Abstract
The neural circuitry of fear likely underlies anxiety and fear-related disorders such as specific and social phobia, panic disorder, and posttraumatic stress disorder. The primary pharmacological treatments currently utilized for these disorders include benzodiazepines, which act on the GABAergic receptor system, and antidepressants, which modulate the monamine systems. However, recent work on the regulation of fear neural circuitry suggests that specific neuropeptide modulation of this system is of critical importance. Recent reviews have examined the roles of the hypothalamic-pituitary-adrenal axis neuropeptides as well as the roles of neurotrophic factors in regulating fear. The present review, instead, will focus on three neuropeptide systems which have received less attention in recent years but which are clearly involved in regulating fear and its extinction. The endogenous opioid system, particularly activating the μ opioid receptors, has been demonstrated to regulate fear expression and extinction, possibly through functioning as an error signal within the amygdala to mark unreinforced conditioned stimuli. The cholecystokinin (CCK) system initially led to much excitement through its potential role in panic disorder. More recent work in the CCK neuropeptide pathway suggests that it may act in concordance with the endogenous cannabinoid system in the modulation of fear inhibition and extinction. Finally, older as well as very recent data suggests that neuropeptide Y (NPY) may play a very interesting role in counteracting stress effects, enhancing extinction, and enhancing resilience in fear and stress preclinical models. Future work in understanding the mechanisms of neuropeptide functioning, particularly within well-known behavioral circuits, are likely to provide fascinating new clues into the understanding of fear behavior as well as suggesting novel therapeutics for treating disorders of anxiety and fear dysregulation.
Keywords: CCK, cannabinoid, NPY, opioid, enkephalin, endorphin, nociceptin, MOR, extinction, phobia, panic, PTSD
Introduction
Anxiety and fear-related disorders are thought to involve dysregulation of the fear system. There are several aspects of the pathology of these disorders that can be modeled in the laboratory. Pre-existing sensitivity involving genetic background and environment can be analyzed using human genome-wide association studies in the human population, knockout and transgenic mice, and environmental manipulations in animal models. Fear acquisition is often modeled with a Pavlovian associative fear learning paradigm to assess freezing behavior in response to a conditioned context or cue. Fear learning can also be assayed using fear-potentiated startle, passive avoidance, and active avoidance. Because the above assays are robust, easily reproducible, and amenable to manipulation, there has been an exponential increase in data contributing to the understanding of fear acquisition. Therefore, for the purpose of this review, we will examine studies employing these assays.
Perhaps the most worthwhile aspect of fear-related disorders to model, in terms of clinical relevance, is the extinction of aversive memories. Resilient individuals likely extinguish fear memories normally, even if they are not conscious of this process. In contrast, those who are vulnerable to fear-related disorders often are unable to normally extinguish aversive memories and continue to have high levels of disruptive, even pathological fear [1]. To overcome anxiety and fear-related pathology, those with fear-related disorders require the aid of professionals in order to extinguish their fear memories – this is known as exposure therapy. Exposure therapy is modeled in the laboratory via an extinction learning paradigm, in which the aversive stimulus is presented repeatedly until inhibition of the fear response is achieved. Because of its face validity, extinction provides an excellent opportunity for bench to bedside translational research. Additionally, enhancing extinction learning or interfering with the consolidation of fear memories may also provide novel therapeutic approaches. Overall, a broader perspective on all aspects of fear will provide a better understanding of anxiety and fear-related disorders.
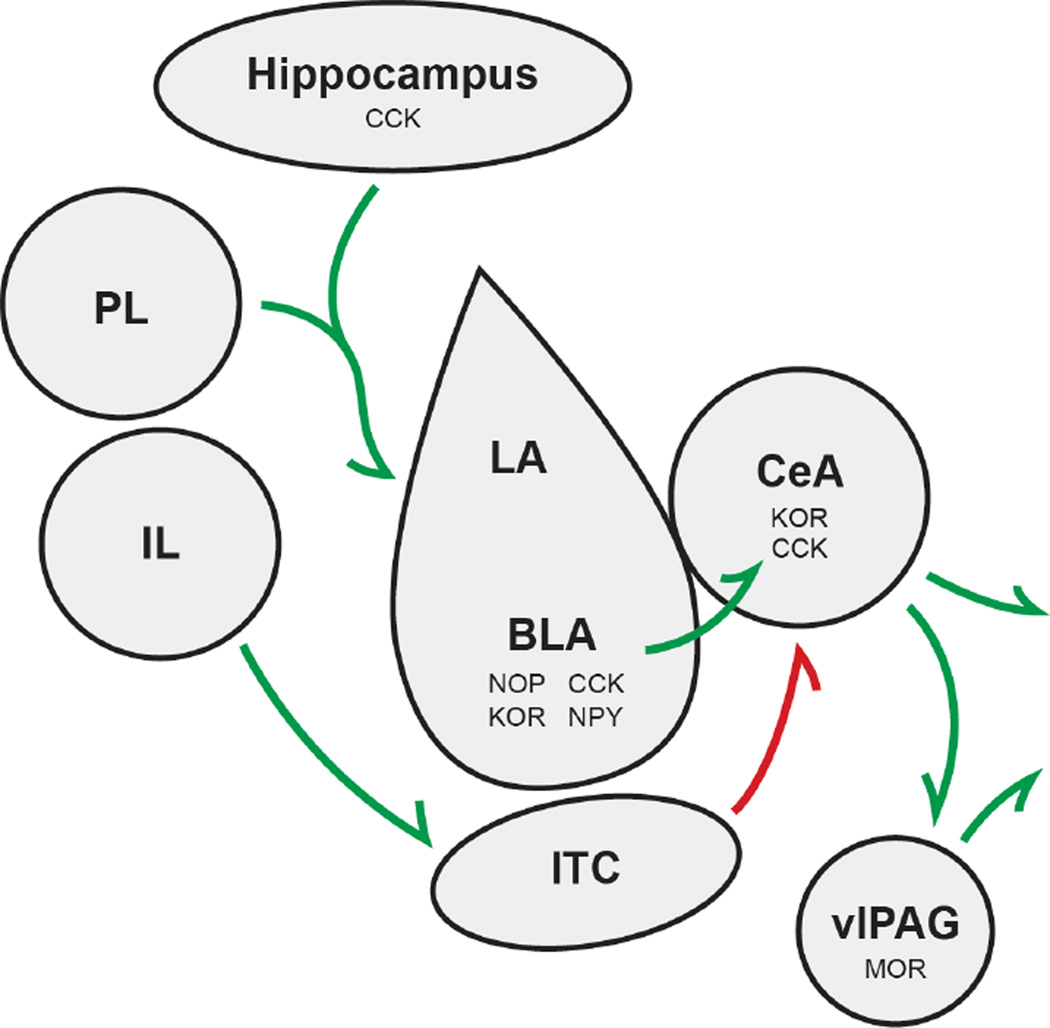
Although sensory cortex, periaqueductal gray, lateral septum, striatum, inferior colliculus, and bed nucleus of the stria terminalis (BNST) have all been implicated in fear, most research has focused on the amygdala, hippocampus, and medial prefrontal cortex (Figure 1). Human imaging studies, as well as pharmacological, lesion, and single unit recordings in animal models have pegged the amygdala as the central fear nucleus. Pathways that convey information about the conditioned (neutral) stimulus and unconditioned (aversive) stimulus are thought to converge at the lateral (LA) and basolateral amygdala (BLA) in associative / Pavlovian learning paradigms. The BLA then sends information to the central amygdala, which controls the expression of fear responses by projecting to brainstem areas. In this model, multiple pairings of the conditioned stimulus and the unconditioned stimulus induce plasticity, resulting in conditioned stimulus-elicited responses at the level of the LA and BLA. Data suggests that extinction is a not an erasure of fear memories, but rather new learning that suppresses fear memories via an inhibitory memory trace. This new learning process may proceed through multiple mechanisms [2]. For review of extinction processes, (see review [6]).
Figure 1. Schematic Diagram of Mammalian Fear Circuitry.
Prelimbic (PL) and Infralimbic (IL) regions of the medial prefrontal cortex, hippocampus, and amygdala (shown are lateral amygdala(LA), basolateral amygdala (BLA), and central amygdala (CeA) subnuclei) are all regions critical to processing fear; green arrows signify excitatory connections, red arrows represent inhibitory connections from the intercalated cell mass (ITC); some of the neuropeptides discussed here and their respective receptors have been demonstrated to act locally within specific nuclei to effect fear and anxiety behavioral output
While the BLA is critical in mediating cued fear conditioning, studies implicate the hippocampus in contextual fear conditioning [3]. It is hypothesized that the hippocampus processes information related to the environment and relays this information to the BLA to be associated with an aversive stimulus. More recent studies have shown medial prefrontal cortex (mPFC) can influence fear learning (see review [4]). The laboratory of Gregory Quirk has shown differential roles for prelimbic and infralimbic subregions of mPFC, where infralimbic activity reduces the expression of conditioned fear while prelimbic activity increases the expression of conditioned fear. The opposing influences of these subregions are thought to occur via activation of different circuits. While the prelimbic subregion sends excitatory input to BLA, the infralimbic projects to a largely GABAergic nucleus adjacent to BLA known as the intercalated mass (ITC) [5]. The ITC then sends inhibitory input to the central amygdala, inhibiting output that will control expression of fear.
While the two major neurotransmitter systems in the brain, GABA and glutamate, figure prominently in the fear system, perhaps the study of neuromodulators will yield the most successful therapeutics for the treatment of fear-related disorders. Most neuropeptides modulate the biochemistry of the cell via activation of G-protein coupled receptors. G-protein coupled receptors interact with three main subtypes of G proteins - Gs, Gq, and Gi, and less often Go. G proteins Gs and Gq are generally thought to enhance excitation, as they activate adenylyl cylase, protein kinases, and cause release of intracellular calcium stores. The G proteins Gi and Go, which often couple to the same receptor, are thought to be mainly inhibitory - they activate inwardly rectifying potassium channels and cause inhibition of adenylyl cyclase. These properties of G-protein coupled receptors make them appealing targets for drug development – they offer finer grade control of neuronal excitation and behavior. In this review, we will discuss behavioral investigations relating to the influence of neuropeptides on fear learning. We will review several of the relevant neuropeptides which have been less examined in recent years, focusing on the opioids, cholecystokinin, and neuropeptide Y. We will not review plasticity-related peptides such as brain derived neurotrophic factor (BDNF), nor corticotrophin releasing factor (CRF), as there are large literatures related to these peptide systems in fear and anxiety models, and have merited reviews of their own.
Opioids
The endogenous opioid peptides that act throughout the brain and periphery include endorphin, enkephalin, dynorphin, and endomorphin. There are three principal classes of opioid receptors – μ, κ, and δ, although up to 17 have been reported. The opioid receptors belong to the super family of G-protein coupled receptors and generally couple to heterotrimeric Gi/Go proteins, although coupling to Gs has also been reported. Activation of the opioid receptors inhibits adenylyl cyclase and voltage-gated calcium channels while stimulating inwardly rectifying potassium channels and phospholipase Cβ [7,8]. Although the opioid system is most recognized for its role in antinociception, many studies now attribute a memory-based function to the opioids as well. Here we review a large body of evidence implicating endogenous opioids, in particular the μ opioid receptor, in fear learning and extinction (Summarized in Table 1).
Table 1.
