Abstract
Purpose of review
Mineral and bone disorders (MBD), inherent complications of moderate and advanced chronic kidney disease (CKD), occur frequently in kidney transplant recipients. However, much confusion exists about clinical application of diagnostic tools and preventive or treatment strategies to correct bone loss or mineral disarrays in transplanted patients. We have reviewed the recent evidence about prevalence and consequences of MBD in kidney transplant recipients and examined diagnostic, preventive and therapeutic options to this end.
Recent findings
Low turnover bone disease occurs more frequently after kidney transplantation according to bone biopsy studies. The risk of fracture is high, especially in the first several months after kidney transplantation. Alterations in minerals (calcium, phosphorus and magnesium) and biomarkers of bone metabolism (PTH, alkaline phosphatase, vitamin D and FGF-23) are observed with varying impact on post-transplant outcomes. Calcineurin inhibitors are linked to osteoporosis, whereas steroid therapy may lead to both osteoporosis and varying degrees of osteonecrosis. Sirolimus and everolimus might have a bearing on osteoblasts proliferation and differentiation or decreasing osteoclast mediated bone resorption. Selected pharmacologic interventions for treatment of MBD in transplant patients include steroid withdrawal, the use of bisphosphonates, vitamin D derivatives, calcimimetics, teriparatide, calcitonin and denosumab.
Summary
MBD following kidney transplantation is common and characterized by loss of bone volume and mineralization abnormalities often leading to low turnover bone disease. Although there are no well-established therapeutic approaches for management of MBD in renal transplant recipients, clinicians should continue individualizing therapy as needed.
Keywords: Renal osteodystrophy, bisphosphonates, fracture, calcineurin inhibitor, adynamic bone
Introduction
Transplantation of solid organs is a common and effective treatment modality for end-stage failure of those organs. Kidney is by far the most frequently transplanted solid organ both in the US and throughout the world.1 Advances in immunosuppressive therapy and transplant techniques over the last decades have improved allograft and patient survival, although long-term survival advantage of some of these agents still remains to be demonstrated.2 Successful transplantation is capable of reversing many complications of end-stage kidney disease; however, disturbances of bone and mineral metabolism, also referred to as “mineral and bone disorders” (MBD), may persist, while new bone disorders may also emerge as a result of transplant related medications. The MBD is inherent features of chronic kidney disease (CKD) and commonly observed both in non-dialysis dependent CKD3 and maintenance dialysis patients.4 Although bone disease has been recognized as a common complication in kidney transplant recipients, the routine application of adequate diagnostic tools and preventive or treatment strategies to correct bone loss or mineral disarrays may often be suboptimal. In this review we summarize the updated information about prevalence and consequences of mineral-and-bone disorders (MBD) in kidney transplant recipients and examine diagnostic, preventive and therapeutic options for these conditions.
Types of Bone Disorders in Kidney Transplant Recipients
The hallmark of MBD is renal osteodystrophy, also known as “kidney bone disease”,4 which is classified into four major groups (Figure 1): (1) high turnover bone disease, (2) adynamic or low turnover bone disease, (3) mixed renal osteodystrophy, and (4) osteomalacia.5,6 Recent evidence suggests that renal osteodystrophy and its primary causes including disordered parathyroid function and disarrays in vitamin D and FGF-23 are related to cardiovascular disease and mortality. Table 1 shows the main characteristics of the 4 traditional types of uremic bone disease.7 The findings from earlier reports on bone abnormalities in patients after renal transplantation are somewhat conflicting.8-15 Heterogeneity of bone lesions has been noted in these early studies,9,13 whereas other studies report a wide range of histopathologic findings including high prevalence of high bone turnover associated with persistence of secondary hyperparathyroidism;8,10,11,16 normal bone formation;9 or low bone turnover (see Table 1).14,15 Prolonged mineralization without osteoid accumulation has been found in some studies as well,8,14,15 whereas frank osteomalacia has been rarely observed in kidney transplant recipients.12,13,17
Figure 1.
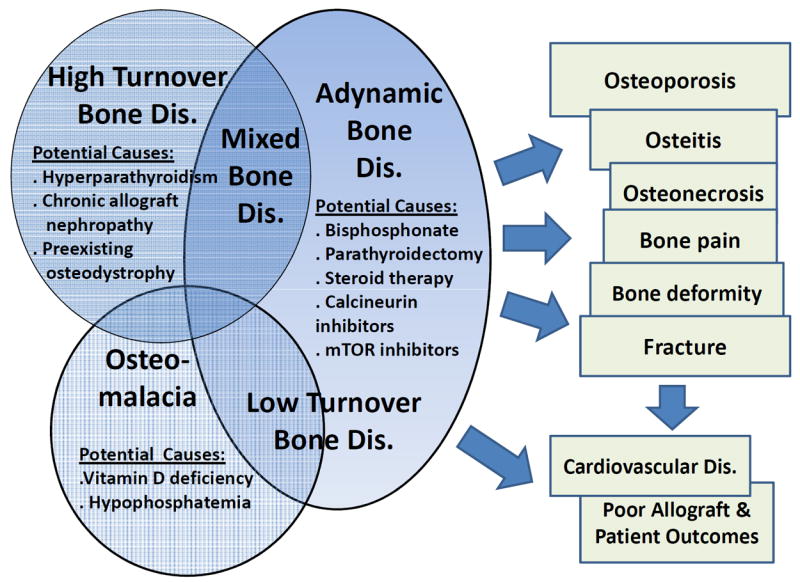
Four main types of renal osteodystrophy in kidney transplant recipients (see also Table 1)
Table 1.
Clinical features of four main categories of bone disease (renal osteodystrophy) in kidney transplant recipients (see also Figure 1)
| Type | Histopathologic features | Biochemical abnormalities | Clinical consequences |
|---|---|---|---|
| Hyperactive (high turnover) bone disease (including osteitisfibrosa) | Marked increase in bone turnover, irregularly shaped trabecules displaying numerous abnormal remodeling sites, and an unusually high number of bone cells with irregular arrangement and shape |
|
|
| Adynamic (low turnover) bone disease | Reduced bone volume and mineralization paralleled by a decrease in bone formation. It is also characterized by presence of few osteoid seams and few osteoblasts. Osteoclast number may be low, normal, or high. |
|
|
| Mixed renal osteodystrophy | Defective mineralization with or without increased bone formation and increased PTH activity in bone. Increased numbers of heterogeneous remodeling sites and an increase in osteoclast number. Bone volume is variable and depends on a dominant pathogenic cause. |
|
|
| Osteomalacia (may also be included under low-turnover category) | Accumulation of unmineralized matrix in which a decrease in mineralization precedes or is more pronounced than the inhibition of collagen deposition. |
|
|
Bone specific AlkPhos is preferred but total (non-specific) AlkPhos can be used after ruling out liver disease or other non-bone sources of circulating AlkPhos.
Findings from Bone Biopsy Studies
In a study by Monier-Faugere et al18 in 56 prevalent kidney transplant patients who underwent bone biopsy, cancellous bone volume/tissue volume was below normal in most patients compared to age- and gender-matched control subjects. Similar bone biopsy findings were reported in a longitudinal study by Cruz et al19 in 20 patients before and then 6 months after kidney transplantation. Pre-transplant bone histomorphometric diagnoses were adynamic bone disease (n=12); mixed bone disease (n=3); mild disease (n=3); and osteitisfibrosa (n=2). After transplantation most patients (n=11) had adynamic bone disease.19 Rojas et al.20 showed that osteoid volume, osteoid thickness, osteoid resorption surface, and osteoclast surface were above the normal range before transplant and remained so approximately 35 days after transplantation; however, osteoid and osteoblast surfaces significantly decreased within 35 days post-transplant.20 There was also inhibition of bone formation and mineralization as well as apoptosis, which correlated with the dose of administered glucocorticoids.20 In contrast to the above findings, a longitudinal study by Lehman et al21 in reported more heterogeneous biopsy findings.
Pooling together the bone biopsy studies in kidney transplant recipients, low turnover bone diseases including a dynamic bone and osteomalacia appear to be common. Most kidney transplant recipients exhibit decreased mineral apposition rate and delayed mineralization which may be accompanied by the dramatic decrease in PTH levels including in patients who had relatively mild bone disease prior to transplantation14 and who received high doses of glucocorticoids.18 Many studies mainly show alterations consistent with a dynamic bone disease and increased deposition of iron in the mineralization front;15 however, some studies suggest decreased bone formation and prolonged mineralization lag time in the presence of persisting bone resorption.8,22,23 Hence, notwithstanding the discrepancies among various bone biopsy studies, the main alteration in bone remodeling after renal transplantation is decrease in bone formation and mineralization in face of persistent bone resorption, which may lead to an imbalance in remodeling favoring resorption. Likewise, the defective bone formation may be a consequence of alterations in osteoblast function, decreased osteoblastogenesis, or increased osteoblast death rate. More bone biopsy studies in larger number of kidney transplant recipients are needed to better understand the combined impact of prior bone disease and immune suppressive regimen on bone histology in this patient population.
Decreased Bone Mineral Density and Osteoporosis
Loss of bone mass after kidney transplantation leading to osteopenia or osteoporosis occurs primarily in the first 12 months, predominantly in cortical bone. The most rapid decrease in bone mineral density (BMD) [not to confuse with MBD] measured by dual-energy X ray absorptiometry occurs in the first 6 months post-transplantation, and seems to slow down thereafter, possibly reflecting reduced corticosteroid dose. BMD has been reported to decrease considerably at a mean of 5.5% to 19.5% during the first 6 months after transplantation,14,24,25 but only 2.6–8.2% between months 6 and 12,26,27 and 0.4-4.5% thereafter.28,29
Risk of Fracture
The overall fracture risk after renal transplantation is 3.6–3.8-fold higher than in healthy individuals,30,31 and is 30% higher during the first 3 years after transplantation than in patients on dialysis.30 In a retrospective study with follow-up time up to 33 years, more advanced age and history of diabetic nephropathy were independent predictors of fracture risk, whereas higher activity status was protective.31 Additional risk factors for fracture in kidney transplant recipients include female gender and combined kidney–pancreas transplantation.32-35 Similar to an increased mortality risk during the first few weeks after kidney transplantation followed by a substantial decline in mortality thereafter when compared to waitlisted dialysis patients, the relative risk of hip fracture was 34% greater in the first few weeks after transplant surgery compared to dialysis patients, but decreased by at least 1% per month until the estimated risk became equal for dialysis and transplant recipients approximately 630 days after transplantation.30 It is important to note that renal transplant recipients are at particular risk of vertebral fracture and that this risk is greater than their risk of lower extremity fractures.31
Mineral Metabolism after Kidney Transplantation
Alterations in mineral metabolism including such biomarkers of bone disease as PTH and alkaline phosphatase are common following successful kidney transplantation. The most recent Kidney Disease Initiative Global Outcomes (KDIGO) guidelines36 proposed periodic monitoring of serum calcium, and phosphorus every 6–12 months, 3–6 months, 1–3 months, in CKD stages 1–3T, 4T, and 5T, respectively, while PTH should also be measured at 3-12 month intervals according to the severity of CKD. Measurement of alkaline phosphatase should be performed annually or more frequently in the presence of elevated PTH according to the same guidelines.
