Abstract
It is not clear why some patients with aspiration advance to acute lung injury or acute respiratory distress syndrome, whereas others do not. The Western diet is high in advanced glycation end products (AGEs) which have been found to be proinflammatory. We hypothesize that dietary AGEs exaggerate the pulmonary inflammatory response following gastric aspiration. CD-1 mice were randomized to receive either a low (LAGE) or a high AGE (HAGE) diet for four weeks. Five hours after intratracheal instillation of acidified small gastric particles, pulmonary function was determined. Polymorphonuclear leukocyte (PMNs) counts, albumin, cytokine/chemokine, and TNF soluble receptor II (TNFsRII) concentrations in the bronchoalveolar lavage (BAL) and lung myeloperoxidase (MPO) activity were measured. Compared to LAGE-fed animals, those fed a HAGE diet had increased lung tissue resistance (p = .017), BAL albumin concentration (p < 0.05), pulmonary PMN counts (p = 0.0045), and lung MPO activity (p = .002) following aspiration. In addition, the plasma levels of TNFsRII were significantly elevated (p < 0.05), while paradoxically levels of keratinocyte chemoattractant (KC) and monocyte chemoattractant protein-1 (MCP-1) were decreased in mice with HAGE diet. In conclusion, a diet high in AGEs exacerbates acute lung injury following gastric aspiration as evidenced by increases in neutrophil infiltration, airway albumin leakage, and decreased pulmonary compliance. This is the first evidence implicating exacerbation of acute inflammatory lung injury by dietary AGEs. Targeting AGEs in the circulatory system may offer a therapeutic strategy for limiting lung injury following gastric aspiration.
Keywords: acute lung injury, gastric aspiration, bronchoalveolar lavage (BAL), advance glycation end-products, pulmonary mechanics, polymorphonuclear leukocyte (PMNs), myeloperoxidase (MPO), cytokines, chemokines
INTRODUCTION
Aspiration related acute lung injury (ALI), and its more severe form,acute respiratory distress syndrome (ARDS), carries a high mortality rate and accounts for up to 20% of all deaths attributed to anesthesia (1). Additionally, gastric aspiration often occurs in the trauma setting, secondary to an altered sensorium and history of recent intake of food and liquids. The consequence of gastric aspiration varies significantly, ranging from a mild, subclinical pneumonitis to ALI and ARDS. It is estimated that about 1/3 of patients with symptomatic gastric aspiration progress to ALI and ARDS (2). However, it is unclear why some patients with gastric aspiration develop ALI/ARDS, whereas, others do not.
Advanced glycation end products (AGEs) are a heterogeneous group of compounds with significant pro-oxidant and pro-inflammatory properties (3). AGEs have been implicated in various chronic diseases characterized by sustained oxidant stress and low-level multi-organ inflammatory injury (4–6). AGEs form spontaneously in the body via non-enzymatic glycol-oxidative reactions between reducing sugars, proteins, and lipids (7). However, a significant quantity of AGEs may also be obtained directly from AGE-rich food. The foods in the diet of Western culture are subject to industrial processes including: heating, irradiation and ionization, to make food safer, flavorful, and colorful. In combination with gross over-nutrition with high fat content, the food has been found to significantly contribute to the production and accumulation of AGEs in the body (8, 9). The interaction of dietary AGEs with structural and cellular components has emerged as a potential mechanism of environmental toxicity for the general adult population. Previous studies have shown that increasing the content of AGEs in the diet can increase circulating levels of AGEs (8, 9). Due to the Western fast food culture, some trauma victims may present with high blood levels of AGEs (10). These considerations led us to hypothesize that pre-injury chronic accumulation of AGEs from the Western diet produce a sub-clinical elevated inflammatory state that results in a more intense inflammatory acute injury. Additionally, since extravasation of fluid, rich in serum proteins, is a hallmark of low pH aspiration lung injuries, increased blood AGE levels could directly stimulate proinflammatory receptor for AGEs (RAGE) in the lung (11). Thus, AGEs represent a potential risk factor for augmenting inflammatory response following a gastric aspiration insult. In the current study, we have examined indices of lung injury, including BAL albumin, pulmonary mechanical compliance, airway resistance, as well as cellular and mediators of the pulmonary inflammatory response, including lung tissue myeloperoxidase (MPO) activity, polymorphonuclear neutrophil (PMN) infiltration, cytokines in a murine model of gastric aspiration and a high-AGE diet.
MATERIALS AND METHODS
Animals with high AGE diet
A semi-purified standard rodent chow (AIN-93G, Bio-Serve, Frenchtown, NJ) was used for this study (12). Prior to packaging, Bio-Serve heated the chow to 100°C for 20–60 sec, as per standard procedure. This chow constituted the low-AGE (LAGE) diet and contained 23 ng AGE/mg. To produce the high-AGE chow (HAGE), the LAGE chow was subjected to an additional heating of 125°C for 30 min that resulted in a chow containing 110 ng AGE/mg. The assay of AGE was performed by MicroCoat Biotechnologie GmbH, D-82347 Bernried, Germany, using photometric ELISA for the quantitative determination of Carboxymethyl Lysine (CML), which corresponds to the level of AGE (13). The two chows contained the same amount of calories, fat, protein and carbohydrates, but the HAGE chow contained a 5-fold increase in AGE content.
Male, specific-pathogen-free CD-1 mice (4-week old, weighing 25–30 g) were purchased from Charles River Laboratories (Wilmington, MA). Animal use and husbandry followed protocols approved by the Institutional Animal Care and Use Committees of the University at Buffalo and the Veterans Administration Western New York Healthcare System and compliant with New York State, Federal, and National Institutes of Health regulations. The animals were acclimated for 1 week, and then were randomized to receive either a LAGE or HAGE diet ad libitum for four weeks. All animals were allowed free access to the chow and water, and three to four animals per cage were housed under a 12-hour light/dark cycle in an animal room. Over a period of 4 weeks, the percent weight gain between the LAGE and HAGE groups was not different (LAGE: 17.52±1.43% vs HAGE: 20.56±1.99%). At the end of 4 weeks of diet, the animals were randomly assigned to one of four groups (n=14–15/group): 1.- LAGE uninjured controls; 2. HAGE uninjured controls; 3. LAGE + gastric aspiration injury; and 4. HAGE + gastric aspiration injury. Anesthesia, 2% halothane in 50% O2, was delivered by nose cone throughout the duration of the surgery.
Gastric aspiration induced lung injury
The aspirate solution used to model gastric aspiration contained 40 mg/mL of small gastric food particles adjusted to a pH of 1.25 with hydrochloric acid (14). Small non-acidified gastric food particles were obtained from the stomach contents of necropsied CD-1 mice in the morning when the stomach was fullest, followed by washing in normal saline and coarse-filtration through gauze to generate a mean particle diameter <10 µm (14, 15). They were then refrigerated in a 100mg/ml solution, and used within 2 weeks. The same batch of particles was used for several experiments. Although several batches of particles were needed to complete this study, we saw little variability between experiments. Following a midline tracheotomy to expose the trachea, the aspirate solution was instilled into the lungs of halothane-anesthetized mice using a 22 gauge needle inserted transtracheally. Just prior to instillation, the chest wall was compressed by hand and rapidly released as the aspirate solution (3.6 mL/kg body weight) and 0.2 mL air-bolus chaser were instilled to facilitate pulmonary distribution. Animals were allowed to recover in 100% O2 following the aspiration until regular, spontaneous breathing was restored. The animals were maintained in room air until sacrificed 5 h later at which time pulmonary function testing and bronchoalveolar lavage (BAL) were performed. This time point was chosen because in our pilot study, we experienced an unacceptable high mortality rate in animals 24 and 48 hours after the aspiration.
Measurement of lung tissue resistance
Pulmonary compliance was measured using the FlexiVent system (SCIREQ Inc., Montreal, Canada) 5 h after instillation of the aspirate solutionand before the animals were sacrificed. FlexiVent is a purposely designed small animal piston pump respirator that serves as a ventilator, as well as measuring device. Prior to each experiment, the pressure transducers were calibrated using a two-point calibration protocol. In order to account for tubing and cannula properties, open and closed calibration of the system was performed for all measurement signals prior to each experiment.