The effect of opioid manipulation on fear/anxiety models
| Authors (Year) |
Manipulation/Drug Type |
Drug Name | Route of Admin |
Species | Behavioral Paradigm |
Observation |
|---|---|---|---|---|---|---|
| Fanselow (1981) | Broad opioid receptor antagonist | naloxone | IP | Rat (female; Long-Evans) | contextual fear conditioning | Enhanced freezing |
| Fanselow et al (1988) | Broad opiod receptor antagonist; not BBB permeable | QNTX (naltrexone methobromide) | IP, ICV | Rat (female; Long-Evans) | contextual fear conditioning | Enhanced freezing with IP, not ICV infusion |
| Fanselow et al (1991) | μ,δ, and κ opiod receptor antagonists | CTOP and naloxonazine (μ), 16-methyl cyprenorphine and naltrindole (δ), nor-binaltorphimine (κ) | ICV | Rat (female; Long-Evans) | contextual fear conditioning | Enhanced freezing with μ receptor antagonists |
| Sanders et al (2005) | μ receptor gene deletion | Mouse (male; C57) | contextual fear conditioning | Slight freezing deficit | ||
| McNally and Westbrook 2003 | Broad opioid receptor antagonist | naloxone | SC | Rat (male; Wistar) | Extinction of cued fear | Impaired extinction learning |
| Zelikowsky and Fanselow (2010) | Broad opioid receptor antagonist | naltrexone | IP | Rat (male; Long-Evans) | overshadowing | Prevention of overshadowing |
| McNally et al (2004) | Broad opioid receptor antagonist | naloxone | SC | Rat (male; Wistar) | Blocking, overexpectation | Prevention of blocking and overshadowing |
| McNally et al (2004) | Broad opioid receptor antagonist | naloxone | vlPAG and dPAG infusion | Rat (male; Wistar) | Extinction of cued fear | Blockage of extinction (vlPAG) |
| McNally et al (2005) | μ,δ, and κ opiod receptor antagonists | CTAP (μ), naltrindole (δ), nor-BNI (κ) | vlPAG infusion | Rat (male; Wistar) | Extinction of cued fear | Blockade of extinction by μ receptor antagonist |
| Roozendaal et al (2007) | NOP agonist | OFQ/N | BLA infusion | Rat (male; Sprague-Dawley) | Inhibitory avoidance retention | Impairment of retention |
| Knoll et al (2007) | κ receptor antagonists | Nor-BNI, JDTic | IP | Rat (male; Sprague-Dawley) | EPM, FPS | Decreased anxiety, decreased conditioned fear |
| Knoll et al (2011) | κ receptor antagonist | JDTic | BLA or CeA infusion | Rat (male; Sprague-Dawley) | EPM, FPS | Decreased anxiety and conditioned fear with BLA and CeA infusion |
Research in the Fanselow laboratory initially demonstrated that pre-treatment with naloxone, an opiate antagonist, increased post-shock freezing levels in rats [9]. This effect was dose and shock intensity-dependent. Notably, naloxone pre-treatment did not enhance freezing to one or zero footshocks, an increase was only observed after multiple footshocks. This suggested that there is release of endorphins to an initial footshock which act as natural analgesics to reduce the aversiveness of subsequent footshocks. A follow up study attempted to determine the locus of naloxone’s effects on freezing behavior. Citing an unpublished study and observing that post-shock freezing is due to Pavlovian conditioning of fear to contextual stimuli, the authors proposed that naloxone may increase freezing by enhancing fear conditioning [10]. To test this, naloxone was administered intraperitoneally (IP) every day before testing, where each animal was placed in one context (A) for four minutes and then subsequently placed in a different context (B) for four minutes. During the first two days termed “adaptation” subjects were simply observed without administration of footshock. The following 12 days, subjects were shocked in one of the two chambers. This was followed by 8 days of extinction. Naloxone enhanced freezing in the chamber associated with footshock during the extinction phase of the experiment, but not during conditioning, when compared to freezing in the neutral chamber. In a second experiment, the authors used a reduced shock intensity and a greater context shift between chambers to examine whether the effects found in the prior experiment were due directly to context or ceiling effects. The authors found that naloxone also enhanced freezing in the conditioned context during acquisition, indicating that naloxone exerts its effects during conditioning as well as extinction [11]. Together these results were consistent with the hypothesis that endogenous opioids are released at the time of an expected fearful or painful stimulus, possibly as an endogenous protective mechanism to a learned fear response.
These initial studies were unable to distinguish between central and peripheral opioid effects on freezing, as the authors used systemic injections of drugs that readily cross the blood brain barrier (BBB). Fanselow et al used an opioid receptor antagonist, QNTX, which is not able to cross the BBB, to specifically characterize opioid effects on freezing in the periphery. Fanselow and colleagues found that intracerebroventricular (ICV), and not systemic, infusion of QNTX enhanced freezing, confirming a central effect of endogenous opioids on fear responses. To dissect which of the three opioid receptors are involved in fear acquisition, the authors administered selective antagonists during fear acquisition in a follow up study. In the first experiment, animals received ICV infusions of vehicle, a μ opioid antagonist, a δ opioid antagonist, or a κ opioid antagonist before conditioning. During conditioning, animals received three successive footshocks in the chamber after a three minute acclimation period. The following day, animals were returned to the chamber and freezing behavior was observed. Treatment with a μ opioid antagonist almost doubled freezing levels compared to vehicle administered animals, mimicking effects observed with pre-treatment of naloxone in other studies. In contrast, freezing was attenuated with administration of a κ receptor antagonist, whereas the δ opioid receptor antagonists exerted no effect on freezing levels. These data suggested that the μ opioid receptor is the primary target of endogenous opioids in reducing fear responses.
To further examine specificity, Fanselow and colleagues assessed the contribution of the μ1 receptor subtype to conditioned freezing by administering a μ1 receptor antagonist, naloxonazine, prior to training. Pre-treatment with naloxonazine caused enhancement of freezing compared to saline controls [13]. They further analyzed μ opioid receptor involvement in fear conditioning using μ opioid receptor (MOR) knockout mice. These mice show enhanced baseline sensitivity to painful stimuli in some tests, such as the tail flick assay and paw pressure test. Notably, no effect of genotype was found with contextual freezing following 5 footshocks when measured 24 hours after fear conditioning. To more sensitively measure differences in learning, the authors administered only a single footshock per day for five days. Freezing behavior pre and post-shock was analyzed each day. There was a slight freezing deficit observed in KOs, with the biggest difference occurring on day 4 and 5. This is surprising, given the pharmacological data showing enhancement of freezing with administration of a μ opioid receptor antagonist. The authors observed no effect of genotype on footshock reactivity [14]. These findings could be due to compensatory changes which may occur in the endogenous opioid system in a developmental knockout of the MOR.
While the initial fear acquisition opioid studies focused on naloxone interactions with unconditioned stimulus intensity, many studies pointed to opioid modulation of learning without the involvement of footshock. McNally and Westbrook set out to investigate the role of opioids in extinction learning based on preliminary reports that proved to be conflicting [15]. In experiment 1, the authors wanted to characterize the effects of opioid receptor antagonism on the extinction of Pavlovian fear conditioning in rats. Instead of contextual fear conditioning, the authors used cued fear conditioning, pairing auditory tone with a brief footshock. Naloxone or vehicle was administered systemically before extinction learning 24 hours after fear conditioning. Naloxone impaired extinction learning suggesting that actions at opioid receptors are critical for the extinction of Pavlovian fear conditioning. Experiment 2 was designed to address the question of peripheral versus central opioid involvement in extinction learning. Rats were fear conditioned and then 24 hours later, prior to extinction learning, they were administered vehicle, naloxone, or naloxone methiodide – a derivative of naloxone that cannot cross the blood brain barrier. Only naloxone was able to inhibit a decrease in the fear response, suggesting that central endogenous opioids are required for extinction modulation.
To make sure that opioid peptides were not involved in some sort of impairment of memory processes, the authors examined the effects of post-extinction injections of naloxone on subsequent cued freezing. Rats were fear conditioned and extinction trained as described, however drugs were administered after extinction learning. Rats were placed in one of four groups receiving either vehicle or naloxone immediately after extinction, naloxone 30 minutes after extinction, or naloxone 120 minutes after extinction. All groups showed an equivalent level of freezing 24 hours later to the conditioned stimulus, suggesting that it is extinction learning and not consolidation of extinction that is critical for opioid involvement, and that administration of naloxone is not involved in memory impairment. In the 4th experiment, the authors demonstrated that opioid receptors regulate the development but not the expression of Pavlovian fear conditioning. Naloxone or vehicle was administered before extinction learning. Naloxone blocked extinction learning as expected. Each group was then administered naloxone or vehicle 24 hours later and tested for expression of fear, yielding four groups – vehicle/vehicle, vehicle/naloxone, naloxone/vehicle, and naloxone/naloxone. Impairment of extinction was observed independently of the presence of naloxone versus vehicle on test, suggesting there is no state-dependent effect on learning. Additionally, injection of naloxone on test did not reverse any extinction. These results reflect similar findings in the Fanselow study suggesting that opioids modulate the learning process. Based on their results, McNally and Westbrook proposed that the endogenous opioids contribute to error correction. To lend support for this hypothesis, McNally and colleagues looked at the effects of naloxone on blocking and overexpectation of fear [15]. Blocking involves two stages. In the first stage, subjects undergo cued fear conditioning to a CS. In the second stage, the same subjects are presented with the CS plus a different, additional CS, as well as the US. Prior conditioning to the original CS will “block” conditioning from accruing to the new CS despite 100% reinforcement. Overexpectation also involves two stages. In the first stage, subjects are conditioned separately to two different CS. In stage two, half of the subjects receive compound presentations of both CS with the US, while the other half of subjects receive additional training to just one CS. Compound training reduces the amount of fear provoked by either CS alone on a subsequent test. McNally et al found that naloxone prevented both blocking and overexpectation [16]. From these data, they suggested that the endogenous opioids may be acting as the error signal that promotes learning during fear conditioning and extinction.
The error correction process occurs when there is a discrepancy between the predicted and actual unconditioned stimulus. When the US is not fully predicted, e.g. during fear conditioning, excitatory learning occurs. This is dependent on repeated pairings of a conditioned stimulus with an unconditioned stimulus. When the US is overpredicted, e.g. during extinction learning, the model proposes that inhibitory learning is occurring. No learning occurs when the US is accurately predicted as when the US has been paired with the CS multiple times [17]. The McNally model predicts that endogenous opioid release represents expected shock input. At the beginning of fear conditioning, the US is not fully predicted and there is no release of opioids. There is a large discrepancy between actual and expected shock and excitatory learning occurs. As CS-US pairings increase, opioids are increasingly released during the CS until the discrepancy between the actual and predicted shock is zero and no further learning occurs. During extinction, there is a large release of endogenous opioids upon presentation of the CS, without reinforcement with shock. Now the discrepancy between expected and actual shock drives inhibitory learning.
Data on the effects of naltrexone in an overshadowing paradigm support the endogenous opioid error signal hypothesis. Overshadowing is similar to blocking in that both suggest fear learning is dependent on the degree to which the US is surprising, i.e. there is a discrepancy between the actual and predicted CS which drives learning. In overshadowing, compound presentation of a light CS and a tone CS with a US reduces the degree to which the light CS can be fear conditioned [18]. Subjects trained with a tone-light compound froze less to light presentation than subjects just trained to light. The more salient CS (tone) and the US build an association rapidly and bring the discrepancy between the predicted and actual shock to zero, preventing further learning of an association between the less salient CS and US. Administration of naltrexone attenuated action of endogenous opioids and rescued responding to the light in compound trained animals, thereby preventing overshadowing [19].
Given the great amount of opioid receptors within the PAG and multiple lines of evidence suggesting PAG influence on freezing, McNally and colleagues used microinjections of an opioid receptor antagonist to determine PAG opioid contribution to extinction learning [13,21,20]. Rats received two tone shock pairings. The following five days, subjects received infusions of vehicle or naloxone into ventrolateral PAG (vlPAG) before extinction learning. Naloxone infusions significantly blocked extinction. Rats were then returned to the test chamber and presented with the CS for ten minutes on the sixth day; no differences were observed between freezing while drug-free. The authors also found no differences in freezing levels on a crossover extinction reinstatement test, indicating that naloxone did not alter expression of an already extinguished conditioned response. The authors further analyzed the effects of naloxone on expression of extinction, by administering two days of extinction training plus drug infusion into the vlPAG. There were significant differences between freezing levels in vehicle versus naloxone groups during the drug-free third day of testing. As the dorsal PAG (dPAG) has also been implicated in freezing, the authors examined the effect of microinjection of naloxone into dPAG on extinction learning. The authors did not observe any blockade of extinction; in fact, they saw an enhancement of extinction on the first day of training. There were no differences in freezing levels between groups on a third drug-free test day, indicating infusion of naloxone in dPAG did not impair development of freezing. Finally, the authors demonstrate dose-dependent impairment of extinction with naloxone infusions into the vlPAG. To dissect which opioid receptor mediates opioid-induced blockade of extinction, McNally and colleagues infused antagonists specific to μ, κ, or δ opioid receptors into the vlPAG. Fear extinction was retarded by infusion of the μ opioid receptor antagonist CTAP into vlPAG prior to extinction training. Given the evidence that activation of opioid receptors can inhibit adenylyl cyclase and decrease intracellular cAMP, the authors next studied the effects of increasing cAMP within vlPAG on extinction behavior. Extinction learning was impaired in a dose-dependent manner by infusion of the membrane permeable cAMP analog 8-Br-cAMP into the vlPAG; however there were no significant differences in extinction behavior with infusion of a PKA activator or an inhibitor of MAPKK/MEK kinase activity c ompared to vehicle [22]. In a separate study, McNally found enhancement of extinction learning with administration of RB101(s), an inhibitor of enkephalin-degrading enzymes [23].