Serum Calcium
There are several factors that may precipitate or worsen hypercalcemia after successful kidney transplantation: (1) persistently elevated serum PTH, (2) correction of hyperphosphatemia; and (3) improved 1,25(OH)2 vitamin D production from the allograft. Although severe hypercalcemia (>3 mmol/l or >12 mg/dL) is rarely observed, hypercalcemic episodes (defined as total serum calcium >2.62 mmol/l or >10.5 mg/dL) were reported in 30% and 12% of renal transplant recipients, 1 year and 5 years after transplantation, respectively.37 In a recent study, post-transplant hypercalcemia was not associated with a specific bone turnover abnormality.38 In one study hyperkalemia appeared to correlate with interstitial micro-calcifications in the renal allograft and poor long-term graft outcomes.39
Serum Phosphorus
Hyperphosphatemia (phosphorus >4.5 mg/dL) is more prevalent in pre-transplant patients, while hypophosphatemia (phosphorus <2.5 mg/dL) is observed much more frequently after renal transplantation especially in the first few weeks postoperatively.17,40-42 Decreased phosphorus reabsorption in the proximal tubule, potentially related to persistently elevated PTH or FGF-23 levels, and a quasi “hungry bone syndrome” seem to be mechanisms responsible for post-transplantation hypophosphatemia. Hypophosphatemia has been associated with severe alterations in bone turnover that include a decrease in osteoblast activity that leads to rickets and osteomalacia.17,43 Several recent studies indicate that post-transplantation hypophosphatemia frequently is independent of PTH,44 suggesting that FGF-23,45-47 or perhaps additional humoral factors (other phosphatonins) contribute to phosphaturia in the early post-transplant period.41,42 Both pre-transplant48 and post-transplant49 serum phosphorous derangements appear to be associated with anemia50 and mortality risk in kidney transplant recipients.
Serum Magnesium
Hypomagnesemia, which is a common condition especially in the first few weeks after kidney transplantation, is also an independent predictor of new onset (de novo) diabetes mellitus in renal transplant recipients.51 Seventy to 80% of serum magnesium is freely filtered at the glomerulus and most (up to 97%) is reabsorbed throughout the nephron. Calcineurin inhibitors including cyclosporine A may interfere with magnesium metabolism leading to decreased magnesium reabsorption, urinary magnesium wasting and hypomagnesaemia in renal transplant recipients receiving these immunosuppressive medications.52 A recent study suggested that low serum magnesium levels were associated with a faster rate of decline in kidney allograft function and increased rates of graft loss in renal transplant recipients with chronic cyclosporine nephropathy.52 Whether hypomagnesemia per se contributes to cyclosporine nephropathy, or whether magnesium supplementation may lessen the cyclosporine nephropathy is not clear.
PTH and Alkaline Phosphatase
PTH levels usually decline rapidly (>50%) during the first 3–6 months after kidney transplantation because of a reduction in functional parathyroid gland mass,53 followed by a more gradual decline probably attributable to the slower involution of these glands.16,37 However, persistently elevated levels of serum PTH despite normalization of renal function have been reported in up to 25% of renal transplant recipients 1 year after transplantation.37,54 These so-called refractory (or tertiary) hyperparathyroidism cases may be the result of monoclonal glandular hyperplasia.55,56 There are several factors which are associated with persistent post-transplant hyperparathyroidism such as prolonged end stage kidney disease prior to transplantation,37,57,58 decreased residual renal function,59 low levels of 1,25(OH)2-and 25(OH) vitamin D, and reduced expression of vitamin D and calcium-sensing receptors and also reduced expression of FGF-23 receptors in the parathyroid gland.54,60-62 Both pre-transplant63 and post-transplant39 serum PTH level are associated with unfavorable outcomes including worse graft function. However, it is important to note that two studies that assessed bone biopsy samples in kidney transplant recipients did not find a correlation between serum PTH levels and bone turnover,18,38 and the diagnostic accuracy of PTH is not quite clear.64 Treatment options for hyperparathyroidism are summarized below. Serum bone-specific alkaline phosphatase is significantly correlated with calcitriol and adequately reflects increased bone formation after renal transplantation.65 Higher levels of alkaline phosphatase, but not PTH in the months prior to kidney transplantation may herald poor post-transplant outcomes (Miklos Z. Molnar and colleagues, personal communication).
Vitamin D and FGF-23
Low serum 25-OH-D levels are common following solid organ transplantation, both during the immediate postoperative period and in long-term graft recipients.66 According to the KDIGO guidelines36 kidney transplant patients should be assessed for the presence of vitamin D deficiency by examining circulating levels of 25-(OH) vitamin D (calcidiol), and vitamin D deficiency and insufficiency should be corrected using treatment strategies recommended for the general population. Even though the level of 1,25(OH)2 vitamin D (calcitriol) usually increases after successful kidney engraftment, it may still remain lower compared to normal population.7 The most important predictor of low 1,25(OH)2 vitamin D levels are immunosuppressive therapy, PTH level and residual renal function.45 The study by Evenepoel et al. found elevated pre-transplantation PTH levels and low post-transplantation levels of FGF23 to be additional predictors of improved post-transplantation 1,25(OH)2 vitamin D levels, although these were weaker than renal graft function.45 FGF-23 per se appears to be a strong and independent predictor of mortality in prevalent kidney transplant recipients.67
Preexisting Osteodystrophy
Virtually all patients who receive a kidney allograft suffer from some degree of preexisting bone disorders. The incidence and prevalence of pre-existing low turnover bone disease may have increased recently, probably due to higher dialysate calcium concentrations (1.75 mmol/L [3.5 mEq/L]), high doses of calcium containing phosphate binders and the potentially overzealous utilization of active vitamin D metabolites. It is not clear whether the pre-existing MBD has significant consequences on post-transplant outcomes; nevertheless, some transplant centers consider dialysis patients with high PTH level as unfavorable candidates for kidney transplantation, which is similar to the policies pertaining to body mass index and obesity that have recently been challenged.68
Effects of Transplantation-Specific Therapies on Bone
Post-transplantation immunosuppressive therapy may have a major impact on the pathogenesis of bone disease.69-72 The role of corticosteroids is well-known. During the first several months after transplantation, rapid bone loss secondary to steroid-induced acceleration in bone remodeling occurs in cancellous bone.14 A study that involved serial bone biopsies at 22 days and 160 days after transplantation showed impaired osteoblastogenesis and early osteoblast apoptosis probably related to steroid therapy.20 The etiology of glucocorticoid induced bone disorder is multi-factorial.73-75 Steroids can be directly toxic to osteoblasts and lead to increased osteoclast activity.75 Other steroid effects include decreased calcium absorption in the gut, reduced gonadal hormone production, diminished insulin-like growth factor–1 (IGF-1) production, decreased sensitivity to PTH, increase in receptor activator of NF-kappa beta ligand (RANKL), and increased osteoclastogenesis.75-77
Calcineurin inhibitors including cyclosporine and tacrolimus have been linked to osteoporosis.78,79 Epidemiologic studies that have examined fracture risk, however, could not establish an association between use of calcineurin inhibitors and fracture risk.31,80 Although mycophenolatemofetil, sirolimus, and azathioprine do not affect bone volume in rodents,81-83 a recent in vitro study suggests sirolimus might interfere with the proliferation and differentiation of osteoblasts,84 while everolimus reduces cancellous bone loss in ovarectomized rats by decreasing osteoclast mediated bone resorption.85 Calcineurin-inhibitor induced pain syndrome may happen as a result of osteonecrosis, along with transient marrow edema.86 These painful conditions, which can be diagnosed by X-ray, radionuclide scan or magnetic resonance imaging are associated with increased intraosseous pressure, compromised vascular supply, marrow edema and the development of a ‘bone compartment syndrome’.86 Steroid therapy is another known risk factor for osteonecrosis in renal transplant recipients.87 Mechanisms may include the differentiation of mesenchymal stem cells to adipocytes causing increased intraosseous pressure and collapse of marrow sinusoids, and increased osteoblast and osteocyte apoptosis. Calcineurin-inhibitors, particularly cyclosporine A, may increase the risk of osteonecrosis because of vasoconstrictive effects, and sirolimus may influence the development of osteonecrosis by potentiating the effects of calcineurin inhibitors or by influencing the lipid profile.86
Chronic Allograft Nephropathy Associated MBD
Gradually failing allografts may lead to post-transplantation CKD stage 3-5T leading to increased risk of worsening or de novo development of hyperparathyroidism, active vitamin D deficiency and the while spectrum of “classic” MBD that is observed in transplant-naïve-CKD patients.3,59 In a study of more than 900 transplant patients PTH exhibited a negative correlation with estimated GFR in CKD stages 3-5T (r = -0.29, P <0.001).59 High PTH values correlate with significant bone loss at the hip and other areas.88 Given recent data that delaying the return to dialysis therapy may be associated with better survival in gradually failing kidney transplant recipients,89,90 higher rates of MBD are to be expected in the prevalent transplant population.
Management of MBD in Kidney Transplant Recipients
Several practice guidelines and expert reviews can be used to lay out pragmatic recommendations for the prevention, diagnosis and management of bone disease and mineral disorders in kidney transplant recipients.36,91,92 To date, no randomized controlled trials in kidney transplant recipients have examined the effect of bone-specific therapies on relevant clinical outcomes, including mortality, quality of life or fracture risk.36 In heart transplant patients alendronate was as effective as calcitriol to prevent bone loss.93 Table 2 shows a list of selected studies pertaining to MBD management in renal transplant recipients. The KDIGO guidelines36 recommend treatment with active vitamin D (calcitriol or alfacalcidiol) or bisphosphonates in the first 12 months after kidney transplant in those with estimated GFR>30 mL/min/1.73 m2 and low bone mineral density, and bone biopsy consideration to guide treatment, specifically before the use of bisphosphonates due to the high incidence of a dynamic bone disease. The Cochrane Database review, however, indicate that no type of MBD treatment was associated with better survival in kidney transplant recipients, although treatment reduces the risk of fractures.92 Selected pharmacologic interventions for treatment of MBD in transplant patients are listed in Table 3 and include steroid withdrawal, bisphosphonates, vitamin D derivatives, calcimimetics, teriparatide, calcitonin and denosumab.
