Abstract
Recent theoretical and empirical developments in human category learning have differentiated an analytic, rule-based system of category learning from a nonanalytic system that integrates information across stimulus dimensions. The researchers applied this theoretical distinction to pigeons’ category learning. Pigeons learned to categorize stimuli varying in the tilt and width of their internal striping. The matched category problems had either a unidimensional (rule-based) or multidimensional (information-integration) solution. Whereas humans and nonhuman primates strongly dimensionalize these stimuli and learn rule-based tasks far more quickly than information-integration tasks, pigeons learned the two tasks equally quickly to the same accuracy level. Pigeons may represent a cognitive system in which the commitment to dimensional analysis and category rules was not strongly made. Their performance could suggest the character of the ancestral vertebrate categorization system from which that of primates emerged.
Keywords: category learning, comparative cognition, pigeons, rules, analytic/nonanalytic cognition, implicit/explicit cognition
Categorization is essential for survival. Consequently, it is a widely studied cognitive adaptation in humans (Ashby & Maddox, 2005; Knowlton & Squire, 1993; Murphy, 2003; Nosofsky, 1987; Posner, Goldsmith, & Welton, 1967; Smith & Minda, 1998) and nonhumans (Cook & Smith, 2006; Herrnstein, Loveland, & Cable, 1976; Lea & Ryan, 1990; Smith, Redford, & Haas, 2008; Wasserman, Kiedinger, & Bhatt, 1988). In the human literature, an influential multiple-systems theoretical perspective (Ashby, Alfonso-Reese, Turken, & Waldron, 1998; Ashby & Ell, 2001; Ashby, Ennis, & Spiering, 2007; Erickson & Kruschke, 1998; E. E. Smith & Grossman, 2008) distinguishes analytic category learning (by which executive attention and working memory derive explicit dimensional rules) from nonanalytic learning (by which behavioral responses are implicitly mapped to unanalyzed perceptual wholes). According to the dominant neuroscience framework, humans’ explicit/analytic system is mediated in part by a broad neural network that includes the anterior cingulate gyrus, prefrontal cortex, the head of the caudate nucleus, and medial temporal lobe structures that subserve declarative memory. This system is related to a broader neural complex serving the executive control of attention (Rossi, Pessoa, Desimone, & Ungerleider, 2009). The implicit/nonanalytic system depends heavily on the striatum and is catalyzed by the reinforcement-mediated strengthening of dopamine-related synapses (Ashby et al., 1998; Ashby & Ell, 2001).
Here we explore the explicit-implicit distinction from a comparative/evolutionary perspective, asking whether pigeons (Columba livia) also have a multiple-systems categorization capacity that includes a rule-privileged dimensional component. We test pigeons with matched category tasks differing only in their analytic/rule-based or nonanalytic/multidimensional solution. Converging findings from multiple laboratories suggest that pigeons have no tendency toward dimensional analysis and rules and instead learn both category tasks equivalently through the same nonanalytic, associative process. The results show that the dual-system, explicit/implicit control of categorization is not a generalized pattern. To the contrary, pigeons may represent a species in which the commitment to dimensional analysis and category rules was not strongly made. Their performance suggests the character of the ancestral vertebrate categorization system from which that of humans emerged. The development of the capacity for dimensional analysis and explicit rule learning could have been an important step in the cognitive evolution of the primate-hominid lineage (Smith, Beran, Crossley, Boomer, & Ashby, 2010; Marcus, Vijayan, Bandi Rao, & Vishton, 1999).
