Abstract
Background
Recent studies show a survival advantage with kidney transplant amongst elderly patients compared to those on dialysis.
Study Design
In our present study we examined and compared the association of expanded donor criteria (ECD) kidney and living kidney donation with outcome of kidney transplant across different ages including elderly recipients.
Setting and Participants
Using the Scientific Registry of Transplant Recipients, we identified 145,470 adult kidney transplanted patients. Mortality and death-censored graft failure risks were estimated by Cox proportional regression analyses over a follow-up period with a median of 3.9 years.
Predictors
ECD kidney and living kidney donation and age compared to others.
Outcomes
Mortality and death-censored graft failure risk.
Results
Patients were 45±16 years old and included 40% women and 19% diabetics. Compared to transplanted patients 55-<65 years old, the fully adjusted death-censored graft failure risk was somewhat higher in patients ≥75 years old (HR, 1.30; 95% CI, 1.09–1.56), 35-<55 years (HR, 1.13; 95% CI, 1.08–1.17) and 18-<35 years (HR, 1.64; 95% CI, 1.57–1.71). Compared to non-ECD kidneys, ECD kidneys were significant predictors of mortality in non-elderly patients (18–<35 years: HR, 1.46 [95% CI, 1.19–1.77]; 35-<55 years: HR, 1.23 [95% CI, 1.14–1.32]; and 55-<65 years: HR, 1.26 [95% CI, 1.15–1.38]) and patients aged 65-<70 years (HR, 1.20; 9% CI, 1.05–1.36); but not in other groups of elderly patients (HRs of 1.12 [95% CI, 0.93–1.36] 70-<75 years and 1.04 [95% CI, 0.74–1.47] for ≥75 years). Similar results were found in risk of graft loss. Compared to deceased donor, living kidney was associated with better survival in all age groups and lower graft loss risk in patients aged <70 years.
Limitations
Unmeasured confounders cannot be adjusted for.
Conclusions
Among deceased donors, the ECD kidneys are not associated with either increased mortality or graft failure in recipients over 70 years. Among all types of donors, the persistent association between living donor kidneys and lower all-cause mortality across all ages suggests that, if possible, elderly patients gain longevity from living donor kidney transplant.
Keywords: elderly, kidney transplantation, mortality, graft failure, living donor
Individuals older than 65 years old, the so-called elderly, make up the fastest growing population group in United States and most developed countries. Based on data in the 2008 report of the United States census bureau, this sector of the population grew from 29.6 million in 1990 to 36.8 million in 2008, equivalent to a 20% incremental growth. As a result of the absolute increase of the elderly in the population distribution, the prevalence and incidence of chronic diseases such as hypertension, diabetes mellitus, kidney disease, coronary artery disease, and heart failure have also increased overall.(1–4) The growth in the population of patients with end stage renal disease (ESRD) during the past 20 years has occurred principally in the elderly.(5) The adjusted incidence rate of ESRD for patients who are older than 75 years was 1744 per million population whereas it was only 127 per million population for those between 20 and 44 years old.(5) In 2006, 49% of the incident ESRD population was older than 65 years, and 26% were ≥75 years.(5) Based on these statistics, one of every 200 US adults older than 75 years is estimated to have ESRD.(5)
Kidney transplant recipients are increasingly older at the time of transplant. In the 2008 Scientific Registry of Transplant Recipients (SRTR), for instance, recipients of deceased non-expanded criteria donor (non-ECD) kidneys in 2007 who were between ages 50 to 64 years and 65 years and older constituted 39 and 13 percent of the total pool, respectively.(6) By comparison, these age groups comprised only 29 and 5 percent of all such recipients in 1993, respectively. Currently, 16,496 ESRD patients older than 65 years are waiting for a kidney transplant; these individuals constitute 18.1% of all listed candidates(6) and need a special pre-transplant evaluation process.(7) The accuracy of previous studies comparing survival after kidney transplant with continued dialysis might have suffered methodological flaws such as selection bias.(8, 9) Subsequent reports that have avoided these methodological problems show that, compared with those who are dialyzed, elderly individuals who receive transplants have a survival advantage, including transplants patients given ECD kidneys.(10–15) Wolfe et al(10) found that among patients aged 60-<75 years who received a primary deceased-donor transplant, the cumulative survival rate, in comparison to maintenance hemodialysis patients, improved after the first year post-transplant, with a projected 4-year increase in life span, along with a 61% reduction in the long-term risk of death. A more granular look at the data shows the projected increase life spans were 4.3 years, 2.8 years, and 1.0 year, for patients aged 60–64 years, 65–69 years, and 70–74 years, respectively.(10) Additionally, with present therapy, elderly patient survival at one, five, and ten years is approximately 80 to 90, 70, and 50 percent, respectively.(16–24) Recent studies show a survival advantage with kidney transplant among the elderly, including the recipients of expanded criteria donor kidneys, in comparison to those patients treated with dialysis.(10–15)
Very few studies have attempted to identify factors capable of predicting outcomes in elderly kidney transplant recipients. According to a 2009 study, acute rejection in the first 90 days and donor age ≥60 years were predictors of lower patient survival; by contrast, delayed graft function, donor age of 60 years or more, and HLA antibodies were associated with greater death-censored graft loss.(25) Time on dialysis prior to transplant was also a significant risk predictor in patients older than 70 years.(25) An article published in 2008 reported that elderly kidney transplant recipients who received organs from living donors aged >55 years had worse 3-year graft survival (86%) but nearly equivalent 3-year patient survival rates (88%) in comparison to counterparts receiving kidneys from living donors ≤55 years.(26)
To our knowledge only a few studies have compared the association of expanded donor criteria (ECD) kidney and living kidney donation with kidney transplant outcomes across different age groups including in the elderly patients. In the present study, we examined the association of ECD kidney and living kidney donation with outcomes from kidney transplant amongst different age ranges with focus on the elderly recipients.
Methods
Patients
We extracted, refined, and examined data from all kidney transplant recipients listed in the SRTR up to December 2006. The SRTR data system includes data on all transplant donors, wait-listed candidates and transplant recipients in the US, which are submitted by members of the Organ Procurement and Transplantation Network. This study was approved by the Institutional Review Committees of Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center. Because of the large sample size, the anonymity of the patients studied and the non-intrusive nature of the research, the requirement for informed consent was waived.