Animals were anesthetized with 2% halothane in 100% O2 and allowed to breathe the mixture for 5 min. The ventral midline neck wound was opened and an 18 gauge steel cannula was inserted into the trachea for approximately 10 mm and secured with a silk suture. Correct placement of the tracheal cannula for two lung ventilation was assessed during the open chest BAL procedure. In all cases, the cannula was observed to be in the trachea and not in a mainstem bronchus. The cannula was connected to the ventilator and default volume-controlled pressure-limited ventilation started with the following settings: tidal volume (TV) 8 ml/kg, positive end expiratory pressure (PEEP) 3 cmH2O, and a frequency of 150 strokes/min. Before actual measurements started, three recruitment maneuvers were performed to standardize lung volumes (inflations to 30 cmH2O over 4 sec with a 4 sec hold). Lung tissue resistance was derived from the respiratory system input impedence determined during a 16 sec multi-frequency forced oscillation technique. This measurement is frequency independent and reflects the energy dissipation in the lung tissues. Lung tissue resistance correlates very strongly with lung elastance (16).
Blood and BAL sampling and processing procedures
Following pulmonary mechanical compliance measurement, the animals were euthanized and a sample of blood was collected with a heparinized syringe from the inferior vena cava and the animal euthanized by transecting the inferior vena cava. A midline incision was then made through the sternum and the lung vasculature was flushed by injecting 20 mL HBSS (with Ca2+, Mg2+) at 37°C into the beating right ventricle. BAL was performed by injecting 5, 1 mL aliquots of 37°C normal saline through the tracheal cannula, collecting the BAL fluid with a syringe, and pooling the recovered aliquots. Recovered BAL was centrifuged at 1500 × g at 4°C for 3 min to pellet cells, and the supernatant was stored at −80°C for albumin and cytokine/chemokine analyses. The cell pellet was resuspended in 4 mL of phosphate buffered normal saline (PBS) + 0.1% sodium azide and the total numbers of BAL-recovered erythrocytes (RBCs) and leukocytes (WBCs) were determined with a Multisizer 3 Coulter Counter (Beckman Coulter, Fullerton, CA). Cytoslides were prepared from the cell suspension by cytocentrifugation (Cytospin 3; Shandon Southern Instruments, Sewickley, PA), stained with Diff-Quik (Baxter, Detroit, MI), and differentially determined by assessing 200 cells by microscopy.
Measurement of plasma AGE concentrations
Measurement of plasma AGE concentrations was performed by MicroCoat GmbH (D-82347 Bernried, Germany), using a carboxymethyl lysine (CML) ELISA to quantitate advanced glycation end products (13). The specimens were pretreated with proteinase K enzymatic digestion.
Whole lung myeloperoxidase activity (MPO) measurement
Whole lung MPO activity was studied as a surrogate measure of PMN-associated pulmonary inflammation. Following BAL, lungs were excised and ice-cold lung homogenate buffer, pH=7.4 (150 mM NaCl, 15 mM Tris base, 1 mM CaCl2•2H2O, 1 mM MgCl2•6H2O containing 500 µM AEBSF HCl, 150 nM aprotinin, 1 µM E-64, 0.5 mM disodium EDTA, and 1 µM leupeptin hemisulfate (protease inhibitor cocktail set I, Calbiochem/EMD Chemicals, Gibbstown, NJ)) was added to a total weight of 3 g (tissue plus buffer). The lungs were then homogenized on ice with a Polytron TP-2000 tissue homogenizer (Brinkman Instruments, Westbury, NY). The tissue homogenate was then centrifuged at 40,000 × g for 10 min at 4°C, and MPO was extracted from the pellet by resuspension in 2 mL phosphate buffer, pH = 6.0, containing 0.5% hexadecyltrimethylammonium bromide and 5 mM EDTA, followed by three freeze-thaw-sonication cycles (1 min, 50% duty cycle Branson Sonifier with microtip probe; Branson Ultrasonics, Danbury, CT). Centrifugation was carried out as beforeand the supernatant was combined with the supernatant from a second MPO extraction of the pellet. MPO activity was measured by combining 10 µL of extracted sample with 300 µL of assay buffer, pH = 6.0, containing 50 mM KH2PO4, 176 mM H202, and 52.5 mM o-dianisidine dihydrochloride in a 96-well microplate (Sarstedt, Newton, NC). Absorbance was recorded at 460 nm for 90 s at 2 s intervals on a SPECTRAmax190 plate reader (Molecular Devices, Sunnyvale, CA). MPO activity was expressed in arbitrary units as the absorbance change per minute over the linear portion of the curve (17).
Albumin concentrations in cell-free BAL
Albumin concentrations (µg/mL) in cell-free BAL were measured as an assessment of pulmonary vasculature permeability injury. Albumin levels were quantitated by direct ELISA with a polyclonal rabbit anti-mouse albumin antibody and a HRP-labeled goat anti-rabbit IgG (BD Biosciences Pharmingen, San Diego, CA) (15). Rat albumin (Sigma, St. Louis, MO) was used as a standard.
Cytokines and chemokines in BAL
Concentrations of tumor necrosis factor-α (TNFα), TNF soluble receptor II (TNF sRII), interleukin (IL)-1β, IL-6, interferon-γ (IFNγ), and IL-10, plus the rodent CXC chemokines keratinocyte chemoattractant (KC), macrophage inflammatory protein-2 (MIP-2), and cytokine-induced neutrophil chemoattractant-1 (CINC-1), and the CC chemokine monocyte chemoattractant protein-1 (MCP-1) were measured in cell-free BAL by sandwich ELISA methodology using reagents from R&D Systems (Minneapolis, MN) and expressed as pg/mL.
Histopathological evaluations
Lung tissue was obtained from normal and injured mice that had not been lavaged or studied for P-V mechanics and processed for histopathological evaluation. Briefly, lungs, thymus, and heart were removed en bloc following tracheal cannulation and flushing of the vasculature with 20 ml of normal saline injected into the right ventricle. The lungs were fixed immediately by insulflation with 10% neutral buffered formalin at 20 cmH2O for 24 h. Lung sections, 4 µm, were prepared and stained with hematoxylin and eosin and assessed for lung injury by experienced pathologists (Drs. James Woytash and Donald Higgs, Department of Pathology, the Erie County Medical Center, Buffalo) blinded to animal group assignments. An objective injury scoring system was used as follows: 0 = no neutrophils per 10 alveoli, 1 = 1–10 neutrophils per 10 alveoli, and 2 = 10–40 neutrophils per 10 alveoli.
Statistical analyses
Values were expressed as mean ± SEM, except as otherwise stated. All analysis was performed using GraphPad Prism 5® (GraphPad Software, La Jolla, CA). Data was analyzed by two-way ANOVA. Following two-way ANOVA, Prism performed the post test Bonferroni’s correction comparing one value with another value within the same treatment (either control or gastric aspiration injury). Comparisons were considered significantly different for p <0.05.