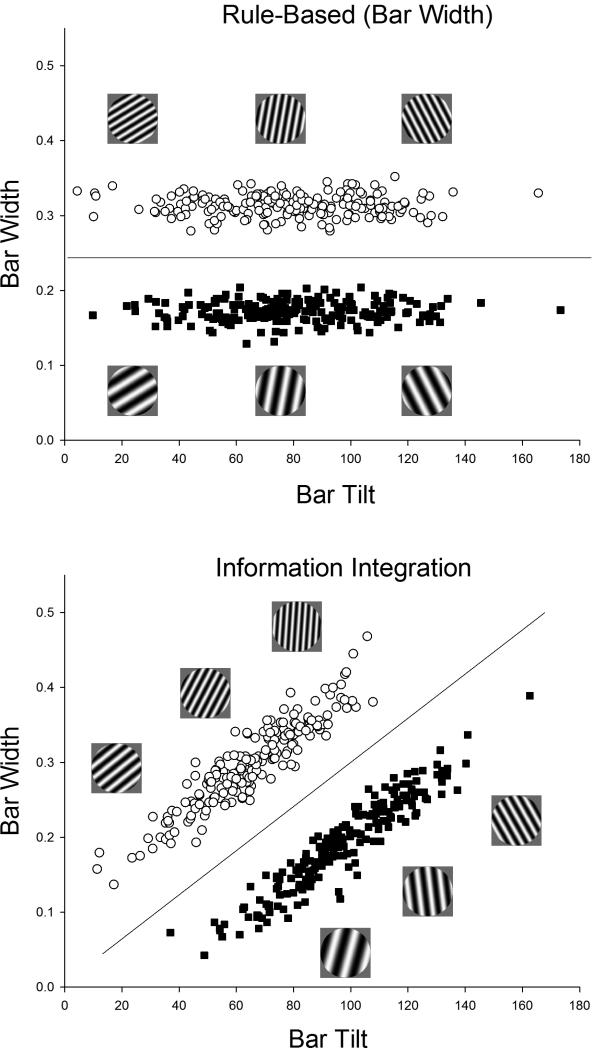
Within the enormous literature on human category learning, strong behavioral evidence for dissociable analytic and nonanalytic categorization systems comes from studies of rule-based (RB) and information-integration (II) categorization tasks. Figure 1 illustrates these tasks using sine-wave gratings varying in the dimensions of bar width and tilt. These dimensions were adopted because they are influential in vision and categorization research, well studied and calibrated in humans, and known to be highly analyzable or separable. In both panels, the open circles and closed squares, respectively, indicate the exact bi-dimensional values of stimuli belonging to Category A or Category B and deserving a Category A or Category B response. In Figure 1 (top), the horizontal category boundary for the RB task shows that only the bar-width dimension carries valid category information. The subject must discover this bar-width solution using feedback provided during successive presentations of single, to-be-categorized instances. In Figure 1 (bottom), the diagonal category boundary for the II task shows that the bar-width and bar-tilt dimensions both contain partially valid category information. The subject must integrate stimulus information across both dimensions to best solve this category problem.
Figure 1.
A rule-based (RB) category structure (top) and an information-integration (II) category structure (bottom). Category tasks are depicted using the USA project's stimulus space. The NZ project examined an analogous stimulus space. In both projects, stimuli were circular sine-wave gratings varying in bar-width and bar-tilt. For each task, three illustrative Category A and Category B stimuli are provided. In addition, the open circles and filled squares illustrate the distribution in width-tilt space of the actual Category A and Category B stimuli presented in the two tasks. The horizontal decision bound (top) shows that only the bar-width dimension carried diagnostic category information, so that a one-dimensional, bar-width decision bound or rule produced optimal performance. The diagonal decision bound (bottom) shows that both the bar-width and bar-tilt dimensions carried partially diagnostic category information, so that information needed to be integrated across dimensions for optimal performance.
The RB and II tasks are elegantly matched in category size, instance variability, category separation, and a priori perceptual difficulty because they are exact geometric rotations of one another through the stimulus space. They differ only in their potential solutions. The RB task potentially affords a dimensional-rule solution whereas the II task depends on a dimensional-integration solution. Consistent with a multiple-systems perspective, humans learn one-dimensional RB categories about 3 to 10 times faster than comparable (i.e., rotated) II categories (e.g., Ashby, Ell, & Waldron, 2003; Maddox, Ashby, & Bohil, 2003), and do so using dimensional analysis, hypotheses, and rules. II tasks, in contrast, are acquired slowly, implicitly, and associatively.
The goal of the present research was to investigate the multiple-systems hypothesis comparatively by asking whether pigeons (Columba livia) share with humans a multiple-systems category-learning competence that includes both analytic, rule-based and nonanalytic, associative systems.