Clinical, Demographic and Laboratory Measures
Demographic data and details of medical history were collected, including information on age, gender, race, ethnicity, type of insurance, presence of diabetes, and dialysis vintage. Dialysis vintage was defined as the duration of time between the first day of dialysis treatment and the day of kidney transplant. Information on the recipient’s serum creatinine, serum albumin, weight and height (for calculation of body mass index (BMI), and information on six co-morbidities: coronary artery disease, chronic obstructive pulmonary disease, hypertension, peptic ulcer, peripheral vascular disease, cerebrovascular disease was also collected. We divided the entire population into six age groups of 18-<35, 35-<55, 55-<65, 65-<70, 70-<75 and ≥75 years. The last 3 groups (≥65 years) were considered the elderly.
Statistical Methods
For survival analysis we used Cox proportional hazard regression models separately across the different age groups. Two predefined outcomes were analyzed: all-cause death and death censored graft failure. Graft failure was defined as re-initiation of dialysis treatment or repeat transplant. In our graft failure analysis, the patients were followed until graft failure or censoring (death or the end of the follow-up period) whichever happened first. Based on the detected association between age groups and death censored graft failure, which appeared to be U-shaped, we tested non-linearity by adding the quadric term of age to the models which already had the linear term. For each analysis, three models were examined based on the level of multivariate adjustment:
An unadjusted model that included mortality or graft failure data and time post-kidney transplant, and entry calendar quarter (year and quarter of kidney transplant date);
Case-mix adjusted models that included age, gender, race-ethnicity (African Americans and other self-categorized African-Americans, Non-Hispanic Whites, Asians, Hispanics and others), diabetes mellitus, dialysis vintage, six previously mentioned co-morbidities and three most recent laboratory measures before transplant: serum creatinine, serum albumin, and BMI.
fully adjusted models which included all of the covariates in the case-mix model as well as the following transplant data: (1) donor type (deceased or living), (2) donor age, (3) panel reactive antibody (PRA) titer (last value prior to transplant), (4) number of HLA mismatches, (5) cold ischemia time, (6) expanded donor criteria (EDC) using standard definition (donor history of hypertension and/or donor serum creatinine > 1.5 mg/dL and/or cause of death in donor is cerebrovascular event) and (7) history of acute rejection. EDC analysis was restricted to deceased donors.
All analyses were carried out with SAS version 9.1, SAS Institute Inc., www.sas.com.
Results
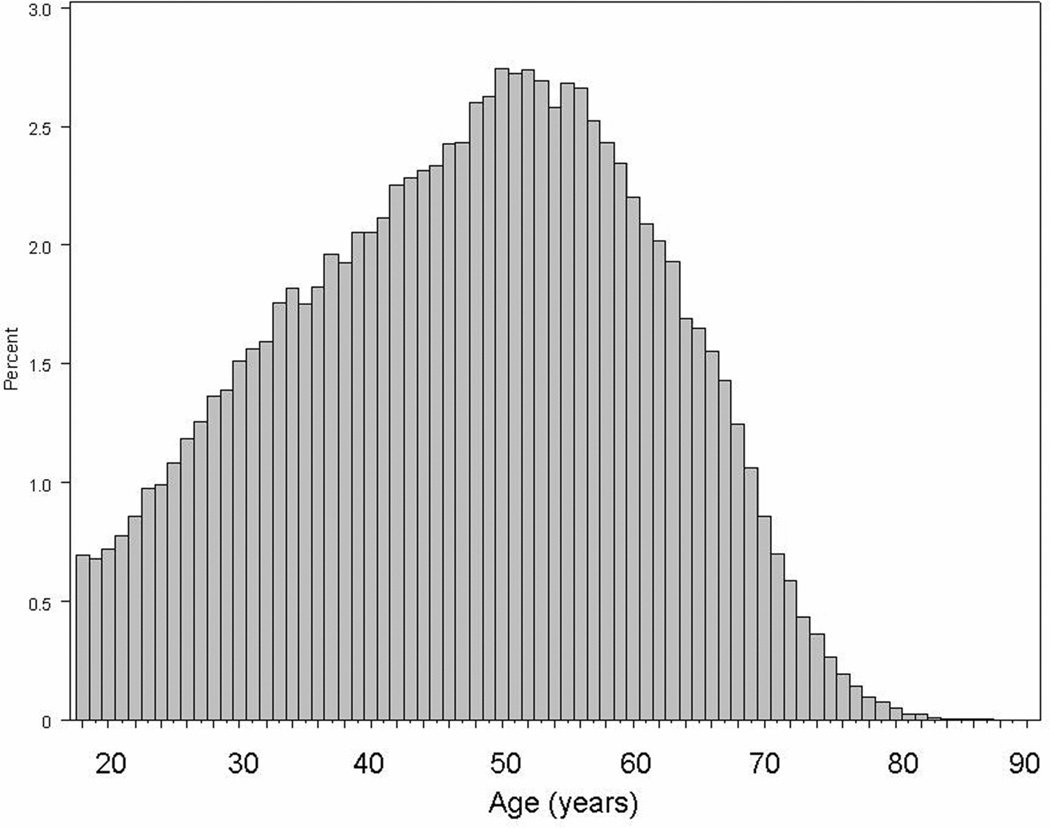
Using the SRTR database we identified 145,470 adult kidney transplanted patients. There were 25,616 deaths (18%) and 22,876 graft failures (16%). The median follow-up time was 1449 (25th–75th percentile, 636–2497) days. Figure 1 shows the age distribution of the 145,470 kidney transplanted patients. Only 11% of patients were older than 65 years. Table 1 shows the clinical, demographic, laboratory, and transplant data of the 145,470 kidney transplanted patients across six different age groups including 3 group younger and 3 older than 65 years. The crude mortality rate was incrementally higher across older groups, whereas the graft failure rate was lower in older groups. Older patients included less women and more non-Hispanic white recipients. The percentage of deceased donors, usage of expanded donor criteria kidneys and older donors was incrementally higher in older groups.
Figure 1.