RESULTS
Plasma AGE concentrations after 4 week of HAGE diet
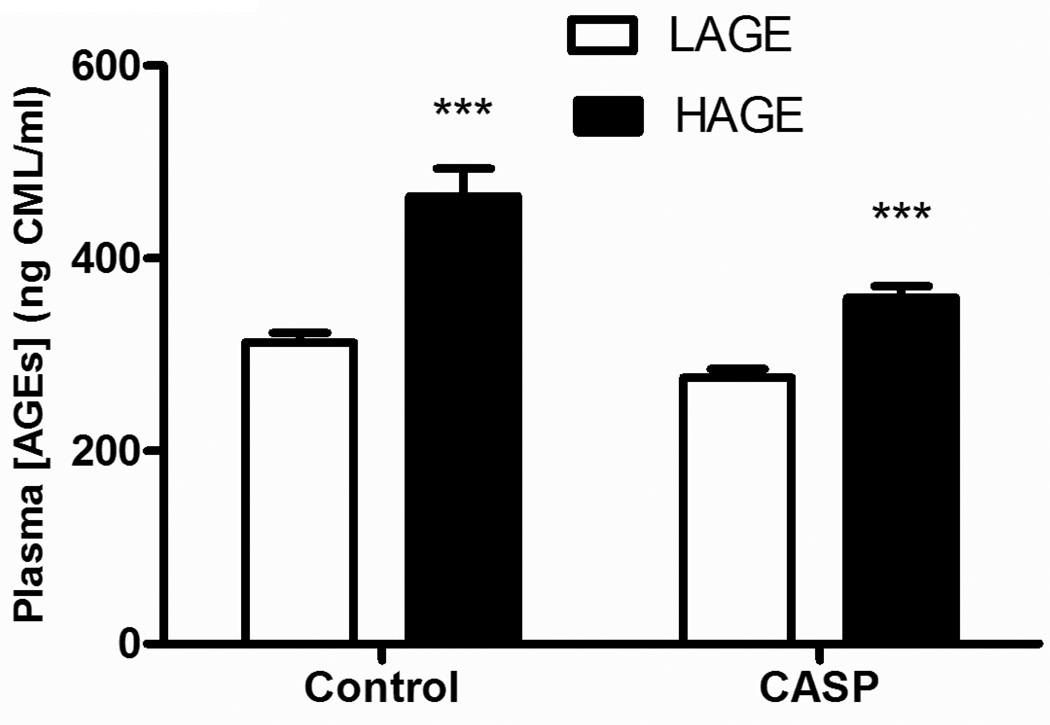
In order to assess the impact of a HAGE diet for 4 week we examined plasma AGE levels. Mice receiving this diet demonstrated an increase in plasma AGE concentration compared to a LAGE diet (controls: 464.8±28.5 vs 312.7±10.0, CASP: 359.5±11.9 vs 276.1±9.0 ng CML/ml, p<0.0001) (Figure 1). Interestingly, the HAGE-fed mice that were injured by gastric aspiration had lower plasma AGE levels than the uninjured control mice (p<0.01). This may reflect the release of soluble AGE receptors from the lung into the circulatory system post injury (18, 19).
Figure 1. Plasma AGE concentrations in mice after 4 week of LAGE or HAGE diet.
Mice fed a HAGE diet demonstrated an elevated plasma AGE concentration compared to LAGE-fed mice (***p < 0.0001), in both uninjured control or gastric aspiration injured animals. Interestingly, plasma AGE concentration was significantly lower in the HAGE animals that had been injured by gastric aspiration compared to the HAGE uninjured control group (p < 0.05).
Lung tissue resistance measurement
In order to assess differences in mechanical impairment of the lung following gastric aspiration in HAGE or LAGE fed mice, we examined lung tissue resistance 5 h post insult. This functional parameter, which quantitatively assesses the level of lung tissue “stiffness” and directly correlates with the lung elastance (16), was increased in injured animals compared with uninjured controls (LAGE: 5.72±1.67 vs 3.26±1.22, HAGE: 12.03±1.67 vs 3.41±1.27 cm H2O/ml, p<0.001). As shown in Figure 2, animals fed a HAGE diet had increased lung tissue resistance at 5 h after aspiration compared to their LAGE counterparts (p<0.05).
Figure 2. Lung tissue resistance 5 h after gastric aspiration in mice following 4 weeks of LAGE or HAGE diet.
As assessed at 5h post-gastric aspiration insult, lung tissue resistance was elevated compared with uninjured controls (p < 0.05), irrespective of diet. However, mice fed a HAGE diet had a greater level of lung tissue resistance 5 h after gastric aspiration injury than their LAGE-fed counterpart (p < 0.05).
Alveolar-capillary integrity, as assessed by BAL albumin concentration
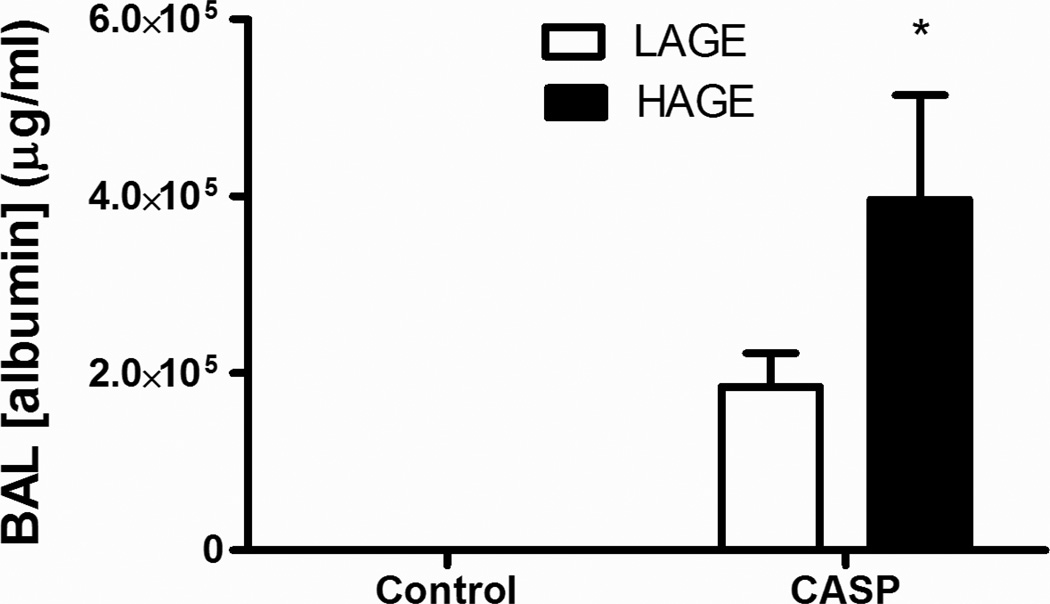
In order to examine the extent of damage to alveolar capillary wall following gastric aspiration in HAGE or LAGE fed mice we assayed airway albumin levels. Gastric aspiration increases BAL albumin levels in both LAGE and HAGE groups compared with controls (LAGE: 194,587±24,400 vs 209±22, HAGE: 245,375±38,675 vs 164±8 µg/ml, p < 0.0001) (Fig. 3). Furthermore, BAL albumin concentrations were higher in the animals fed a HAGE diet following gastric aspiration compared to similarly injured mice fed a LAGE diet (245,375±38,675 vs 194,587±24,400, p < 0.05) (Fig. 3).
Fig. 3. Albumin concentration in BAL 5 h after gastric aspiration from mice with 4 weeks of LAGE or HAGE diet.
Lung injury caused by gastric aspiration impaired the alveolar-capillary integrity as assessed by the increased albumin concentrations in cell-free BAL, in either LAGE or HAGE groups (p < 0.0001 vs uninjured controls). Five hours after gastric aspiration, the albumin concentration in BAL was higher in HAGE group, as compared with LAGE group (p < 0.05).
Airway Leukocyte Recruitment
Infiltration of polymorphonuclear neutrophils (PMNs) into the pulmonary airspaces is a hallmark of gastric aspiration, as observed in BAL leukocyte counts in our murine model (LAGE: 1,870,800±193,008 vs 9,143±4,103, HAGE: 2,606,700±290,186 vs 1,565±731 cells/ml, p<0.0001, compared to uninjured control mice) (Fig 4a). More importantly, PMN infiltration was higher in the HAGE-fed group than the LAGE-fed mice (p<0.01, Fig 4a). Conversely, BAL alveolar macrophages (aMØ) counts following gastric aspiration lung injury was decreased in HAGE-fed mice compared with the LAGE-fed group (222,000±35,240 vs 534,300±9,100 cells/ml, p<0.01, Fig 4b).
Fig. 4. Leukocyte numbers in BAL from mice with LAGE or HAGE diets.