Several human studies mirror results observed by McNally and colleagues. In a 1988 study, Kelly Egan and John Carr found that simple phobics who received intravenous injection of naloxone prior to systematic desensitization treatment did not show a reduction in symptomatology (measured by the SCL-90 Global Severity Index), nor a reduction in the number of feared items endorsed as eliciting much or very much fear (Fear Survey Schedule) [24]. Studies by Peter de Jong and Thomas Merluzzi also demonstrate blockade of extinction in spider phobics with administration of naltrexone [25].
In an effort to identify more subtypes of the classical opioid receptors, the Opioid Receptor Like 1 (ORL1) was discovered, alternatively known as the nociceptin or orphanin FQ receptor [26], which we will refer to as the NOP receptor. Although NOP shares a high degree of structural homology with the δ, μ, and κ opioid receptors, it bears no pharmacological homology with the classic opioid receptors. As the BLA expresses a high density of NOP receptors and drugs that act on NOP alter levels of norepinephrine within the BLA, Roozendaal and colleagues decided to look at the activation of NOP and its effects on step-through latency in the inhibitory avoidance retention test [27]. Immediate post-training infusion of the heptadecapeptide orphanin FQ/nociceptin (OFQ/N) into the BLA induced a dose-dependent impairment of retention. This impairment of retention was replicated when an optimal dose of OFQ/N was infused 3 hours post-training, but not 6 hours – suggesting that OFQ/N modulates consolidation of learning. Post-training infusions of the NOP receptor antagonist into the BLA enhanced retention latencies and co-administration with a beta-adrenergic receptor antagonist, atenolol, blocked this memory enhancement. Atenolol administered alone had no influence on retention latencies. This supports an earlier finding by Manabe and colleagues who showed that deletion of the NOP receptor increased step-through latencies [28]. The Roozendaal study also supports data from the Grottick group showing increased latency on step-through retention using OFQ/N peptide knockout mice [29]. These mice also exhibited enhanced fear conditioning, however the authors did not address whether this was contextual versus cued fear conditioning [29]. To get at effects of OFQ/N on fear conditioning, Fornari and colleagues administered OFQ/N peptide ICV before context and cued fear conditioning. Rats showed impaired context and cued fear conditioning with high doses of OFQ/N, but only an impairment of context conditioning with lower doses. The authors suggest the impairment of cued conditioning at higher doses could be due to non-specific effects. Interestingly, they found no effects on conditioning with post-training infusions of the peptide [30].
While studies have demonstrated the importance of amygdala NOP in fear learning, recent evidence has also proven κ opioid receptors (KOR) to be critical at the same locus. Systemic treatment with KOR antagonists attenuated fear-potentiated startle without affecting baseline startle [31]. A follow up study by the same group found that this inhibition of fear-potentiated startle is specific to basolateral and central amygdala, as determined by site-specific infusions of KOR antagonists. The same group also found increased KOR mRNA in the BLA after fear conditioning and decreased mRNA after extinction training [32].
Altogether, the large body of evidence examining the role of the opioids in fear and anxiety points to a highly critical role played by the endogenous opioid systems in a potential error signal. The model predicts that endogenous opioid release represents expected shock input and the discrepancy between actual shock input and predicted shock input drives learning. This effect has been localized to the ventrolateral PAG. As the opioid system is so divergent, including multiple isoforms of the receptor with various natural ligands at several different levels of the brain, it will be very interesting to narrow in on how the opioid system orchestrates specific functions within the fear response and fear modulation cascade.
Cholecystokinin (CCK)
Cholecystokinin (CCK) was originally isolated in the gastrointestinal system, but is found extensively throughout the nervous system, with particularly high concentrations distributed throughout the limbic system [33]. CCK is synthesized as a 115 amino acid preprohormone and is converted into multiple isoforms. The predominant form of CCK in the CNS is a sulfated octapeptide, CCK-8S, however, CCK-8 nonsulphated, CCK-5, and CCK-4 isoforms exist in lesser concentrations within the brain [34,35]. There are two CCK receptors – CCK-A and CCK-B. Their designations refer to their primary localization, “A” for alimentary and “B” for brain, although CCK-B is found in the stomach and vagus nerve and CCK-A receptor distribution in the brain is wider than originally thought [36,37]. Both receptors belong to the super family of G-protein coupled receptors, and couple to Gq. CCK-A has a high affinity for sulphated CCK-8 (CCK-8S), where CCK-B is equally selective for CCK-8S, non-sulphated CCK-8 (CCK-8N), CCK-4, and CCK-5 [38,39,40].
Initial behavioral studies showed impairment of acquisition of active avoidance with IP administration of sulphated and non-sulphated CCK-8. Both versions of the peptide were also able to enhance extinction of active avoidance [41]. In a separate study, the authors found no effect of IP injection with CCK-8S or CCK-8N on step-through passive avoidance during the first learning trial. However, when CCK was administered immediately after the first learning trial, latencies significantly increased, suggesting a role for CCK in memory consolidation. The authors were able to replicate these effects with CCK ICV infusion [42]. However, according to a review by the Belcheva group, the Fekete studies and other early reports may be slightly contradictory in their proposed roles for CCK due to their use of high doses [43]. Nevertheless, data has continuously supported the idea that CCK plays a crucial role in anxiety and fear (Summarized in Table 2). CCK-8S and CCK-8N have been shown to increase anxiety-like behavior in elevated plus maze, the marble burying test, light-dark test, and open field test. Pharmacological experiments seem to implicate the CCK-B receptor in mediating these effects (for review, see [44]).
Table 2.
Modulation of the cholecystokinin system in fear/anxiety models
| Authors (Year) |
Manipulation/Drug Type |
Drug Name | Route of Admin |
Species | Behavioral Paradigm |
Observation |
|---|---|---|---|---|---|---|
| Fekete et al (1984) | CCK receptor agonist | CCK-8S, CCK-8N | IP, ICV | Rat (male; Sprague-Dawley) | Active avoidance | Impairment of acquisition; enhancement of extinction |
| Fekete et al (1981) | CCK receptor agonist | CCK-8S, CCK-8N | IP, ICV | Rat (male; CFY) | Passive avoidance | Enhancement of retention |
| Fendt et al (1995) | CCK receptor agonist | CCK-8 | PnC infusion | Rat (male; Wistar) | ASR | Enhanced ASR |
| Josselyn et al (1995) | CCK-B antagonist | L-365,260 | IP | Rat (male; Wistar) | FPS | Attenuated FPS |
| Frankland et al (1996) | CCK-B agonist | Pentagastrin | ICV | Rat (Wistar) | ASR | Potentiation of ASR |
| Frankland et al (1997) | CCK-B agonist and CCK-B antagonist | Pentagastrin and PD135158 | ICV (pentagastrin) and intra-BLA (PD-135158) | Rat (Wistar) | ASR | Blockade of potentiation caused by pentagastrin |
| Chhatwal et al (2009) | CCK-B agonist | Pentagastrin | ICV | Rat (male; Sprague-Dawley) | Extinction of FPS | Blockade of extinction |
| Chhatwal et al (2009) | Cb1 antagonist and CCK-B antagonist | SR151716a (Cb1 antagonist) and CR2945 (CCK-B antagonist) | IP | Rat (male; Sprague-Dawley) | Extinction of FPS | CR2945 reverses blockade of extinction by SR141716a |
| Izumi et al (1996) | CCK-B antagonist | LY288513 | SC | Rat (male; Sprague-Dawley) | Conditioned fear stress | Blockade of acquisition and expression |
| Tsutsumi et al (1999) | CCK-B antagonist | PD135158 | Rat (male; Wistar) | Conditioned fear stress | Blockade of acquisition and expression | |
| Raud et al (2005) | CCK-B gene deletion | Mouse (female; C57) | Dark-light box exploration; EPM | Anxiolytic phenotype | ||
| Chen et al (2006) | Forebrain CCK-B overexpression | Mouse | OFT; conditioned fear stress | Anxiogenic phenotype; enhanced freezing |
A report by Claude de Montigny sparked a flurry of interest in CCK when it was found that intravenous (IV) injection of CCK-4 caused panic attacks in healthy subjects. Based on reports of benzodiazepine antagonism of CCK behavioral effects, de Montigny hypothesized that administration of CCK should induce anxiety in human subjects. The author selected the CCK-4 isoform based on chemical properties allowing blood brain barrier passage and maximal activation of central receptors with minimal peripheral activation. De Montigny also includes an anecdote from a personal communication with JF Rehfeld, who reported “a very unpleasant anxiety” immediately after self-administration. This panicogenic effect found by de Montigny was blocked with pre-treatment of lorazepam, but not meprobamate, or naloxone [45]. This study was followed up by Bradwejn and colleagues, who found that IV CCK-4 induced panic attacks in all subjects previously diagnosed with panic disorder. Panic disorder is a type of anxiety disorder characterized by repeated attacks of intense fear that something bad will occur when not expected. In a second controlled study, Bradwejn found that patients with panic disorder were more sensitive to the panicogenic effect of CCK-4 compared to healthy controls. Although this was not a complete dose-response study with administration of two doses, the results suggest a dose-response effect for duration and time onset until symptoms. The authors suggest that the threshold for panic attack may be lower in those with panic disorder [46]. Importantly, the authors found that pre-treatment with a CCK-B receptor antagonist, L-365,260, blocked CCK-4 induced panic attacks in a separate study [47]. Jim Abelson and Randolph Neese found a similar sensitivity in patients with panic disorder compared to healthy controls with IV administration of pentagastrin, a synthetic peptide identical to CCK-4 [48]. Positron emission tomography studies conducted on patients experiencing CCK-4 induced panic attacks show regional cerebral blood flow (rCBF) changes in anterior cingulate gyrus, the claustrum-insular-amygdala region, and cerebellar vermis [49,50]. Kennedy and Bradwejn found evidence supporting an association between panic disorder and CCK-B, suggesting that a single nucleotide polymorphism in the coding region may confer susceptibility to the disorder [51]. Recently, the Estivill group found several human microRNAs that are associated with panic disorder. Micro-RNAs are endogenous small non-coding RNAs that bind to target mRNAs, fine tuning gene expression via translational repression, degradation, and deadenylation [52]. Luciferase assays showed miR-488 and and miR-148 reduced luciferase activity of CCK-B [53].
Given the increasing amount of data attributing fear and anxiety type properties to CCK, Markus Fendt used the acoustic startle response model to further characterize CCK mechanism of action [54]. The acoustic startle response pathway is elegantly simple, with inputs from the auditory nerve sending information to the pontine reticular formation (PnC) which project to spinal cord and muscle [55]. The PnC receives inputs from the amygdala, central gray, and laterodorsal tegmental area. The authors found that infusion of CCK-8 (the authors do not specify whether they used the sulfated or non-sulfated form of the octapeptide) into PnC potentiated the acoustic startle response. They also found that CCK increased tone evoked activity in PnC neurons by about 30%. In the discussion, the authors suggest that CCK-containing projection neurons from the central amygdala or the midbrain central gray are capable of releasing CCK into the PnC, mediating excitatory effects.
In parallel with the above work, Sheena Josselyn and colleagues found that systemic L-365,260, a CCK-B antagonist, attenuated fear-potentiated startle, but did not alter baseline startle [56]. A follow up study by the same group showed that ICV administration of pentagastrin enhanced acoustic startle, without affecting locomotion [57]. They found a similar behavioral effect with intra-amygdala infusions of pentagastrin, not attributable to changes in locomotion. This potentiation was mildly attenuated with systemic pre-treatment with L-365,260. Infusion of a different CCK-B antagonist into the amygdala blocked potentiation of startle caused by systemic injection of pentagastrin [58]. These findings suggest that the potentiation of startle is mediated by CCK-B in the amygdala, however it does not rule out the contribution of CCK-B in other regions, such as PnC, as suggested by Fendt.