Distribution of age in 145,470 kidney transplant patients
Table 1.
selected clinical and laboratory values of Incremental categories of age of kidney transplant recipients
| Non-elderly | Elderly | p-for trend | |||||
|---|---|---|---|---|---|---|---|
| 18–<35 y | 35–<55 y | 55–<65 y | 65–<70 y | 70–<75 y | >=75 y | ||
| No. of recipients | 29,404 (20) | 67,552 (46) | 32,847 (23) | 10,101 (7) | 4,271 (3) | 1,295 (1) | <0.001 |
| Cohort time (× 1,000 person-years) | 174.3 | 329.5 | 127.0 | 34.1 | 13.0 | 3.2 | <0.001 |
| No. of Deaths* | 3050 [10] | 10258 [15] | 7259 [22] | 2759 [27] | 1178 [28] | 397 [31] | <0.001 |
| Deaths per 10,000 person-years | 174.9 | 311.3 | 571.6 | 809.4 | 905.5 | 1226.3 | <0.001 |
| No. of Graft failures* | 6306 [21] | 9271 [14] | 3500 [11] | 1014 [10] | 397 [9] | 126 [10] | <0.001 |
| Graft failures per 10,000 person-years | 361.7 | 281.3 | 275.6 | 297.5 | 305.1 | 389.2 | <0.001 |
| Acute rejection episode | 1900 (23) | 3409 (15) | 1393 (11) | 365 (9) | 180 (9) | 59 (9) | <0.001 |
| Graft thrombosis | 55 (7) | 121 (7) | 52 (6) | 16 (5) | 3 (2) | 2 (4) | 0.3 |
| Age (years) | 27±5 | 45±6 | 59±3 | 67±1 | 72±1 | 77±2 | <0.001 |
| women | 42 | 40 | 39 | 38 | 34 | 27 | <0.001 |
| Race | |||||||
| Caucasian | 55 | 57 | 60 | 64 | 70 | 79 | <0.001 |
| Hispanic) | 15 | 12 | 11 | 10 | 9 | 6 | <0.001 |
| Asian-American) | 4 | 4 | 4 | 4 | 4 | 3 | 0.05 |
| African-American) | 23 | 24 | 23 | 19 | 16 | 11 | <0.001 |
| Diabetes mellitus | 7 | 19 | 30 | 29 | 23 | 15 | <0.001 |
| BMI (kg/m2) | 24.9±5.5 | 27.0±5.6 | 27.7±5.3 | 27.3±4.9 | 26.8±4.5 | 26.3±4.3 | <0.001 |
| Time on waiting list (days)** | 566 [361, 136–802] | 626 [407, 154–908] | 604 [408, 159–883] | 585 [406, 160–852] | 593 [414, 161–883] | 553 [388, 174–788] | <0.001 |
| Comorbidities: | |||||||
| Angina | 3 | 9 | 18 | 22 | 21 | 21 | <0.001 |
| Cerebro-vascular disease | 1 | 2 | 3 | 4 | 4 | 3 | <0.001 |
| COPD | 1 | 1 | 1 | 2 | 2 | 1 | <0.001 |
| Hypertension | 78 | 83 | 84 | 85 | 84 | 85 | <0.001 |
| Peripheral vascular disease | 1 | 3 | 6 | 6 | 5 | 4 | <0.001 |
| Peptic ulcer | 3 | 4 | 6 | 6 | 6 | 6 | <0.001 |
| Recipient serum creatinine (mg/dL) | 9.2±3.8 | 8.4±3.5 | 7.5±3.0 | 7.0±2.7 | 6.9±2.6 | 6.6±2.5 | <0.001 |
| Recipient serum albumin (g/dL) | 3.90±0.71 | 3.86±0.68 | 3.82±0.62 | 3.84±0.59 | 3.86±0.60 | 3.90±0.52 | <0.001 |
| Number of HLA mismatch | 2.9±1.8 | 3.3±1.9 | 3.4±1.8 | 3.4±1.8 | 3.5±1.8 | 3.6±1.7 | <0.001 |
| PRA (%) ** | 8 [0, 0–2] | 9 [0, 0–2] | 7 [0, 0–1] | 7 [0, 0–1] | 6 [0, 0–0] | 4 [0, 0–0] | <0.001 |
| No. with PRA =0% | 11836 (73) | 34347 (72) | 18981 (75) | 6046 (74) | 2674 (78) | 843 (78) | <0.001 |
| Donor age (years) | 35±14 | 38±14 | 40±15 | 43±16 | 45±16 | 47±17 | <0.001 |
| Living Donor | 63 | 45 | 36 | 31 | 28 | 24 | <0.001 |
| Cold Ischemia time (hours)** | 9.0 [2.4, 1.0– 17.0] | 12.4 [12.0, 1.0–21.1] | 14.0 [14.0, 2.2–22.0] | 14.6 [15.0, 4.0–22.0] | 15.2 [15.0, 6.4–22.0] | 16.0 [16.0, 8.0–23.0] | <0.001 |
| Using ECD kidney | 7 | 12 | 22 | 30 | 36 | 43 | <0.001 |
Data include 145,470 recipients between January 1, 1998 and December 31, 2006. Unless otherwise indicated, categorical values shown as number (percentage) or percentage; continuous variables as mean ± SD.
Values in brackets indicate the crude death rate or crude graft failure rate in the indicated group during the 6 years of observation.