One possibility is that a multiple-systems organization for categorization exists broadly across vertebrates. Categorization could be an important enough cognitive capacity to deserve and receive redundant expression within cognition, and a multiple-systems architecture might have emerged early in the vertebrate lineage. Moreover, a rule-preferring system could be a particularly adaptive component within the overall categorization system for solving certain types of category problems or for flexibly maneuvering on demand among alternative solutions to category problems. If so, then pigeons would exhibit something like the RB/II performance differences that humans show, demonstrating an important continuity in categorization across vertebrate species with 250 million years of phylogenetic separation.
However, by another feasible cognitive organization, pigeons could exclusively learn by gradually associating response outputs to unanalyzed regions of perceptual space. In this case, stimuli would be treated integrally as unitary wholes, with dimensional analysis, attentional focusing, and rule formation held in abeyance during associative learning. Then, RB and II tasks would be equivalent to one another, and as a result equally coherent and learnable for pigeons. It is an intriguing theoretical possibility that pigeons represent a species in which the principal cognitive commitment was made toward associating responses to stimulus wholes and not toward dimensional analysis and rules. This would demonstrate an important discontinuity between the category-learning system of humans and pigeons, the latter possibly typifying the phylogenetically older vertebrate categorization system.
Given the importance of this theoretical issue, and the potential power of the multiple-systems framework to inform it, laboratories in New Zealand (NZ-Canterbury) and the United States (USA-Tufts) independently contrasted these possibilities by giving pigeons RB/II tasks instantiated using sine-wave gratings varying in bar width and tilt. Upon discovering the strong convergence in the use of this common framework across the laboratories, the independent investigations were combined for joint presentation here.
Method
Naïve pigeons (11-USA; 6-NZ), maintained at 80-85% of free-feeding weight, were tested using a two-alternative symbolic matching-to-sample choice procedure using touchscreen-equipped LCD monitors. To-be-categorized stimuli were presented through a window in each chamber's front panel. These stimuli were circular sine-wave gratings varying in bar width/tilt. Choice stimuli were located to each side of the stimulus. These choice stimuli were illuminated following observing responses to the to-be-categorized stimulus. Response assignments were counterbalanced across birds and tasks. A single response to the correct or incorrect choice produced food reinforcement or a timeout, respectively, followed by an inter-trial interval. A central food hopper in the front panel delivered the grain reinforcements for correct choices. White noise masked external sounds. Daily sessions contained approximately equal numbers of samples from each category selected randomly from the available pool. Training continued until each bird reached criterial performance and began their next RB or II acquisition.
Table 1 summarizes USA-NZ procedural differences. These procedural differences resulted from the independent planning and conduct of the original projects. They underscore the strong convergence of the pattern of findings across the NZ and USA projects.
Table 1.
Procedural differences—USA and NZ projects.
| Contrast | USA | NZ |
|---|---|---|
| Number of Birds | 11 | 6 |
| RB Task First | 6 | 3 |
| II Task First | 5 | 3 |
| Screen Resolution | 1024 × 786 | 640 × 480 |
| Choice Assignment | Visual | Spatial / Visual |
| Choice Colors | Blue / Red | Green / Red |
| Stimuli per Category | 200 | 40 |
| Stimulus Size | 3 × 3 cm | 9.5 × 12 cm |
| Task | Simultaneous | Zero-Delay |
| Warning Signal | Yes | No |
| Trial-Start Response | Yes | No |
| Observing Response | Variable 13-15 pecks | Fixed 5 pecks |
| Sample Illuminated during Response | Yes | No |
| Inter-Trial Interval | 3 s | 9 s |
| Timeout | Dark 8 s | Flashing 10 s |
| Reinforcement Time | 2.5 s | 3 s |
| Grain | Mixed | Wheat |
| Daily Trials | 80 | 90 |
| Criterial Performance | 6 sessions ≥ 85% | 4 sessions ≥ 80% |
Categories created by the randomization technique (Ashby & Gott, 1988) were defined by bivariate-normal distributions in the bar-width/bar-tilt stimulus space. Table 2 lists the distributional parameters for the category structures tested. Two RB tasks were tested (bar width with a horizontal optimal decision bound; bar tilt with a vertical decision bound). One II task was tested in which the optimal decision bound was the stimulus space's major diagonal. The II task was simply a 45° rotation of the RB tasks (see Figure 1). Nine birds were tested first with an RB task (USA—3 tilt, 3 width; NZ—3 width). Eight birds were tested first with the II task (USA-5, NZ-3). The respective learning criteria were six non-consecutive sessions of accuracy ≥ 85% (USA) and four non-consecutive sessions of accuracy ≥ 80% (NZ) for the two laboratories. Subsequently, birds were switched to new category structures and retrained so that all birds experienced both RB and II tasks.