Our laboratory has also shown involvement of the CCK system in extinction learning, suggesting that the effect of CCK may be dependent on endocannabinoid activation. Pentagastrin administered ICV dose-dependently impaired extinction of fear-potentiated startle [59]. Previous studies have firmly established a specific role in extinction learning for the endocannabinoids. Antagonism of the cannabinoid 1 receptor (Cb1) blocks extinction of aversive memories across several different paradigms, with a groundbreaking study by the Marsicano study demonstrating that global knockout of Cb1 receptor blocks fear extinction [60]. Interestingly, the Cb1-expressing neurons within the amygdala are highly overlapping with CCK-expressing neurons [61]. Hippocampal data suggested that Cb1 activation prevents presynaptic release of CCK. On the heels of this data, Chhatwal and colleagues demonstrated that blockade of fear extinction with a systemic Cb1 antagonist was reversed with intra-amygdala infusion of a CCK-B antagonist [59]. These results suggest that the effects of endogenous cannabinoid activation in mediating extinction of fear may be through the prevention of presynaptic CCK release, which may normally serve to maintain fear responses and impair extinction.
Given the role of CCK-B in fear and acoustic startle responses, the Vaccarino group hypothesized that perhaps individual behavioral differences were associated with individual differences in the CCK system. The authors measured fear-potentiated startle responses, acoustic startle responses, and percent time spent in the open arm of an elevated plus maze. Animals were split into high and low responding groups based on mean startle response and on anxiety-like responses in the elevated plus maze. Using autoradiography, the authors found less binding of a CCK-B specific radiolabeled ligand in the BLA and CeA of high fear-potentiated startle responders. They also found less binding in the BLA, but not CeA, in high anxiety-like responders. They saw no differences in binding between low and high acoustic startle responders. Given the large body of evidence suggesting that increased CCK peptide contributes to high anxiety/fear states, the authors suggest that decreased binding of CCK-B in high responders may be due to receptor down-regulation in response to increased activity [62].
Other groups, however, have produced data that conflicts with the results of Vaccarino. Harro and colleagues separated rats into “anxious” and “non-anxious” groups according to time spent in the open arms of an elevated plus maze. They observed decreased numbers of CCK receptors in hippocampus of anxious rats compared to non-anxious rats and increased number of CCK receptors in frontal cortex of anxious rats compared to non-anxious rats [63]. When rats are socially isolated, the authors noted a decrease in their exploratory behavior, as well as an increase in CCK receptor binding in the frontal cortex, but not hippocampus [64]. Another group found increased CCK receptor binding in hippocampus in a group of “anxious” rats, as assigned by their behavior in the elevated plus maze assay [65]. These early studies do not differentiate between CCK-A and CCK-B receptor binding, and none of the binding studies so far have included correlational analyses. Additionally, baseline levels of stress may differ between studies, accounting for differences in binding levels. Nevertheless, these studies are interesting as they contribute to the prediction that dysregulation of the CCK system may play a substantial role in the pathology of fear-related and anxiety disorders.
Around this time, the Koyama group tested the effects of three non-peptide CCK receptor antagonists on rat fear behavior assayed by conditioned fear stress. Rats were individually subjected to five minutes of inescapable footshock – 2.5 mA of scrambled shock presented for 30 seconds on an interval schedule. Twenty-four hours after footshock the animals were returned to the original chamber and observed for five minutes. Aside from administering a particularly intense and lengthy footshock, conditioned fear stress is nearly identical to contextual fear conditioning. LY288513, a CCK-B antagonist, blocked acquisition of conditioned freezing when administered systemically 30 minutes prior to the footshock conditioning procedure. LY288513 also blocked expression of conditioned fear when administered 30 minutes prior to re-exposure to the conditioned context. LY288513 did not seem to alter consolidation, as administration 5 minutes after conditioning did not affect expression of freezing the following day. A CCK-A antagonist, lorglumide, had no effect on the acquisition of fear, however, it blocked expression of fear at the highest dose administered [66]. Another group found a similar effect of rats with PD135158, a different CCK-B antagonist, in the conditioned fear stress paradigm. PD135158 blocked acquisition and expression of conditioned fear but not fear consolidation [67]. In a follow-up study, this same group found differences in the conditioned fear stress paradigm following continuous administration of ICV saline, CCK-B antisense, and CCK-B sense oligonucleotides. CCK-B antisense significantly suppressed the expression of conditioned fear, without affecting motor behavior. Autoradiography showed decreased binding in rats infused with CCK-B antisense [68].
Several knockout mouse models have been used to explore the role of CCK-B in fear and anxiety. Raud and colleagues found that CCK-B receptor knockout mice have an anxiolytic phenotype as assayed by dark-light box exploration paradigm and elevated plus maze. There were no significant differences between genotypes in expression of context and or cued fear conditioning, however neither acquisition nor extinction behavior were analyzed [69]. The Tang group overexpressed CCK-B in the mouse forebrain using a tTA/tetO-inducible transgenic approach. The authors propose that CCKergic tone is dependent on receptor number and that enhanced CCKergic tone plays a role in anxiogenesis. The authors used doxycycline to inhibit transgene expression. Mutant mice (increased CCK-B density) spent less time and made fewer entries into the center of an open field chamber, but exhibited no motor deficits. Doxycycline treatment, which should ‘turn-off’ the inducible CCKB overexpression, reversed this phenotype. CCK-B overexpression also resulted in increased expression of freezing in the conditioned fear stress paradigm. This result supports prior findings that systemic treatment with CCK-B antagonists blocks expression of conditioned fear stress. Because of previous reports suggesting an antagonistic relationship between GABA and CCK, the authors repeated the open-field test and conditioned fear stress test with administration of diazepam. They found that treatment with diazepam in mutant (CCK-B overexpressing) mice reversed anxiety-like behavior measured by the open-field test. Diazepam also reversed the increase in expression of conditioned freezing observed in mutant mice [70]. A follow up study by the Tang group examined the role of CCK-B in mild versus intense contextual fear conditioning. CCK-B overexpression mutants showed impaired expression of contextual freezing with one trial of footshock compared to wild-types. There was an enhanced fear response observed in these same mice with 36 trials of footshock as compared to wild-type. In order to study whether the increased fear response following 36 trials of footshock was relevant to an anxiety-like phenotype, three groups of mutant mice were subjected to no footshock, one trial of footshock, or 36 trials of footshock and were examined by the open-field test. Together with naïve wild-type mice, they found an interaction between the transgene and extensive, but not mild, stress in the anxiogenesis observed. An elevated plus maze test revealed similar results. This study suggests that increased expression of CCK-B disables the turning point from enhancement to impairment of fear memory in response to stress. By testing six groups of wild-type mice to 1, 3, 6, 12, 24, or 36 footshocks in context and cued fear conditioning, they observed a typical inverted “U” shaped freezing curve, where there is an initial enhancement of freezing as the number of trials increases. An impairment of freezing began at 12 trials and decreased further with 24 and 36 footshocks. This “U” curve was not observed in mutant mice with CCK-B overexpression, who exhibited a linear increase in freezing behavior [71].
A large amount of research has been driven by cholecystokinin’s dramatic panic-inducing effects on humans. Numerous studies have demonstrated CCK to be anxiolytic, utilizing specific pharmacological agents to suggest that this anxiety phenotype is mediated via CCK-B. Additional studies have found that CCK-B agonists potentiate acoustic startle response and block extinction of conditioned fear. Further analysis has shown that these effects may be specific to the amygdala and dependent on cannabinoid receptors. Given new data suggesting more extensive CNS localization of CCK-A, it will be interesting to explore CCK-A’s role in anxiety and fear [37].
Neuropeptide Y
Neuropeptide Y (NPY) is a 36 amino acid peptide initially discovered as part of the pancreatic polypeptide family [72]. Immunocytochemistry and radioimmunoassay show NPY to be the most highly concentrated and widely expressed peptide in the mammalian brain [73], exceeding those of cholecystokinin (CCK) and somatostatin. In particular, NPY is notably dense in the cortical, limbic and hypothalamic regions, in particular, basal ganglia, hippocampus, hypothalamus, amygdala, nucleus accumbens, cortex, PAG, and lower brain stem [73,74,75].
With the highest levels of NPY mRNA being found in the hypothalamic arcuate nucleus [76], extensive studies have shown NPY to be critical in stimulating food intake and regulating energy stores (see review [77] [78]. Additionally, NPY is also found to target the paraventricular nucleus (PVN), where it stimulates synthesis of corticotropin-releasing factor (CRF) [79] and induces (hypothalamic-pituitary-adrenal) HPA axis stress responses. [80,81,82,83]. Additionally, literature indicates the role of NPY in circadian rhythms [84], epilepsy [85], addiction [86], reproduction [87], immune regulation [88], neuroprotection [89] and anxiety and fear [90] (Summarized in Table 3).
Table 3.
The effect of NPY manipulation on fear/anxiety models
| Authors (Year) |
Manipulation/Drug Type |
Drug Name | Route of Admin |
Species | Behavioral Paradigm |
Observation |
|---|---|---|---|---|---|---|
| Flood et al (1989) | NPY receptor agonist | NPY | Local infusion | Mouse (male; CD-1) | Footshock avoidance T-maze | Impairment of retention with amygdalar and hippocampal infusion |
| Nakajima et al (1994) | NPY receptor agonist | NPY | ICV | Mouse (male; ddY) | Step-down passive avoidance | Enhanced consolidation and retrieval |
| Broqua et al (1995) | Y1 receptor agonist | [Leu31, Pro34]-NPY | ICV | Rat (male; Sprague-Dawley and Long-Evans) | FPS | Inhibition of FPS |
| Karlsson et al (2005) | NPY receptor agonist | NPY | ICV | Mouse (male; C57Bl/6) | Cued and contextual fear conditioning | Inhibition of cued and context freezing on test |
| Gutman et al (2008) | NPY receptor agonist | NPY | BLA infusion | Rat (male; Sprague-Dawley) | FPS and ASR | Inhibition of FPS; no effect on ASR |
| Gutman et al (2008) | NPY receptor agonist | NPY | ICV | Rat (male; Sprague-Dawley) | Extinction of FPS | Enhancement of extinction of FPS |
| Gutman et al (2008) | Y1 receptor antagonist | BIBO 3304 | BLA infusion | Rat (male; Sprague-Dawley) | Extinction of FPS | Blockade of extinction of FPS |
| Pickens et al (2009) | NPY receptor agonist | NPY | ICV | Rat (male; Long-Evans) | Fear incubation | Reduced expression of incubated fear |
| Fendt et al (2009) | NPY receptor agonist | NPY | Amygdala infusion | Mouse (DBA/1J) | FPS and expression of fear conditioning | Reduced freezing and FPS on expression test |
There are six known receptors for NPY designated Y1 through Y6 [91], and their effects are mediated by G-protein-coupled downstream signaling [92]. Among these subunit variants, the Y1, Y2, Y4, and Y5 are functional subtypes located in the human brain (Holmes et al. 2003), and are activated by the three peptides in the neuropeptide Y hormone family: NPY, pancreatic polypeptide, and peptide YY [93]. NPY receptors are expressed differentially in many areas of the brain [94] and in particular, with mRNA expression of Y1, Y2, Y4, and Y5 observed in the amygdala, including the basolateral amygdala.
The expression of NPY-immunoreactive cells have been identified in the amygdala of rat [75] and humans [95,96]. mRNA expression from four functional Y-receptor subtypes (NPY Y1, Y2, Y4, and Y5) has also been observed in the amygdala, including the basolateral amygdala. In contrast, the central amygdala only expresses NPY Y1 and Y5 receptor mRNA [97,98,99]. Overall, this positions NPY as a prime candidate for the regulation of emotional and learning and memory of fear. The literature indicates NPY to have a major role in regulating anxiety. Intracerebro-ventricular (ICV) or intra-amygdala infusion of NPY leads to an anxiolytic behavioral profile in several animal models [100–106]. The anxiolytic behavioral effects of NPY seems to be mediated primarily through the Y1 receptor [91,105,107–109]. Overexpression of NPY in the amygdala attenuated behavioral responses to stress and reduced anxiety-like behavior on the elevated plus maze, while the Y1 antagonist BIBP 3226 also enhanced anxiety [110]. Additionally, Y2 and Y5 receptors have also been implicated [111,112]. Further, Sajdyk et al. found that injections of NPY into the BLA blocked the anxiogenic effects of a chemical or physical stressor, an effect that persisted for 8 weeks after a series of NPY infusions into the BLA [113]. Also, ten days of repeated daily stressors caused behavioral habituation and an upregulation of amygdala NPY expression [114] – thus NPY may act as a buffer promoting a behavioral adaptation to stress. It was found that acute restraint stress reduced anxiogenic responses on the elevated plus maze for WT but not transgenic rats overexpressing NPY [115]. Furthermore, another study examined expression of NPY during recovery from a chronic variable stress (CVS) model of repetitive trauma in rats. ELISA for NPY peptide was reduced in the amygdala 7 days after CVS, while a significant increase in prefrontal NPY was observed at the same recovery time-point [116].