Table 2.
Overview of relevant clinical trials and observational studies related to MBD management in kidney transplant patients
| Study (first author, publication year) | Type of study | Intervention | Number of patients | Outcome/comments |
|---|---|---|---|---|
|
| ||||
|
Bisphosphonates (see also
Fig 2)
| ||||
| Kovac et al.94, 2000 | RCT | Alendronate + calcium + Vit D vs | 12 | BMD increment in lumbar spine in Alendronate group and decrement in control group |
| Calcium + Vit D | ||||
|
| ||||
| Giannini et al.95, 2001 | RCT | Alendronate + calcium + calcitriol vs | 40 | BMD increases in Alendronate arm, but not in control arm. |
| Calcium + calcitriol | ||||
|
| ||||
| Jeffery et al.96, 2003 | RCT | Alendronate and calcium vs calcitriol and calcium | 117 | One year of treatment with alendronate or calcitriol, both with calcium supplementation, resulted in significant increases in BMD at the lumbar spine and femur, with a trend toward alendronate being more effective at the spine. |
|
| ||||
| Haas et al.97, 2003 | RCT | Zoledronate + calcium vs placebo + | 20 | BMD increment in lumbar spine in Zoledronate group and decrement in control group |
| Calcium | ||||
|
| ||||
| Schwarz et al.98, 2004 (follow up of study by Haas et al.97, 2003) | RCT | Zoledronate + calcium vs placebo + | 20 | The early bone-sparing effect of short-term Zoledronate therapy confers no sustained benefit versus placebo at three year post-transplantation. |
| Calcium | ||||
|
| ||||
| Fan et al.99, 2003 | RCT | Pamidronate + calcium + Vit D vs | 25 | BMD preserved in lumbar spine in Pamidronate group and decrement in control group |
| Calcium + Vit D | ||||
|
| ||||
| Coco et al.100, 2003 | RCT | Pamidronate + calcium + Vit D vs | 59 | BMD preserved in lumbar spine in Pamidronate group and decrement in control group |
| Calcium + Vit D | ||||
|
| ||||
| Walsh et al.101, 2009 | RCT | Pamidronate + calcium + Vit D vs | 93 | BMD preserved in lumbar spine in Pamidronate group and decrement in control group |
| Calcium + Vit D | ||||
|
| ||||
| Torregrosa et al.102, 2011 | RCT | Pamidronate + calcium + Vit D vs | 39 | Pamidronate significantly reduced spinal bone loss, but no significant benefit was found for the incidence of fractures. |
| Calcium + Vit D | ||||
|
| ||||
| Grotz et al.103, 2001 | RCT | Ibandronate + calcium vs | 72 | BMD preserved in Ibandronate group and decrement in control group |
| Calcium | ||||
|
| ||||
| Torregrosa et al.104, 2010 | RCT | Risedronate + calcium + Vit D vs | 101 | Administration of risedronate immediately after renal transplantation contributes to an improved BMD, particularly in the femoral neck at 6-month follow-up, without major side effects. |
| Calcium + Vit D | ||||
|
| ||||
| Nowacka-Cieciura et al.105, 2006 | Observational | Alendronate/Risedronate vs drug free | 66 | BMD preserved in treated group |
|
| ||||
|
Vitamin D derivatives and vitamin D receptor activators
| ||||
| Cueto-Manzano et al.106, 2000 | RCT | Calcium + Calcitriol vs drug free | 30 | 1,25-dihydroxyvitamin D3 and calcium carbonate did not significantly improve bone loss in long-term renal transplant recipients. However, significant osteoclast suppression and a trend to maintain trabecular bone volume and wall thickness as well as improve the axial BMD were observed in the treatment group. |
|
| ||||
| De Sevaux et al.107, 2002 | RCT | Calcium + Calcitriol vs drug free | 111 | Treatment with a low dose of active vitamin D and calcium partially prevents bone loss at the lumbar spine and proximal femur during the first 6 months after transplantation |
|
| ||||
| El-Agroudy et al.108, 2003 | RCT | Alfacalcidol vs placebo | 40 | In treated group BMD increased and PTH decreased, whereas BMD decreased in control group |
|
| ||||
| Torres et al.109, 2004 | RCT | Calcium + Calcitriol vs Calcium | 86 | Therapy with low-dose calcium supplements during 1 year, plus intermittent calcitriol for 3 months after transplantation, is safe, decreases PTH levels more rapidly, and prevents bone loss at the proximal femur; a more pronounced effect is seen in recipients with at least one at-risk allele of the VDR genotype |
|
| ||||
| Josephson et al.110, 2004 | RCT | Calcium + Calcitriol vs Calcium vs placebo | 64 | BMD decrement was detected in placebo group, whereas BMD was small increased and preserved in treated group |
|
| ||||
| Perez et al.111, 2010 | RCT | Paricalcitol vs drug free | 42 | Profile of urinary peptides was changed due to treatment with paricalcitol |
|
| ||||
|
Calcimimetics
| ||||
| Kruse et al.56, 2005 | Observational | Cinacalcet vs drug free | 14 | Serum calcium decreased and normalized, whereas serum PTH and phosphate levels did not change significantly |
|
| ||||
| Serra et al.112, 2005 | Observational | Cinacalcet vs drug free | 11 | Serum calcium and PTH decreased, whereas serum phosphate increased |
|
| ||||
| Szwarc et al.113, 2006 | Observational | Cinacalcet vs drug free | 9 | Serum calcium, phosphate and PTH did not change |
|
| ||||
| Srinivas et al.114, 2006 | Observational | Cinacalcet vs drug free | 11 | Serum calcium decreased, whereas serum phosphate increased and PTH did not change |
|
| ||||
| Bergua et al.115, 2007 | Observational | Cinacalcet vs drug free | 13 | Serum calcium and PTH decreased, whereas serum phosphate increased |
|
| ||||
| Bergua et al.116, 2008 | Observational | Cinacalcet vs drug free | 9 | Serum calcium, creatinine and PTH decreased, whereas radial BMD increased |
|
| ||||
| Lopez et al.117, 2009 | Observational | Cinacalcet vs drug free | 29 | Serum calcium decreased, whereas serum phosphate increased and PTH did not change |
|
| ||||
| Borchhardt et al.118, 2010 | Observational | Cinacalcet vs drug free | 10 | While cinacalcet might decrease bone formation rate, it did not change bone volume, and bone mineral density of the femur increased |
|
| ||||
| Cho et al.119, 2010 | Observational | Cinacalcet vs drug free | 23 | Cinacalcet therapy was associated with significant reduction of serum calcium compared to control. Cinacalcet therapy was associated with greater BMD increase at the hip over the 36-month post-transplant period. |
|
| ||||
| Copley et al.120, 2010 | Observational | Cinacalcet vs drug free | 41 | Serum calcium and PTH decreased, whereas serum phosphate increased, but estimated GFR did not change |
|
| ||||
| Schwarz et al.121, 2011 | Observational | Cinacalcet vs drug free | 58 | Serum calcium, estimated GFR and PTH decreased, whereas serum phosphate increased |
|
| ||||
| Pinho et al.122, 2011 | Observational | Cinacalcet vs drug free | 18 | Serum calcium and PTH decreased, whereas estimated GFR did not change |
Table 3.
Pharmacologic agents used for the management of MBD in kidney transplant patients.
| Vitamin D preparation | Type | Serum calcium & phosphorus | Serum PTH and alkaline phosphatase | Availability (and brand name in the USA/Canada) |
|---|---|---|---|---|
|
| ||||
| Bisphosphonates* | ||||
|
| ||||
| Alendronate | N-containing | ↓ | ↓ | Fosamax™ |
|
| ||||
| Pamidronate | N-containing | ↓ | ↓ | APD™, Aredia™ |
|
| ||||
| Zoledronate | N-containing | ↓ | ↓ | Zometa™, Aclasta™ |
|
| ||||
| Risedronate | N-containing | ↓ | ↓ | Actonel™ |
|
| ||||
| ibandronate | N-containing | ↓ | ↓ | Boniva™ |
|
| ||||
| Nutritional Vitamin D | ||||
|
| ||||
| Ergocalciferol | D2, prepro- hormone, inactive | ↑ | ? | Generic (Drisdol™) |
|
| ||||
| Cholecalciferol | D3, prepro- hormone, inactive | ↑ | ? | Generic (Calciol, Vitamin D3) |
|
| ||||
| 25(OH)D (calcidiol, calcifediol) | D3, prehormone | ↑ | ? | Currently not yet available in the USA (Calderol™) |
|
| ||||
| Vitamin D Receptor Activators | ||||
|
| ||||
| 1-alfa-calcidiol | 1-α(OH)D3, missing 25(OH) | ↑ | ? | Not available in the USA (one-alpha™) |
|
| ||||
| Doxercalciferol | 1-α(OH)D2, missing 25(OH) | ↔ to ↑ | ↓ | PO & IV (Hectoral™) |
|
| ||||
| Calcitriol | D3 hormone, non-selective VDRA | ↑ | ↔ to ↓ | IV and PO (Calcijex™, Rocaltrol™) |
|
| ||||
| Vitamin D Mimetics | ||||
|
| ||||
| Paricalcitol | D2, 19-Nor, selective VDRA | ↔ to ↑ | ↓ | IV & PO (Zemplar™) |
|
| ||||
| Maxacalcitol | 1,25-dihydroxy-22-oxa-vitamin D3 selective VDRA | ↔ to ↑ | ↓ | Not available in the USA (currently only in Japan) |
|
| ||||
| Calcimimetics | ||||
|
| ||||
| Cinacalcet | Calcimimetic (calcium sensing receptor activator) | Ca: ↓ | ↓ | Only in PO form (Sensipar/Mimpara™) |
| P: ↓ in ESRD | ||||
| P: ↑ in NDD-CKD | ||||
|
| ||||
| Other agents | ||||
|
| ||||
| Teriparatide | Recombinant PTH 1-34 | Ca: ↑ | ↑ | Injectable (Forteo™) |
| P: ↓in non-CKD | ||||
|
| ||||
| Calcitonin | Salmon-calcitonin | ↓ | ↓ | Miacalcin, Fortical, Calcimar (injectable and nasal) |
|
| ||||
| RANK-ligand inhibitors | ||||
|
| ||||
| Denosumab | IgG2 monoclonal antibody inhibiting osteoclastic bone resorption | Ca: ↓ | ↓ | Prolia™ (60 mg SC q 6 mo) No data in RTR |
CKD: chronic kidney disease, HD: hemodialysis; PTH: parathyroid hormone. 25(OH)D: 25-hydroxyvitamin D; 1,25(OH)2D: 1,25-dihydroxyvitamin D; AP: alkaline phosphatase, MVDRA: vitamin D receptor activators, (OH): hydroxyl; RTR: renal transplant recipients
Other bishosphonates include non-nitrogenous (non-N-containing) bisphosphonates Etidronate (Didronel™), Clodronate (Bonefos™, Loron™), and Tiludronate (Skelid™); and other nitrogenous (N-containing) bisphosphonates include Neridronate and Olpadronate.