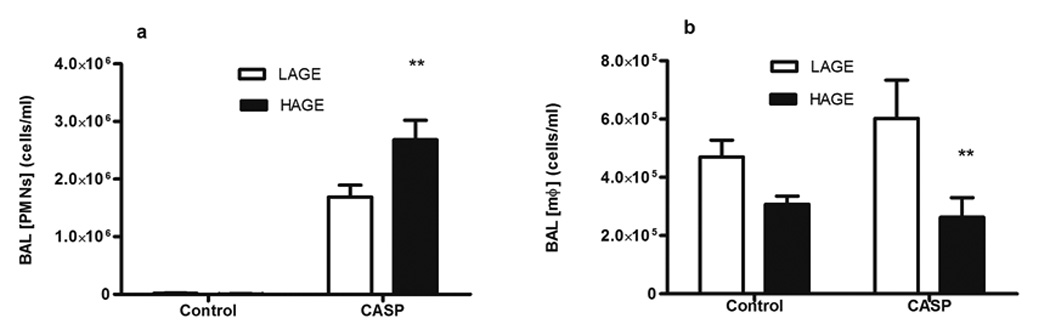
A. PMN counts were significantly higher in all injured groups. However, HAGE animals had higher PMN counts than the LAGE animals after gastric aspiration (p < 0.01). B. Macrophage counts in the BAL exhibited a completely different pattern, as compared to that of PMN. In animals with gastric aspiration, the dietary AGEs decreased the numbers of alveolar macrophage numbers, as compared with LAGE group (p < 0.01). However, gastric aspiration did not affect the macrophage counts.
Myeloperoxidase (MPO) levels in the lung homogenate
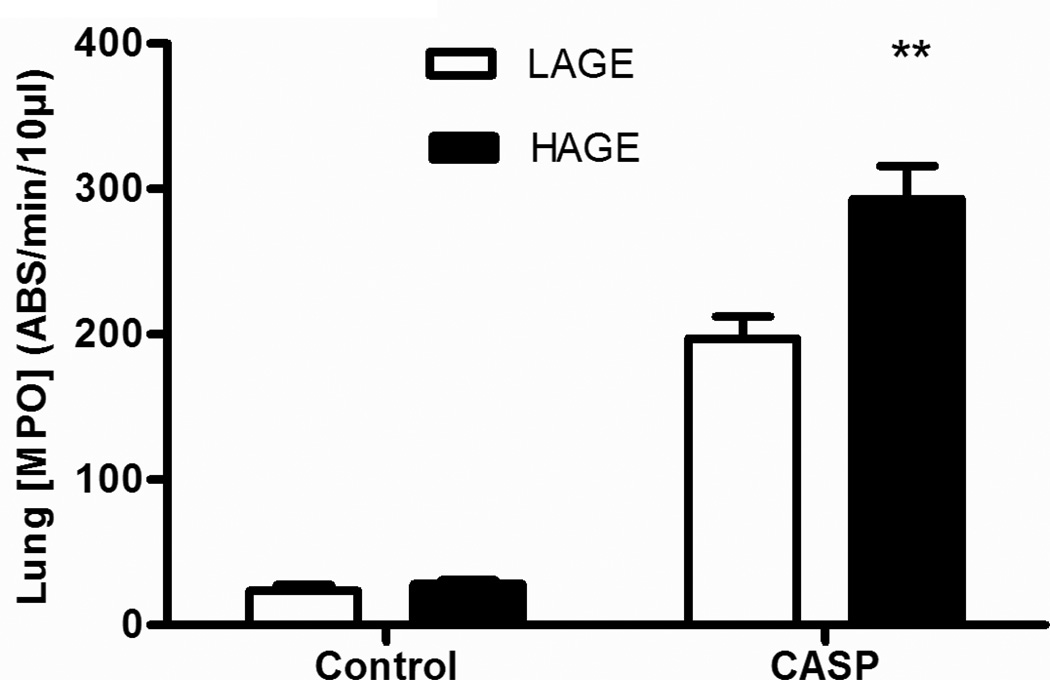
The lung MPO activity is a “footprint” of PMN pulmonary infiltration (Fig. 5) in both the airways and lung interstitium. Whole lung MPO activity in gastric aspiration-injured animals was increased compared with uninjured controls at 5 h post-injury (p<0.001). In the absence gastric aspiration-induced injury, feeding animals with HAGE diet did not increase the lung MPO activity. However, in animals injured by gastric aspiration, whole lung MPO activity was increased in the HAGE group compared with the LAGE group (287.5 ± 14.4 vs 209.7 ± 13 ABS/min/10µl, p < 0.0001). These findings are consistent with changes of BAL PMN numbers (Fig. 4a).
Fig. 5. Myeloperoxidase (MPO) activity in whole lung homogenates from mice with LAGE or HAGE diet.
Gastric aspiration increased the pulmonary MPO activity in all animals, as compared to uninjured controls (p < 0.01). This increased enzymatic activity was more pronounced in HAGE animals than the LAGE animals (p < 0.02).
Cytokine levels in cell-free BAL
Gastric aspiration elaborated distinct cytokine patterns compared with uninjured controls, as assessed in the cell-free BAL. Concentrations of the proinflammatory cytokines, IL-1β and IL-6, were increased in mice 5 h following gastric aspiration, compared with uninjured controls (Table 1). However, the composition of the animals’ diet did not demonstrate a main effect on IL-1β and IL-6 BAL levels following gastric aspiration.
Table 1.
BAL IL-1β and IL-6 concentrations 5 h after gastric aspiration in mice with LAGE or HAGE diet
| Uninjured controls | Gastric aspiration-injured | p value | |||
|---|---|---|---|---|---|
| LAGE | HAGE | LAGE | HAGE | ||
| IL-1β | 29.1±7.6 | 34.1±10.5 | 2034.1±542.5 | 1398.8±405.6 | * |
| IL-6 | 7.8±1.6 | 10.7±1.6 | 366.9±97.2 | 314.6±74.6 | * |
Data are expressed as mean ± SEM, with n = 14–15 mice in each group. Data was analyzed by two-way ANOVA.
p < 0.0001 gastric aspiration vs controls. Dietary AGEs did not affect the IL-1β or IL-6 concentration in the BAL when compared within their respective injury groups.
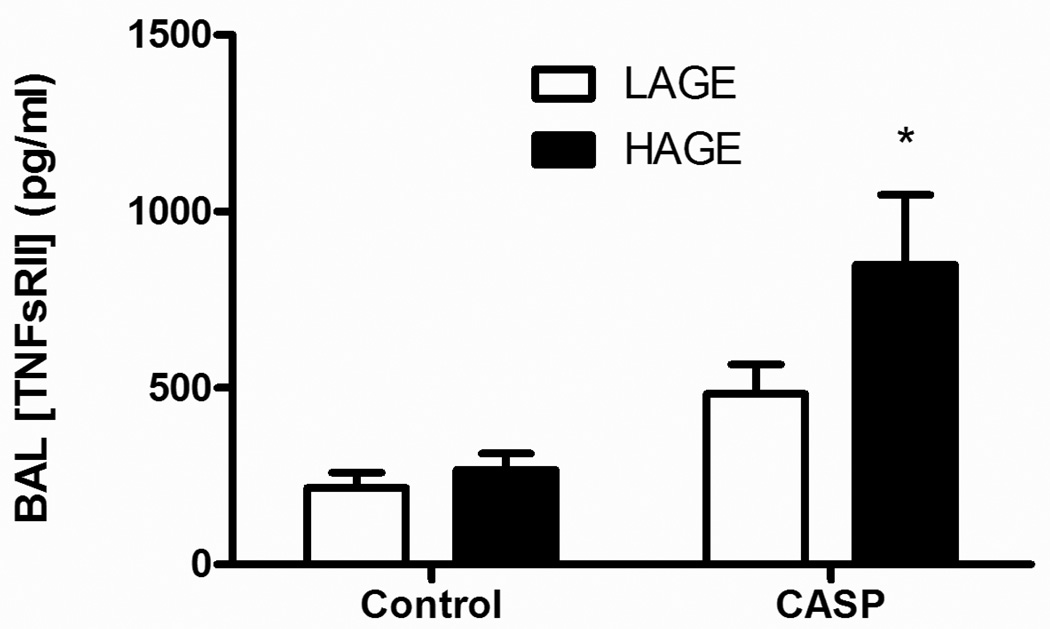
BAL TNFα concentrations were not different between uninjured controls and animals, who received a gastric aspiration insult regardless of the diet composition at 5 h post-injury (data not shown). However, the BAL TNF soluble receptor II (TNFsRII) and levels were increased in animals following gastric aspiration, as compared with uninjured controls (p < 0.0002). Additionally, the levels were greater in mice on the HAGE diet than the LAGE group, 5 h following gastric aspiration (849.4 ± 197.4 vs 483.2 ± 83.1 pg/ml, p < 0.05, Fig. 6).