Neuropeptide Y is implicated in affecting learning and memory through different processes. Following footshock avoidance training in rats, post-training injections of NPY into the amygdala and hippocampus impaired memory retention for footshock avoidance in a T-maze, whereas injection into the rostral hippocampus and septum improved retention [100]. Furthermore, third ventricular injections of NPY improved consolidation and retrieval in a step-down passive avoidance test [117]. In NPY Y2 receptor knockout mice, deficits were observed in the probe trial of the Morris Water Maze task and in an object recognition test [118].
NPY is ideally expressed and localized to modulate fear learning circuitry, as NPY colocalizes with GABA in local circuit neurons of the BLA [119] and likely exerts inhibitory control on BLA projection neurons. Additionally, the NPY Y1 receptor is robustly expressed in the BLA [99]. Throughout the BLA, Y1r-immunoreactivity was predominately found on soma with negligible fiber staining. High levels of co-expression of Y1r (99.9%) in CaMKII-immunoreactive cells were seen, suggesting that these receptors colocalize on pyramidal cells. Further, it suggests that NPY may influence BLA output by directly regulating the activity of these projection neurons. Additionally, Y1r-immunoreactivity was also colocalized with the interneuronal marker, parvalbumin. Parvalbumin interneurons participate in feed forward inhibition of BLA pyramidal cells, representing the largest number of Y1r expressing interneurons in the BLA (but only 4% of the total neuronal population). Therefore NPY could modulate the activity of the BLA via actions on both projection cells and interneuron cell populations.
One report found that ICV injections of NPY did not affect startle amplitude, however it dose-dependently inhibited fear-potentiated startle. Central administration of Y1 agonist increased time in the open arms of the EPM and inhibited FPS, while no such effects were seen with a Y2 agonist [103]. These data indicates NPY to be anxiolytic, but possibly playing important role in blunting fear responsiveness as well.
Additional mouse studies have investigated central administration of NPY, Y1, Y2 and Y5 receptor agonists and a Y1 receptor antagonist on heart rate after fear conditioning [120]. With ICV injections 15 min before cued memory recall test, NPY induced bradycardia and blunted the stress-induced tachycardic response. Additionally, Y1 receptor antagonist BIBO 3304 blocked the NPY- and Y1-receptor agonist-induced suppression of conditioned tachycardia without affecting basal HR. The tachycardia elicited by both conditioned and unconditioned stressor was effectively attenuated by the Y1 receptor agonist. These results suggest NPY mediates central inhibition of sympathetic response, through a specific contribution of Y1, but not Y2 and Y5 receptors, to modulate emotional responses. In another experiment, ICV NPY (0.5, 1.0 nmol) produced clear anxiolytic-like effects in the elevated plus-maze and light. NPY (0.5 nmol) also increased locomotor activity in the open field test. In the fear conditioning paradigm, NPY administered prior to training reduced freezing to context (0.5, 1.0 nmol) and auditory cue (1.0 nmol) [121] 24 and 48 hours later.
Work from our group found that ICV administration of NPY inhibits baseline acoustic startle and expression of fear potentiated startle (FPS) [122]. Intra-BLA infusions of NPY also inhibited FPS but did not attenuate acoustic startle, while there was no effect of NPY infused into the medial amygdala on fear responses. In contrast, expression of fear was not affected by infusions of a Y1 antagonist (BIBO 3304) into the BLA. Central NPY activation was found to enhance extinction of FPS, and extinction of contextual fear - consistent with the fear expression data. Moreover, infusion of a NPY Y1 antagonist BIBO 3304 into BLA blocks extinction of FPS following conditioned fear in rats [122].
Another report utilized conditioned fear in the passive avoidance test, and found that following fear conditioning in rats, there was increased NPY-like immunoreactivity in the amygdala, hypothalamus, nucleus accumbens, while there was decreased NPY-like immunoreactivity in the frontal cortex [123]. Moreover, diazepam and buspirone dose-dependently inhibited passive avoidance and attenuated the fear induced changes in NPY immunoreactivity. Buspirone attenuated the fear-induced changes in NPY-expression in all regions studied. In the amygdala, the effect of diazepam was dose-dependent. The effect of diazepam on both behavior and NPY-LI was antagonized by flumazenil. Apart from supporting the role of the NPY system in fear and anxiety, the results of this study suggest that NPY is involved in the anxiolytic effects of diazepam and buspirone and that the effect of diazepam is mediated by benzodiazepine receptors.
Using a model of fear incubation, (where mass fear conditioning - 100 tone-shock pairings over 10 days) it was found that both incubated and non-incubated fear responses were attenuated by central administration of NPY [124]. In contrast, D-Phe CRF(12–41), MTIP, BIBO3304, or BIIE0246 had no effect on conditioned fear at the different time points. Another report found that intra-amygdala injections of NPY decreased the expression of conditioned fear measured by conditioned freezing and fear-potentiated startle [125]. Additionally, these NPY effects were not replicated by intra-amygdala injections of the Y1R agonists Y-28 or Y-36, and co-infusion of the Y1R antagonist BIBO 3304 did not block the NPY effects. Moreover, Y1R-deficient mice were also fear conditioned and no significant differences between wild type and mutant littermates in fear expression (freezing) were found. Finally, when NPY was injected into the amygdala of Y1R-deficient mice, the local infusion of NPY had no effect on reducing fear.
Most recently, Verma and colleagues performed fear conditioning and extinction on NPY knockout mice as well as Y receptor knockout mice (Y1, Y2, Y4 and Y1/Y2 double KO) using a discriminative delay fear-conditioning paradigm. NPYKO mice acquired higher freezing levels and showed increased expression and impaired extinction of conditioned fear [126]. Y1-KO mice show faster conditioning and delayed extinction, whereas Y2-KO mice are similar to wildtype mice. In contrast, Y1/Y2 double KO mice exhibited enhanced fear acquisition and impaired between-session extinction, indicating an important role of Y2 receptors in these processes. Interestingly, Y4-KO mice showed normal fear conditioning but impaired extinction. Similarly, adeno-associated viral (AAV) vector-mediated over-expression of NPY in the BLA of NPY-KO mice normalized the increased fear acquisition of NPY-KO mice. In addition, extinction was significantly improved after AAV-induced over-expression of NPY in the BLA of NPY-KO mice [126].
Overall the literature consistently demonstrates that NPY within the BLA has an inhibitory role in fear acquisition and facilitates extinction of conditioned fear. Y1R does not appear to be involved in the mediation of the observed intra-amygdala NPY effects suggesting that these effects are mediated via other NPY receptors. However, Y1R may be more important for fear extinction circuitry in the BLA. These effects seem to be mediated predominantly in the BLA. However, the knockout studies suggest the Y1 receptor may modulate the acquisition of fear (in regions other than the amygdala), whereas extinction may involve Y1 and Y4 receptors. Future studies may further dissect in which regions of the brain NPY is likely regulating fear learning and extinction, as well as the specific NPY receptors involved.
NPY is also thought be an important factor in resilience or development of psychiatric disease states. Abnormally low levels of plasma and cerebrospinal fluid levels of NPY have been found in patients with depression and anxiety disorders [127,128]. Further data indicates that genetic variations of NPY predispose certain individuals to have low NPY levels, which can increase responsiveness to aversive stimuli in the mPFC and anterior cingulate resulting in greater risk to depression and other affective disorders [129]. These findings further for the idea that NPY may be critical to the control of normal emotional responses.
An interesting comparison study investigated resiliency during military survival training (uncontrollable stress / trauma) in terms of neuropeptide regulation [130]. They compared Special Forces soldiers versus non-Special Forces soldiers, with the hypothesis that enhanced levels of NPY will be associated with resilience against developing stress and trauma related pathology such as PTSD. Interestingly Special Forces had greater increases in plasma NPY levels following interrogation stress, while NPY levels also returned to baseline much more rapidly. In contrast, the non-Special Forces soldiers also had lower levels of NPY compared to Special Forces 24 hours after the trauma exposure. Although this is only correlational data, the higher and more prolonged NPY levels identified in the resilient Special Forces implicate NPY in an important role in controlling stress and fear responsiveness.
PTSD patients are known to have augmented sympathetic responses. Administration of yohimbine, a noradrenergic α(2)-antagonist, has been found to enhance sympathetic responses and PTSD symptoms. Another study found that PTSD patients had lower baseline plasma NPY levels and a blunted increase in NPY following yohimbine administration, compared to healthy controls [128]. Additionally, the baseline NPY levels were also negatively correlated with combat exposure scale scores and PTSD symptoms. Overall, the findings are consistent with prior data and suggest that combat stress-induced decreases in plasma NPY may mediate, in part, the noradrenergic system hyper-reactivity observed in combat-related PTSD. The persistence of this decrease in plasma NPY may contribute to symptoms of hyperarousal and the expression of exaggerated alarm reactions, anxiety reactions, or both in combat veterans with PTSD.
Consistent with these data, the Yehuda laboratory also found that high levels of NPY are found following trauma in individuals who do not go on to develop PTSD [131]. These data are consistent with the previously mentioned increases in NPY expression following fear training in animal models and further support the idea that NPY may be important for resiliency and is protective against the development of fear and trauma related pathology. Consistent evidence in the literature suggests that NPY likely promotes resilience because it blunts fear expression and/or enhances extinction of conditioned fear [122].
Discussion/Conclusion
In summary, CCK, opioids and NPY systems each have potent effects on modulating fear and anxiety circuitry in combination with effects on stress responsiveness. While NPY is anxiolytic, and within the BLA has an inhibitory role in fear acquisition and facilitates extinction of conditioned fear, the CCK system is anxiogenic and is critical in the amygdala to drive fear expression or blunt extinction. The opioid system seems to be pivotal for fear acquisition and extinction, driving learning by contributing to error correction. This does not rule out interactions between systems, but suggests unique subpopulations of neurons within the amygdala that may be more specific to on and off of fear expression and extinction. Long term changes in expression are implicated in potential differences in resilience or susceptibility to PTSD, panic attacks or other anxiety disorders. As some of the most abundantly expressed neuropeptides in the brain (CCK and NPY) this makes for attractive drug targets for future pharmacological approaches.
As mentioned, extinction of fear, modeled in the laboratory, is quite similar procedurally to real world inhibition of aversive memories via exposure therapy. Both involve repeated presentations of the fear-inducing stimulus until the fear behavior is inhibited. As exposure therapy is currently the most effective and prescribed treatment for those with fear-related disorders, learning more about extinction from a basic science perspective is of great interest. For example, D-cycloserine (DCS) as an adjunct to exposure therapy has had promising success in augmenting the treatment of phobias and social anxiety [132]. DCS, a partial agonist of the NMDA receptor, was initially found to facilitate extinction learning of conditioned fear in the laboratory [133], and then translated to extinction studies in humans [134]. In this way, studies of conditioned fear and the neuropeptides in the laboratory may be the first step in translating these indications from the bench to the clinic. The neuropeptides are particularly appealing with respect to their modulatory properties – drugs targeting the various neuropeptide systems might be expected to shift extinction learning curves without the danger of neuronal over-excitation. CCK, the opioids, and NPY have each been shown to exhibit some system dysregulation in fear-related disorders, specifically PTSD, specific phobias, and panic disorder. Given the demonstrated role these neuropeptides play in fear related disorders and the ease of bench to bedside translation, it is expected that future therapeutic strategies will likely exploit these systems.
Highlights.
This review focuses on 3 neuropeptide systems involved in fear and its extinction.
The endogenous opioid system, the CCK system, and the NPY system.
They offer new insight into fear-related disorders such as phobia, panic, and ptsd.
They may point to novel therapeutics for treating anxiety and fear disorders.
