular formula | [M+H]+ m/z | Reference | ||
|---|---|---|---|---|---|---|---|
| Mean measured mass (Da) | Theoretical exact mass (Da) | Mass error (ppm) | |||||
| 1 | 1.79 | Magnoflorine | C20H23NO4 | 342.1703 | 342.1705 | −0.6 | [13–15] |
| 2 | 3.32 | Groenlandicine | C19H15NO4 | 322.1064 | 322.1079 | −4.7 | [14] |
| 3 | 3.81 | Berberastine | C20H17NO5 | 352.1184 | 352.1185 | −0.3 | [14] |
| 4 | 3.71 | Lincangenine/stephabine | C21H21NO5 | 368.1506 | 368.1498 | 2.2 | [16] |
| 5 | 4.02 | Demethyleneberberine | C19H17NO4 | 324.1220 | 324.1236 | −4.9 | [17] |
| 6 | 4.32 | Lycoranine B | C18H13NO4 | 308.0903 | 308.0923 | −6.5 | [18] |
| 7 | 4.50 | Jatrorrhizine/columbamine | C20H19NO4 | 338.1386 | 338.1392 | −1.8 | [13, 14] |
| 8 | 4.66 | Epiberberine | C20H17NO4 | 336.1228 | 336.1236 | −2.4 | [13, 14] |
| 9 | 4.95 | Coptisine | C19H13NO4 | 320.0915 | 320.0923 | −2.5 | [13, 14] |
| 10 | 5.81 | Thalifendine/berberrubine | C18H15NO4 | 322.1070 | 322.1079 | −2.8 | [14] |
| 11 | 6.68 | Palmatine | C21H21NO4 | 352.1556 | 352.1549 | 2.0 | [13, 14] |
| 12 | 7.01 | Berberine | C20H17NO4 | 336.1236 | 336.1236 | 0 | [13, 14] |
| 13 | 8.90 | Dihydrochelerythrine | C21H19NO4 | 350.1394 | 350.1392 | 0.6 | [19] |
Alkaloids comprise the main compounds in C. chinensis Franch., including berberine, palmatine, coptisine, epiberberine, jatrorrhizine, columbamine, and magnoflorine. Berberine, palmatine, coptisine, epiberberine, and jatrorrhizine are the five main alkaloids in C. chinensis Franch., with their contents range from 5% to 10% in total [1]. Among them, berberine, coptisine, epiberberine, jatrorrhizine are the chemical indicators in evaluating the quality of C. chinensis Franch. in China pharmacopoeia [1]. Although the compounds, like magnoflorine and columbamine, with very lower contents, are difficult to identify in chromatographs [20], they help explain the pharmacological effects of C. chinensis Franch. Therefore, identifying these compounds is significant. In our study, the UPLC-qTOF/MS provides the information of 13 compounds in C. chinensis Franch.
Except lincangenine/stephabine, lycoranine B, and dihydrochelerythrine, all these identified compounds have been reported in C. chinensis Franch. in [13–15, 21] with significant bioactivities. And all the identified compounds are protoberberine alkaloids with the similar structures chemically. Lincangenine/stephabine and lycoranine B have been isolated from Stephania suberosa and Lycoris radiata, respectively [16, 18]. And dihydrochelerythrine could be transformed for berberine chemically [19]. These references show a higher possibility for the existence of lincangenine/stephabine, lycoranine B, and dihydrochelerythrine in C. chinensis Franch. And they are the first time to be reported in C. chinensis Franch. Lincangenine and stephabine are isomeric compounds, and they both have the possibility to exist in C. chinensis Franch. Jatrorrhizine/columbamine and thalifendine/berberrubine are the isomeric compounds, respectively. And they could all exist in C. chinensis Franch. because they could transform into each other with biosynthesized way [22].
3.3. Dihydrochelerythrine Contained in Crude and Processed C. chinensis Franch.
Dihydrochelerythrine was detected in this study. Figure 2 presents the relative content (based on the peak area) in crude and processed C. chinensis Franch. It shows that the relative content of dihydrochelerythrine in crude C. chinensis Franch. is very lower than other samples. And the relative content of dihydrochelerythrine in Cu HL is the highest. It is possible that the auxiliary material or heating process could enhance the transformation of dihydrochelerythrine from berberine. In our previous study, the transformation of berberine to berberrubine, palmatine to palmatine, could be increased with an acidic condition. And the result in this study confirm the general rule of transformation with protoberberine alkaloids in processed C. chinensis Franch. that acidic condition in processing could enhance the protoberberine transformation. Dihydrochelerythrine has been reported with many pharmacological activities, such as antiparasitic and antitumor effects [23, 24]. And the reason for increasing the content of dihydrochelerythrine in processed C. chinensis Franch. would be explored further.