Steroid withdrawal or avoidance
The rationale for minimizing corticosteroid exposure is compelling and based on well-established risks of osteoporosis, avascular necrosis and other side effects. Some studies found beneficial effects of early tapering of prednisolone on BMD.123,124 In contrast, however, randomized controlled trials have shown that steroid withdrawal, when carried out weeks to months after kidney transplantation, is associated with an increased risk of acute rejection.125,126 Hence, the current KDIGO guidelines36 do not currently recommend steroid withdrawal and avoidance as a routine course of action.
Bisphosphonates
Bisphosphonates (also known as diphosphonates) consist of two phosphonate (PO3) groups and are used to prevent bone mass loss and to treat osteoporosis and other osteopenic conditions. Figure 2 shows an overview of the results of the bisphosphonates studies in kidney transplant recipients. In four studies of multiple doses of pamidronate during the initial months after renal transplantation, prevention of bone loss occurred even after treatment was discontinued.99-102 Most, if not all, studies suggest that pamidronate administration prevents bone loss shortly after transplantation, although low turnover bone disease may develop or worsen in many patients.99,100 Similar results were found when alendronate was administered.94,105 Intravenous ibandronate was used by Grotz et al.103 in 80 randomly assigned transplant recipients at a dose of 1 mg immediately before the transplant and 2 mg at 3, 6, and 9 months after transplantation and demonstrated prevention of bone loss, spinal deformation, and loss of body height during the first year after kidney transplantation.103 Another randomized controlled trial of 20 kidney transplant recipients showed that zoledronate improved the calcium content of cancellous bone.97 As to whether this early short-term intervention exhibits a sustained bone-sparing effect later in time, in another study zoledronate therapy conferred no sustained benefit versus placebo at 3 year post-transplantation.98 Weekly oral risedronate immediately after renal transplantation can improve BMD, particularly in the femoral neck at 6-month follow-up, without major side effects.104 Hence, bisphosphonate therapy may significantly improve bone mineral density at the femoral neck and lumbar spine and reduce the risk of acute rejection and might reduce the risk of fracture,127 although bisphosphonates do not appear to have any effect on patient survival or graft loss.92
Figure 2.
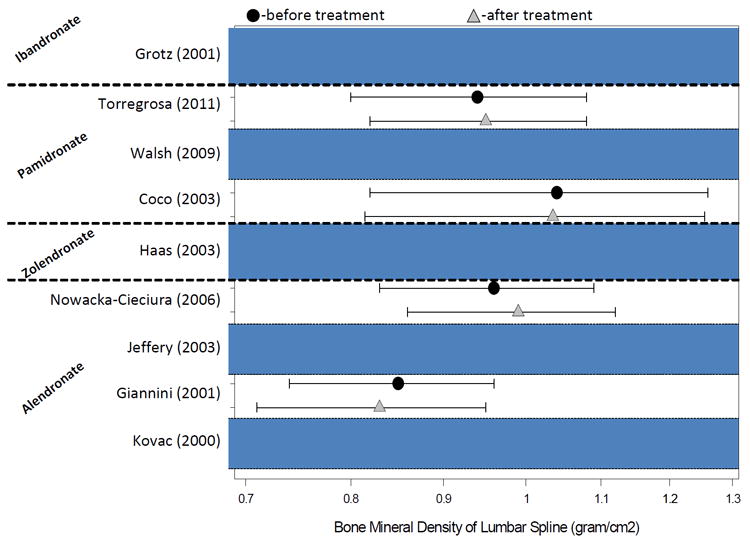
Overview of the results of the bisphosphonates studies in kidney transplant recipients (see also Table 2)
Vitamin D derivatives and D-mimetics with or without calcium supplement
Several forms of vitamin D derivatives and their therapeutic classification are shown in Table 3. In a well-controlled, blinded study, Josephson et al110 showed that kidney transplant recipients who were given calcium and calcitriol had significantly less bone loss in the lumbar spine and increased BMD in the distal radius and femoral neck compared with transplant patients given calcium alone or placebo. The treated patients did not develop significant hypercalcemia or deterioration of kidney function during the two years of the study.110 Torres et al reported that therapy with low-dose calcium supplements during 1 year, plus intermittent calcitriol for 3 months after transplantation was safe, decreased PTH levels more rapidly, and prevented bone loss at the proximal femur.109 Compared to placebo, calcidiol and oral calcium increased BMD at the lumbar spine and femoral neck.107,108,128 Paricalcitol, a selective vitamin D receptor activator, also known as D-mimetic,4,129 is indicated in the prevention and treatment of secondary hyperparathyroidism. Preliminary results of a randomized controlled trial showed that changes in the profile of urinary peptides occurred due to treatment with paricalcitol;111 however, no study has assessed the association between bone fracture, BMD or outcomes and administration of paricalcitol.
Calcimimetics
In the past several years the calcimimetic agent cinacalcet has been frequently evaluated for the treatment of hypercalcemia in renal graft patients with ongoing refractory hyperparathyroidism. As shown in some post-transplant trials cinacalcet successfully corrects elevated serum calcium and PTH levels with no negative effect on renal function,114,115,120,121 and it appears to be safe in kidney transplant recipients.117,122 A favorable effect of cinacalcet on BMD in renal transplant patients was reported by several small studies (see Table 2).116,118,119 Interestingly, cinacalcet might also have favorable effect on blood pressure in kidney transplant recipients, but not on outcomes.130
Other potential MBD treatment modalities
Another therapeutic agent studied in patients after kidney transplantation is teriparatide, a recombinant human PTH. A recent trial showed that teriparatide administered to kidney transplant patients for 6 months was safe but did not alter BMD in the lumbar spine or distal radius compared with the placebo group.131 However, BMD at the femoral neck remained stable in those given teriparatide, compared with a decrease in the placebo group. In addition, after 6 months, no significant differences between the two groups were detected in fractures, bone histology, vitamin D levels, PTH levels, kidney function, or serologic bone markers.131 Teriparatide can be considered as an alternative treatment of MBD in kidney transplant patients with low PTH and refractory hypocalcemia.132 Another potential therapeutic agent is calcitonin;, although it has no effect on mortality, graft loss and risk of fracture in patients after kidney transplantation.92,133,134 Exercise training and hormonal therapy are other potential interventions. The effect of regular exercise or hormone replacement therapy on bone loss or risk of fracture has not yet examined in kidney transplant recipient, although data from other solid organ transplant patients are promising.135,136 Denosumab, a RANK-ligand inhibitor for treatment of post-menopausal osteoporosis;4 can theoretically reduce osteoclasticresorption of trabecular structures and, hence, be used for treatment of osteonecrosis, but currently there is no human data. Early stages of osteonecrosis are generally managed conservatively or with core decompression accompanied by bone grafting and more recently the injection of bone morphogenic protein, while iloprost to improve blood flow combined with bisphosphonates deserve further studies.86
Conclusions
Mineral and bone disorders following kidney transplantation are common and characterized by loss of bone volume and mineralization abnormalities leading to low turnover bone disease in most patients. There are several contributing factors including pre-existing osteodystrophy, transplantation-specific therapies and reduced renal function due to chronic allograft nephropathy. At this time there are no well-established therapeutic approaches that would provide bone preserving or anabolic effects with high degree of certainty. However, vitamin D analogues and bisphosphonates are often used for treatment of MBD after kidney transplantation. Whereas more studies are needed to examine the effects of different therapeutic interventions on bone disorders after kidney transplantation, clinicians should continue to individualize therapy according to their expertise and best judgment.
Key bullet points.
Mineral and bone disorders following kidney transplantation are common and characterized by loss of bone volume and mineralization abnormalities leading to low turnover bone disease in most of these patients.
At this time there are no well-established therapeutic approaches that would provide bone preserving or anabolic effects with high degree of certainty in renal transplant recipients.
However, vitamin D analogous and bisphosphonates are often used for treatment of mineral and bone disorders after kidney transplantation.
More studies are needed to examine the effects of different therapeutic interventions on bone disorders after kidney transplantation, clinicians should continue to individualize therapy according to their expertise and best judgment.
Acknowledgments
Funding Source:
The study was supported by Dr.Kalantar-Zadeh’s research grants from the National Institute of Diabetes, Digestive and Kidney Disease of the National Institute of Health (R01 DK078106 and K24 DK091419), and a philanthropic grant from Mr. Harold Simmons. MZM received grants from the National Developmental Agency (KTIA-OTKA-EU 7KP-HUMAN-MB08-A-81231) from the Research and Technological Innovation Fund, and is recipient of the Hungarian Eötvös Scholarship (MÖB/77-2/2012).
None.
Footnotes
Relevant Potential Conflict of Interest:
Dr.Kalantar-Zadeh has received grants and/or honoraria from Abbott, Amgen, DaVita, Fresenius-Kabi, Genzyme, Otsuka, and Shire.
