Abstract
Transient Receptor Potential Canonical (TRPC) channels have been implicated in several aspects of cardiorenal physiology including regulation of blood pressure, vasoreactivity, vascular remodeling, and glomerular filtration. Gain and loss of function studies also support the role of TRPC channels in adverse remodeling associated with cardiac hypertrophy and heart failure. This review discusses TRP channels in the cardiovascular and glomerular filtration systems and their role in disease pathogenesis. We describe the regulation of gating of TRPC channels in the cardiorenal system as well as the influence on activation of these channels by the underlying cytoskeleton and scaffolding proteins. We then focus on the role of TRP channels in the pathogenesis of adverse cardiac remodeling and as potential therapeutic targets in the treatment of heart failure.
Keywords: Transient receptor potential channels, Transient receptor potential canonical channels, TRPC channels, Calcium, Cytoskeleton, Scaffolding proteins, Hypertension, Mechanotransduction, Vascular reactivity, Vascular remodeling, Hypertrophy, Heart failure, Podocyte, Focal segmental glomerulosclerosis, Cardiorenal system, End-stage kidney disease
Introduction
Hypertension has long been recognized as a risk factor for cardiorenal diseases including chronic renal insufficiency, atherosclerosis, stroke and cardiomyopathy (1). It has become clear that the cellular response to the hemodynamic and neurohormonal changes associated with hypertension result in maladaptive tissue remodeling and subsequent tissue damage (2). A variety of medications have long been used as effective agents to lower blood pressure, but often fail to prevent damage in certain tissues (3). Thus, an improved understanding of the pathophysiologic mechanism of cellular damage in response to hypertension would potentially lead to novel therapeutic strategies aimed at preventing underlying tissue damage. In particular, we have focused on transient receptor potential (TRPC) channels which are non-selective cation channels that are frequently implicated in hypertension (4-5). How TRPC channel activation is altered in hypertension is likely to involve changes in the manner in which TRPC associate in large protein complexes. Commonly TRPC channels associate with the cytoskeleton in order to link changes in membrane cationic flux with alteration in the actin-based cytoskeleton (6-8). This review will discuss the role of TRP channels in the cardiovascular and glomerular filtration systems and their role in disease pathogenesis. We describe the role of mechanotransduction in the regulation of gating of TRP channels as well as the influence on activation of these channels by the underlying cytoskeleton and scaffolding proteins.
TRPC Channels - Overview
TRPC channels are G-protein coupled receptor (GPCR) activated ion channels that include seven family members which can be divided into sub-groups based on their activation mechanism (9). TRPC channels are found in virtually every tissue and are activated by diverse chemical and environmental cues including temperature, mechanical forces and various ligands (10). TRPC channels are non-selective meaning that opening of the channel leads to the influx of Na and Ca2+ into the cytosol. TRPC channels are classified by sub-groups based on their gating mechanism: TRPC2 is a pseudogene in humans, TRPC3/6/7 represent one group and the TRPC4/5 channels are a second sub-group (9). TRPC1 is often considered as part of the TRPC4/5 subgroup although its activation mechanism may be distinct and remains unclear (11). An important form of regulation of TRPC channels is the delivery of the channel to the cell surface. Most often TRPC3/6/7 channels reside in vesicles underneath the cell membrane and are directed to the cell surface upon activation of a receptor tyrosine kinase and GPRC activation. Considerable evidence suggests that Gq-dependent production of diacylglycerol (DAG) is the mechanism triggering TRPC3/6/7 activation (12-14). Once in the surface membrane the channels are constitutively active and rapidly conduct the Na/Ca2+ current. Alternatively TRPC4/5 channels are tethered in the membrane via scaffold proteins utilizing PDZ domains and are activated in response to hydrolysis of phosphoinositol phosphate (PIP2) (8, 15). In addition to forming homo-oligomers, TRP channels have been shown to form hetero-oligomers with other TRPC channels as well as with members of the TRPV and TRPP channel families (16-19). An important limitation is the lack of specific pharmacologic tools such as subtype specific inhibitors (20). It is likely that as these compounds are developed, we will develop a greater appreciation for the physiologic function of these channels.