Figure 2.
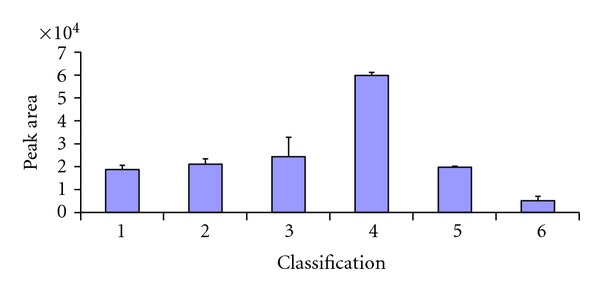
(1) Jiang HL, (2) Yu HL, (3) Jiu HL, (4) Cu HL, (5) Yan HL, (6) crude C. chinensis Franch. The relative content of dihydrochelerythrine in crude and processed C. chinensis Franch (N = 3).
3.4. Confirmation of TCM Processing Theories with Crude and Processed C. chinensis Franch.
There are 5 kinds of processed C. chinensis Franch.: Jiu HL, Jiang HL, Yu HL, Cu HL, and Yan HL. Crude C. chinensis Franch. and 5 different processed products were analyzed by PCA. Figure 3 shows the differences of crude and processed C. chinensis Franch. In the PCA, crude and 5 processed C. chinensis Franch. have been separated clearly. Jiu HL, Jiang HL, and Yu HL (PAT) are clustered into one side, and Cu HL and Yan HL (PST) are clustered into the other side. Among them, Jiu HL and Jiang HL are positioned in positive region; crude, Cu HL and Yan HL are positioned in the negative region, while Jiu HL group is in the middle of these two regions.
Figure 3.
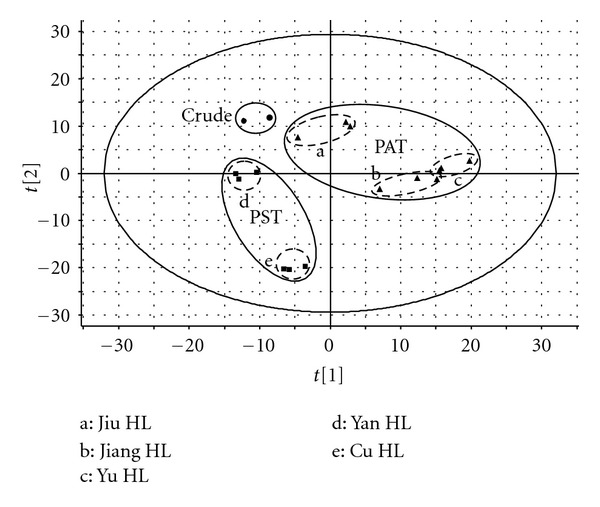
PCA of crude and processed C. chinensis Franch.
According to the TCM theories, all the TCMs could be grouped into 4 classifications, which are cold, heat, warm, and cool. And C. chinensis Franch. is in the classification of “cold,” and it belongs to the extreme cold level. This contribution of C. chinensis Franch. could lead to some side-effects if patients take it for a long period of time or some patients with special physiques take it. Therefore, the contribution of C. chinensis Franch. should be modified to fit the specific requirement in clinic. On one side, C. chinensis Franch. should be adjusted into a little “warm” to eliminate the side-effect of the extreme cold character. On the other side, C. chinensis Franch. could be modified into more “cold” to meet the extremely heat syndrome in clinic. The former one should be treated as the method of PAT, while the latter one should be treated as the method of PST. Among the PAT, Jiang HL and Yu HL could transform more of C. chinensis Franch. into the “warm” side, while Jiu HL could transform less compared with Jiang HL and Yu HL. And this could be confirmed in the PCA that Jiang HL and Yu HL are in the positive region while Jiu HL is in the middle of the two regions. And crude, Cu HL and Yan HL belong to the contribution of “cold” with the positions of negative region in PCA. Thus, the result of PCA conforms with the traditional processing theories and illustrates the methods of PAT and PST.
Our experiment shows the differences in the 5 processed C. chinensis Franch. which can be identified by UPLC-qTOF and analyzed by Markerlynx software. And this method could demonstrate the TCM theories markedly. This is the first time to elucidate TCM processing theories by modern technology.