References
- 1.United States Renal Data System (USRDS) USRDS 2011 Annual Data Report. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2011. [Google Scholar]
- 2.Bunnapradist S, Kalantar-Zadeh K. Does the Use of mTOR Inhibitors Increase Long-Term Mortality in Kidney Recipients? American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011 Nov 4; doi: 10.1111/j.1600-6143.2011.03829.x. epub ahead of print 2012. [DOI] [PubMed] [Google Scholar]
- 3.Kovesdy CP, Kalantar-Zadeh K. Bone and mineral disorders in pre-dialysis CKD. International urology and nephrology. 2008;40(2):427–440. doi: 10.1007/s11255-008-9346-7. [DOI] [PubMed] [Google Scholar]
- 4.Kalantar-Zadeh K, Shah A, Duong U, Hechter RC, Dukkipati R, Kovesdy CP. Kidney bone disease and mortality in CKD: revisiting the role of vitamin D, calcimimetics, alkaline phosphatase, and minerals. Kidney Int Suppl. 2010 Aug;(117):S10–21. doi: 10.1038/ki.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hruska KA, Teitelbaum SL. Renal osteodystrophy. N Engl J Med. 1995 Jul 20;333(3):166–174. doi: 10.1056/NEJM199507203330307. [DOI] [PubMed] [Google Scholar]
- 6.Wang M, Hercz G, Sherrard DJ, Maloney NA, Segre GV, Pei Y. Relationship between intact 1-84 parathyroid hormone and bone histomorphometric parameters in dialysis patients without aluminum toxicity. Am J Kidney Dis. 1995 Nov;26(5):836–844. doi: 10.1016/0272-6386(95)90453-0. [DOI] [PubMed] [Google Scholar]
- 7.Malluche HH, Monier-Faugere MC, Herberth J. Bone disease after renal transplantation. Nature reviews. Nephrology. 2010 Jan;6(1):32–40. doi: 10.1038/nrneph.2009.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carlini RG, Rojas E, Arminio A, Weisinger JR, Bellorin-Font E. What are the bone lesions in patients with more than four years of a functioning renal transplant? Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 1998;13(Suppl 3):103–104. doi: 10.1093/ndt/13.suppl_3.103. [DOI] [PubMed] [Google Scholar]
- 9.Sanchez CP, Salusky IB, Kuizon BD, et al. Bone disease in children and adolescents undergoing successful renal transplantation. Kidney international. 1998 May;53(5):1358–1364. doi: 10.1046/j.1523-1755.1998.00866.x. [DOI] [PubMed] [Google Scholar]
- 10.Briner VA, Thiel G, Monier-Faugere MC, et al. Prevention of cancellous bone loss but persistence of renal bone disease despite normal 1,25 vitamin D levels two years after kidney transplantation. Transplantation. 1995 May 27;59(10):1393–1400. doi: 10.1097/00007890-199505270-00006. [DOI] [PubMed] [Google Scholar]
- 11.Torres A, Machado M, Concepcion MT, et al. Influence of vitamin D receptor genotype on bone mass changes after renal transplantation. Kidney international. 1996 Nov;50(5):1726–1733. doi: 10.1038/ki.1996.492. [DOI] [PubMed] [Google Scholar]
- 12.Felsenfeld AJ, Gutman RA, Drezner M, Llach F. Hypophosphatemia in long-term renal transplant recipients: effects on bone histology and 1,25-dihydroxycholecalciferol. Mineral and electrolyte metabolism. 1986;12(5-6):333–341. [PubMed] [Google Scholar]
- 13.Bonomini V, Feletti C, Di Felice A, Buscaroli A. Bone remodelling after renal transplantation (RT) Advances in experimental medicine and biology. 1984;178:207–216. doi: 10.1007/978-1-4684-4808-5_29. [DOI] [PubMed] [Google Scholar]
- 14.Julian BA, Laskow DA, Dubovsky J, Dubovsky EV, Curtis JJ, Quarles LD. Rapid loss of vertebral mineral density after renal transplantation. N Engl J Med. 1991 Aug 22;325(8):544–550. doi: 10.1056/NEJM199108223250804. [DOI] [PubMed] [Google Scholar]
- 15.Velasquez-Forero F, Mondragon A, Herrero B, Pena JC. Adynamic bone lesion in renal transplant recipients with normal renal function. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 1996;11(Suppl 3):58–64. doi: 10.1093/ndt/11.supp3.58. [DOI] [PubMed] [Google Scholar]
- 16.Parfitt AM. Hypercalcemic hyperparathyroidism following renal transplantation: differential diagnosis, management, and implications for cell population control in the parathyroid gland. Mineral and electrolyte metabolism. 1982 Aug;8(2):92–112. [PubMed] [Google Scholar]
- 17.Moorhead JF, Wills MR, Ahmed KY, Baillod RA, Varghese Z, Tatler GL. Hypophosphataemic osteomalacia after cadaveric renal transplantation. Lancet. 1974 Apr 20;1(7860):694–697. doi: 10.1016/s0140-6736(74)92902-x. [DOI] [PubMed] [Google Scholar]
- 18.Monier-Faugere MC, Mawad H, Qi Q, Friedler RM, Malluche HH. High prevalence of low bone turnover and occurrence of osteomalacia after kidney transplantation. Journal of the American Society of Nephrology : JASN. 2000 Jun;11(6):1093–1099. doi: 10.1681/ASN.V1161093. [DOI] [PubMed] [Google Scholar]
- 19.Cruz EA, Lugon JR, Jorgetti V, Draibe SA, Carvalho AB. Histologic evolution of bone disease 6 months after successful kidney transplantation. Am J Kidney Dis. 2004 Oct;44(4):747–756. [PubMed] [Google Scholar]
- 20.Rojas E, Carlini RG, Clesca P, et al. The pathogenesis of osteodystrophy after renal transplantation as detected by early alterations in bone remodeling. Kidney international. 2003 May;63(5):1915–1923. doi: 10.1046/j.1523-1755.2003.00938.x. [DOI] [PubMed] [Google Scholar]
- 21.Lehmann G, Ott U, Stein G, Steiner T, Wolf G. Renal osteodystrophy after successful renal transplantation: a histomorphometric analysis in 57 patients. Transplantation proceedings. 2007 Dec;39(10):3153–3158. doi: 10.1016/j.transproceed.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Carlini RG, Rojas E, Weisinger JR, et al. Bone disease in patients with long-term renal transplantation and normal renal function. Am J Kidney Dis. 2000 Jul;36(1):160–166. doi: 10.1053/ajkd.2000.8289. [DOI] [PubMed] [Google Scholar]
- 23.Cueto-Manzano AM, Konel S, Hutchison AJ, et al. Bone loss in long-term renal transplantation: histopathology and densitometry analysis. Kidney international. 1999 May;55(5):2021–2029. doi: 10.1046/j.1523-1755.1999.00445.x. [DOI] [PubMed] [Google Scholar]
- 24.Lippuner K, Casez JP, Horber FF, Jaeger P. Effects of deflazacort versus prednisone on bone mass, body composition, and lipid profile: a randomized, double blind study in kidney transplant patients. The Journal of clinical endocrinology and metabolism. 1998 Nov;83(11):3795–3802. doi: 10.1210/jcem.83.11.5235. [DOI] [PubMed] [Google Scholar]
- 25.Mikuls TR, Julian BA, Bartolucci A, Saag KG. Bone mineral density changes within six months of renal transplantation. Transplantation. 2003 Jan 15;75(1):49–54. doi: 10.1097/00007890-200301150-00009. [DOI] [PubMed] [Google Scholar]
- 26.Nam JH, Moon JI, Chung SS, et al. Pamidronate and calcitriol trial for the prevention of early bone loss after renal transplantation. Transplantation proceedings. 2000 Nov;32(7):1876. doi: 10.1016/s0041-1345(00)01898-4. [DOI] [PubMed] [Google Scholar]
- 27.Brandenburg VM, Politt D, Ketteler M, et al. Early rapid loss followed by long-term consolidation characterizes the development of lumbar bone mineral density after kidney transplantation. Transplantation. 2004 May 27;77(10):1566–1571. doi: 10.1097/01.tp.0000131990.13277.28. [DOI] [PubMed] [Google Scholar]
- 28.Pichette V, Bonnardeaux A, Prudhomme L, Gagne M, Cardinal J, Ouimet D. Long-term bone loss in kidney transplant recipients: a cross-sectional and longitudinal study. Am J Kidney Dis. 1996 Jul;28(1):105–114. doi: 10.1016/s0272-6386(96)90138-9. [DOI] [PubMed] [Google Scholar]
- 29.Cruz DN, Wysolmerski JJ, Brickel HM, et al. Parameters of high bone-turnover predict bone loss in renal transplant patients: a longitudinal study. Transplantation. 2001 Jul 15;72(1):83–88. doi: 10.1097/00007890-200107150-00017. [DOI] [PubMed] [Google Scholar]
- 30.Ball AM, Gillen DL, Sherrard D, et al. Risk of hip fracture among dialysis and renal transplant recipients. JAMA : the journal of the American Medical Association. 2002 Dec 18;288(23):3014–3018. doi: 10.1001/jama.288.23.3014. [DOI] [PubMed] [Google Scholar]
- 31.Vautour LM, Melton LJ, 3rd, Clarke BL, Achenbach SJ, Oberg AL, McCarthy JT. Long-term fracture risk following renal transplantation: a population-based study. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2004 Feb;15(2):160–167. doi: 10.1007/s00198-003-1532-y. [DOI] [PubMed] [Google Scholar]
- 32.Grotz WH, Mundinger FA, Rasenack J, et al. Bone loss after kidney transplantation: a longitudinal study in 115 graft recipients. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 1995 Nov;10(11):2096–2100. [PubMed] [Google Scholar]
- 33.Chiu MY, Sprague SM, Bruce DS, Woodle ES, Thistlethwaite JR, Jr, Josephson MA. Analysis of fracture prevalence in kidney-pancreas allograft recipients. Journal of the American Society of Nephrology : JASN. 1998 Apr;9(4):677–683. doi: 10.1681/ASN.V94677. [DOI] [PubMed] [Google Scholar]
- 34.Nisbeth U, Lindh E, Ljunghall S, Backman U, Fellstrom B. Fracture frequency after kidney transplantation. Transplantation proceedings. 1994 Jun;26(3):1764. [PubMed] [Google Scholar]
- 35.Nisbeth U, Lindh E, Ljunghall S, Backman U, Fellstrom B. Increased fracture rate in diabetes mellitus and females after renal transplantation. Transplantation. 1999 May 15;67(9):1218–1222. doi: 10.1097/00007890-199905150-00004. [DOI] [PubMed] [Google Scholar]
- 36.KDIGO clinical practice guideline for the care of kidney transplant recipients. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2009 Nov;9(Suppl 3):S1–155. doi: 10.1111/j.1600-6143.2009.02834.x. [DOI] [PubMed] [Google Scholar]
- 37.Evenepoel P, Claes K, Kuypers D, Maes B, Bammens B, Vanrenterghem Y. Natural history of parathyroid function and calcium metabolism after kidney transplantation: a single-centre study. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2004 May;19(5):1281–1287. doi: 10.1093/ndt/gfh128. [DOI] [PubMed] [Google Scholar]