Fig. 6. Levels of TNF soluble receptor II in cell-free BAL 5 h after gastric aspiration in mice with LAGE or HAGE diet.
The TNF soluble receptor II (TNFsRII) levels were higher in animals with gastric aspiration, as compared with uninjured controls (p < 0.0002). The levels were even higher in the HAGE group than in the LAGE group, 5 h after gastric aspiration (p < 0.05).
Chemokine concentrations in cell-free BAL
Elevated concentrations of chemokines in BAL (MIP-1α and MIP-2) were observed in gastric aspiration-injured animals, when compared with controls (p < 0.0001). However, diet did not affect BAL concentrations of these chemokines in uninjured controls or in gastric aspiration-injured animals.
Interestingly, BAL concentrations of concentrations of the CXC neutrophil chemoattractant, KC (Fig. 7a) and the monotactic CC chemokine, MCP-1 Fig. 7b) were lower in the HAGE-fed animals compared to the LAGE-fed (KC: p < 0.01, MCP-1: p < 0.05) following gastric aspiration.
Fig. 7. Levels of the CXC neutrophil chemoattractant, i.e., keratinocyte chemoattractant (KC), and the CC chemokine monocyte chemoattractant protein-1 (MCP-1) in cell-free BAL 5 h after gastric aspiration in mice with LAGE or HAGE diet.
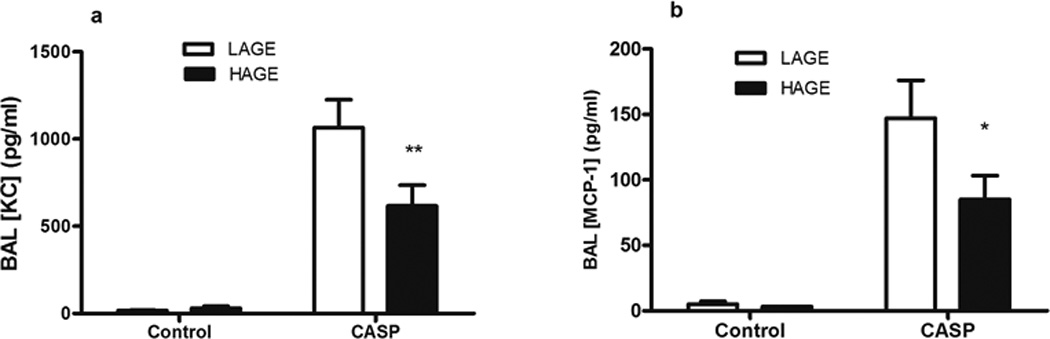
Levels of KC (Fig. 7a) and MCP-1 (Fig 7b) were elevated most prominently in mice with gastric aspiration-induced lung injury (p < 0.0001 vs uninjured controls), but their levels in the cell-free BAL were lower in HAGE animals after gastric aspiration, as compared to LAGE animals (KC: p < 0.01, MCP-1: p < 0.05).
Histopathology
Lungs were obtained from normal uninjured control mice and gastric aspiration-injured mice fed with a LAGE or HAGE diet and processed for a “blinded” histopathological examination. In normal mouse lungs, the alveolar walls are very thin and the alveoli contain only occasional alveolar macrophages, with an objective injury score of 0 (Fig. 8A). Lungs obtained from mice fed a LAGE diet and injured by gastric aspirationdemonstrated areas of alveolar disruption with significant hemorrhage, with an objective injury score of 1 (Fig. 8B), consistent with the classic picture of acute lung injury seen clinically. Gastric aspiration injury induced a more pronounced histopathological change in the lungs from HAGE-fed mice, with an objective injury score of 2 (Fig. 8C).
Fig. 8. Representative light photomicrographs of H–E stained lung sections prepared from LAGE- or HAGE-fed mice 5 h following gastric aspiration.
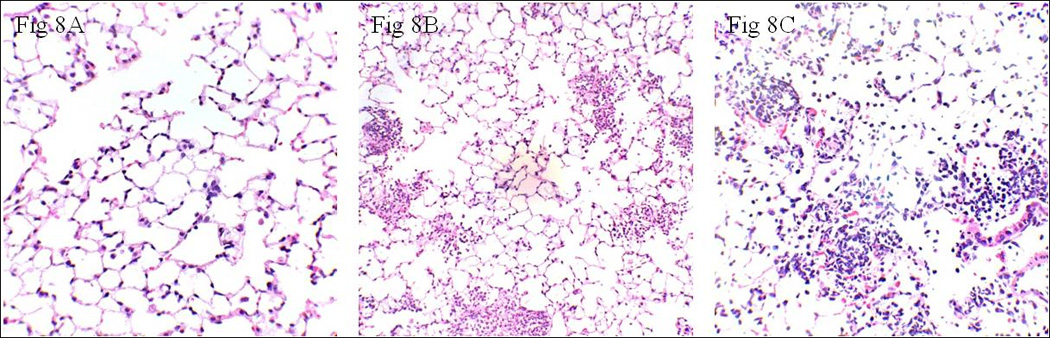
A: In animals with LAGE diet and without gastric aspiration, no significant neutrophil emigration was observed. The objective injury score was 0. B: In the gastric aspiration injury group with a low AGE diet, patchy neutrophilic infiltrates, septal congestion, and exudate formation were observed. Note that the alveolar walls are thin. The objective injury score was 1. C: These pathologic changes were more pronounced after the gastric aspiration injury in animals with a 4-week high AGE diet, with an objective injury score of 2. Note the thickened alveolar walls with intramural neutrophils. (Objective injury scoring: 0 = No neutrophils per 10 alveoli, 1 = 1–10 neutrophils per 10 alveoli, and 2 = 10–40 neutrophils per 10 alveoli).
DISCUSSION
There are two principal findings in this study. First, diet induced increased blood AGE levels are associated with a more intense pulmonary inflammatory response following gastric aspiration, as evidenced by the elevated number of PMNs in BAL and whole lung homogenate MPO enzymatic activity, as well as increased expression of TNFsRII. Secondly, the increased inflammatory response, due to diet induced high circulating AGE levels, is associated with more severe lung injury involving impaired the alveolar-capillary integrity and worsening of pulmonary mechanics. The results of these experiments support our hypothesis that dietary AGEs enhance the pulmonary inflammatory response following gastric aspiration and may be an independent risk factor for whether the aspiration insult progresses into a more severe form of acute lung injury. To our knowledge, this is the first demonstration of a potential relationship between dietary-induced increases in circulatory AGEs and severity of acute inflammatory lung injury following gastric aspiration, a common condition seen in trauma and ICU patients.
The rationale for hypothesizing that dietary AGEs are associated with the pulmonary inflammatory response and mechanical impairment after gastric aspiration was based on the recent data of AGEs in human and animal studies (3, 4, 6). AGEs are a heterogeneous group of compounds deriving from the non-enzymatic glycation and oxidation between proteins or lipids and aldose sugars. Although AGEs can be obtained by endogenous production through non-enzymatic food glucose-protein interactions, they are also formed in food during heating (i.e, cooking). The modern Western diet, with industrial processes aimed to make food safer, flavorful and colorful by heating, irradiation and ionization, contributes to the production and accumulation of AGEs in the circulation. A plethora of evidence suggests that AGEs are involved in a vicious cycle of inflammation, generation of reactive oxygen species (ROS), amplified production of AGEs, more inflammation, and so on. AGE are implicated in the mechanisms of diabetes, chronic renal failure, atherosclerosis, rheumatoid arthritis, aging process, neurodegeneration, inflammatory response, and oxidative stress (4–6, 12, 20), as well as the development of metabolic syndrome state (21, 22). AGEs are ligand to RAGE, or receptor for advanced glycation end products (23). AGE-RAGE interaction activates nuclear factor-kappa B (NF-κB). NF-κB is a critical factor transducing a variety of inflammatory and pro- or anti-apoptotic signals in the cell. In addition to the ligation of RAGE, AGEs is linked to increased generation of ROS by decreasing activities of superoxide dismutase and catalase, diminishing glutathione stores, and activation of Protein Kinase C. The direct link of inflammation to AGE formation was suggested by studies in which the activation of myeloperoxidase pathways was shown to directly generate CML-AGEs (24). A recent study by Tikellis (9) showed that a Western diet was associated with cardiac hypertrophy, inflammation, mitochondrial dependent superoxide production, and cardiac AGE accumulation.