Role of the Cytoskeleton and Scaffolding Proteins in TRPC Channel Regulation
Growth factor stimulation results in translocation of TRPC channels to the plasma membrane via a process involving cytoskeletal rearrangements regulated by Rho GTPase activity (8). In turn, calcium influx through TRPC channels regulates cytoskeletal reorganization important for cellular migration (21). Evidence also suggests an interdependence between TRPC channel activation and cytoskeletal signaling during muscle remodeling. Activation of TRPC1 in differentiating myotubes has been shown to be influenced by the formation of stress fibers in response to stimulation with sphingosine 1-phosphate: a bioactive lipid which has also been shown to promote satellite cell renewal and differentiation in muscle damaged by eccentric contraction (22-23). TRP channel activation has also been shown to influence the activity of cofilin, a key regulator of actin remodeling in neurons (21).
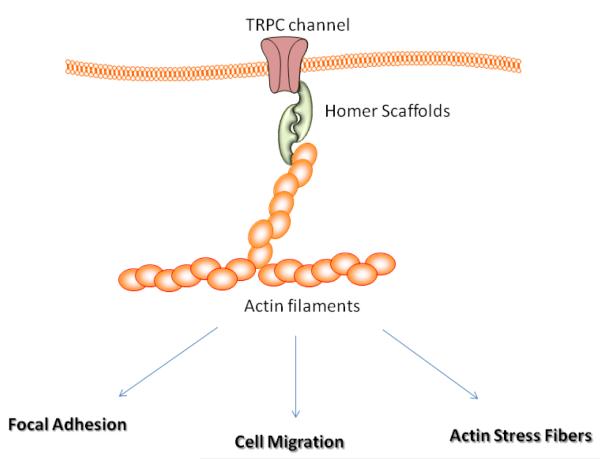
What is clear from these recent studies in neurons and muscle cells is that TRPC channels interact with cytoskeletal elements, either directly or through scaffold proteins, such as the homer proteins (Figure 1) (24). Homer proteins are Ena Vasodilator Homology (EVH1) domain containing proteins that scaffold TRPC channels. We found that Homer 1 isoforms are the predominant Homer isoforms in skeletal muscle. Mice lacking Homer 1 exhibited a myopathy characterized by decreased muscle fiber cross-sectional area and decreased skeletal muscle force generation (25). Homer 1 knockout myotubes displayed increased basal TRP current density and spontaneous cation influx. This spontaneous cation influx in Homer 1 knockout myotubes was associated with a marked reduction in the stiffness of the cytoskeleton of the homer-1 knockout cells as determined by atomic force microscopy. Moreover, diminished Homer 1 expression in mouse models of Duchenne’s muscular dystrophy suggested that loss of Homer 1 scaffolding of TRPC channels may contribute to the increased TRPC channel activity observed in mdx myofibers and therefore a reduction in the pre-stress of the muscle fiber (25-26). Whether Homer-1 proteins serve a similar function to link TRPC activity with cytoskeletal stiffness in other cell type such as podocytes and neurons remains an important question.
Fig. 1.
Schematic model depicting TRPC channel interaction with cytoskeleton. Homer proteins scaffolds the channel in the cell membrane. Homer proteins can then interact with actin filaments through direct interaction.
Role of TRPC Channels in Renal Function and the Progression to End Stage Kidney Disease
An important complication of longstanding hypertension is the development of significant kidney damage, often necessitating renal replacement therapy as hemodialysis or transplantation. Unlike the cardiovascular complications of hypertension, tight blood pressure control has not been found to be renal protective except in patients exhibiting substantial proteinuria (27). It is therefore important to understand the pathogenic mechanism underlying hypertensive renal disease. Interestingly, TRPC channels are widely expressed in the adult kidney including the podocyte, renal fibroblasts, tubular cells and certainly in the vascular smooth muscle cells (28). In particular TRPC6 channels influence the filtration barrier function of podocytes in the glomerulus. Mutations in TRPC6 have been reported in several families with an inherited form of focal segmental glomerulosclerosis (FSGS) (29-30). Here, activating mutations disrupt the barrier function and result in substantial proteinuria and eventual end stage kidney disease. In contrast, mice lacking TRPC6 are resistant to the nephrotoxic effects of angiotensin II, a neurohormone that causes hypertension and vasoconstriction (31). It is important to point out that TRPC channels have been shown to alter the organization of the actin cytoskeleton in the podocytes which may represent an important pathogenic mechanism in focal segmental glomerulosclerosis (FSGS) (32). In this case, excessive TRPC channel currents remodel the actin cytoskeleton which can alter the adhesive properties of the foot processes (33). It will be important to know if a similar mechanism is activated in the glomerulus subjected to hypertension. In this way, inhibitors of TRPC channels may ameliorate the damage caused to the nephron by elevated glomerular pressure.