mean [median, 25th–75th percentile]
COPD: Chronic obstructive pulmonary disease; HLA: Human leukocyte antibody; PRA: panel reactive antibody (last value prior to transplant); BMI: Body Mass Index; ECD, expanded criteria donor
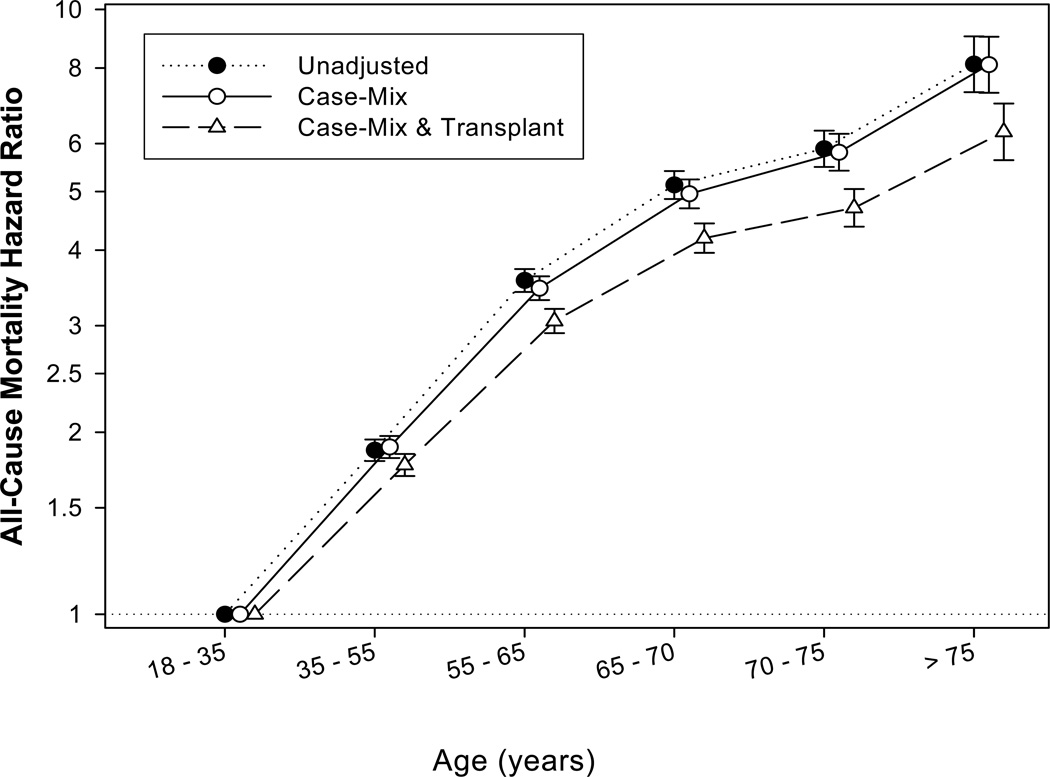
As shown in Figure 2 we examined the association between age groups and all cause mortality using 18-<35 years as the reference group. Compared to patients in the 18-<35 years range, patients aged 65-<70, 70-<75 and ≥75 years old had risks of all-cause mortality that were 4 times (HR, 4.19 95% CI, 3.96–4.43), 5 times (HR, 4.70; 95% CI, 4.37–5.05) and 6 times (HR, 6.27; 95% CI, 5.63–6.99) greater, respectively (p-for trend <0.001).
Figure 2.
Hazard ratios (95% confidence intervals) of all-cause mortality using Cox regression analyses in 145,470 kidney transplant patients (reference category: 18-<35 years)
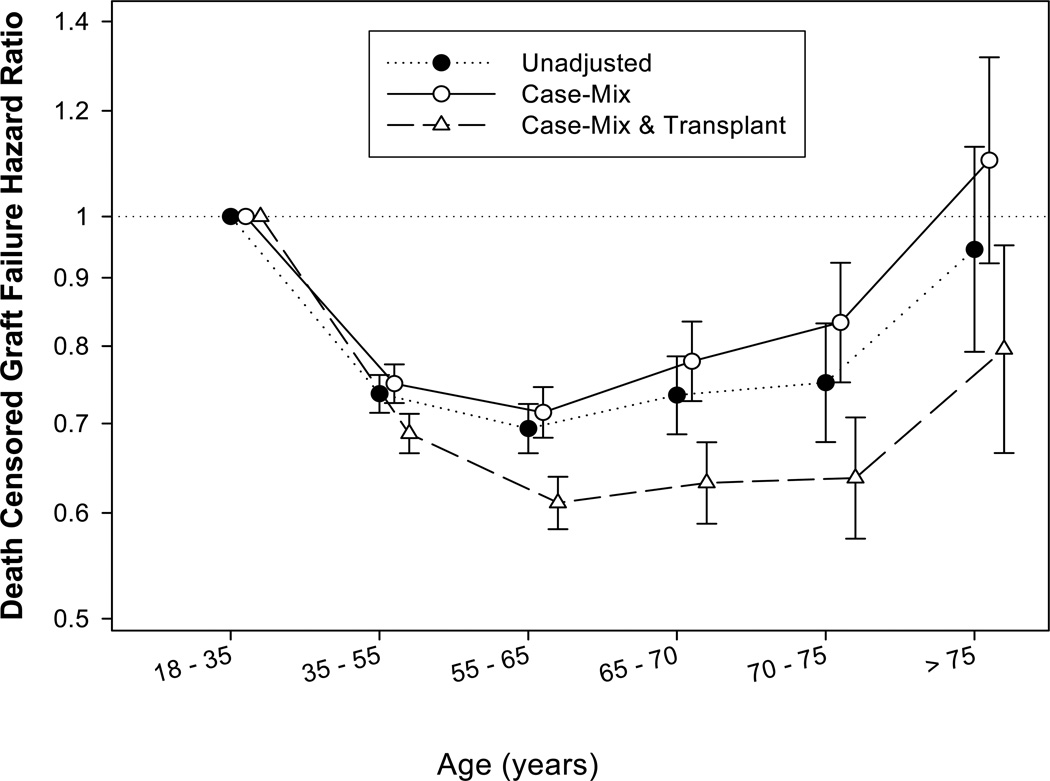
Figure 3 shows the association between age groups and death censored graft failure using 18-<35 years as the reference group. The association between age groups and death-censored graft failure was U-shaped. This observation was supported by the regression analysis where age as a quadratic term was a significant (p<0.001) predictor of death-censored graft failure when this term was added to a model in addition to the linear terms (data not shown). Compared to patients aged 18-<35 years (reference), the fully adjusted death-censored graft failure risk was lower in patients aged 65-<70 years (by 37%; HR, 0.63; 95% CI, 0.59–0.68), 70-<75 years (by 36%; HR, 0.64; 95% CI, 0.57–0.71) and ≥75 years old (by 20%; HR, 0.80; 95% CI, 0.67–0.95).
Figure 3.
Hazard ratios (95% confidence intervals) of death censored graft failure using Cox regression analyses in 145,470 kidney transplant patients (reference category: 18-<35 years)
Table 2 shows predictors of all-cause mortality in different age groups. Compared to non-ECD kidney, ECD kidney was significant predictor of mortality in younger patients, i.e., 46%, 23%, 26%, and 20% higher death risk in 18-<35, 35-<55,55-<65 and 65-<70 years old patients, respectively, but not significantly so for older patients (in the fully adjusted model, HR of 1.12 [95% CI, 0.93–1.36] for 70-<75 years and HR of 1.04 [95% CI, 0.74–1.47] for ≥75 years). Age was a strong effect modifier of the association between ECD kidney and mortality (p for interaction term<0.001). Compared to deceased donor kidney, living kidney donation was associated with greater survival across all age groups (Table 2), although subtle differences were observed across age groups (age-donor interaction p-value <0.001). Additional analyses using the subgroup of non-ECD of the deceased donors as the reference confirmed the above findings, although the power was somewhat mitigated (see Table 2).