4. Conclusion
Forms of processed C. chinensis Franch. have been recorded in the long history of TCM. Their curative effects have also been verified in clinical setting. The chemical analysis of processed C. chinensis Franch. via UPLC-qTOF/MS demonstrates significant differences. Such information demonstrates the significance of processed C. chinensis Franch. as well as of PAT and PST drugs. The processing technology helps create new pharmacological drugs that possess distinctive clinical effects. This result, therefore, is a new finding in traditional alternative medicine.
Acknowledgment
This work was supported by the National Natural Science Foundation of China (81274013, 81130069), the program for Changjiang Scholars and Innovative Research Team in University of Ministry of Education of China (IRT1150), the Chinese National S&T Special Project on Major New Drug Innovation (2011ZX09307-002-01), Selected Program of Personnel Department for Oversea Scholar.
Abbreviations
- UPLC-qTOF/MS:
Ultra-performance liquid chromatography-quadrupole time of flight mass spectrometry
- PCA:
Principal component analysis
- TCM:
Traditional Chinese medicine
- PST:
Processing synergy theory
- PAT:
Processing antagonism theory
- Jiu HL:
Processed C. chinensis Franch. with rice wine
- Jiang HL:
Processed C. chinensis Franch. with Zingiber officinales Rosc.
- Yu HL:
Processed C. chinensis Franch. with Euodia rutaecarpa (Juss.) Benth
- Cu HL:
Processed C. chinensis Franch. with vinegar
- Yan HL:
Processed C. chinensis Franch. with salt.
References
- 1. State Pharmacopoeia Committee. Pharmacopoeia of People's Republic of China (2010 version), pp. 285, 2010.
- 2.Kou JP, Wu Y, Wang QZ, et al. Studies on the sedative and hypnotic activities of JiaotaiWan prepared by raw or wine-processed Coptidis Rhizoma . Pharmacology and Clinics of Chinese Materia Medica. 2007;23(5):15–17. [Google Scholar]
- 3.Jiang J, Jia XB, Lu XH, et al. Empirical study of Fructus Evodiae processed Rhizoma Coptidis’s synergistic effect on breadboard gastric ulcer of rats. China Jounral of Traditional Chinese Medicine and Pharmacy. 2010;25(12):2130–2132. [Google Scholar]
- 4.Li JC, Meng XL, Cui R, et al. Comparison in pharmacodynamic effects of different types of processed Rhizoma Coptis on mouse diabetes. Chinese Traditional Patent Medicine. 32(11):1922–1925. [Google Scholar]
- 5.Zhou SH, Pan WJ, Xiao XH, et al. Biothermokinetic studies on four properties of traditional Chinese materia medica-Comparison of different preparation properties of Coptidis Rhizoma by microcalorimetry. Chinese Traditional and Herbal Drugs. 2004;35(11):1230–1232. [Google Scholar]
- 6.Yang C, Qiu X, Kong LD. Effects of different processing products of Rhizoma Coptidis on scavenging oxygen free radical and anti lipid peroxidation. Journal of Nanjing University. 2001;37(5):559–663. [Google Scholar]
- 7.Liu LH, Chen ZL. Analysis of four alkaloids of Coptis chinensis in rat plasma by high performance liquid chromatography with electrochemical detection. Analytica Chimica Acta. 2012;737:99–104. doi: 10.1016/j.aca.2012.05.054. [DOI] [PubMed] [Google Scholar]
- 8.Yang F, Zhang T, Zhang R, Ito Y. Application of analytical and preparative high-speed counter-current chromatography for separation of alkaloids from Coptis chinensis Franch. Journal of Chromatography A. 1998;829(1-2):137–141. doi: 10.1016/s0021-9673(98)00776-6. [DOI] [PubMed] [Google Scholar]
- 9.Yan D, Jin C, Xiao XH, Dong XP. Antimicrobial properties of berberines alkaloids in Coptis chinensis Franch by microcalorimetry. Journal of Biochemical and Biophysical Methods. 2008;70(6):845–849. doi: 10.1016/j.jbbm.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 10.Giri P, Kumar GS. Self-structure induction in single stranded poly(A) by small molecules: studies on DNA intercalators, partial intercalators and groove binding molecules. Archives of Biochemistry and Biophysics. 2008;474(1):183–192. doi: 10.1016/j.abb.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 11.Islam MM, Suresh Kumar G. RNA targeting by small molecule alkaloids: studies on the binding of berberine and palmatine to polyribonucleotides and comparison to ethidium. Journal of Molecular Structure. 2008;875(1–3):382–391. [Google Scholar]