Role of TRPC Channels in Vascular Reactivity and the Progression of Vascular Disease
Poorly controlled hypertension results in vascular injury and remodeling and is a major risk factor for the development of atherosclerosis. Hypertensive arterial remodeling involves both smooth muscle cell (SMC) hypertrophy and migration, results in changes in arterial compliance, and contributes to aneurysm formation in mouse models and humans (34). The renin-angiotensin system is a key regulator of blood pressure and hypertensive arterial remodeling. Angiotensin II, in addition to promoting hypertension by increasing vascular tone, promotes SMC migration and proliferation (35). TRPC channels have been implicated in the modulation of calcium influx during agonist stimulation of the vasculature and in the pathophysiology of hypertension (4, 36). TRPC channel expression is upregulated in monocytes from spontaneously hypertensive rats and in patients with essential hypertension (37-38). TRPC6 deficient mice may exhibit elevated blood pressure and enhanced agonist-induced contractility of isolated aortic rings and cerebral arteries. This increase in vascular reactivity observed in mice lacking TRPC6 was found to be due to an upregulation of TRPC3 with constitutive activity in vascular smooth muscle (39). Chronic stimulation with angiotensin II results in arterial remodeling characterized by medial expansion (34). Spontaneously hypertensive rats exhibit increased expression of TRPC3 in SMCs and knockdown of TRPC3 by siRNA inhibited calcium influx in response to angiotensin II stimulation (40). Angiotensin II stimulation has also been shown to result in upregulation of TRPC1 in human coronary artery smooth muscle cells, and silencing of TRPC1 resulted in a significant reduction of angiotensin II-mediated SMC hypertrophy (41).
Expression of TRPC channels is upregulated in response to arterial injury and in the transition of SMCs from a contractile to migratory phenotype (42-43). Human vein samples obtained during coronary artery bypass grafting, which developed neointimal hyperplasia when cultured in vitro, exhibited increased expression of TRPC1 in the neointima. Treatment with a targeted antibody to TRPC1 both reduced neointimal growth in cultured human vein samples and blocked calcium entry and proliferation of SMCs in culture (44). Oxidized LDL, which induces inflammation and promotes atherosclerosis, also augmented surface expression of TRPC1 in SMCs in an actin cytoskeleton-dependent fashion by promoting its translocation to the cell surface (45).
Role of TRPC Channels in Maladaptive Hypertrophy and the Progression to Heart Failure
In hypertensive patients, the heart is subjected chronically to increased load which can precipitate cardiac hypertrophy, and, if left untreated, heart failure. Several lines of evidence from multiple laboratories have demonstrated that TRPC channels may play a role in the pathogenesis of cardiac hypertrophy (26). A working model has been that increased TRPC channel activity enhances calcineurin signaling to drive hypertrophic signaling in various models of cardiac hypertrophy and failure. Cardiac expression of TRPC1, TRPC3 and TRPC6 have been found to be upregulated in response to pressure overload and TRPC channels are localized to the costamere of cardiomyocytes (46-49). Bush et. al. showed that TRPC3 expression is upregulated in multiple rodent models of pathological cardiac hypertrophy, whereas TRPC5 expression is increased in failing human hearts (49). Cardiac-specific overexpression of TRPC3 in mice was associated with increased calcineurin/NFAT activity, increased hypertrophy in response to pressure overload or treatment with phenylephrine plus angiotensin II, and increased propensity to heart failure progression (46). These adverse effects on cardiac remodeling after pressure overload stimulation were associated with TRPC3 overexpression and were blocked by targeted disruption of calcineurin (26, 46).