Table 2.
Population parameters for the distributions tested in the category tasks.
| Rule-Based (Bar Width) | ||||
|---|---|---|---|---|
| Category A | Category B | |||
| Tilt | Width | Tilt | Width | |
| USA | ||||
| Minimum | 4.41 | 0.279 | 9.79 | 0.128 |
| Maximum | 165.58 | 0.351 | 173.31 | 0.204 |
| Standard Deviation | 27.91 | 0.014 | 26.96 | 0.013 |
| Mean | 78.76 | 0.313 | 79.97 | 0.172 |
| NZ | ||||
| Minimum | -26.67 | 0.037 | -6.96 | 0.048 |
| Maximum | 119.26 | 0.042 | 109.36 | 0.052 |
| Standard Deviation | 33.86 | 0.009 | 29.65 | 0.009 |
| Mean | 41.91 | 0.040 | 41.38 | 0.049 |
| Rule-Based (Bar Tilt) | ||||
|---|---|---|---|---|
| Category A | Category B | |||
| Tilt | Width | Tilt | Width | |
| USA | ||||
| Minimum | 41.02 | 0.012 | 91.38 | 0.046 |
| Maximum | 70.35 | 0.503 | 116.02 | 0.510 |
| Standard Deviation | 5.13 | 0.082 | 4.94 | 0.086 |
| Mean | 55.97 | 0.243 | 104.57 | 0.247 |
| Information Integration | ||||
|---|---|---|---|---|
| Category A | Category B | |||
| Tilt | Width | Tilt | Width | |
| USA | ||||
| Minimun | 11.34 | 0.136 | 36.95 | 0.042 |
| Maximum | 107.77 | 0.468 | 162.60 | 0.388 |
| Standard Deviation | 18.55 | 0.055 | 20.62 | 0.060 |
| Mean | 65.31 | 0.299 | 97.61 | 0.192 |
| NZ | ||||
| Minimum | 5.15 | 0.025 | -2.43 | 0.036 |
| Maximum | 107.35 | 0.058 | 80.09 | 0.063 |
| Standard Deviation | 23.65 | 0.016 | 21.13 | 0.015 |
| Mean | 52.08 | 0.041 | 31.21 | 0.047 |
Results
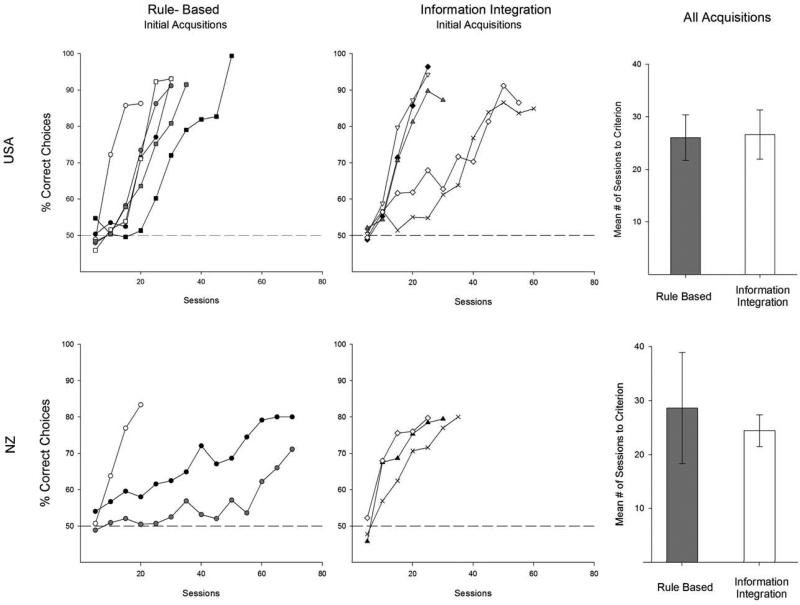
Figure 2 shows the results. The leftmost panels show mean choice accuracy across sessions for each pigeon during their first RB or II acquisition. Pigeons significantly improved over sessions, but showed no RB/II difference in their speed of learning. The II task—profoundly more difficult for humans and nonhuman primates—was learned just as quickly as either RB task. Pigeons (USA) took on average 30.3 ± 3.7 (mean ± SEM) sessions and 36.4 ± 7.3 sessions to reach criterion on the RB and II task, respectively. Pigeons (NZ) took on average 42.5 ± 23.5 sessions and 28.0 ± 2.1 sessions to reach criterion on the RB and II task, respectively. Combined, these means were 33.4 ± 5.6 sessions (RB) and 33.2 ± 4.6 sessions (II). Two NZ birds experienced RB learning difficulty and one bird never reached criterion (this latter bird was not included in subsequent analyses). Neither of these birds had any greater difficulty learning the subsequent II task. Two USA birds were slightly slower II learners than the majority of birds, but one of these was equally slow during later RB learning. Statistical comparisons (ANOVAs, t tests, p < .05) confirmed the improvements across sessions and the absence of RB/II learning-speed differences for each laboratory separately and for the two laboratories combined.
Figure 2.