- 12.Chen J, Wang F, Liu J, Lee FSC, Wang X, Yang H. Analysis of alkaloids in Coptis chinensis Franch by accelerated solvent extraction combined with ultra performance liquid chromatographic analysis with photodiode array and tandem mass spectrometry detections. Analytica Chimica Acta. 2008;613(2):184–195. doi: 10.1016/j.aca.2008.02.060. [DOI] [PubMed] [Google Scholar]
- 13.Luo X, Chen B, Yao S. Simultaneous analysis of protoberberine, indolequinoline and quinolone alkaloids in coptis-evodia herb couple and the Chinese herbal preparations by high-performance liquid chromatography-electrospray mass spectrometry. Talanta. 2005;66(1):103–110. doi: 10.1016/j.talanta.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 14.Liu Q, Qiu S, Yu H, Ke Y, Jin Y, Liang X. Selective separation of structure-related alkaloids in Rhizoma Coptidis with “click” binaphthyl stationary phase and their structural elucidation with liquid chromatography-mass spectrometry. Analyst. 2011;136(20):4357–4365. doi: 10.1039/c1an15444c. [DOI] [PubMed] [Google Scholar]
- 15.Hung TM, Lee JP, Min BS, et al. Magnoflorine from CoptidisRhizoma protects high density lipoprotein during oxidant stress. Biological and Pharmaceutical Bulletin. 2007;30(6):1157–1160. doi: 10.1248/bpb.30.1157. [DOI] [PubMed] [Google Scholar]
- 16.Patra A, Montgomery CT, Freyer AJ, et al. The protoberberine alkaloids of Stephania suberosa. Phytochemistry. 1987;26(2):547–549. [Google Scholar]
- 17.Li Y, Ren G, Wang YX, et al. Bioactivities of berberine metabolites after transformation through CYP450 isoenzymes. Journal of Translational Medicine. 2011;9(62):1–10. doi: 10.1186/1479-5876-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang L, Zhang XQ, Yin ZQ, Wang Y, Ye WC. Two new amaryllidaceae alkaloids from the bulbs of Lycoris radiata. Chemical and Pharmaceutical Bulletin. 2009;57(6):610–611. doi: 10.1248/cpb.57.610. [DOI] [PubMed] [Google Scholar]
- 19.Hanaoka M, Motonishi T, Mukai C. Chemical transformation of protoberberines. Part 9. A biomimetic synthesis of oxychelerythrine, dihydrochelerythrine, and chelerythrine from berberine. Journal of the Chemical Society, Perkin Transactions 1. 1986:2253–2256. [Google Scholar]
- 20.Kuang YH, Zhu JJ, Wang ZM, Zhang QW. Simultaneous quantitative analysis of five alkaloids in rhizoma of Coptis chinensis by multi-components assay by single marker. Chinese Pharmaceutical Journal. 2009;44(5):390–394. [Google Scholar]
- 21.Jung HA, Min BS, Yokozawa T, Lee JH, Kim YS, Choi JS. Anti-Alzheimer and antioxidant activities of CoptidisRhizoma alkaloids. Biological and Pharmaceutical Bulletin. 2009;32(8):1433–1438. doi: 10.1248/bpb.32.1433. [DOI] [PubMed] [Google Scholar]
- 22.Rueffer M, Zenk MH. Columbamine, the central intermediate in the late stages of protoberberine biosynthesis. Tetrahedron Letters. 1986;27(8):923–924. [Google Scholar]
- 23.Vrba J, Doležel P, Vičar J, Modrianský M, Ulrichová J. Chelerythrine and dihydrochelerythrine induce G1 phase arrest and bimodal cell death in human leukemia HL-60 cells. Toxicology in Vitro. 2008;22(4):1008–1017. doi: 10.1016/j.tiv.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 24.Yao JY, Zhou ZM, Li XL, et al. Antiparasitic efficacy of dihydrosanguinarine and dihydrochelerythrine from Macleaya microcarpa against Ichthyophthirius multifiliis in richadsin (Squaliobarbus curriculus) Veterinary Parasitology. 2011;183(1-2):8–13. doi: 10.1016/j.vetpar.2011.07.021. [DOI] [PubMed] [Google Scholar]