TRPC6 expression was found to be increased in mouse models of cardiomyopathy due to constitutive calcineurin activity or pressure overload. Cardiac specific overexpression of TRPC6 in transgenic mice was associated with increased NFAT-dependent gene expression, heightened sensitivity to pressure overload, a propensity for heart failure, and increased lethality in a dose dependent manner (48). The TRPC6 promoter contains two conserved NFAT sites which were required for its activation in response to calcineurin/NFAT signaling, and TRPC6 overexpression in cardiomyocytes increased the expression of the calcineurin-dependent RCAN1 gene in vivo. Thus TRPC6 appears to form a positive regulatory circuit in the calcineurin/NFAT pathway that promotes pressure-overload hypertrophy and the transition to heart failure (48, 50).
We recently reported that TRPC1 is required for the development of pressure-overload hypertrophy (51). Aortic banding results in increased TRPC1 expression and an increase in a non-selective cationic current which is absent in cardiomyocytes from TRPC1 KO mice. TRPC1 KO mice fail to manifest evidence of maladaptive cardiac hypertrophy and maintain preserved cardiac function when subjected to pressure overload. Loss of TRPC1 results in decreased dephosphorylation and activation of NFAT in cardiomyocytes subjected to pressure overload suggesting that decreased signaling through the calcineurin/NFAT pathway confers protection from pressure overload hypertrophy in these mice (51). We speculate that TRPC channels are present in the costamere to contribute to the adhesion strengthening response and prevent membrane damage to the cardiomyocytes under increased hemodynamic load. It is easy to see from these studies that increased wall stress on cardiomyocytes (associated with hypertension) activates TRPC channel activity and accelerates downstream Ca2+ dependent signaling events and drive cardiac hypertrophy.
Conclusions
TRPC channels play an influential role in several aspects of cardiorenal physiology including podocyte function/glomerular filtration, vascular reactivity and remodeling, as well as maladaptive cardiac hypertrophy and heart failure. Evidence supports the notion that TRPC channels sense and respond to changes in tension within the cytoskeleton. Increased TRPC channel activity that occurs with increased pressure can activate signal transduction cascades which, in turn, can result in maladaptive cytoskeletal remodeling contributing to the pathophysiology of diverse processes such as hypertensive arterial remodeling and podocyte dysfunction in familial FSGS. Therapeutic strategies aimed at inhibiting TRPC channel activity may therefore influence not only blood pressure control, but also blunt the maladaptive signaling occurring in the various target organs and therefore limit organ damage.
Acknowledgments
This work was supported by Award Number R01HL093470 (PBR) from the National Heart, Lung, and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health. Additional support includes a MDA research award (PBR), a HHMI Physician Scientist Early Career Award (JAS), American Heart Association Beginning Grant-in-Aid (JAS), and a Mandel Foundation Award (PBR, JAS).
Footnotes
Disclosure No potential conflicts of interest relevant to this article were reported.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Black HR, Elliott WJ. Hypertension : a companion to Braunwald’s heart disease. Saunders/Elsevier; Philadelphia: 2007. [Google Scholar]
- 2.Adams KF., Jr. Pathophysiologic role of the renin-angiotensin-aldosterone and sympathetic nervous systems in heart failure. Am J Health Syst Pharm. 2004;61(Suppl 2):S4–13. doi: 10.1093/ajhp/61.suppl_2.S4. [DOI] [PubMed] [Google Scholar]