Table 2.
all-cause mortality using Cox regression analyses
| Type of Donor Kidney | Non-elderly) | Elderly) | ||||
|---|---|---|---|---|---|---|
| 18–<35 y | 35–<55 y | 55–<65 y | 65–<70 y | 70–<75 y | ≥75 y | |
| ECD vs. non-ECD kidney | ||||||
| Unadjusted Case-mix model* Fully adjusted model** |
1.95 (1.60– 2.38) 1.55 (1.33– 1.80) 1.46 (1.19– 1.77) |
1.60 (1.49–1.71) 1.35 (1.21– 1.49) 1.23 (1.14– 1.32) |
1.54 (1.44–1.64) 1.46 (1.34– 1.59) 1.26 (1.15– 1.38) |
1.42 (1.29– 1.57) 1.43 (1.29–1.59) 1.20 (1.05–1.36) |
1.48 (1.29– 1.70) 1.45 (1.25–1.68) 1.12 (0.93– 1.36) |
1.37 (1.09– 1.72) 1.34 (1.07– 1.69) 1.04 (0.74– 1.47) |
| Living vs. deceased donor | ||||||
| Unadjusted Case-mix model* Fully adjusted model** |
0.57 (0.51– 0.63) 0.56 (0.50– 0.62) 0.65 (0.55– 0.77) |
0.52 (0.50– 0.55) 0.54 (0.51– 0.57) 0.65 (0.59– 0.71) |
0.55 (0.52– 0.58) 0.57 (0.54– 0.60) 0.70 (0.64– 0.76) |
0.60 (0.55– 0.66) 0.60 (0.55– 0.66) 0.69 (0.60– 0.79) |
0.59 (0.51– 0.68) 0.58 (0.50– 0.67) 0.70 (0.57– 0.86) |
0.46 (0.35– 0.61) 0.45 (0.34– 0.61) 0.64 (0.44– 0.93) |
| Living vs. non-ECD deceased kidney | ||||||
| Unadjusted Case-mix model* Fully adjusted model** |
0.60 (0.54– 0.67) 0.58 (0.52– 0.65) 0.66 (0.55– 0.78) |
0.56 (0.53– 0.59) 0.56 (0.53– 0.59) 0.67 (0.62– 0.73) |
0.61 (0.57– 0.65) 0.61 (0.57– 0.65) 0.72 (0.65– 0.79) |
0.62 (0.60– 0.73) 0.65 (0.59– 0.72) 0.69 (0.58– 0.82) |
0.67 (0.58– 0.79) 0.65 (0.56– 0.77) 0.72 (0.55– 0.94) |
0.51 (0.38– 0.69) 0.48 (0.35– 0.67) 0.64 (0.40– 1.02) |
Values shown are Hazard ratios (95% confidence intervals); analysis done in in 145,470 kidney transplant recipients over the age of 18 years.
Footnote: Abbreviations:
ECD: Expanded criteria donor
case mix model was adjusted for : age, gender, race-ethnicity, diabetes mellitus, dialysis vintage, six co-morbidities and three laboratory measures: serum creatinine, serum albumin, and BMI.
fully adjusted models was adjusted for all of the covariates in the case-mix model as well as donor type (deceased or living), donor age, panel reactive antibody (PRA) titer (last value prior to transplant), number of HLA mismatches, cold ischemia time, expanded donor criteria (EDC) and history of acute rejection.
As shown in Table 3 we also examined predictors of death-censored graft failure within the age groups. Compared to the non-ECD kidneys, the ECD kidneys were significant predictors of graft loss in younger patients, i.e., 40%, 31%, 38% and 36% increased risk of graft loss in 18-<35, 35-<55, 55-<65 and 65-<70 years old patients, respectively, but not significantly so in older patients, although similar trends were observed in the elderly as well (see Table 3). Age was an effect modifier of the association between ECD kidney and graft loss (p for interaction <0.001). Compared to the deceased kidney, living kidney donation was significantly associated with better graft survival in non-elderly patient, i.e., 22%, 29%, 32% and 49% lower graft failure in 18-<35, 35-<55, 55-<65 and 65-<70 years old patients. The effect modifying role of age was consistent with a significant statistical interaction (p<0.001). Similar results were found when the subgroup of non-ECD of the deceased donors was used as the reference (see Table 3).
Table 3.
death-censored graft failure using Cox regression analyses
| Type of Donor Kidney |
Non-elderly | Elderly) | ||||
|---|---|---|---|---|---|---|
| 18–<35y | 35–<55 y | 55–<65 years | 65–<70 years | 70–<75 years | ≥75 years | |
| ECD vs. non-ECD kidney | ||||||
| Unadjusted Case-mix model* Fully adjusted model** |
1.72 (1.51– 1.96) 1.57 (1.41– 1.75) 1.40 (1.25– 1.58) |
1.72 (1.60– 1.84) 1.56 (1.44– 1.69) 1.31(1.22– 1.40) |
2.12 (1.95– 2.30) 2.02 (1.84– 2.21) 1.38 (1.24– 1.53) |
2.00 (1.72– 2.31) 2.00 (1.72– 2.32) 1.36 (1.09– 1.71) |
2.04 (1.64– 2.54) 2.11 (1.69– 2.64) 1.32 (0.96– 1.82) |
1.81 (1.21– 2.70) 1.94 (1.29– 2.93) 1.45 (0.67– 3.15) |
| Living vs. deceased donor | ||||||
| Unadjusted Case-mix model* Fully adjusted model** |
0.61 (0.58– 0.65) 0.68 (0.64– 0.73) 0.78 (0.70– 0.87) |
0.52 (0.49– 0.54) 0.60 (0.57– 0.63) 0.71 (0.66– 0.77) |
0.49 (0.45– 0.53) 0.53 (0.49– 0.58) 0.68 (0.61– 0.77) |
0.45 (0.38– 0.53) 0.46 (0.39– 0.55) 0.51 (0.41– 0.64) |
0.42 (0.32– 0.56) 0.43 (0.32– 0.57) 0.71 (0.48–1.04) |
0.46 (0.28– 0.78) 0.49 (0.29–0.83) 1.17 (0.61–2.25) |
| Living vs. non-ECD deceased kidney | ||||||
| Unadjusted Case-mix model* Fully adjusted model** |
0.64 (0.60– 0.69) 0.68 (0.63– 0.72) 0.80 (0.72– 0.88) |
0.56 (0.53– 0.59) 0.62 (0.59– 0.66) 0.74 (0.68– 0.80) |
0.59 (0.54– 0.65) 0.63 (0.57– 0.69) 0.72 (0.63– 0.82) |
0.56 (0.47– 0.67) 0.57 (0.47– 0.68) 0.53 (0.40– 0.70) |
0.56 (0.41– 0.75) 0.55 (0.41–0.75) 0.70 (0.44–1.12) |
0.55 (0.31– 0.97) 0.56 (0.31–1.01) 1.13 (0.52–2.48) |
Values shown are Hazard ratios (95% confidence intervals); analysis done in in 145,470 kidney transplant recipients over the age of 18 years.