Most of the studies have focused on various organ systems except the lung, the organ where, interestingly, the concentration of the receptors for AGEs (RAGE) is the highest in the body (25). We hypothesize that the lung is a vulnerable target for the adverse biological consequences of AGEs/RAGE ligation and activation. This would be particularly true for low pH lung injury in which a hallmark of the pathogenesis is extravasation of proteins from the circulatory system to the airways (26, 27). The preexisting elevated blood AGE levels could directly stimulate RAGE and activates NF-κB in the lung.
In our study, we demonstrated that a high AGE diet significantly increased the blood AGE concentration, consistent with the previous studies in animal models and humans which demonstrated a significant intestinal absorption of food-derived AGE and covalent deposition of AGEs in the kidney and liver (10, 28). Furthermore, our current study demonstrated for the first time the association between high blood AGE levels and augmented pulmonary inflammatory response. We have clearly demonstrated that an acute lung injury induced by gastric aspiration increased the number of PMNs in BAL, as compared with uninjured control animals. Furthermore, the PMN counts were higher after gastric aspiration in animals with high AGE diet than in the LAGE group. Neutrophils have been implicated as cellular mediators of acute lung injury characteristic of ALI/ ARDS. Clinical studies, as well as animal models of ARDS, have demonstrated sequestration of large numbers of neutrophils in the lung microvasculature and accumulation of neutrophil proteases in BAL fluid and serum (29). In addition, neutrophil activation was shown to result in endothelial cell injury in vitro and to induce lung capillary leak in vivo by elastase-dependent mechanisms (30). The quantification of neutrophils in BAL fluid reflects recruitment of neutrophils into the airspaces of the lungs. This positive correlation between the dietary AGEs and the pulmonary PMN activation in our study was supported by a recent human study by Gupta et al (31), who investigated the involvement of AGEs in PMN-mediated reactive oxygen species (ROS) generation and the associated oxygen species in type II diabetic mellitus patients. PMNs isolated from diabetic patients exhibited a higher level of respiratory burst and produced increased amounts of superoxide anion, as compared to the controls (31), due to the higher blood levels of AGEs in diabetic patients. The AGE-PMN interaction possibly upregulated NADPH oxidase, leading to enhanced ROS generation and, thus contributes to the pathogenesis acute lung injury after gastric aspiration.
Our experiment also revealed that the whole lung homogenate MPO activity, a marker for granulocyte activity and a measure of pulmonary leukostasis (32), significantly increased in our experiment 5 h after gastric aspiration. MPO is a glycoprotein expressed in all cells of the myeloid lineage, but found most abundantly in azurophilic granules of neutrophils. MPO is released by activated neutrophils and, as such, measurement of MPO in whole lung homogenates reflects accumulation of neutrophils in the lungs, providing a useful complement to the measurement of neutrophil migration from the blood to the airspaces.
Change in alveolar-capillary membrane permeability is one of the critical parameters that define the pathophysiology of ALI in humans and animals. During the genesis of ALI/ARDS, the selective barrier function of the pulmonary endothelium and/or epithelium is lost due to injury or dysfunction. This is manifested by leakage of protein-rich fluid from the vascular space into the interstitial and/or alveolar space, reflecting the accumulation of extravascular protein primarily from an increase in permeability. The albumin concentration in the BAL was significantly increased after gastric aspiration. This increase was even higher in animals fed with high AGE diet. This finding is consistent with the exaggerated pulmonary inflammatory response and a worsening of impaired pulmonary capillary permeability, potentially resulting from the pro-inflammatory effects of AGEs.
There are two groups of macrophages in the airway, i.e. resident and recruited alveolar macrophages. Normally, resident alveolar macrophages were shown to have a prolonged lifespan with minimal replacement by bone marrow-derived cells. During acute lung injury, inflammatory monocytes migrate from the circulation into the airspaces, resulting in a robust accumulation of the recruited macrophages. In our animal model of gastric aspiration with HAGE diet, the number of BAL macrophages was significantly lower than in the LAGE animals. Previous study has demonstrated that in the lungs, alveolar macrophages migrate to the bronchopulmonary lymph nodes during the acute response to bacterial infection (33). This could be a potential cause of decreased macrophage counts in BAL in the case of pulmonary inflammation induced by gastric aspiration, although different from bacterial infection. We speculate that this also could be due to the late peak of the expansion of the recruited macrophages pool after gastric aspiration in the animals with HAGE diet. This phenomenon may also explain the low KC and MCP-1 levels in the BAL after gastric aspiration in the HAGE animals, since the resident tissue macrophages are the source of the major neutrophil chemoattractants, e.g. KC, and MIP-2 (34). The exact mechanisms that underlie the lower macrophage pool remain to be elucidated.
The role of anti-inflammatory cytokines in the pathogenesis of ALI/ARDS has been the focus of intense investigation. On the basis of several experimental studies, the proinflammatory cytokine, TNFα, is an important early mediator of ALI (35). Previous data has shown that TNFα levels in the plasma, BAL, and pulmonary edema fluid have not been consistently correlated with clinical outcomes in patients at risk for or with ALI (35, 36). This result may be due, in part, to the lack of consideration of the interplay between TNFα and the surface receptor II (TNFsRII) that mediates its inflammatory effects. In response to inflammatory stimuli, including TNFα, TNFsRII are shed from the cell surface. These soluble receptors act as “decoy” receptors and bind to circulating TNFα thereby inhibiting TNF bioactivity by preventing cell surface receptor binding. Elevation of circulating levels of these soluble receptors is therefore a more sensitive indicator of the proinflammatory state than TNFα concentrations assayed during a specific window of time (37). Thus, the lack of correlation of TNFα levels with the neutrophil infiltration and elevated activity of MPO in our current study is most likely related to the increase in TNFsRII. Therefore, levels of TNFsRII themselves may be a better indication of the biological impact of TNFα. Previous findings from other laboratories have demonstrated that TNFsRII are increased in the circulation of patients with trauma (38, 39). Although these studies were limited, each suggested that there is an association between TNFsRII in the plasma and morbidity and mortality.
The lung tissue resistance is derived from part of the pulmonary pressure volume loop, and a subcomponent of the actual compliance measurement. Lung tissue resistance itself is the sum of two components - the flow-dependent frictional forces of lung tissue, and the elastic properties of the lung independent of flow resistance, with the latter component much more important than the former (16). Our current study demonstrated that a high AGE diet was associated with an increased lung tissue resistance. This mechanical impairment suggests an increased stiffness of lung due to pulmonary edema, increased lung cellularity, and/or surfactant deficiency (33). This is most likely secondary to the inflammatory change with the breakdown of the alveolar-capillary integrity and with the leakage of the albumin into the alveoli. Serum proteins also inactivate surfactant function as previously observed in our model (15, 40). Impaired lung compliance due to pulmonary edema and atelectasis is a hallmark of ALI/ARDS.
One of the limitations to this study is that we did not examine the extent or degree of pulmonary injury at the later time points following gastric aspiration. Further study with different aspiration doses is needed to investigate the dynamic change of cytokines/chemokines in BAL, as well in blood, after gastric aspiration. Systemic cytokine levels, i.e., those in the serum, could be higher in animals with high AGE diet and after acid aspiration. However, this was not the focus of our current study, which investigated the effect of high AGE diet on pulmonary (local) inflammatory response, as well as on pulmonary mechanics. Secondly, we did not assess the expression of RAGE in the lung following a high AGE diet. Ligation of AGEs with RAGE is the putative mechanism for generating the augmented acute inflammatory changes observed in these experiments. Nevertheless, the primary goal in the current study was to provide insight into the effect of high dietary AGEs on the pulmonary inflammatory response after gastric aspiration-induced pulmonary injury, findings that could potentially lead to new therapeutic strategies to be tested laboratory-based and later patient-oriented investigations.