The leftmost panels show mean accuracy across sessions for individual pigeons tested first in a rule-based (RB) task. The central panels show mean accuracy across sessions for individual pigeons tested first in an information-integration (II) task. The rightmost panels show the mean number of sessions to criterion for all RB and II acquisitions for 16 pigeons. The top and bottom rows of panels, respectively, show results from the USA and NZ projects.
There was also no evidence that RB tasks supported higher terminal accuracy levels than II tasks. Comparisons of mean accuracy over the last 10 sessions for each task revealed no significant advantage of the RB task relative to the II task (USA: RB=84.85% ± .39, II=87.15% ± .93, NZ: RB=79.2% ± .10, II=77.0% ± 1.3). Thus, for these pigeons, the II task presented no greater learning challenge than the RB task.
Figure 2's rightmost panels show the combined results from all acquisitions for the 16 pigeons reaching criterion. Even with multiple acquisitions and experience with both kinds of tasks, the II task was still no more difficult for the pigeons than the RB task. The mean number of sessions to criterion was equivalent across tasks. The terminal accuracy over the last 10 sessions for all acquisitions was also equivalent across tasks (USA: RB=84.95% ± .5, II=86.54% ± .7, NZ: RB=76.4% ± 2.0, II=76.5% ± .1). Statistical comparisons combining all acquisitions confirmed that pigeons’ RB/II performance was equivalent in both speed of learning and terminal accuracy.
Specific-item memorization was not a factor in pigeons’ discrimination performance. Pigeons (USA only) were tested for discrimination transfer with novel tilt-width stimuli chosen from within the same elliptical distributions that defined the categories (i.e., the distributions of open circles and filled squares in Figure 1), but from different locations than the training stimuli. Pigeons were tested immediately after reaching criterion with any task. Two different sets of 20 novel stimuli (10 from each category) were tested over four sessions. Each session's 20 non-reinforced transfer probe trials were randomly placed within otherwise normal sessions. The pigeons showed perfect transfer. They had statistically equivalent mean accuracies for novel stimuli (91.02% ± 1.4) and training stimuli (90.1% ± .72). There were also no RB/II performance differences for novel stimuli (RB-91.01% ± 1.42; II-91.0% ± 1.97). Current exemplar-memorization or exemplar-generalization accounts of performance would predict an accuracy advantage for old, training items compared to new, transfer items. Thus, the pigeons were apparently responding based on the general perceptual distributions that defined the categories in stimulus space and that encompassed both training and novel, transfer stimuli.