- 38.Borchhardt K, Sulzbacher I, Benesch T, Fodinger M, Sunder-Plassmann G, Haas M. Low-turnover bone disease in hypercalcemic hyperparathyroidism after kidney transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2007 Nov;7(11):2515–2521. doi: 10.1111/j.1600-6143.2007.01950.x. [DOI] [PubMed] [Google Scholar]
- 39.Gwinner W, Suppa S, Mengel M, et al. Early calcification of renal allografts detected by protocol biopsies: causes and clinical implications. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2005 Aug;5(8):1934–1941. doi: 10.1111/j.1600-6143.2005.00938.x. [DOI] [PubMed] [Google Scholar]
- 40.Rosenbaum RW, Hruska KA, Korkor A, Anderson C, Slatopolsky E. Decreased phosphate reabsorption after renal transplantation: Evidence for a mechanism independent of calcium and parathyroid hormone. Kidney international. 1981 Apr;19(4):568–578. doi: 10.1038/ki.1981.54. [DOI] [PubMed] [Google Scholar]
- 41.Levi M. Post-transplant hypophosphatemia. Kidney international. 2001 Jun;59(6):2377–2387. doi: 10.1046/j.1523-1755.2001.00755.x. [DOI] [PubMed] [Google Scholar]
- 42.Green J, Debby H, Lederer E, Levi M, Zajicek HK, Bick T. Evidence for a PTH-independent humoral mechanism in post-transplant hypophosphatemia and phosphaturia. Kidney international. 2001 Sep;60(3):1182–1196. doi: 10.1046/j.1523-1755.2001.0600031182.x. [DOI] [PubMed] [Google Scholar]
- 43.Wilkins GE, Granleese S, Hegele RG, Holden J, Anderson DW, Bondy GP. Oncogenic osteomalacia: evidence for a humoral phosphaturic factor. The Journal of clinical endocrinology and metabolism. 1995 May;80(5):1628–1634. doi: 10.1210/jcem.80.5.7745010. [DOI] [PubMed] [Google Scholar]
- 44.Bhan I, Shah A, Holmes J, et al. Post-transplant hypophosphatemia: Tertiary ‘Hyper-Phosphatoninism’? Kidney international. 2006 Oct;70(8):1486–1494. doi: 10.1038/sj.ki.5001788. [DOI] [PubMed] [Google Scholar]
- 45.Evenepoel P, Naesens M, Claes K, Kuypers D, Vanrenterghem Y. Tertiary ‘hyperphosphatoninism’ accentuates hypophosphatemia and suppresses calcitriol levels in renal transplant recipients. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2007 May;7(5):1193–1200. doi: 10.1111/j.1600-6143.2007.01753.x. [DOI] [PubMed] [Google Scholar]
- 46*.Trombetti A, Richert L, Hadaya K, et al. Early post-transplantation hypophosphatemia is associated with elevated FGF-23 levels. European journal of endocrinology / European Federation of Endocrine Societies. 2011 May;164(5):839–847. doi: 10.1530/EJE-10-1150. This recent study examined the hypothesis that high FGF-23 levels early after transplantation contribute to the onset of hypophosphatemia, independently of parathyroid hormone (PTH) and other factors regulating phosphate metabolism. They concluded that in early post-transplant period, elevated FGF-23 may contribute to hypophosphatemia in addition to PTH. [DOI] [PubMed] [Google Scholar]
- 47.Evenepoel P, Meijers BK, de Jonge H, et al. Recovery of hyperphosphatoninism and renal phosphorus wasting one year after successful renal transplantation. Clinical journal of the American Society of Nephrology : CJASN. 2008 Nov;3(6):1829–1836. doi: 10.2215/CJN.01310308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48**.Sampaio MS, Molnar MZ, Kovesdy CP, et al. Association of pretransplant serum phosphorus with posttransplant outcomes. Clinical journal of the American Society of Nephrology : CJASN. 2011 Nov;6(11):2712–2721. doi: 10.2215/CJN.06190611. A recent study of 9,384 kidney transplant recipients showing pretransplant phosphorus levels 7.5 to <9.5 mg/dl and >/=9.5 mg/dl were associated with increased risk of functional graft failure and increased risk of all-cause and cardiovascular deaths, respectively, when compared with 3.5 to <5.5 mg/dl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Connolly GM, Cunningham R, McNamee PT, Young IS, Maxwell AP. Elevated serum phosphate predicts mortality in renal transplant recipients. Transplantation. 2009 Apr 15;87(7):1040–1044. doi: 10.1097/TP.0b013e31819cd122. [DOI] [PubMed] [Google Scholar]
- 50**.Kovesdy CP, Mucsi I, Czira ME, et al. Association of serum phosphorus level with anemia in kidney transplant recipients. Transplantation. 2011 Apr 27;91(8):875–882. doi: 10.1097/TP.0b013e3182111edf. This observational cohort study finds higher serum phosphorus is independently associated with post-transplant anemia in kidney transplant recipients. [DOI] [PubMed] [Google Scholar]
- 51.Van Laecke S, Van Biesen W, Verbeke F, De Bacquer D, Peeters P, Vanholder R. Posttransplantation hypomagnesemia and its relation with immunosuppression as predictors of new-onset diabetes after transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2009 Sep;9(9):2140–2149. doi: 10.1111/j.1600-6143.2009.02752.x. [DOI] [PubMed] [Google Scholar]
- 52.Mazzola BL, Vannini SD, Truttmann AC, et al. Long-term calcineurin inhibition and magnesium balance after renal transplantation. Transplant international : official journal of the European Society for Organ Transplantation. 2003 Feb;16(2):76–81. doi: 10.1007/s00147-002-0479-9. [DOI] [PubMed] [Google Scholar]
- 53.Bonarek H, Merville P, Bonarek M, et al. Reduced parathyroid functional mass after successful kidney transplantation. Kidney international. 1999 Aug;56(2):642–649. doi: 10.1046/j.1523-1755.1999.00589.x. [DOI] [PubMed] [Google Scholar]
- 54.Reinhardt W, Bartelworth H, Jockenhovel F, et al. Sequential changes of biochemical bone parameters after kidney transplantation. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 1998 Feb;13(2):436–442. doi: 10.1093/oxfordjournals.ndt.a027843. [DOI] [PubMed] [Google Scholar]
- 55.Messa P, Sindici C, Cannella G, et al. Persistent secondary hyperparathyroidism after renal transplantation. Kidney international. 1998 Nov;54(5):1704–1713. doi: 10.1046/j.1523-1755.1998.00142.x. [DOI] [PubMed] [Google Scholar]
- 56.Kruse AE, Eisenberger U, Frey FJ, Mohaupt MG. The calcimimetic cinacalcet normalizes serum calcium in renal transplant patients with persistent hyperparathyroidism. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2005 Jul;20(7):1311–1314. doi: 10.1093/ndt/gfh924. [DOI] [PubMed] [Google Scholar]
- 57.Torres A, Lorenzo V, Salido E. Calcium metabolism and skeletal problems after transplantation. Journal of the American Society of Nephrology : JASN. 2002 Feb;13(2):551–558. doi: 10.1681/ASN.V132551. [DOI] [PubMed] [Google Scholar]
- 58.Lewin E. Involution of the parathyroid glands after renal transplantation. Current opinion in nephrology and hypertension. 2003 Jul;12(4):363–371. doi: 10.1097/00041552-200307000-00004. [DOI] [PubMed] [Google Scholar]
- 59.Ambrus C, Molnar MZ, Czira ME, et al. Calcium, phosphate and parathyroid metabolism in kidney transplanted patients. International urology and nephrology. 2009 Dec;41(4):1029–1038. doi: 10.1007/s11255-009-9631-0. [DOI] [PubMed] [Google Scholar]
- 60.Caravaca F, Fernandez MA, Cubero J, Aparicio A, Jimenez F, Garcia MC. Are plasma 1,25-dihydroxyvitamin D3 concentrations appropriate after successful kidney transplantation? Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 1998;13(Suppl 3):91–93. doi: 10.1093/ndt/13.suppl_3.91. [DOI] [PubMed] [Google Scholar]
- 61.Drueke TB. Primary and secondary uraemic hyperparathyroidism: from initial clinical observations to recent findings. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 1998 Jun;13(6):1384–1387. doi: 10.1093/ndt/13.6.1384. [DOI] [PubMed] [Google Scholar]
- 62.Krajisnik T, Olauson H, Mirza MA, et al. Parathyroid Klotho and FGF-receptor 1 expression decline with renal function in hyperparathyroid patients with chronic kidney disease and kidney transplant recipients. Kidney international. 2010 Nov;78(10):1024–1032. doi: 10.1038/ki.2010.260. [DOI] [PubMed] [Google Scholar]
- 63.Roodnat JI, van Gurp EA, Mulder PG, et al. High pretransplant parathyroid hormone levels increase the risk for graft failure after renal transplantation. Transplantation. 2006 Aug 15;82(3):362–367. doi: 10.1097/01.tp.0000228923.75739.88. [DOI] [PubMed] [Google Scholar]
- 64**.Kovesdy CP, Molnar MZ, Czira ME, et al. Diagnostic accuracy of serum parathyroid hormone levels in kidney transplant recipients with moderate-to-advanced CKD. Nephron Clinical practice. 2011;118(2):c78–85. doi: 10.1159/000320318. This recent observational study, almost 500 CKD stage 3 patients and 150 CKD stage 4 patients were examined to determine the sensitivity and specificity of the Kidney/Dialysis Outcome Quality Initiative-recommended PTH levels in detecting elevated serum beta-CrossLaps or osteocalcin levels. In conclusion, currently applied cutoffs for PTH in kidney transplant recipients with CKD stages 3 and 4 do not appear to adequately detect increased biochemical markers of bone turnover. Diagnostic uncertainty exists in patients with CKD stage 3 and PTH between 35 and 140 pg/ml, and CKD stage 4 and PTH between 70 and 240 pg/ml. [DOI] [PubMed] [Google Scholar]
- 65.Withold W, Friedrich W, Degenhardt S. Serum bone alkaline phosphatase is superior to plasma levels of bone matrix proteins for assessment of bone metabolism in patients receiving renal transplants. Clinicachimicaacta; international journal of clinical chemistry. 1997 May 28;261(2):105–115. doi: 10.1016/s0009-8981(97)06519-4. [DOI] [PubMed] [Google Scholar]