- 3.Davis BR, Kostis JB, Simpson LM, Black HR, Cushman WC, Einhorn PT, Farber MA, Ford CE, Levy D, Massie BM, Nawaz S. Heart failure with preserved and reduced left ventricular ejection fraction in the antihypertensive and lipid-lowering treatment to prevent heart attack trial. Circulation. 2008;118:2259–2267. doi: 10.1161/CIRCULATIONAHA.107.762229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brayden JE, Earley S, Nelson MT, Reading S. Transient receptor potential (TRP) channels, vascular tone and autoregulation of cerebral blood flow. Clin Exp Pharmacol Physiol. 2008;35:1116–1120. doi: 10.1111/j.1440-1681.2007.04855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Firth AL, Remillard CV, Yuan JX. TRP channels in hypertension. Biochim Biophys Acta. 2007;1772:895–906. doi: 10.1016/j.bbadis.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Becker D, Bereiter-Hahn J, Jendrach M. Functional interaction of the cation channel transient receptor potential vanilloid 4 (TRPV4) and actin in volume regulation. Eur J Cell Biol. 2009;88:141–152. doi: 10.1016/j.ejcb.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Clark K, Middelbeek J, van Leeuwen FN. Interplay between TRP channels and the cytoskeleton in health and disease. Eur J Cell Biol. 2008;87:631–640. doi: 10.1016/j.ejcb.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 8.Bezzerides VJ, Ramsey IS, Kotecha S, Greka A, Clapham DE. Rapid vesicular translocation and insertion of TRP channels. Nat Cell Biol. 2004;6:709–720. doi: 10.1038/ncb1150. [DOI] [PubMed] [Google Scholar]
- 9.Montell C. Physiology, phylogeny, and functions of the TRP superfamily of cation channels. Sci STKE. 2001;2001:RE1. doi: 10.1126/stke.2001.90.re1. [DOI] [PubMed] [Google Scholar]
- 10.Riccio A, Medhurst AD, Mattei C, Kelsell RE, Calver AR, Randall AD, Benham CD, Pangalos MN. mRNA distribution analysis of human TRPC family in CNS and peripheral tissues. Brain Res Mol Brain Res. 2002;109:95–104. doi: 10.1016/s0169-328x(02)00527-2. [DOI] [PubMed] [Google Scholar]
- 11.Wes PD, Chevesich J, Jeromin A, Rosenberg C, Stetten G, Montell C. TRPC1, a human homolog of a Drosophila store-operated channel. Proc Natl Acad Sci U S A. 1995;92:9652–9656. doi: 10.1073/pnas.92.21.9652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hofmann T, Obukhov AG, Schaefer M, Harteneck C, Gudermann T, Schultz G. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature. 1999;397:259–263. doi: 10.1038/16711. [DOI] [PubMed] [Google Scholar]
- 13.Boulay G, Zhu X, Peyton M, Jiang M, Hurst R, Stefani E, Birnbaumer L. Cloning and expression of a novel mammalian homolog of Drosophila transient receptor potential (Trp) involved in calcium entry secondary to activation of receptors coupled by the Gq class of G protein. J Biol Chem. 1997;272:29672–29680. doi: 10.1074/jbc.272.47.29672. [DOI] [PubMed] [Google Scholar]
- 14.Dietrich A, Mederos y Schnitzler M, Kalwa H, Storch U, Gudermann T. Functional characterization and physiological relevance of the TRPC3/6/7 subfamily of cation channels. Naunyn-Schmiedebergs Archives of Pharmacology. 2005;371:257–265. doi: 10.1007/s00210-005-1052-8. [DOI] [PubMed] [Google Scholar]
- 15.Tang Y, Tang J, Chen Z, Trost C, Flockerzi V, Li M, Ramesh V, Zhu MX. Association of mammalian trp4 and phospholipase C isozymes with a PDZ domain-containing protein, NHERF. J Biol Chem. 2000;275:37559–37564. doi: 10.1074/jbc.M006635200. [DOI] [PubMed] [Google Scholar]
- 16.Ma X, Nilius B, Wong JW, Huang Y, Yao X. Electrophysiological properties of heteromeric TRPV4-C1 channels. Biochim Biophys Acta. 2011;1808:2789–2797. doi: 10.1016/j.bbamem.2011.07.049. [DOI] [PubMed] [Google Scholar]
- 17.Strubing C, Krapivinsky G, Krapivinsky L, Clapham DE. TRPC1 and TRPC5 form a novel cation channel in mammalian brain. Neuron. 2001;29:645–655. doi: 10.1016/s0896-6273(01)00240-9. [DOI] [PubMed] [Google Scholar]
- 18.Schaefer M. Homo- and heteromeric assembly of TRP channel subunits. Pflugers Arch. 2005;451:35–42. doi: 10.1007/s00424-005-1467-6. [DOI] [PubMed] [Google Scholar]
- 19.Bai CX, Giamarchi A, Rodat-Despoix L, Padilla F, Downs T, Tsiokas L, Delmas P. Formation of a new receptor-operated channel by heteromeric assembly of TRPP2 and TRPC1 subunits. EMBO reports. 2008;9:472–479. doi: 10.1038/embor.2008.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ito S, Kume H, Naruse K, Kondo M, Takeda N, Iwata S, Hasegawa Y, Sokabe M. A novel Ca2+ influx pathway activated by mechanical stretch in human airway smooth muscle cells. Am J Respir Cell Mol Biol. 2008;38:407–413. doi: 10.1165/rcmb.2007-0259OC. [DOI] [PubMed] [Google Scholar]