Discussion
The present results provide the crucial new observation that pigeons show no tendency to learn rules or to apply dimensional analysis when tested with highly controlled and diagnostic category tasks. In sharp contrast to humans and nonhuman primates, the pigeons showed complete indifference to the task's rotation in perceptual space, learning RB and II tasks equally quickly and to the same level of accuracy. Note that this result also suggests that there is no inherent difficulty difference between the two categorization tasks shown in Figure 1. The fact that humans find the dimensional task in the top panel so much easier must therefore be due to differences in how they learn the two tasks rather than a fundamental difference in the two tasks themselves.
The indifference of the pigeons to the task's rotation in perceptual space is exactly the same indifference that humans show to task rotations within non-separable/integral perceptual spaces (Foard & Kemler Nelson, 1984; L. B. Smith & Kemler Nelson, 1978). Pigeons’ data pattern is also similar to that produced by humans’ implicit-striatal category-learning system that is judged to be nonanalytic. Indeed, all aspects of their performance are consistent with a cognitive organization by which they gradually associate behavioral responses to unanalyzed stimulus wholes or to regions of perceptual space, while holding in abeyance stimulus analysis, selective attention, and rule formation. The interpretation emphasizing a response to unanalyzed stimulus wholes has much in common with the proposals of Pearce (1994) and others that animals may treat multi-dimensional stimuli as unitary, configural wholes. The interpretation emphasizing a response to a region of perceptual space owes much to the idea of receptive fields in the perceptual system. Higher-order receptors could be receiving bi-dimensional (tilt-width) inputs, and could be tuned to be maximally responsive to optimal joint values along the two dimensions. Activation would degrade for less optimal stimulation, but, importantly, the higher-order unit would not need or have any appreciation of which dimensional input was non-optimal and by how much.
A non-analytic perceptual system of this kind could still express some phenomena of dimensional emphasis and preferential dimensional responding. For example, if the task contingencies made only one of two stimulus dimensions relevant, pigeons should and do gradually learn to base generalization along the rewarded axis through the stimulus space (e.g. the RB task here). Even non-dimensional, unanalyzed generalization gradients would support this adaptive response pattern. And if the task contingencies were flipped midway during performance, pigeons should and do gradually learn to reorient their discriminatory axis, as the gradients of adaptive generalization shifted (e.g., Leith & Maki, 1975). In just the same way, humans can learn to respond to either axis of variation in stimulus spaces involving dimensions that are clearly integral for them. Even nonanalytic systems can learn, gradually and associatively, to orient or reorient the decision boundary within a perceptual space, and thus they can sometimes respond undimensionally though implicitly (Wills et al., 2009). Though evidence consistent with attentional learning to dimensions by pigeons has been found (e.g., Mackintosh & Little, 1969), these results may sometimes be explained by invoking simpler and nonanalytic perceptual or associative processes.
Therefore, it is important to define carefully the capacity that pigeons did not show in the present tasks. The inferential power of the matched and diagnostic RB and II tasks is that they allow the concurrent evaluation of rule-based and associative task solution within a common, mutually controlling, and well-understood empirical/theoretical framework. By rotating the dimensional axis of category tasks, from RB to II, one can ask whether the minds of different species are dimensionally polarized. If so, then the dimensional task orientation admits strong and rapid learning, just as a polarizing filter will strongly admit light when it finds the axis of the light's polarization. The minds of humans and macaques at least are strongly dimensionalized in this way. In contrast, the pigeons learn both tasks at the same speed and to the same level, and thus most likely in the same, associative way with no dimensional privilege. Thus, the cognitive system of pigeons appears not to be dimensionally polarized in the sense of rapidly appreciating unidimensional task solutions or in the sense of using what one might call proto-rules.
Our conclusion about pigeons’ cognitive system is not constrained by the power of our methods. There were essentially no RB-II differences in learning rates or learning levels, even though we separately tested relatively large numbers of birds in two different and independent experiments (NZ and USA). In sharp contrast, the RB-II performance differences shown by macaques and humans are immediately identifiable, in small subject samples and even individual learners. There is no hint in the present data that a larger sample of pigeons would have produced any RB-II performance differences, much less one the size seen in humans and macaques. Nor are our results constrained by some narrow choice of particular methodologies. There were many methodological differences between the procedures in the NZ and USA laboratories, yet the identical pattern of results obtained.