- 66*.Stein EM, Shane E. Vitamin D in organ transplantation. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2011 Jul;22(7):2107–2118. doi: 10.1007/s00198-010-1523-8. Recent review about the role of Vitamin D in solid organ tranplant patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67**.Wolf M, Molnar MZ, Amaral AP, et al. Elevated fibroblast growth factor 23 is a risk factor for kidney transplant loss and mortality. Journal of the American Society of Nephrology : JASN. 2011 May;22(5):956–966. doi: 10.1681/ASN.2010080894. First prospective study with almost 1,000 prevalent kidney transplant recipients showing that FGF23 is and independent and significant predictor of mortality and graft loss. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Duong U, Mehrotra R, Kovesdy CP, et al. Comparing mortality risk of minerals, PTH and alkaline phosphatase over 6 years in 12,422 chronic peritoneal dialysis (CPD) patients. Am J Kidney Dis; NKF Spring Clinical Meetings; April 26-30, 2011; Las Vegas, NV. 2011. abstract NKF. [Google Scholar]
- 69.Movsowitz C, Epstein S, Fallon M, Ismail F, Thomas S. Cyclosporin-A in vivo produces severe osteopenia in the rat: effect of dose and duration of administration. Endocrinology. 1988 Nov;123(5):2571–2577. doi: 10.1210/endo-123-5-2571. [DOI] [PubMed] [Google Scholar]
- 70.Epstein S. Post-transplantation bone disease: the role of immunosuppressive agents and the skeleton. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 1996 Jan;11(1):1–7. doi: 10.1002/jbmr.5650110102. [DOI] [PubMed] [Google Scholar]
- 71.Bone histology in renal transplant patients receiving cyclosporin. Lancet. 1988 May 7;1(8593):1048–1049. [PubMed] [Google Scholar]
- 72.Dumoulin G, Hory B, Nguyen NU, et al. Lack of evidence that cyclosporine treatment impairs calcium-phosphorus homeostasis and bone remodeling in normocalcemic long-term renal transplant recipients. Transplantation. 1995 Jun 27;59(12):1690–1694. doi: 10.1097/00007890-199506270-00008. [DOI] [PubMed] [Google Scholar]
- 73.Canalis E, Delany AM. Mechanisms of glucocorticoid action in bone. Annals of the New York Academy of Sciences. 2002 Jun;966:73–81. doi: 10.1111/j.1749-6632.2002.tb04204.x. [DOI] [PubMed] [Google Scholar]
- 74.Canalis E. Mechanisms of glucocorticoid-induced osteoporosis. Current opinion in rheumatology. 2003 Jul;15(4):454–457. doi: 10.1097/00002281-200307000-00013. [DOI] [PubMed] [Google Scholar]
- 75.Lukert BP, Raisz LG. Glucocorticoid-induced osteoporosis: pathogenesis and management. Annals of internal medicine. 1990 Mar 1;112(5):352–364. doi: 10.7326/0003-4819-112-5-352. [DOI] [PubMed] [Google Scholar]
- 76.Cunningham J. Pathogenesis and prevention of bone loss in patients who have kidney disease and receive long-term immunosuppression. Journal of the American Society of Nephrology : JASN. 2007 Jan;18(1):223–234. doi: 10.1681/ASN.2006050427. [DOI] [PubMed] [Google Scholar]
- 77.Cunningham J. Posttransplantation bone disease. Transplantation. 2005 Mar 27;79(6):629–634. doi: 10.1097/01.tp.0000149698.79739.ef. [DOI] [PubMed] [Google Scholar]
- 78.Ugur A, Guvener N, Isiklar I, Turan M, Erdal R, Haberal M. Osteoporosis after renal transplantation: single center experience. Transplantation. 2001 Mar 15;71(5):645–649. doi: 10.1097/00007890-200103150-00011. [DOI] [PubMed] [Google Scholar]
- 79.Marcen R, Caballero C, Pascual J, et al. Lumbar bone mineral density in renal transplant patients on neoral and tacrolimus: a four-year prospective study. Transplantation. 2006 Mar 27;81(6):826–831. doi: 10.1097/01.tp.0000203557.36884.e3. [DOI] [PubMed] [Google Scholar]
- 80.Patel S, Kwan JT, McCloskey E, et al. Prevalence and causes of low bone density and fractures in kidney transplant patients. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2001 Oct;16(10):1863–1870. doi: 10.1359/jbmr.2001.16.10.1863. [DOI] [PubMed] [Google Scholar]
- 81.Joffe I, Katz I, Sehgal S, et al. Lack of change of cancellous bone volume with short-term use of the new immunosuppressant rapamycin in rats. Calcified tissue international. 1993 Jul;53(1):45–52. doi: 10.1007/BF01352014. [DOI] [PubMed] [Google Scholar]
- 82.Bryer HP, Isserow JA, Armstrong EC, et al. Azathioprine alone is bone sparing and does not alter cyclosporin A-induced osteopenia in the rat. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 1995 Jan;10(1):132–138. doi: 10.1002/jbmr.5650100119. [DOI] [PubMed] [Google Scholar]
- 83.Dissanayake IR, Goodman GR, Bowman AR, et al. Mycophenolatemofetil: a promising new immunosuppressant that does not cause bone loss in the rat. Transplantation. 1998 Jan 27;65(2):275–278. doi: 10.1097/00007890-199801270-00025. [DOI] [PubMed] [Google Scholar]
- 84.Singha UK, Jiang Y, Yu S, et al. Rapamycin inhibits osteoblast proliferation and differentiation in MC3T3-E1 cells and primary mouse bone marrow stromal cells. Journal of cellular biochemistry. 2008 Feb 1;103(2):434–446. doi: 10.1002/jcb.21411. [DOI] [PubMed] [Google Scholar]
- 85.Kneissel M, Luong-Nguyen NH, Baptist M, et al. Everolimus suppresses cancellousbone loss, bone resorption, and cathepsin K expression by osteoclasts. Bone. 2004 Nov;35(5):1144–1156. doi: 10.1016/j.bone.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 86.Elder GJ. From marrow oedema to osteonecrosis: common paths in the development of post-transplant bone pain. Nephrology (Carlton) 2006 Dec;11(6):560–567. doi: 10.1111/j.1440-1797.2006.00708.x. [DOI] [PubMed] [Google Scholar]
- 87.Paydas S, Balal M, Demir E, Sertdemir Y, Erken U. Avascular osteonecrosis and accompanying anemia, leucocytosis, and decreased bone mineral density in renal transplant recipients. Transplantation proceedings. 2011 Apr;43(3):863–866. doi: 10.1016/j.transproceed.2011.02.072. [DOI] [PubMed] [Google Scholar]
- 88.Akaberi S, Lindergard B, Simonsen O, Nyberg G. Impact of parathyroid hormone on bone density in long-term renal transplant patients with good graft function. Transplantation. 2006 Sep 27;82(6):749–752. doi: 10.1097/01.tp.0000230130.50451.78. [DOI] [PubMed] [Google Scholar]
- 89.Molnar MZ, Ojo AO, Bunnapradist S, Kovesdy CP, Kalantar-Zadeh K. Timing of dialysis initiation in transplant-naive and failed transplant patients. Nature reviews Nephrology. 2012 Feb 28; doi: 10.1038/nrneph.2012.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Molnar MZ, Streja E, Kovesdy CP, et al. Estimated Glomerular Filtration Rate at Re-Initiation of Dialysis and Mortality in Failed Kidney Transplant Recipients. Nephrol Dial Transplant. 2012 doi: 10.1093/ndt/gfs004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kasiske BL, Zeier MG, Chapman JR, et al. KDIGO clinical practice guideline for the care of kidney transplant recipients: a summary. Kidney international. 2010 Feb;77(4):299–311. doi: 10.1038/ki.2009.377. [DOI] [PubMed] [Google Scholar]
- 92.Palmer SC, McGregor DO, Strippoli GF. Interventions for preventing bone disease in kidney transplant recipients. Cochrane Database Syst Rev. 2007;(3) doi: 10.1002/14651858.CD005015.pub3. CD005015. [DOI] [PubMed] [Google Scholar]
- 93.Shane E, Addesso V, Namerow PB, et al. Alendronate versus calcitriol for the prevention of bone loss after cardiac transplantation. N Engl J Med. 2004 Feb 19;350(8):767–776. doi: 10.1056/NEJMoa035617. [DOI] [PubMed] [Google Scholar]
- 94.Kovac D, Lindic J, Kandus A, Bren AF. Prevention of bone loss with alendronate in kidney transplant recipients. Transplantation. 2000 Nov 27;70(10):1542–1543. doi: 10.1097/00007890-200011270-00028. [DOI] [PubMed] [Google Scholar]
- 95.Giannini S, D’Angelo A, Carraro G, et al. Alendronate prevents further bone loss in renal transplant recipients. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2001 Nov;16(11):2111–2117. doi: 10.1359/jbmr.2001.16.11.2111. [DOI] [PubMed] [Google Scholar]
- 96.Jeffery JR, Leslie WD, Karpinski ME, Nickerson PW, Rush DN. Prevalence and treatment of decreased bone density in renal transplant recipients: a randomized prospective trial of calcitriol versus alendronate. Transplantation. 2003 Nov 27;76(10):1498–1502. doi: 10.1097/01.TP.0000092523.30277.13. [DOI] [PubMed] [Google Scholar]
- 97.Haas M, Leko-Mohr Z, Roschger P, et al. Zoledronic acid to prevent bone loss in the first 6 months after renal transplantation. Kidney international. 2003 Mar;63(3):1130–1136. doi: 10.1046/j.1523-1755.2003.00816.x. [DOI] [PubMed] [Google Scholar]
- 98.Schwarz C, Mitterbauer C, Heinze G, Woloszczuk W, Haas M, Oberbauer R. Nonsustained effect of short-term bisphosphonate therapy on bone turnover three years after renal transplantation. Kidney international. 2004 Jan;65(1):304–309. doi: 10.1111/j.1523-1755.2004.00369.x. [DOI] [PubMed] [Google Scholar]
- 99.Fan SL, Kumar S, Cunningham J. Long-term effects on bone mineral density of pamidronate given at the time of renal transplantation. Kidney international. 2003 Jun;63(6):2275–2279. doi: 10.1046/j.1523-1755.2003.00012.x. [DOI] [PubMed] [Google Scholar]
- 100.Coco M, Glicklich D, Faugere MC, et al. Prevention of bone loss in renal transplant recipients: a prospective, randomized trial of intravenous pamidronate. Journal of the American Society of Nephrology : JASN. 2003 Oct;14(10):2669–2676. doi: 10.1097/01.asn.0000087092.53894.80. [DOI] [PubMed] [Google Scholar]
- 101.Walsh SB, Altmann P, Pattison J, et al. Effect of pamidronate on bone loss after kidney transplantation: a randomized trial. Am J Kidney Dis. 2009 May;53(5):856–865. doi: 10.1053/j.ajkd.2008.11.036. [DOI] [PubMed] [Google Scholar]
- 102.Torregrosa JV, Fuster D, Monegal A, et al. Efficacy of low doses of pamidronate in osteopenic patients administered in the early post-renal transplant. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2011 Jan;22(1):281–287. doi: 10.1007/s00198-010-1197-2. [DOI] [PubMed] [Google Scholar]
- 103.Grotz W, Nagel C, Poeschel D, et al. Effect of ibandronate on bone loss and renal function after kidney transplantation. Journal of the American Society of Nephrology : JASN. 2001 Jul;12(7):1530–1537. doi: 10.1681/ASN.V1271530. [DOI] [PubMed] [Google Scholar]