In summary, the results of this study strongly support the hypothesis that high circulatory AGEs levels secondary to diet exacerbate acute lung injury following gastric aspiration. These findings imply an important pathogenic interaction between AGEs and the acute inflammatory response in the lung following gastric aspiration. Due to our Western fast food culture, many trauma victims may present with preexisting high blood levels of AGEs. This may help explain why some patients with gastric aspiration develop ALI/ARDS whereas others do not. Pre-injury increases in circulatory AGEs from the Western diet could render the host susceptible to elevated levels of inflammation causing inappropriate and exaggerated inflammatory responses following gastric aspiration. Thus, the present study provides a rationale for further studies of the potential role of RAGE in the lung, and a potential benefit of restricting high dietary AGE intake in attenuating lung injury following gastric aspiration in trauma. Thus, the present study provides a rationale for further studies of the potential role of RAGE in the lung, and a potential benefit of lowering blood AGE levels in attenuating lung injury following gastric aspiration in trauma. To this end, there are three possible approaches: 1. Restricting high dietary AGE intake. 2. Blocking RAGE to avoid RAGE-AGE interaction, by administration of anti-RAGE antibody. 3. Competitive binding of RAGE ligands by administration of decoy receptors, i.e., sRAGE.
Table 2.
Concentrations of chemokines, MIP-1α and MIP-2, in the cell-free BAL 5 h after gastric aspiration in mice with LAGE or HAGE diet
| Uninjured controls | Gastric aspiration-injured | p value | |||
|---|---|---|---|---|---|
| LAGE | HAGE | LAGE | HAGE | ||
| MIP-1α | 4.4±0.79 | 4.3±1.6 | 248.3±45.8 | 209.5±54.5 | * |
| MIP-2 | 54.5±25.3 | 31.9±2.1 | 676.8±66.2 | 484.2±122.0 | * |
Data are expressed as mean ± SEM, with n = 14–15 mice in each group. Data was analyzed by two-way ANOVA.
p < 0.0001 gastric aspiration vs controls. Dietary AGEs did not affect the MIP-1α or MIP-2 concentration in the BAL when compared within their respective injury groups.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the support of NIH grants, HL048889 and AI084410 (PRK, BAD). The authors also gratefully acknowledge the expertise of Drs. James Woytash and Donald Higgs in interpreting the histopathology, and the excellent editorial assistance of Ms. Elizabeth Tona.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This work was presented in part at the 5th Annual Academic Surgical Congress, February 3–5, 2010, San Antonio, TX.
REFERENCES
- 1.Marik PE. Aspiration pneumonitis and aspiration pneumonia. N Engl J Med. 2001;344(9):665–671. doi: 10.1056/NEJM200103013440908. [DOI] [PubMed] [Google Scholar]
- 2.Michelet P, Couret D, Bregeon F, Perrin G, D'Journo XB, Pequignot V, Vig V, Auffray JP. Early onset pneumonia in severe chest trauma: a risk factor analysis. J Trauma. 2010;68(2):395–400. doi: 10.1097/TA.0b013e3181a601cb. [DOI] [PubMed] [Google Scholar]
- 3.Ulrich P, Cerami A. Protein glycation, diabetes, and aging. Recent Prog Horm Res. 2001:561–521. doi: 10.1210/rp.56.1.1. [DOI] [PubMed] [Google Scholar]
- 4.Ramasamy R, Vannucci SJ, Yan SS, Herold K, Yan SF, Schmidt AM. Advanced glycation end products and RAGE: a common thread in aging, diabetes, neurodegeneration, and inflammation. Glycobiology. 2005;15(7):16R–28R. doi: 10.1093/glycob/cwi053. [DOI] [PubMed] [Google Scholar]
- 5.Peppa M, Uribarri J, Cai W, Lu M, Vlassara H. Glycoxidation and inflammation in renal failure patients. Am J Kidney Dis. 2004;43(4):690–695. doi: 10.1053/j.ajkd.2003.11.022. [DOI] [PubMed] [Google Scholar]
- 6.Van Nguyen C. Toxicity of the AGEs generated from the Maillard reaction: On the relationship of food-AGEs and biological-AGEs. Molecular nutrition & food research. 2006;50(12):1140–1149. doi: 10.1002/mnfr.200600144. [DOI] [PubMed] [Google Scholar]
- 7.Thornalley PJ. The enzymatic defence against glycation in health, disease and therapeutics: a symposium to examine the concept. Biochem Soc Trans. 2003;31(Pt 6):1341–1342. doi: 10.1042/bst0311341. [DOI] [PubMed] [Google Scholar]
- 8.Bengmark S. Impact of nutrition on ageing and disease. Current opinion in clinical nutrition and metabolic care. 2006;9(1):2–7. doi: 10.1097/01.mco.0000171129.29278.26. [DOI] [PubMed] [Google Scholar]
- 9.Tikellis C, Thomas MC, Harcourt BE, Coughlan MT, Pete J, Bialkowski K, Tan A, Bierhaus A, Cooper ME, Forbes JM. Cardiac inflammation associated with a Western diet is mediated via activation of RAGE by AGEs. Am J Physiol Endocrinol Metab. 2008;295(2):E323–E330. doi: 10.1152/ajpendo.00024.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uribarri J, Cai W, Sandu O, Peppa M, Goldberg T, Vlassara H. Diet-derived advanced glycation end products are major contributors to the body's AGE pool and induce inflammation in healthy subjects. Ann N Y Acad Sci. 2005:1043461–1043466. doi: 10.1196/annals.1333.052. [DOI] [PubMed] [Google Scholar]
- 11.Kupferminc MJ, Peaceman AM, Aderka D, Wallach D, Socol ML. Soluble tumor necrosis factor receptors and interleukin-6 levels in patients with severe preeclampsia. Obstetrics and gynecology. 1996;88(3):420–427. doi: 10.1016/0029-7844(96)00179-2. [DOI] [PubMed] [Google Scholar]
- 12.Lin RY, Choudhury RP, Cai W, Lu M, Fallon JT, Fisher EA, Vlassara H. Dietary glycotoxins promote diabetic atherosclerosis in apolipoprotein E-deficient mice. Atherosclerosis. 2003;168(2):213–220. doi: 10.1016/s0021-9150(03)00050-9. [DOI] [PubMed] [Google Scholar]
- 13.Boehm BO, Schilling S, Rosinger S, Lang GE, Lang GK, Kientsch-Engel R, Stahl P. Elevated serum levels of N(epsilon)-carboxymethyl-lysine, an advanced glycation end product, are associated with proliferative diabetic retinopathy and macular oedema. Diabetologia. 2004;47(8):1376–1379. doi: 10.1007/s00125-004-1455-y. [DOI] [PubMed] [Google Scholar]
- 14.Raghavendran K, Davidson BA, Mullan BA, Hutson AD, Russo TA, Manderscheid PA, Woytash JA, Holm BA, Notter RH, Knight PR. Acid and particulate-induced aspiration lung injury in mice: importance of MCP-1. Am J Physiol Lung Cell Mol Physiol. 2005;289(1):L134–L143. doi: 10.1152/ajplung.00390.2004. [DOI] [PubMed] [Google Scholar]
- 15.Davidson BA, Knight PR, Wang Z, Chess PR, Holm BA, Russo TA, Hutson A, Notter RH. Surfactant alterations in acute inflammatory lung injury from aspiration of acid and gastric particulates. Am J Physiol Lung Cell Mol Physiol. 2005;288(4):L699–L708. doi: 10.1152/ajplung.00229.2004. [DOI] [PubMed] [Google Scholar]
- 16.Bachofen H, Duc G. Lung tissue resistance in healthy children. Pediatric research. 1968;2(2):119–124. doi: 10.1203/00006450-196803000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Davidson BA, Knight PR, Helinski JD, Nader ND, Shanley TP, Johnson KJ. The role of tumor necrosis factor-alpha in the pathogenesis of aspiration pneumonitis in rats. Anesthesiology. 1999;91(2):486–499. doi: 10.1097/00000542-199908000-00024. [DOI] [PubMed] [Google Scholar]