Acknowledgements
Support was provided by NIH (F32MH085443, R01DA01962), the Center for Behavioral Neuroscience (NSF agreement IBN-987675), the Burroughs Wellcome Fund, and by an NIH/NCRR base grant (P51RR000165) to Yerkes National Primate Research Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jovanovic T, Ressler KJ. How the neurocircuitry and genetics of fear inhibition may inform our understanding of PTSD. Am J Psychiatry. 2010;167:648–662. doi: 10.1176/appi.ajp.2009.09071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quirk GJ, Pare D, Richardson R, Herry C, Monfils MH, et al. Erasing fear memories with extinction training. J Neurosci. 2010;30:14993–14997. doi: 10.1523/JNEUROSCI.4268-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goosens KA. Hippocampal regulation of aversive memories. Curr Opin Neurobiol. 2011;21:460–466. doi: 10.1016/j.conb.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quirk GJ, Garcia R, Gonzalez-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry. 2006;60:337–343. doi: 10.1016/j.biopsych.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 5.Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- 6.Myers KM, Davis M. Mechanisms of fear extinction. Mol Psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- 7.Hughes J, Kosterlitz HW. Opioid Peptides: introduction. Br Med Bull. 1983;39:1–3. doi: 10.1093/oxfordjournals.bmb.a071781. [DOI] [PubMed] [Google Scholar]
- 8.Waldhoer M, Bartlett SE, Whistler JL. Opioid receptors. Annu Rev Biochem. 2004;73:953–990. doi: 10.1146/annurev.biochem.73.011303.073940. [DOI] [PubMed] [Google Scholar]
- 9.Fanselow MS, Bolles RC. Naloxone and shock-elicited freezing in the rat. J Comp Physiol Psychol. 1979;93:736–744. doi: 10.1037/h0077609. [DOI] [PubMed] [Google Scholar]
- 10.Erhman RN, Josephson J, Schull J, Sparich C. An assessment of the behavioral effects of the endorphin system with instrumental and classical conditioning paradigms. Paper presented at the meeting of the Eastern Psychological Association; New York. 1979. [Google Scholar]
- 11.Fanselow MS. Naloxone and Pavlovian fear conditioning. Learning and Motivation. 1981;12:398–419. [Google Scholar]
- 12.Hammer GD, Kapp BS. The effects of naloxone administered into the periaqueductal gray on shock-elicited freezing behavior in the rat. Behav Neural Biol. 1986;46:189–195. doi: 10.1016/s0163-1047(86)90668-0. [DOI] [PubMed] [Google Scholar]
- 13.Fanselow MS, Kim JJ, Young SL, Calcagnetti DJ, DeCola JP, et al. Differential effects of selective opioid peptide antagonists on the acquisition of pavlovian fear conditioning. Peptides. 1991;12:1033–1037. doi: 10.1016/0196-9781(91)90056-u. [DOI] [PubMed] [Google Scholar]
- 14.Sanders MJ, Kieffer BL, Fanselow MS. Deletion of the mu opioid receptor results in impaired acquisition of Pavlovian context fear. Neurobiol Learn Mem. 2005;84:33–41. doi: 10.1016/j.nlm.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 15.McNally GP, Westbrook RF. Opioid receptors regulate the extinction of Pavlovian fear conditioning. Behav Neurosci. 2003;117:1292–1301. doi: 10.1037/0735-7044.117.6.1292. [DOI] [PubMed] [Google Scholar]
- 16.McNally GP, Pigg M, Weidemann G. Blocking, unblocking, and overexpectation of fear: a role for opioid receptors in the regulation of Pavlovian association formation. Behav Neurosci. 2004;118:111–120. doi: 10.1037/0735-7044.118.1.111. [DOI] [PubMed] [Google Scholar]
- 17.Rescorla RA, Wagner AR. A Theory of Pavlovian Conditioning: Variations in the Effectiveness of Reinforcement and Nonreinforcement. New York: Appleton-Century-Crofts; 1972. [Google Scholar]
- 18.Mackintosh NJ, Reese B. One-Trial Overshadowing. Quarterly Journal of Experimental Psychology. 1979;31:519–526. [PubMed] [Google Scholar]
- 19.Zelikowsky M, Fanselow MS. Opioid regulation of Pavlovian overshadowing in fear conditioning. Behav Neurosci. 2010;124:510–519. doi: 10.1037/a0020083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McNally GP, Pigg M, Weidemann G. Opioid receptors in the midbrain periaqueductal gray regulate extinction of pavlovian fear conditioning. J Neurosci. 2004;24:6912–6919. doi: 10.1523/JNEUROSCI.1828-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carrive P. The periaqueductal gray and defensive behavior: functional representation and neuronal organization. Behav Brain Res. 1993;58:27–47. doi: 10.1016/0166-4328(93)90088-8. [DOI] [PubMed] [Google Scholar]
- 22.McNally GP, Lee BW, Chiem JY, Choi EA. The midbrain periaqueductal gray and fear extinction: opioid receptor subtype and roles of cyclic AMP, protein kinase A, and mitogen-activated protein kinase. Behav Neurosci. 2005;119:1023–1033. doi: 10.1037/0735-7044.119.4.1023. [DOI] [PubMed] [Google Scholar]
- 23.McNally GP. Facilitation of fear extinction by midbrain periaqueductal gray infusions of RB101(S), an inhibitor of enkephalin-degrading enzymes. Behav Neurosci. 2005;119:1672–1677. doi: 10.1037/0735-7044.119.6.1672. [DOI] [PubMed] [Google Scholar]
- 24.Egan KJ, Carr JE, Hunt DD, Adamson R. Endogenous opiate system and systematic desensitization. J Consult Clin Psychol. 1988;56:287–291. doi: 10.1037//0022-006x.56.2.287. [DOI] [PubMed] [Google Scholar]
- 25.Arntz A, Merckelbach H, de Jong P. Opioid antagonist affects behavioral effects of exposure in vivo. J Consult Clin Psychol. 1993;61:865–870. doi: 10.1037//0022-006x.61.5.865. [DOI] [PubMed] [Google Scholar]
- 26.Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, et al. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377:532–535. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- 27.Roozendaal B, Lengvilas R, McGaugh JL, Civelli O, Reinscheid RK. Orphanin FQ/nociceptin interacts with the basolateral amygdala noradrenergic system in memory consolidation. Learn Mem. 2007;14:29–35. doi: 10.1101/lm.403607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manabe T, Noda Y, Mamiya T, Katagiri H, Houtani T, et al. Facilitation of long-term potentiation and memory in mice lacking nociceptin receptors. Nature. 1998;394:577–581. doi: 10.1038/29073. [DOI] [PubMed] [Google Scholar]
- 29.Higgins GA, Kew JN, Richards JG, Takeshima H, Jenck F, et al. A combined pharmacological and genetic approach to investigate the role of orphanin FQ in learning and memory. Eur J Neurosci. 2002;15:911–922. doi: 10.1046/j.1460-9568.2002.01926.x. [DOI] [PubMed] [Google Scholar]
- 30.Fornari RV, Soares JC, Ferreira TL, Moreira KM, Oliveira MG. Effects of nociceptin/orphanin FQ in the acquisition of contextual and tone fear conditioning in rats. Behav Neurosci. 2008;122:98–106. doi: 10.1037/0735-7044.122.1.98. [DOI] [PubMed] [Google Scholar]
- 31.Knoll AT, Meloni EG, Thomas JB, Carroll FI, Carlezon WA., Jr Anxiolytic-like effects of kappa-opioid receptor antagonists in models of unlearned and learned fear in rats. J Pharmacol Exp Ther. 2007;323:838–845. doi: 10.1124/jpet.107.127415. [DOI] [PubMed] [Google Scholar]
- 32.Knoll AT, Muschamp JW, Sillivan SE, Ferguson D, Dietz DM, et al. Kappa opioid receptor signaling in the basolateral amygdala regulates conditioned fear and anxiety in rats. Biol Psychiatry. 2011;70:425–433. doi: 10.1016/j.biopsych.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vanderhaeghen JJ, Signeau JC, Gepts W. New peptide in the vertebrate CNS reacting with antigastrin antibodies. Nature. 1975;257:604–605. doi: 10.1038/257604a0. [DOI] [PubMed] [Google Scholar]
- 34.Rehfeld JF. Neuronal cholecystokinin: one or multiple transmitters? J Neurochem. 1985;44:1–10. doi: 10.1111/j.1471-4159.1985.tb07105.x. [DOI] [PubMed] [Google Scholar]
- 35.Derrien M, McCort-Tranchepain I, Ducos B, Roques BP, Durieux C. Heterogeneity of CCK-B receptors involved in animal models of anxiety. Pharmacol Biochem Behav. 1994;49:133–141. doi: 10.1016/0091-3057(94)90467-7. [DOI] [PubMed] [Google Scholar]
- 36.Hill DR, Campbell NJ, Shaw TM, Woodruff GN. Autoradiographic localization and biochemical characterization of peripheral type CCK receptors in rat CNS using highly selective nonpeptide CCK antagonists. J Neurosci. 1987;7:2967–2976. doi: 10.1523/JNEUROSCI.07-09-02967.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mercer LD, Beart PM. Immunolocalization of CCK1R in rat brain using a new anti-peptide antibody. Neurosci Lett. 2004;359:109–113. doi: 10.1016/j.neulet.2004.01.045. [DOI] [PubMed] [Google Scholar]
- 38.Lotti VJ, Chang RS. A new potent and selective non-peptide gastrin antagonist and brain cholecystokinin receptor (CCK-B) ligand: L-365,260. Eur J Pharmacol. 1989;162:273–280. doi: 10.1016/0014-2999(89)90290-2. [DOI] [PubMed] [Google Scholar]
- 39.Schafer U, Harhammer R, Boomgaarden M, Sohr R, Ott T, et al. Binding of cholecystokinin-8 (CCK-8) peptide derivatives to CCKA and CCKB receptors. J Neurochem. 1994;62:1426–1431. doi: 10.1046/j.1471-4159.1994.62041426.x. [DOI] [PubMed] [Google Scholar]
- 40.Fink H, Rex A, Voits M, Voigt JP. Major biological actions of CCK--a critical evaluation of research findings. Exp Brain Res. 1998;123:77–83. doi: 10.1007/s002210050546. [DOI] [PubMed] [Google Scholar]
- 41.Fekete M, Lengyel A, Hegedus B, Penke B, Zarandy M, et al. Further analysis of the effects of cholecystokinin octapeptides on avoidance behaviour in rats. Eur J Pharmacol. 1984;98:79–91. doi: 10.1016/0014-2999(84)90111-0. [DOI] [PubMed] [Google Scholar]
- 42.Kadar T, Fekete M, Telegdy G. Modulation of passive avoidance behaviour of rats by intracerebroventricular administration of cholecystokinin octapeptide sulfate ester and nonsulfated cholecystokinin octapeptide. Acta Physiol Acad Sci Hung. 1981;58:269–274. [PubMed] [Google Scholar]
- 43.Hadjiivanova C, Belcheva S, Belcheva I. Cholecystokinin and learning and memory processes. Acta Physiol Pharmacol Bulg. 2003;27:83–88. [PubMed] [Google Scholar]
- 44.Wang H, Wong PT, Spiess J, Zhu YZ. Cholecystokinin-2 (CCK2) receptor-mediated anxiety-like behaviors in rats. Neurosci Biobehav Rev. 2005;29:1361–1373. doi: 10.1016/j.neubiorev.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 45.de Montigny C. Cholecystokinin tetrapeptide induces panic-like attacks in healthy volunteers. Preliminary findings. Arch Gen Psychiatry. 1989;46:511–517. doi: 10.1001/archpsyc.1989.01810060031006. [DOI] [PubMed] [Google Scholar]
- 46.Bradwejn J, Koszycki D, Shriqui C. Enhanced sensitivity to cholecystokinin tetrapeptide in panic disorder. Clinical and behavioral findings. Arch Gen Psychiatry. 1991;48:603–610. doi: 10.1001/archpsyc.1991.01810310021005. [DOI] [PubMed] [Google Scholar]
- 47.Bradwejn J, Koszycki D, Couetoux du Tertre A, van Megen H, den Boer J, et al. The panicogenic effects of cholecystokinin-tetrapeptide are antagonized by L-365,260, a central cholecystokinin receptor antagonist, in patients with panic disorder. Arch Gen Psychiatry. 1994;51:486–493. doi: 10.1001/archpsyc.1994.03950060050005. [DOI] [PubMed] [Google Scholar]
- 48.Abelson JL, Nesse RM. Cholecystokinin-4 and panic. Arch Gen Psychiatry. 1990;47:395. doi: 10.1001/archpsyc.1990.01810160095016. [DOI] [PubMed] [Google Scholar]