Footnote: Abbreviations:
ECD: Expanded criteria donor
case mix model was adjusted for : age, gender, race-ethnicity, diabetes mellitus, dialysis vintage, six co-morbidities and three laboratory measures: serum creatinine, serum albumin, and BMI.
fully adjusted models was adjusted for all of the covariates in the case-mix model as well as donor type (deceased or living), donor age, panel reactive antibody (PRA) titer (last value prior to transplant), number of HLA mismatches, cold ischemia time, expanded donor criteria (EDC) and history of acute rejection.
Discussion
In 145,470 kidney transplant recipients, older age was associated with an incrementally higher risk of death. The association between age and death-censored graft failure was U-shaped, indicating the highest risk of graft failure in both young adults (18-<35 years) and very old patients (≥75 years) and the lowest risk in patients aged 55-<75 years. In the patients older than 70 years, the ECD kidney was not a significant predictor of death or graft loss; however, in patients younger than 70 years ECD was a strong predictor of poor outcomes. Living donor kidney was associated with better patient survival in all age groups, while patients younger than 70 years also exhibited the advantage of lower risk of graft loss.
Compared to patients 18-<35 years old, elderly patients had higher risk of mortality. These results are not surprising as age is an important predictor of death in both the ESRD and non-ESRD populations.(27)
The association between age groups and graft failure was also U-shaped. Compared to patients aged 18-<35 years (reference), patient 65-<70 years, 70-<75 years and ≥75 years had a 37%, 36% and 20%, respectively, lower risk of graft failure, whereas the risk was similar in patients aged 55-<75 years. Survival in the elderly kidney transplant recipient is currently excellent. With present therapy, patient survival at one, five, and ten years is approximately 85, 70, and 50 percent, respectively.(16–24) Although these data are promising, elderly patients may not live long enough to suffer graft failure. In elderly transplanted recipients, death with functioning graft due to cardiovascular disease,(21) infection, or malignancy(28) was the most common cause of graft loss.(29) Moreover, kidney function predicts non-cardiovascular mortality from multiple causes in the elderly.(30) Another potential explanation for greater graft survival in patients ≥75 years is that activation of the immune system in old patients is lower than in younger counterparts. For example, acute rejection is less common in older transplant recipients, presumably on account of an immune system that is less active.(24). The most common cause of graft failure after the first year post transplant is chronic renal allograft nephropathy.(31) However multiple factors (alloantigen-dependent and alloantigen-independent) seem to be involved in the pathogenesis of chronic graft dysfunction, (32). Data from both experimental models and humans indicate an important role for many elements of the immune system in the pathogenesis of chronic renal allograft nephropathy.(33–36) Decreased activation of the alloantigen-dependent factors of chronic renal allograft nephropathy might contribute to longer graft life in elderly patients.
In contrast to patients aged <70 years, ECD kidneys did not correlate with all-cause mortality in patients older than 70 years. Similar associations were found with graft loss. This is an important consideration in the selection of a suitable kidney for elderly patients. The use of ECD kidneys has grown steadily, making up 16% of all deceased donor transplants in 2003.(37) The use of ECD kidneys has been even more common in the elderly. Elderly ESRD patients received 688 ECD kidneys (33%) of a total of 2078 deceased donor kidneys received them.(14) Elderly recipients of ECD kidneys had a significantly (25%) lower mortality risk compared with similar aged chronic dialysis patients on the transplant waiting list.(14) Thus, based on data from the current study and the reported literature data, EDC kidney transplant for elderly patients might be recommended.
In all patients, kidneys from living donors were important predictors of greater survival. Living donor kidneys remain the best choice for all those awaiting transplant. (38, 39) However, in the last decade, a rising number of patients older than 60 years have been transplanted with deceased donor kidneys.(40) Our results confirm findings from a previous study (25) that living donors are the optimal choice for elderly ESRD patients. In our study, only kidneys from living donors were an important predictor of patient survival in the very elderly patients.
Our study is notable for its large sample size and for the several important transplant covariates that were accounted for in the multivariate analyses. As with all registry-based observational studies, these results are subject to certain limitations. Like all observational studies, our study also cannot prove causality. Immunosuppressive and other regimens, which have potential impacts on patient and graft survival, were not available in the database. Nonetheless, in the fully adjusted model, we controlled for a number of important variables.
In conclusion, similar to the general population, among the kidney transplant recipients older age is associated with higher risk of death. The association between age and (death censored) graft failure is somewhat U-shaped. In the elderly patients aged over 70 years, EDC kidney is not predictor of death or graft loss; however, in patients younger than 70 years, EDC was a strong predictor of both increased death and graft loss rates. Living donor kidney appears associated with greater survival across all age groups including the elderly, although the significantly lower graft loss rate is observed mainly among those younger than 70 years. Hence, our study suggests that the elderly ESRD patients gain years of life if they receive a kidney transplant, in particular from a living donor. Given other data indicating that elderly transplant recipients have a 41% lower overall risk of death compared with waitlisted candidates,(14) elderly ESRD patients should be transplanted with a living donor kidney if possible. However, among those elderly individuals over 70 years who expect deceased kidneys, receiving ECD kidneys exhibit virtually the same survival and graft failure outcomes. These findings need to be verified in additional studies.