- 21.Wen Z, Han L, Bamburg JR, Shim S, Ming GL, Zheng JQ. BMP gradients steer nerve growth cones by a balancing act of LIM kinase and Slingshot phosphatase on ADF/cofilin. J Cell Biol. 2007;178:107–119. doi: 10.1083/jcb.200703055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Formigli L, Sassoli C, Squecco R, Bini F, Martinesi M, Chellini F, Luciani G, Sbrana F, Zecchi-Orlandini S, Francini F, Meacci E. Regulation of transient receptor potential canonical channel 1 (TRPC1) by sphingosine 1-phosphate in C2C12 myoblasts and its relevance for a role of mechanotransduction in skeletal muscle differentiation. J Cell Sci. 2009;122:1322–1333. doi: 10.1242/jcs.035402. [DOI] [PubMed] [Google Scholar]
- 23.Sassoli C, Formigli L, Bini F, Tani A, Squecco R, Battistini C, Zecchi-Orlandini S, Francini F, Meacci E. Effects of S1p on Skeletal Muscle Repair/Regeneration during Eccentric Contraction. J Cell Mol Med. 2010 doi: 10.1111/j.1582-4934.2010.01250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuan JP, Kiselyov K, Shin DM, Chen J, Shcheynikov N, Kang SH, Dehoff MH, Schwarz MK, Seeburg PH, Muallem S, Worley PF. Homer binds TRPC family channels and is required for gating of TRPC1 by IP3 receptors. Cell. 2003;114:777–789. doi: 10.1016/s0092-8674(03)00716-5. [DOI] [PubMed] [Google Scholar]
- •25.Stiber JA, Zhang ZS, Burch J, Eu JP, Zhang S, Truskey GA, Seth M, Yamaguchi N, Meissner G, Shah R, Worley PF, Williams RS, Rosenberg PB. Mice lacking Homer 1 exhibit a skeletal myopathy characterized by abnormal transient receptor potential channel activity. Mol Cell Biol. 2008;28:2637–2647. doi: 10.1128/MCB.01601-07. This paper shows that Homer 1 isoforms serve as regulatory scaffolds for TRPC1 channels in striated muscle.
- 26.Stiber JA, Seth M, Rosenberg PB. Mechanosensitive channels in striated muscle and the cardiovascular system: not quite a stretch anymore. J Cardiovasc Pharmacol. 2009;54:116–122. doi: 10.1097/FJC.0b013e3181aa233f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bloch MJ, Basile J. African American patients with hypertensive chronic kidney disease receive no benefit on kidney disease progression from the currently recommended blood pressure goal of <130/80 mm Hg unless there is significant proteinuria at baseline: long-term follow-up of the AASK study. J Clin Hypertens (Greenwich) 2011;13:214–216. doi: 10.1111/j.1751-7176.2010.00409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goel M, Sinkins WG, Zuo CD, Estacion M, Schilling WP. Identification and localization of TRPC channels in the rat kidney. Am J Physiol Renal Physiol. 2006;290:F1241–1252. doi: 10.1152/ajprenal.00376.2005. [DOI] [PubMed] [Google Scholar]
- 29.Winn MP, Conlon PJ, Lynn KL, Farrington MK, Creazzo T, Hawkins AF, Daskalakis N, Kwan SY, Ebersviller S, Burchette JL, Pericak-Vance MA, Howell DN, Vance JM, Rosenberg PB. A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science. 2005;308:1801–1804. doi: 10.1126/science.1106215. [DOI] [PubMed] [Google Scholar]
- 30.Reiser J, Polu KR, Moller CC, Kenlan P, Altintas MM, Wei C, Faul C, Herbert S, Villegas I, Avila-Casado C, McGee M, Sugimoto H, Brown D, Kalluri R, Mundel P, Smith PL, Clapham DE, Pollak MR. TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat Genet. 2005;37:739–744. doi: 10.1038/ng1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •31.Eckel J, Lavin PJ, Finch EA, Mukerji N, Burch J, Gbadegesin R, Wu G, Bowling B, Byrd A, Hall G, Sparks M, Zhang ZS, Homstad A, Barisoni L, Birbaumer L, Rosenberg P, Winn MP. TRPC6 Enhances Angiotensin II-induced Albuminuria. J Am Soc Nephrol. 2011 doi: 10.1681/ASN.2010050522. This paper shows that despite developing similar levels of hypertension in response to chronic angiotensin II infusion as WT, mice lacking TRPC6 developed significantly less albuminuria: suggesting that TRPC6 adversely influences podocyte function.