- 104.Torregrosa JV, Fuster D, Gentil MA, et al. Open-label trial: effect of weekly risedronate immediately after transplantation in kidney recipients. Transplantation. 2010 Jun 27;89(12):1476–1481. doi: 10.1097/TP.0b013e3181dc13d0. [DOI] [PubMed] [Google Scholar]
- 105.Nowacka-Cieciura E, Cieciura T, Baczkowska T, et al. Bisphosphonates are effective prophylactic of early bone loss after renal transplantation. Transplantation proceedings. 2006 Jan-Feb;38(1):165–167. doi: 10.1016/j.transproceed.2005.12.041. [DOI] [PubMed] [Google Scholar]
- 106.Cueto-Manzano AM, Konel S, Freemont AJ, et al. Effect of 1,25-dihydroxyvitamin D3 and calcium carbonate on bone loss associated with long-term renal transplantation. Am J Kidney Dis. 2000 Feb;35(2):227–236. doi: 10.1016/s0272-6386(00)70331-3. [DOI] [PubMed] [Google Scholar]
- 107.De Sevaux RG, Hoitsma AJ, Corstens FH, Wetzels JF. Treatment with vitamin D and calcium reduces bone loss after renal transplantation: a randomized study. Journal of the American Society of Nephrology : JASN. 2002 Jun;13(6):1608–1614. doi: 10.1097/01.asn.0000016082.70875.36. [DOI] [PubMed] [Google Scholar]
- 108.El-Agroudy AE, El-Husseini AA, El-Sayed M, Ghoneim MA. Preventing bone loss in renal transplant recipients with vitamin D. Journal of the American Society of Nephrology : JASN. 2003 Nov;14(11):2975–2979. doi: 10.1097/01.asn.0000093255.56474.b4. [DOI] [PubMed] [Google Scholar]
- 109.Torres A, Garcia S, Gomez A, et al. Treatment with intermittent calcitriol and calcium reduces bone loss after renal transplantation. Kidney international. 2004 Feb;65(2):705–712. doi: 10.1111/j.1523-1755.2004.00432.x. [DOI] [PubMed] [Google Scholar]
- 110.Josephson MA, Schumm LP, Chiu MY, Marshall C, Thistlethwaite JR, Sprague SM. Calcium and calcitriol prophylaxis attenuates posttransplant bone loss. Transplantation. 2004 Oct 27;78(8):1233–1236. doi: 10.1097/01.tp.0000137937.44703.42. [DOI] [PubMed] [Google Scholar]
- 111.Perez V, Sanchez A, Bayes B, et al. Effect of paricalcitol on the urinary peptidome of kidney transplant patients. Transplantation proceedings. 2010 Oct;42(8):2924–2927. doi: 10.1016/j.transproceed.2010.07.077. [DOI] [PubMed] [Google Scholar]
- 112.Serra AL, Schwarz AA, Wick FH, Marti HP, Wuthrich RP. Successful treatment of hypercalcemia with cinacalcet in renal transplant recipients with persistent hyperparathyroidism. Nephrology, dialysis, transplantation :official publication of the European Dialysis and Transplant Association - European Renal Association. 2005 Jul;20(7):1315–1319. doi: 10.1093/ndt/gfh925. [DOI] [PubMed] [Google Scholar]
- 113.Szwarc I, Argiles A, Garrigue V, et al. Cinacalcet chloride is efficient and safe in renal transplant recipients with posttransplant hyperparathyroidism. Transplantation. 2006 Sep 15;82(5):675–680. doi: 10.1097/01.tp.0000232452.80018.ad. [DOI] [PubMed] [Google Scholar]
- 114.Srinivas TR, Schold JD, Womer KL, et al. Improvement in hypercalcemia with cinacalcet after kidney transplantation. Clinical journal of the American Society of Nephrology : CJASN. 2006 Mar;1(2):323–326. doi: 10.2215/CJN.00500705. [DOI] [PubMed] [Google Scholar]
- 115.Bergua C, Torregrosa JV, Cofan F, Oppenheimer F. Cinacalcet for the treatment of hypercalcemia in renal transplanted patients with secondary hyperparathyroidism. Transplantation proceedings. 2007 Sep;39(7):2254–2255. doi: 10.1016/j.transproceed.2007.07.079. [DOI] [PubMed] [Google Scholar]
- 116.Bergua C, Torregrosa JV, Fuster D, Gutierrez-Dalmau A, Oppenheimer F, Campistol JM. Effect of cinacalcet on hypercalcemia and bone mineral density in renal transplanted patients with secondary hyperparathyroidism. Transplantation. 2008 Aug 15;86(3):413–417. doi: 10.1097/TP.0b013e31817c13e1. [DOI] [PubMed] [Google Scholar]
- 117.Lopez V, Toledo R, Sola E, et al. Treatment with cinacalcet in 29 kidney transplant patients with persistent hyperparathyroidism. Transplantation proceedings. 2009 Jul-Aug;41(6):2394–2395. doi: 10.1016/j.transproceed.2009.06.055. [DOI] [PubMed] [Google Scholar]
- 118.Borchhardt KA, Diarra D, Sulzbacher I, Benesch T, Haas M, Sunder-Plassmann G. Cinacalcet decreases bone formation rate in hypercalcemic hyperparathyroidism after kidney transplantation. American journal of nephrology. 2010;31(6):482–489. doi: 10.1159/000304180. [DOI] [PubMed] [Google Scholar]
- 119.Cho ME, Duan Z, Chamberlain CE, Reynolds JC, Ring MS, Mannon RB. Cinacalcet improves bone density in post-kidney transplant hyperparathyroidism. Transplantation proceedings. 2010 Nov;42(9):3554–3558. doi: 10.1016/j.transproceed.2010.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Copley JB, Germain M, Stern L, et al. Evaluation of cinacalcet HCl treatment after kidney transplantation. Transplantation proceedings. 2010 Sep;42(7):2503–2508. doi: 10.1016/j.transproceed.2010.04.052. [DOI] [PubMed] [Google Scholar]
- 121.Schwarz A, Merkel S, Leitolf H, Haller H. The effect of cinacalcet on bone remodeling and renal function in transplant patients with persistent hyperparathyroidism. Transplantation. 2011 Mar 15;91(5):560–565. doi: 10.1097/TP.0b013e3182079431. [DOI] [PubMed] [Google Scholar]
- 122.Pinho LR, Ribeiro Santos MJ, Pestana Vasconcelos M. Cinacalcet in the treatment of persistent hyperparathyroidism after kidney transplantation. Clinical nephrology. 2011 Mar;75(3):263–268. doi: 10.5414/cnp75263. [DOI] [PubMed] [Google Scholar]
- 123.Nikkel LE, Mohan S, Zhang A, et al. Reduced fracture risk with early corticosteroid withdrawal after kidney transplant. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012 Mar;12(3):649–659. doi: 10.1111/j.1600-6143.2011.03872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.van den Ham EC, Kooman JP, Christiaans ML, van Hooff JP. The influence of early steroid withdrawal on body composition and bone mineral density in renal transplantation patients. Transplant international : official journal of the European Society for Organ Transplantation. 2003 Feb;16(2):82–87. doi: 10.1007/s00147-002-0488-8. [DOI] [PubMed] [Google Scholar]
- 125.Kasiske BL, Chakkera HA, Louis TA, Ma JZ. A meta-analysis of immunosuppression withdrawal trials in renal transplantation. Journal of the American Society of Nephrology : JASN. 2000 Oct;11(10):1910–1917. doi: 10.1681/ASN.V11101910. [DOI] [PubMed] [Google Scholar]
- 126.Pascual J, Quereda C, Zamora J, Hernandez D. Steroid withdrawal in renal transplant patients on triple therapy with a calcineurin inhibitor and mycophenolatemofetil: a meta-analysis of randomized, controlled trials. Transplantation. 2004 Nov 27;78(10):1548–1556. doi: 10.1097/01.tp.0000140969.43761.1f. [DOI] [PubMed] [Google Scholar]
- 127.Stein EM, Ortiz D, Jin Z, McMahon DJ, Shane E. Prevention of fractures after solid organ transplantation: a meta-analysis. The Journal of clinical endocrinology and metabolism. 2011 Nov;96(11):3457–3465. doi: 10.1210/jc.2011-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Talalaj M, Gradowska L, Marcinowska-Suchowierska E, Durlik M, Gaciong Z, Lao M. Efficiency of preventive treatment of glucocorticoid-induced osteoporosis with 25-hydroxyvitamin D3 and calcium in kidney transplant patients. Transplantation proceedings. 1996 Dec;28(6):3485–3487. [PubMed] [Google Scholar]
- 129.Noori N, Kalantar-Zadeh K, Kovesdy CP, Rachelle B, Benner D, Kopple JD. Association of dietary phosphorus to protein ratio with mortality in hemodialysis patients. Am J Kidney Dis. 2010;(suppl issue) doi: 10.2215/CJN.08601209. abstract NKF Spring Clinical Meeting. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zitt E, Woess E, Mayer G, Lhotta K. Effect of cinacalcet on renal electrolyte handling and systemic arterial blood pressure in kidney transplant patients with persistent hyperparathyroidism. Transplantation. 2011 Oct 27;92(8):883–889. doi: 10.1097/TP.0b013e31822d87e8. [DOI] [PubMed] [Google Scholar]
- 131.Cejka D, Benesch T, Krestan C, et al. Effect of teriparatide on early bone loss after kidney transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2008 Sep;8(9):1864–1870. doi: 10.1111/j.1600-6143.2008.02327.x. [DOI] [PubMed] [Google Scholar]
- 132.Nogueira EL, Costa AC, Santana A, et al. Teriparatide efficacy in the treatment of severe hypocalcemia after kidney transplantation in parathyroidectomized patients: a series of five case reports. Transplantation. 2011 Aug 15;92(3):316–320. doi: 10.1097/TP.0b013e3182247b98. [DOI] [PubMed] [Google Scholar]
- 133.El-Husseini AA, El-Agroudy AE, El-Sayed MF, Sobh MA, Ghoneim MA. Treatment of osteopenia and osteoporosis in renal transplant children and adolescents. Pediatric transplantation. 2004 Aug;8(4):357–361. doi: 10.1111/j.1399-3046.2004.00191.x. [DOI] [PubMed] [Google Scholar]
- 134.Grotz WH, Rump LC, Niessen A, et al. Treatment of osteopenia and osteoporosis after kidney transplantation. Transplantation. 1998 Oct 27;66(8):1004–1008. doi: 10.1097/00007890-199810270-00007. [DOI] [PubMed] [Google Scholar]
- 135.Mitchell MJ, Baz MA, Fulton MN, Lisor CF, Braith RW. Resistance training prevents vertebral osteoporosis in lung transplant recipients. Transplantation. 2003 Aug 15;76(3):557–562. doi: 10.1097/01.TP.0000076471.25132.52. [DOI] [PubMed] [Google Scholar]
- 136.Braith RW, Magyari PM, Fulton MN, Aranda J, Walker T, Hill JA. Resistance exercise training and alendronate reverse glucocorticoid-induced osteoporosis in heart transplant recipients. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2003 Oct;22(10):1082–1090. doi: 10.1016/s1053-2498(02)01184-1. [DOI] [PubMed] [Google Scholar]