- 49.Benkelfat C, Bradwejn J, Meyer E, Ellenbogen M, Milot S, et al. Functional neuroanatomy of CCK4-induced anxiety in normal healthy volunteers. Am J Psychiatry. 1995;152:1180–1184. doi: 10.1176/ajp.152.8.1180. [DOI] [PubMed] [Google Scholar]
- 50.Javanmard M, Shlik J, Kennedy SH, Vaccarino FJ, Houle S, et al. Neuroanatomic correlates of CCK-4-induced panic attacks in healthy humans: a comparison of two time points. Biol Psychiatry. 1999;45:872–882. doi: 10.1016/s0006-3223(98)00348-5. [DOI] [PubMed] [Google Scholar]
- 51.Kennedy JL, Bradwejn J, Koszycki D, King N, Crowe R, et al. Investigation of cholecystokinin system genes in panic disorder. Mol Psychiatry. 1999;4:284–285. doi: 10.1038/sj.mp.4000507. [DOI] [PubMed] [Google Scholar]
- 52.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 53.Muinos-Gimeno M, Espinosa-Parrilla Y, Guidi M, Kagerbauer B, Sipila T, et al. Human microRNAs miR-22, miR-138-2, miR-148a, and miR-488 are associated with panic disorder and regulate several anxiety candidate genes and related pathways. Biol Psychiatry. 2011;69:526–533. doi: 10.1016/j.biopsych.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 54.Fendt M, Koch M, Kungel M, Schnitzler HU. Cholecystokinin enhances the acoustic startle response in rats. Neuroreport. 1995;6:2081–2084. doi: 10.1097/00001756-199510010-00030. [DOI] [PubMed] [Google Scholar]
- 55.Davis M, Gendelman DS, Tischler MD, Gendelman PM. A primary acoustic startle circuit: lesion and stimulation studies. J Neurosci. 1982;2:791–805. doi: 10.1523/JNEUROSCI.02-06-00791.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Josselyn SA, Frankland PW, Petrisano S, Bush DE, Yeomans JS, et al. The CCKB antagonist, L-365,260, attenuates fear-potentiated startle. Peptides. 1995;16:1313–1315. doi: 10.1016/0196-9781(95)02013-m. [DOI] [PubMed] [Google Scholar]
- 57.Frankland PW, Josselyn SA, Bradwejn J, Vaccarino FJ, Yeomans JS. Intracerebroventricular infusion of the CCKB receptor agonist pentagastrin potentiates acoustic startle. Brain Res. 1996;733:129–132. doi: 10.1016/0006-8993(96)00756-1. [DOI] [PubMed] [Google Scholar]
- 58.Frankland PW, Josselyn SA, Bradwejn J, Vaccarino FJ, Yeomans JS. Activation of amygdala cholecystokininB receptors potentiates the acoustic startle response in the rat. J Neurosci. 1997;17:1838–1847. doi: 10.1523/JNEUROSCI.17-05-01838.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chhatwal JP, Gutman AR, Maguschak KA, Bowser ME, Yang Y, et al. Functional interactions between endocannabinoid and CCK neurotransmitter systems may be critical for extinction learning. Neuropsychopharmacology. 2009;34:509–521. doi: 10.1038/npp.2008.97. [DOI] [PubMed] [Google Scholar]
- 60.Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, et al. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- 61.Mascagni F, McDonald AJ. Immunohistochemical characterization of cholecystokinin containing neurons in the rat basolateral amygdala. Brain Res. 2003;976:171–184. doi: 10.1016/s0006-8993(03)02625-8. [DOI] [PubMed] [Google Scholar]
- 62.Wunderlich GR, Raymond R, DeSousa NJ, Nobrega JN, Vaccarino FJ. Decreased CCK(B) receptor binding in rat amygdala in animals demonstrating greater anxiety-like behavior. Psychopharmacology (Berl) 2002;164:193–199. doi: 10.1007/s00213-002-1181-4. [DOI] [PubMed] [Google Scholar]
- 63.Harro J, Kiivet RA, Lang A, Vasar E. Rats with anxious or non-anxious type of exploratory behaviour differ in their brain CCK-8 and benzodiazepine receptor characteristics. Behav Brain Res. 1990;39:63–71. doi: 10.1016/0166-4328(90)90121-t. [DOI] [PubMed] [Google Scholar]
- 64.Vasar E, Peuranen E, Harro J, Lang A, Oreland L, et al. Social isolation of rats increases the density of cholecystokinin receptors in the frontal cortex and abolishes the anti-exploratory effect of caerulein. Naunyn Schmiedebergs Arch Pharmacol. 1993;348:96–101. doi: 10.1007/BF00168543. [DOI] [PubMed] [Google Scholar]
- 65.Koks S, Vasar E, Soosaar A, Lang A, Volke V, et al. Relation of exploratory behavior of rats in elevated plus-maze to brain receptor binding properties and serum growth hormone levels. Eur Neuropsychopharmacol. 1997;7:289–294. doi: 10.1016/s0924-977x(97)00034-5. [DOI] [PubMed] [Google Scholar]
- 66.Izumi T, Inoue T, Tsuchiya K, Hashimoto S, Ohmori T, et al. Effect of the selective CCKB receptor antagonist LY288513 on conditioned fear stress in rats. Eur J Pharmacol. 1996;300:25–31. doi: 10.1016/0014-2999(95)00859-4. [DOI] [PubMed] [Google Scholar]
- 67.Tsutsumi T, Akiyoshi J, Isogawa K, Kohno Y, Hikichi T, et al. Suppression of conditioned fear by administration of CCKB receptor antagonist PD135158. Neuropeptides. 1999;33:483–486. doi: 10.1054/npep.1999.0766. [DOI] [PubMed] [Google Scholar]
- 68.Tsutsumi T, Akiyoshi J, Hikichi T, Kiyota A, Kohno Y, et al. Suppression of conditioned fear by administration of CCKB receptor antisense oligodeoxynucleotide into the lateral ventricle. Pharmacopsychiatry. 2001;34:232–237. doi: 10.1055/s-2001-18030. [DOI] [PubMed] [Google Scholar]
- 69.Raud S, Innos J, Abramov U, Reimets A, Koks S, et al. Targeted invalidation of CCK2 receptor gene induces anxiolytic-like action in light-dark exploration, but not in fear conditioning test. Psychopharmacology (Berl) 2005;181:347–357. doi: 10.1007/s00213-005-2255-x. [DOI] [PubMed] [Google Scholar]
- 70.Chen Q, Nakajima A, Meacham C, Tang YP. Elevated cholecystokininergic tone constitutes an important molecular/neuronal mechanism for the expression of anxiety in the mouse. Proc Natl Acad Sci U S A. 2006;103:3881–3886. doi: 10.1073/pnas.0505407103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen Q, Tang M, Mamiya T, Im HI, Xiong X, et al. Bi-directional effect of cholecystokinin receptor-2 overexpression on stress-triggered fear memory and anxiety in the mouse. PLoS One. 2010;5 doi: 10.1371/journal.pone.0015999. e15999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tatemoto K, Carlquist M, Mutt V. Neuropeptide Y--a novel brain peptide with structural similarities to peptide YY and pancreatic polypeptide. Nature. 1982;296:659–660. doi: 10.1038/296659a0. [DOI] [PubMed] [Google Scholar]
- 73.Allen YS, Adrian TE, Allen JM, Tatemoto K, Crow TJ, et al. Neuropeptide Y distribution in the rat brain. Science. 1983;221:877–879. doi: 10.1126/science.6136091. [DOI] [PubMed] [Google Scholar]
- 74.Adrian TE, Allen JM, Bloom SR, Ghatei MA, Rossor MN, et al. Neuropeptide Y distribution in human brain. Nature. 1983;306:584–586. doi: 10.1038/306584a0. [DOI] [PubMed] [Google Scholar]
- 75.Chronwall BM, DiMaggio DA, Massari VJ, Pickel VM, Ruggiero DA, et al. The anatomy of neuropeptide-Y-containing neurons in rat brain. Neuroscience. 1985;15:1159–1181. doi: 10.1016/0306-4522(85)90260-x. [DOI] [PubMed] [Google Scholar]
- 76.Morris BJ. Neuronal localisation of neuropeptide Y gene expression in rat brain. J Comp Neurol. 1989;290:358–368. doi: 10.1002/cne.902900305. [DOI] [PubMed] [Google Scholar]
- 77.Beck B. Neuropeptide Y in normal eating and in genetic and dietary-induced obesity. Philos Trans R Soc Lond B Biol Sci. 2006;361:1159–1185. doi: 10.1098/rstb.2006.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kuo LE, Kitlinska JB, Tilan JU, Li L, Baker SB, et al. Neuropeptide Y acts directly in the periphery on fat tissue and mediates stress-induced obesity and metabolic syndrome. Nat Med. 2007;13:803–811. doi: 10.1038/nm1611. [DOI] [PubMed] [Google Scholar]
- 79.Haas DA, George SR. Neuropeptide Y-induced effects on hypothalamic corticotropin-releasing factor content and release are dependent on noradrenergic/adrenergic neurotransmission. Brain Res. 1989;498:333–338. doi: 10.1016/0006-8993(89)91112-8. [DOI] [PubMed] [Google Scholar]
- 80.Hanson ES, Dallman MF. Neuropeptide Y (NPY) may integrate responses of hypothalamic feeding systems and the hypothalamo-pituitary-adrenal axis. J Neuroendocrinol. 1995;7:273–279. doi: 10.1111/j.1365-2826.1995.tb00757.x. [DOI] [PubMed] [Google Scholar]
- 81.White BD, Dean RG, Edwards GL, Martin RJ. Type II corticosteroid receptor stimulation increases NPY gene expression in basomedial hypothalamus of rats. Am J Physiol. 1994;266:R1523–R1529. doi: 10.1152/ajpregu.1994.266.5.R1523. [DOI] [PubMed] [Google Scholar]
- 82.King PJ, Widdowson PS, Doods HN, Williams G. Regulation of neuropeptide Y release by neuropeptide Y receptor ligands and calcium channel antagonists in hypothalamic slices. J Neurochem. 1999;73:641–646. doi: 10.1046/j.1471-4159.1999.0730641.x. [DOI] [PubMed] [Google Scholar]
- 83.Pomonis JD, Levine AS, Billington CJ. Interaction of the hypothalamic paraventricular nucleus and central nucleus of the amygdala in naloxone blockade of neuropeptide Y-induced feeding revealed by c-fos expression. J Neurosci. 1997;17:5175–5182. doi: 10.1523/JNEUROSCI.17-13-05175.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yannielli PC, Harrington ME. Neuropeptide Y in the mammalian circadian system: effects on light-induced circadian responses. Peptides. 2001;22:547–556. doi: 10.1016/s0196-9781(01)00356-4. [DOI] [PubMed] [Google Scholar]
- 85.Baraban SC. Neuropeptide Y and epilepsy: recent progress, prospects and controversies. Neuropeptides. 2004;38:261–265. doi: 10.1016/j.npep.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 86.Thiele TE, Sparta DR, Hayes DM, Fee JR. A role for neuropeptide Y in neurobiological responses to ethanol and drugs of abuse. Neuropeptides. 2004;38:235–243. doi: 10.1016/j.npep.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 87.Kalra SP, Kalra PS. NPY--an endearing journey in search of a neurochemical on/off switch for appetite, sex and reproduction. Peptides. 2004;25:465–471. doi: 10.1016/j.peptides.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 88.Groneberg DA, Folkerts G, Peiser C, Chung KF, Fischer A. Neuropeptide Y (NPY) Pulm Pharmacol Ther. 2004;17:173–180. doi: 10.1016/j.pupt.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 89.Silva AP, Xapelli S, Grouzmann E, Cavadas C. The putative neuroprotective role of neuropeptide Y in the central nervous system. Curr Drug Targets CNS Neurol Disord. 2005;4:331–347. doi: 10.2174/1568007054546153. [DOI] [PubMed] [Google Scholar]
- 90.Heilig M. The NPY system in stress, anxiety and depression. Neuropeptides. 2004;38:213–224. doi: 10.1016/j.npep.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 91.Wahlestedt C, Reis DJ. Neuropeptide Y-related peptides and their receptors--are the receptors potential therapeutic drug targets? Annu Rev Pharmacol Toxicol. 1993;33:309–352. doi: 10.1146/annurev.pa.33.040193.001521. [DOI] [PubMed] [Google Scholar]
- 92.Michel MC, Lewejohann K, Farke W, Bischoff A, Feth F, et al. Regulation of NPY/NPY Y1 receptor/G protein system in rat brain cortex. Am J Physiol. 1995;268:R192–R200. doi: 10.1152/ajpregu.1995.268.1.R192. [DOI] [PubMed] [Google Scholar]