- 32.Moller CC, Wei C, Altintas MM, Li J, Greka A, Ohse T, Pippin JW, Rastaldi MP, Wawersik S, Schiavi S, Henger A, Kretzler M, Shankland SJ, Reiser J. Induction of TRPC6 channel in acquired forms of proteinuric kidney disease. Journal of the American Society of Nephrology. 2007;18:29–36. doi: 10.1681/ASN.2006091010. [DOI] [PubMed] [Google Scholar]
- 33.Moller CC, Flesche J, Reiser J. Sensitizing the Slit Diaphragm with TRPC6 ion channels. Journal of the American Society of Nephrology. 2009;20:950–953. doi: 10.1681/ASN.2008030329. [DOI] [PubMed] [Google Scholar]
- 34.Sparks MA, Parsons KK, Stegbauer J, Gurley SB, Vivekanandan-Giri A, Fortner CN, Snouwaert J, Raasch EW, Griffiths RC, Haystead TA, Le TH, Pennathur S, Koller B, Coffman TM. Angiotensin II type 1A receptors in vascular smooth muscle cells do not influence aortic remodeling in hypertension. Hypertension. 2011;57:577–585. doi: 10.1161/HYPERTENSIONAHA.110.165274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shin EY, Lee CS, Park MH, Kim DJ, Kwak SJ, Kim EG. Involvement of betaPIX in angiotensin II-induced migration of vascular smooth muscle cells. Exp Mol Med. 2009;41:387–396. doi: 10.3858/emm.2009.41.6.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soboloff J, Spassova M, Xu W, He LP, Cuesta N, Gill DL. Role of endogenous TRPC6 channels in Ca2+ signal generation in A7r5 smooth muscle cells. J Biol Chem. 2005;280:39786–39794. doi: 10.1074/jbc.M506064200. [DOI] [PubMed] [Google Scholar]
- 37.Liu D, Scholze A, Zhu Z, Kreutz R, Wehland-von-Trebra M, Zidek W, Tepel M. Increased transient receptor potential channel TRPC3 expression in spontaneously hypertensive rats. Am J Hypertens. 2005;18:1503–1507. doi: 10.1016/j.amjhyper.2005.05.033. [DOI] [PubMed] [Google Scholar]
- 38.Liu D, Scholze A, Zhu Z, Krueger K, Thilo F, Burkert A, Streffer K, Holz S, Harteneck C, Zidek W, Tepel M. Transient receptor potential channels in essential hypertension. J Hypertens. 2006;24:1105–1114. doi: 10.1097/01.hjh.0000226201.73065.14. [DOI] [PubMed] [Google Scholar]
- 39.Dietrich A, Mederos YSM, Gollasch M, Gross V, Storch U, Dubrovska G, Obst M, Yildirim E, Salanova B, Kalwa H, Essin K, Pinkenburg O, Luft FC, Gudermann T, Birnbaumer L. Increased vascular smooth muscle contractility in TRPC6-/- mice. Mol Cell Biol. 2005;25:6980–6989. doi: 10.1128/MCB.25.16.6980-6989.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu D, Yang D, He H, Chen X, Cao T, Feng X, Ma L, Luo Z, Wang L, Yan Z, Zhu Z, Tepel M. Increased transient receptor potential canonical type 3 channels in vasculature from hypertensive rats. Hypertension. 2009;53:70–76. doi: 10.1161/HYPERTENSIONAHA.108.116947. [DOI] [PubMed] [Google Scholar]
- 41.Takahashi Y, Watanabe H, Murakami M, Ohba T, Radovanovic M, Ono K, Iijima T, Ito H. Involvement of transient receptor potential canonical 1 (TRPC1) in angiotensin II-induced vascular smooth muscle cell hypertrophy. Atherosclerosis. 2007;195:287–296. doi: 10.1016/j.atherosclerosis.2006.12.033. [DOI] [PubMed] [Google Scholar]