- 18.Uchida T, Shirasawa M, Ware LB, Kojima K, Hata Y, Makita K, Mednick G, Matthay ZA, Matthay MA. Receptor for advanced glycation end-products is a marker of type I cell injury in acute lung injury. Am J Respir Crit Care Med. 2006;173(9):1008–1015. doi: 10.1164/rccm.200509-1477OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen MJ, Carles M, Brohi K, Calfee CS, Rahn P, Call MS, Chesebro BB, West MA, Pittet JF. Early release of soluble receptor for advanced glycation endproducts after severe trauma in humans. J Trauma. 2010;68(6):1273–1278. doi: 10.1097/TA.0b013e3181db323e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Beijnum JR, Buurman WA, Griffioen AW. Convergence and amplification of toll-like receptor (TLR) and receptor for advanced glycation end products (RAGE) signaling pathways via high mobility group B1 (HMGB1) Angiogenesis. 2008;11(1):91–99. doi: 10.1007/s10456-008-9093-5. [DOI] [PubMed] [Google Scholar]
- 21.Lander HM, Tauras JM, Ogiste JS, Hori O, Moss RA, Schmidt AM. Activation of the receptor for advanced glycation end products triggers a p21(ras)-dependent mitogen-activated protein kinase pathway regulated by oxidant stress. The Journal of biological chemistry. 1997;272(28):17810–17814. doi: 10.1074/jbc.272.28.17810. [DOI] [PubMed] [Google Scholar]
- 22.Norata GD, Garlaschelli K, Grigore L, Tibolla G, Raselli S, Redaelli L, Buccianti G, Catapano AL. Circulating soluble receptor for advanced glycation end products is inversely associated with body mass index and waist/hip ratio in the general population. Nutrition Metabolism and Cardiovascular Diseases. 2009;19(2):129–134. doi: 10.1016/j.numecd.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 23.van Zoelen MA, Yang H, Florquin S, Meijers JC, Akira S, Arnold B, Nawroth PP, Bierhaus A, Tracey KJ, van der Poll T. Role of toll-like receptors 2 and 4, and the receptor for advanced glycation end products in high-mobility group box 1-induced inflammation in vivo. Shock. 2009;31(3):280–284. doi: 10.1097/SHK.0b013e318186262d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson MM, Requena JR, Crowley JR, Thorpe SR, Heinecke JW. The myeloperoxidase system of human phagocytes generates Nepsilon-(carboxymethyl)lysine on proteins: a mechanism for producing advanced glycation end products at sites of inflammation. The Journal of clinical investigation. 1999;104(1):103–113. doi: 10.1172/JCI3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tur-Kaspa I, Maor Y, Weissenberg R, Madgar I, Aderka D, Dor J, Mashiach S, Wallach D. High levels of soluble p55-TNF receptors in seminal and prostatic fluids of normal and infertile men. The Journal of urology. 1996;155(4):1436–1438. [PubMed] [Google Scholar]
- 26.Kennedy TP, Johnson KJ, Kunkel RG, Ward PA, Knight PR, Finch JS. Acute acid aspiration lung injury in the rat: biphasic pathogenesis. Anesthesia and analgesia. 1989;69(1):87–92. [PubMed] [Google Scholar]
- 27.Grimbert FA, Parker JC, Taylor AE. Increased pulmonary vascular permeability following acid aspiration. Journal of applied physiology: respiratory, environmental and exercise physiology. 1981;51(2):335–345. doi: 10.1152/jappl.1981.51.2.335. [DOI] [PubMed] [Google Scholar]
- 28.Koschinsky T, He CJ, Mitsuhashi T, Bucala R, Liu C, Buenting C, Heitmann K, Vlassara H. Orally absorbed reactive glycation products (glycotoxins): an environmental risk factor in diabetic nephropathy. Proc Natl Acad Sci U S A. 1997;94(12):6474–6479. doi: 10.1073/pnas.94.12.6474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weiland JE, Davis WB, Holter JF, Mohammed JR, Dorinsky PM, Gadek JE. Lung neutrophils in the adult respiratory distress syndrome. Clinical and pathophysiologic significance. Am Rev Respir Dis. 1986;133(2):218–225. doi: 10.1164/arrd.1986.133.2.218. [DOI] [PubMed] [Google Scholar]
- 30.Anderson BO, Brown JM, Bensard DD, Grosso MA, Banerjee A, Patt A, Whitman GJ, Harken AH. Reversible lung neutrophil accumulation can cause lung injury by elastase-mediated mechanisms. Surgery. 1990;108(2):262–267. discussion 267-8. [PubMed] [Google Scholar]
- 31.Gupta A, Tripathi AK, Tripathi RL, Madhu SV, Banerjee BD. Advanced glycosylated end products-mediated activation of polymorphonuclear neutrophils in diabetes mellitus and associated oxidative stress. Indian journal of biochemistry & biophysics. 2007;44(5):373–378. [PubMed] [Google Scholar]
- 32.Goldblum SE, Wu KM, Jay M. Lung myeloperoxidase as a measure of pulmonary leukostasis in rabbits. Journal of applied physiology: respiratory, environmental and exercise physiology. 1985;59(6):1978–1985. doi: 10.1152/jappl.1985.59.6.1978. [DOI] [PubMed] [Google Scholar]
- 33.Sircar S. Mechanics of Pulmonary Ventilation. In: Sircar S, editor. Principles of Medical Physiolog. New York: Geroge Thieme Verlag; 2008. pp. 314–327. [Google Scholar]
- 34.De Filippo K, Henderson RB, Laschinger M, Hogg N. Neutrophil chemokines KC and macrophage-inflammatory protein-2 are newly synthesized by tissue macrophages using distinct TLR signaling pathways. J Immunol. 2008;180(6):4308–4315. doi: 10.4049/jimmunol.180.6.4308. [DOI] [PubMed] [Google Scholar]
- 35.Tracey KJ, Lowry SF, Cerami A. Cachetin/TNF-alpha in septic shock and septic adult respiratory distress syndrome. Am Rev Respir Dis. 1988;138(6):1377–1379. doi: 10.1164/ajrccm/138.6.1377. [DOI] [PubMed] [Google Scholar]
- 36.Pittet JF, Mackersie RC, Martin TR, Matthay MA. Biological markers of acute lung injury: prognostic and pathogenetic significance. Am J Respir Crit Care Med. 1997;155(4):1187–1205. doi: 10.1164/ajrccm.155.4.9105054. [DOI] [PubMed] [Google Scholar]
- 37.Aderka D. The potential biological and clinical significance of the soluble tumor necrosis factor receptors. Cytokine & growth factor reviews. 1996;7(3):231–240. doi: 10.1016/s1359-6101(96)00026-3. [DOI] [PubMed] [Google Scholar]
- 38.Hensler T, Sauerland S, Bouillon B, Raum M, Rixen D, Helling HJ, Andermahr J, Neugebauer EA. Association between injury pattern of patients with multiple injuries and circulating levels of soluble tumor necrosis factor receptors, interleukin-6 and interleukin-10, and polymorphonuclear neutrophil elastase. J Trauma. 2002;52(5):962–970. doi: 10.1097/00005373-200205000-00023. [DOI] [PubMed] [Google Scholar]
- 39.Parsons PE, Matthay MA, Ware LB, Eisner MD. Elevated plasma levels of soluble TNF receptors are associated with morbidity and mortality in patients with acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2005;288(3):L426–L431. doi: 10.1152/ajplung.00302.2004. [DOI] [PubMed] [Google Scholar]
- 40.Raghavendran K, Davidson BA, Knight PR, Wang Z, Helinski J, Chess PR, Notter RH. Surfactant dysfunction in lung contusion with and without superimposed gastric aspiration in a rat model. Shock. 2008;30(5):508–517. doi: 10.1097/SHK.0b013e3181673fc5. [DOI] [PMC free article] [PubMed] [Google Scholar]