- 93.Lindner D, Stichel J, Beck-Sickinger AG. Molecular recognition of the NPY hormone family by their receptors. Nutrition. 2008;24:907–917. doi: 10.1016/j.nut.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 94.Dumont Y, Fournier A, St-Pierre S, Quirion R. Comparative characterization and autoradiographic distribution of neuropeptide Y receptor subtypes in the rat brain. J Neurosci. 1993;13:73–86. doi: 10.1523/JNEUROSCI.13-01-00073.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Walter A, Mai JK, Jimenez-Hartel W. Mapping of neuropeptide Y-like immunoreactivity in the human forebrain. Brain Res Bull. 1990;24:297–311. doi: 10.1016/0361-9230(90)90084-d. [DOI] [PubMed] [Google Scholar]
- 96.Caberlotto L, Fuxe K, Hurd YL. Characterization of NPY mRNA-expressing cells in the human brain: co-localization with Y2 but not Y1 mRNA in the cerebral cortex, hippocampus, amygdala, and striatum. J Chem Neuroanat. 2000;20:327–337. doi: 10.1016/s0891-0618(00)00107-1. [DOI] [PubMed] [Google Scholar]
- 97.Parker RM, Herzog H. Regional distribution of Y-receptor subtype mRNAs in rat brain. Eur J Neurosci. 1999;11:1431–1448. doi: 10.1046/j.1460-9568.1999.00553.x. [DOI] [PubMed] [Google Scholar]
- 98.Wolak ML, DeJoseph MR, Cator AD, Mokashi AS, Brownfield MS, et al. Comparative distribution of neuropeptide Y Y1 and Y5 receptors in the rat brain by using immunohistochemistry. J Comp Neurol. 2003;464:285–311. doi: 10.1002/cne.10823. [DOI] [PubMed] [Google Scholar]
- 99.Rostkowski AB, Teppen TL, Peterson DA, Urban JH. Cell-specific expression of neuropeptide Y Y1 receptor immunoreactivity in the rat basolateral amygdala. J Comp Neurol. 2009;517:166–176. doi: 10.1002/cne.22143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Flood JF, Baker ML, Hernandez EN, Morley JE. Modulation of memory processing by neuropeptide Y varies with brain injection site. Brain Res. 1989;503:73–82. doi: 10.1016/0006-8993(89)91706-x. [DOI] [PubMed] [Google Scholar]
- 101.Heilig M, McLeod S, Koob GK, Britton KT. Anxiolytic-like effect of neuropeptide Y (NPY), but not other peptides in an operant conflict test. Regul Pept. 1992;41:61–69. doi: 10.1016/0167-0115(92)90514-u. [DOI] [PubMed] [Google Scholar]
- 102.Heilig M. Antisense inhibition of neuropeptide Y (NPY)-Y1 receptor expression blocks the anxiolytic-like action of NPY in amygdala and paradoxically increases feeding. Regul Pept. 1995;59:201–205. doi: 10.1016/0167-0115(95)00103-i. [DOI] [PubMed] [Google Scholar]
- 103.Broqua P, Wettstein JG, Rocher MN, Gauthier-Martin B, Junien JL. Behavioral effects of neuropeptide Y receptor agonists in the elevated plus-maze and fear-potentiated startle procedures. Behav Pharmacol. 1995;6:215–222. [PubMed] [Google Scholar]
- 104.Britton KT, Southerland S, Van Uden E, Kirby D, Rivier J, et al. Anxiolytic activity of NPY receptor agonists in the conflict test. Psychopharmacology (Berl) 1997;132:6–13. doi: 10.1007/s002130050313. [DOI] [PubMed] [Google Scholar]
- 105.Sajdyk TJ, Vandergriff MG, Gehlert DR. Amygdalar neuropeptide Y Y1 receptors mediate the anxiolytic-like actions of neuropeptide Y in the social interaction test. Eur J Pharmacol. 1999;368:143–147. doi: 10.1016/s0014-2999(99)00018-7. [DOI] [PubMed] [Google Scholar]
- 106.Kokare DM, Dandekar MP, Chopde CT, Subhedar N. Interaction between neuropeptide Y and alpha-melanocyte stimulating hormone in amygdala regulates anxiety in rats. Brain Res. 2005;1043:107–114. doi: 10.1016/j.brainres.2005.02.038. [DOI] [PubMed] [Google Scholar]
- 107.Heilig M, Soderpalm B, Engel JA, Widerlov E. Centrally administered neuropeptide Y (NPY) produces anxiolytic-like effects in animal anxiety models. Psychopharmacology (Berl) 1989;98:524–529. doi: 10.1007/BF00441953. [DOI] [PubMed] [Google Scholar]
- 108.Kask A, Kivastik T, Rago L, Harro J. Neuropeptide Y Y1 receptor antagonist BIBP3226 produces conditioned place aversion in rats. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23:705–711. doi: 10.1016/s0278-5846(99)00029-9. [DOI] [PubMed] [Google Scholar]
- 109.Wieland HA, Willim KD, Entzeroth M, Wienen W, Rudolf K, et al. Subtype selectivity and antagonistic profile of the nonpeptide Y1 receptor antagonist BIBP 3226. J Pharmacol Exp Ther. 1995;275:143–149. [PubMed] [Google Scholar]
- 110.Primeaux SD, Wilson SP, Cusick MC, York DA, Wilson MA. Effects of altered amygdalar neuropeptide Y expression on anxiety-related behaviors. Neuropsychopharmacology. 2005;30:1589–1597. doi: 10.1038/sj.npp.1300705. [DOI] [PubMed] [Google Scholar]
- 111.Sajdyk TJ, Schober DA, Gehlert DR. Neuropeptide Y receptor subtypes in the basolateral nucleus of the amygdala modulate anxiogenic responses in rats. Neuropharmacology. 2002;43:1165–1172. doi: 10.1016/s0028-3908(02)00234-4. [DOI] [PubMed] [Google Scholar]
- 112.Sajdyk TJ, Schober DA, Smiley DL, Gehlert DR. Neuropeptide Y-Y2 receptors mediate anxiety in the amygdala. Pharmacol Biochem Behav. 2002;71:419–423. doi: 10.1016/s0091-3057(01)00679-7. [DOI] [PubMed] [Google Scholar]
- 113.Sajdyk TJ, Johnson PL, Leitermann RJ, Fitz SD, Dietrich A, et al. Neuropeptide Y in the amygdala induces long-term resilience to stress-induced reductions in social responses but not hypothalamic-adrenal-pituitary axis activity or hyperthermia. J Neurosci. 2008;28:893–903. doi: 10.1523/JNEUROSCI.0659-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Thorsell A, Carlsson K, Ekman R, Heilig M. Behavioral and endocrine adaptation, and up-regulation of NPY expression in rat amygdala following repeated restraint stress. Neuroreport. 1999;10:3003–3007. doi: 10.1097/00001756-199909290-00024. [DOI] [PubMed] [Google Scholar]
- 115.Thorsell A, Michalkiewicz M, Dumont Y, Quirion R, Caberlotto L, et al. Behavioral insensitivity to restraint stress, absent fear suppression of behavior and impaired spatial learning in transgenic rats with hippocampal neuropeptide Y overexpression. Proc Natl Acad Sci U S A. 2000;97:12852–12857. doi: 10.1073/pnas.220232997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.McGuire JL, Larke LE, Sallee FR, Herman JP, Sah R. Differential Regulation of Neuropeptide Y in the Amygdala and Prefrontal Cortex during Recovery from Chronic Variable Stress. Front Behav Neurosci. 2011;5:54. doi: 10.3389/fnbeh.2011.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nakajima M, Inui A, Teranishi A, Miura M, Hirosue Y, et al. Effects of pancreatic polypeptide family peptides on feeding and learning behavior in mice. J Pharmacol Exp Ther. 1994;268:1010–1014. [PubMed] [Google Scholar]
- 118.Redrobe JP, Dumont Y, Herzog H, Quirion R. Characterization of neuropeptide Y,Y(2) receptor knockout mice in two animal models of learning and memory processing. J Mol Neurosci. 2004;22:159–166. doi: 10.1385/JMN:22:3:159. [DOI] [PubMed] [Google Scholar]
- 119.McDonald AJ, Pearson JC. Coexistence of GABA and peptide immunoreactivity in non-pyramidal neurons of the basolateral amygdala. Neurosci Lett. 1989;100:53–58. doi: 10.1016/0304-3940(89)90659-9. [DOI] [PubMed] [Google Scholar]
- 120.Tovote P, Meyer M, Beck-Sickinger AG, von Horsten S, Ove Ogren S, et al. Central NPY receptor-mediated alteration of heart rate dynamics in mice during expression of fear conditioned to an auditory cue. Regul Pept. 2004;120:205–214. doi: 10.1016/j.regpep.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 121.Karlsson RM, Holmes A, Heilig M, Crawley JN. Anxiolytic-like actions of centrally-administered neuropeptide Y,but not galanin, in C57BL/6J mice. Pharmacol Biochem Behav. 2005;80:427–436. doi: 10.1016/j.pbb.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 122.Gutman AR, Yang Y, Ressler KJ, Davis M. The role of neuropeptide Y in the expression and extinction of fear-potentiated startle. J Neurosci. 2008;28:12682–12690. doi: 10.1523/JNEUROSCI.2305-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Krysiak R, Obuchowicz E, Herman ZS. Conditioned fear-induced changes in neuropeptide Y-like immunoreactivity in rats: the effect of diazepam and buspirone. Neuropeptides. 2000;34:148–157. doi: 10.1054/npep.2000.0804. [DOI] [PubMed] [Google Scholar]
- 124.Pickens CL, Adams-Deutsch T, Nair SG, Navarre BM, Heilig M, et al. Effect of pharmacological manipulations of neuropeptide Y and corticotropin-releasing factor neurotransmission on incubation of conditioned fear. Neuroscience. 2009;164:1398–1406. doi: 10.1016/j.neuroscience.2009.09.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fendt M, Burki H, Imobersteg S, Lingenhohl K, McAllister KH, et al. Fear-reducing effects of intra-amygdala neuropeptide Y infusion in animal models of conditioned fear: an NPY Y1 receptor independent effect. Psychopharmacology (Berl) 2009;206:291–301. doi: 10.1007/s00213-009-1610-8. [DOI] [PubMed] [Google Scholar]
- 126.Verma D, Tasan RO, Mietzsch M, Weger S, Heilbronn R, Herzog H, Sperk G. Reduced fear conditioning after viral vector mediated neuropeptide Y administration into the basolateral amygdala. BMC Pharmacology. 2011;11 [Google Scholar]
- 127.Heilig M, Zachrisson O, Thorsell A, Ehnvall A, Mottagui-Tabar S, et al. Decreased cerebrospinal fluid neuropeptide Y (NPY) in patients with treatment refractory unipolar major depression: preliminary evidence for association with preproNPY gene polymorphism. J Psychiatr Res. 2004;38:113–121. doi: 10.1016/s0022-3956(03)00101-8. [DOI] [PubMed] [Google Scholar]
- 128.Rasmusson AM, Hauger RL, Morgan CA, Bremner JD, Charney DS, et al. Low baseline and yohimbine-stimulated plasma neuropeptide Y (NPY) levels in combat-related PTSD. Biol Psychiatry. 2000;47:526–539. doi: 10.1016/s0006-3223(99)00185-7. [DOI] [PubMed] [Google Scholar]
- 129.Mickey BJ, Zhou Z, Heitzeg MM, Heinz E, Hodgkinson CA, et al. Emotion processing, major depression, and functional genetic variation of neuropeptide Y. Arch Gen Psychiatry. 2011;68:158–166. doi: 10.1001/archgenpsychiatry.2010.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Morgan CA, 3rd, Wang S, Southwick SM, Rasmusson A, Hazlett G, et al. Plasma neuropeptide-Y concentrations in humans exposed to military survival training. Biol Psychiatry. 2000;47:902–909. doi: 10.1016/s0006-3223(99)00239-5. [DOI] [PubMed] [Google Scholar]
- 131.Yehuda R, Brand S, Yang RK. Plasma neuropeptide Y concentrations in combat exposed veterans: relationship to trauma exposure, recovery from PTSD, and coping. Biol Psychiatry. 2006;59:660–663. doi: 10.1016/j.biopsych.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 132.Norberg MM, Krystal JH, Tolin DF. A meta-analysis of D-cycloserine and the facilitation of fear extinction and exposure therapy. Biol Psychiatry. 2008;63:1118–1126. doi: 10.1016/j.biopsych.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 133.Walker DL, Ressler KJ, Lu KT, Davis M. Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of D-cycloserine as assessed with fear-potentiated startle in rats. J Neurosci. 2002;22:2343–2351. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, et al. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry. 2004;61:1136–1144. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]