- 42.Bergdahl A, Gomez MF, Wihlborg AK, Erlinge D, Eyjolfson A, Xu SZ, Beech DJ, Dreja K, Hellstrand P. Plasticity of TRPC expression in arterial smooth muscle: correlation with store-operated Ca2+ entry. Am J Physiol Cell Physiol. 2005;288:C872–880. doi: 10.1152/ajpcell.00334.2004. [DOI] [PubMed] [Google Scholar]
- 43.Berra-Romani R, Mazzocco-Spezzia A, Pulina MV, Golovina VA. Ca2+ handling is altered when arterial myocytes progress from a contractile to a proliferative phenotype in culture. Am J Physiol Cell Physiol. 2008;295:C779–790. doi: 10.1152/ajpcell.00173.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumar B, Dreja K, Shah SS, Cheong A, Xu SZ, Sukumar P, Naylor J, Forte A, Cipollaro M, McHugh D, Kingston PA, Heagerty AM, Munsch CM, Bergdahl A, Hultgardh-Nilsson A, Gomez MF, Porter KE, Hellstrand P, Beech DJ. Upregulated TRPC1 channel in vascular injury in vivo and its role in human neointimal hyperplasia. Circ Res. 2006;98:557–563. doi: 10.1161/01.RES.0000204724.29685.db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ingueneau C, Huynh UD, Marcheix B, Athias A, Gambert P, Negre-Salvayre A, Salvayre R, Vindis C. TRPC1 is regulated by caveolin-1 and is involved in oxidized LDL-induced apoptosis of vascular smooth muscle cells. J Cell Mol Med. 2009;13:1620–1631. doi: 10.1111/j.1582-4934.2008.00593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakayama H, Wilkin BJ, Bodi I, Molkentin JD. Calcineurin-dependent cardiomyopathy is activated by TRPC in the adult mouse heart. FASEB J. 2006;20:1660–1670. doi: 10.1096/fj.05-5560com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guinamard R, Bois P. Involvement of transient receptor potential proteins in cardiac hypertrophy. Biochim Biophys Acta. 2007;1772:885–894. doi: 10.1016/j.bbadis.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 48.Kuwahara K, Wang Y, McAnally J, Richardson JA, Bassel-Duby R, Hill JA, Olson EN. TRPC6 fulfills a calcineurin signaling circuit during pathologic cardiac remodeling. J Clin Invest. 2006;116:3114–3126. doi: 10.1172/JCI27702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bush EW, Hood DB, Papst PJ, Chapo JA, Minobe W, Bristow MR, Olson EN, McKinsey TA. Canonical transient receptor potential channels promote cardiomyocyte hypertrophy through activation of calcineurin signaling. J Biol Chem. 2006;281:33487–33496. doi: 10.1074/jbc.M605536200. [DOI] [PubMed] [Google Scholar]
- 50.Onohara N, Nishida M, Inoue R, Kobayashi H, Sumimoto H, Sato Y, Mori Y, Nagao T, Kurose H. TRPC3 and TRPC6 are essential for angiotensin II-induced cardiac hypertrophy. Embo J. 2006;25:5305–5316. doi: 10.1038/sj.emboj.7601417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••51.Seth M, Zhang ZS, Mao L, Graham V, Burch J, Stiber J, Tsiokas L, Winn M, Abramowitz J, Rockman HA, Birnbaumer L, Rosenberg P. TRPC1 channels are critical for hypertrophic signaling in the heart. Circ Res. 2009;105:1023–1030. doi: 10.1161/CIRCRESAHA.109.206581. This paper shows that mice lacking TRPC1 fail to manifest evidence of maladaptive cardiac hypertrophy and maintain preserved cardiac function when subjected to pressure overload.