Acknowledgement
We acknowledge SRTR for providing the database for this research.
Support: The study was supported by research grant 0655776Y from the American Heart Association grant to Dr Kalantar-Zadeh. Dr Kalantar-Zadeh’s other funding sources include the National Institute of Diabetes, Digestive and Kidney Disease of the National Institute of Health (R01 DK078106); a research grant from DaVita Clinical Research, and a philanthropic grant from Mr. Harold Simmons. Dr Molnar received grants from the National Developmental Agency (KTIA-OTKA-EU 7KP-HUMAN-MB08-A-81231) from the Research and Technological Innovation Fund, and was also supported by Hungarian Kidney Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The findings of this manuscript were included in an oral presentation during the American Society of Nephrology (ASN) Kidney Week, November 8–13, 2011, Philadelphia, PA.
Financial Disclosure: Dr. Krishnan is an employee of DaVita. Dr. Kalantar-Zadeh is the medical director of DaVita Harbor-UCLA/MFI in Long Beach, CA. The remaining authors declare that they have no relevant financial interests.
References
- 1.Goldberg RJ, Spencer FA, Farmer C, Meyer TE, Pezzella S. Incidence and hospital death rates associated with heart failure: a community-wide perspective. Am J Med. 2005;118(7):728–734. doi: 10.1016/j.amjmed.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 2.Cowie CC, Rust KF, Ford ES, Eberhardt MS, Byrd-Holt DD, Li C, et al. Full accounting of diabetes and pre-diabetes in the U.S. population in 1988–1994 and 2005–2006. Diabetes Care. 2009;32(2):287–294. doi: 10.2337/dc08-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ong KL, Cheung BM, Man YB, Lau CP, Lam KS. Prevalence, awareness, treatment, and control of hypertension among United States adults 1999–2004. Hypertension. 2007;49(1):69–75. doi: 10.1161/01.HYP.0000252676.46043.18. [DOI] [PubMed] [Google Scholar]
- 4.Paraskevas KI, Bessias N, Koupidis SA, Tziviskou E, Mikhailidis DP, Oreopoulos DG. Incidence of end-stage renal disease in the elderly: a steadily rising global socioeconomic epidemic. Int Urol Nephrol. 2010;42(2):523–525. doi: 10.1007/s11255-009-9691-1. [DOI] [PubMed] [Google Scholar]
- 5.US Renal Data System: USRDS 2008 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2008. [Google Scholar]
- 6.The 2008 SRTR report on the state of transplantation. [cited 2010 February]; Available from: www.ustransplant.org/annual_reports.
- 7.Hartmann EL, Wu C. The evolving challenge of evaluating older renal transplant candidates. Adv Chronic Kidney Dis. 2010;17(4):358–367. doi: 10.1053/j.ackd.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 8.Taube DH, Winder EA, Ogg CS, Bewick M, Cameron JS, Rudge CJ, et al. Successful treatment of middle aged and elderly patients with end stage renal disease. British medical journal (Clinical research ed. 1983;286(6383):2018–2020. doi: 10.1136/bmj.286.6383.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fauchald P, Albrechtsen D, Leivestad T, Berg KJ, Talseth T, Flatmark A. Renal replacement therapy in elderly patients. Transpl Int. 1988;1(3):131–134. doi: 10.1007/BF00348834. [DOI] [PubMed] [Google Scholar]
- 10.Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. The New England journal of medicine. 1999;341(23):1725–1730. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 11.Johnson DW, Herzig K, Purdie D, Brown AM, Rigby RJ, Nicol DL, et al. A comparison of the effects of dialysis and renal transplantation on the survival of older uremic patients. Transplantation. 2000;69(5):794–799. doi: 10.1097/00007890-200003150-00020. [DOI] [PubMed] [Google Scholar]
- 12.Oniscu GC, Brown H, Forsythe JL. How great is the survival advantage of transplantation over dialysis in elderly patients? Nephrol Dial Transplant. 2004;19(4):945–951. doi: 10.1093/ndt/gfh022. [DOI] [PubMed] [Google Scholar]
- 13.Giessing M, Budde K, Fritsche L, Slowinski T, Tuerk I, Schoenberger B, et al. "Old-for-old" cadaveric renal transplantation: surgical findings, perioperative complications and outcome. European urology. 2003;44(6):701–708. doi: 10.1016/s0302-2838(03)00380-4. [DOI] [PubMed] [Google Scholar]
- 14.Rao PS, Merion RM, Ashby VB, Port FK, Wolfe RA, Kayler LK. Renal transplantation in elderly patients older than 70 years of age: results from the Scientific Registry of Transplant Recipients. Transplantation. 2007;83(8):1069–1074. doi: 10.1097/01.tp.0000259621.56861.31. [DOI] [PubMed] [Google Scholar]
- 15.Savoye E, Tamarelle D, Chalem Y, Rebibou JM, Tuppin P. Survival benefits of kidney transplantation with expanded criteria deceased donors in patients aged 60 years and over. Transplantation. 2007;84(12):1618–1624. doi: 10.1097/01.tp.0000295988.28127.dd. [DOI] [PubMed] [Google Scholar]
- 16.Velez RL, Brinker KR, Vergne-Marini PJ, Nesser DA, Long DL, Trevino G, et al. Renal transplantation with cyclosporine in the elderly population. Transplantation proceedings. 1991;23(2):1749–1752. [PubMed] [Google Scholar]
- 17.Morris GE, Jamieson NV, Small J, Evans DB, Calne R. Cadaveric renal transplantation in elderly recipients: is it worthwhile? Nephrol Dial Transplant. 1991;6(11):887–892. doi: 10.1093/ndt/6.11.887. [DOI] [PubMed] [Google Scholar]
- 18.Doyle SE, Matas AJ, Gillingham K, Rosenberg ME. Predicting clinical outcome in the elderly renal transplant recipient. Kidney international. 2000;57(5):2144–2150. doi: 10.1046/j.1523-1755.2000.00066.x. [DOI] [PubMed] [Google Scholar]
- 19.Saudan P, Berney T, Leski M, Morel P, Bolle JF, Martin PY. Renal transplantation in the elderly: a long-term, single-centre experience. Nephrol Dial Transplant. 2001;16(4):824–828. doi: 10.1093/ndt/16.4.824. [DOI] [PubMed] [Google Scholar]
- 20.Bentas W, Jones J, Karaoguz A, Tilp U, Probst M, Scheuermann E, et al. Renal transplantation in the elderly: surgical complications and outcome with special emphasis on the Eurotransplant Senior Programme. Nephrol Dial Transplant. 2008;23(6):2043–2051. doi: 10.1093/ndt/gfm912. [DOI] [PubMed] [Google Scholar]
- 21.Oniscu GC, Brown H, Forsythe JL. How old is old for transplantation? Am J Transplant. 2004;4(12):2067–2074. doi: 10.1111/j.1600-6143.2004.00622.x. [DOI] [PubMed] [Google Scholar]
- 22.Fabrizii V, Winkelmayer WC, Klauser R, Kletzmayr J, Saemann MD, Steininger R, et al. Patient and graft survival in older kidney transplant recipients: does age matter? J Am Soc Nephrol. 2004;15(4):1052–1060. doi: 10.1097/01.asn.0000120370.35927.40. [DOI] [PubMed] [Google Scholar]
- 23.Kauffman HM, McBride MA, Cors CS, Roza AM, Wynn JJ. Early mortality rates in older kidney recipients with comorbid risk factors. Transplantation. 2007;83(4):404–410. doi: 10.1097/01.tp.0000251780.01031.81. [DOI] [PubMed] [Google Scholar]
- 24.Tesi RJ, Elkhammas EA, Davies EA, Henry ML, Ferguson RM. Renal transplantation in older people. Lancet. 1994;343(8895):461–464. doi: 10.1016/s0140-6736(94)92698-0. [DOI] [PubMed] [Google Scholar]
- 25.Heldal K, Hartmann A, Leivestad T, Svendsen MV, Foss A, Lien B, et al. Clinical outcomes in elderly kidney transplant recipients are related to acute rejection episodes rather than pretransplant comorbidity. Transplantation. 2009;87(7):1045–1051. doi: 10.1097/TP.0b013e31819cdddd. [DOI] [PubMed] [Google Scholar]
- 26.Gill J, Bunnapradist S, Danovitch GM, Gjertson D, Gill JS, Cecka M. Outcomes of kidney transplantation from older living donors to older recipients. Am J Kidney Dis. 2008;52(3):541–552. doi: 10.1053/j.ajkd.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 27.United States Renal Data System. Excerpts from USRDS 2009 Annual Data Report. U.S. Department of Health and Human Services. The National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. Am J Kidney Dis. 2010;55(Suppl 1):S1. [Google Scholar]
- 28.Sato K, Ogawa K, Onumata O, Aso K, Nakayama Y, Yoshida K, et al. Cause of death in renal transplant patients: a comparison between azathioprine and ciclosporin. Surg Today. 2001;31(8):681–687. doi: 10.1007/s005950170070. [DOI] [PubMed] [Google Scholar]
- 29.Cecka JM. The UNOS renal transplant registry. Clin Transpl. 2001:1–18. [PubMed] [Google Scholar]
- 30.Fried LF, Katz R, Sarnak MJ, Shlipak MG, Chaves PH, Jenny NS, et al. Kidney function as a predictor of noncardiovascular mortality. J Am Soc Nephrol. 2005;16(12):3728–3735. doi: 10.1681/ASN.2005040384. [DOI] [PubMed] [Google Scholar]
- 31.Chapman JR, O'Connell PJ, Nankivell BJ. Chronic renal allograft dysfunction. J Am Soc Nephrol. 2005;16(10):3015–3026. doi: 10.1681/ASN.2005050463. [DOI] [PubMed] [Google Scholar]
- 32.Tullius SG, Tilney NL. Both alloantigen-dependent and -independent factors influence chronic allograft rejection. Transplantation. 1995;59(3):313–318. [PubMed] [Google Scholar]
- 33.Chandraker A, Azuma H, Nadeau K, Carpenter CB, Tilney NL, Hancock WW, et al. Late blockade of T cell costimulation interrupts progression of experimental chronic allograft rejection. J Clin Invest. 1998;101(11):2309–2318. doi: 10.1172/JCI2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Theruvath TP, Saidman SL, Mauiyyedi S, Delmonico FL, Williams WW, Tolkoff-Rubin N, et al. Control of antidonor antibody production with tacrolimus and mycophenolate mofetil in renal allograft recipients with chronic rejection. Transplantation. 2001;72(1):77–83. doi: 10.1097/00007890-200107150-00016. [DOI] [PubMed] [Google Scholar]
- 35.Sijpkens YW, Joosten SA, Wong MC, Dekker FW, Benediktsson H, Bajema IM, et al. Immunologic risk factors and glomerular C4d deposits in chronic transplant glomerulopathy. Kidney international. 2004;65(6):2409–2418. doi: 10.1111/j.1523-1755.2004.00662.x. [DOI] [PubMed] [Google Scholar]
- 36.Joosten SA, Sijpkens YW, van Ham V, Trouw LA, van der Vlag J, van den Heuvel B, et al. Antibody response against the glomerular basement membrane protein agrin in patients with transplant glomerulopathy. Am J Transplant. 2005;5(2):383–393. doi: 10.1111/j.1600-6143.2005.00690.x. [DOI] [PubMed] [Google Scholar]
- 37.Organ Procurement and Transplantation Network/Scientific Registry of Transplant Recipients Annual Report 1994–2003. HHS/HRSA/HSB/DOT; UNOS; URREA. 2004 [Google Scholar]
- 38.Meier-Kriesche HU, Kaplan B. Waiting time on dialysis as the strongest modifiable risk factor for renal transplant outcomes: a paired donor kidney analysis. Transplantation. 2002;74(10):1377–1381. doi: 10.1097/00007890-200211270-00005. [DOI] [PubMed] [Google Scholar]
- 39.Mange KC, Joffe MM, Feldman HI. Effect of the use or nonuse of long-term dialysis on the subsequent survival of renal transplants from living donors. The New England journal of medicine. 2001;344(10):726–731. doi: 10.1056/NEJM200103083441004. [DOI] [PubMed] [Google Scholar]
- 40.Jassal SV, Krahn MD, Naglie G, Zaltzman JS, Roscoe JM, Cole EH, et al. Kidney transplantation in the elderly: a decision analysis. J Am Soc Nephrol. 2003;14(1):187–196. doi: 10.1097/01.asn.0000042166.70351.57. [DOI] [PubMed] [Google Scholar]