However, our interpretation is somewhat constrained because we tested only one dimensional pair (tilt-width). This choice was based on the qualitative separability of these dimensions for humans and on the strong, converging, RB privilege found in humans, macaques, and apparently even in capuchin monkeys (Smith et al., in preparation). In pigeons, single cell recordings have found that both spatial frequency and orientation are selectively tuned in different cells (e.g., Hardy & Jassik-Gerschenfeld, 1979) and there is no physiological or behavioral evidence that these dimensions are unitary or integral in any way that differs fundamentally from that seen in other vertebrate species. Nonetheless, it should be stated that we have not shown that pigeons’ categorization is exclusively nonanalytic across all dimensional pairings. We have shown that it is nonanalytic for the dimensional combination that shows the best-documented dimensional privilege in humans and nonhuman primates.
A reviewer suggested it might be of interest to exaggerate the separability of our tilt and width dimensions, by instantiating the two dimensional values in separate stimulus circles. This might cause the RB task to be selectively facilitated and/or the II task to be selectively impaired. But even if one or both of these results occurred, note that it would be difficult to know why. One possibility is that the II task might now require a difficult cognitive integration over multiple looks or fixations. Another is that the relevant dimension in the RB task might be isolable through gaze aversion—aiming the receptors toward one stimulus or the other.
This thought experiment calls to mind recent work by Pearce, Esber, George, and Haselgrove (2008). They found that pigeons were more able to attend selectively to a relevant dimension when it was carried in a separate stimulus, but less able to when it was carried in the same stimulus along with an irrelevant dimension. They concluded that the seeming attentional changes in the former case were a consequence of preliminary receptor-exposure acts, and did not represent more central changes in attention to dimensions. They even expressed their theoretical disappointment that these changes were mediated by peripheral orienting responses, and not by central processes. Their findings and interpretation converge strongly with the present findings and interpretation.
A unitary, exclusively nonanalytic category system would have some distinct advantages for pigeons. Such a system could have a neural economy that might especially suit nervous systems constrained in size by the weight limitations of flight. Moreover, pigeons could avoid strategy competition during category learning and avoid the maladaptive, adventitious rules that humans often invent and exhibit during learning (Jitsumori, 1993). They might also be adept at learning non-linear category boundaries that would defeat a rule-based system. There is a parsimony, breadth and power to a category-learning system that always, simply associates responses to stimuli, without overlaying axes, dimensions, and rules. We therefore suggest that pigeons’ category learning could illuminate a phylogenetically ancient associative categorization system that is widely distributed across the vertebrates.
In turn, one considers when and why in vertebrate evolution the privilege of dimensional analysis and category rules emerged. Rule-based category systems are not a species-unique human endowment, grounded in humans’ language, symbolic functioning, or frontal-cortical brain development. Macaques (Macaca mulatta) also learn RB tasks faster and to higher terminal performance levels than they do II tasks, probably because they perceive analytically the stimulus dimensions composing the stimuli (Smith et al., 2010). Macaques present an illuminating comparative contrast to pigeons. They also help date the phylogenetic emergence of dimensionally-analytic categorization. This emergence in the primates was surely gradual—related research (Smith, Minda, & Washburn, 2004) shows that macaques do not have the full suite of dimensionally analytic categorization abilities.
The multiple-systems organization also has distinct advantages. It allows for economical, quickly learned, and easy-to-generalize category representations (i.e., rules). It brings cognitive flexibility and attentional agility arising from dimensional analysis. Perhaps most important, it opens up the possibilities for cognitive analysis, rules, inferences, symbolic representations, and eventually even language. Therefore, the privilege that developed regarding explicit dimensional analysis and category rules may have been among the crucial pre-adaptations that promoted cognitive evolution within the primate-hominid lineage.
Acknowledgments
The preparation of this article was supported by NSF Grant 718804, NIMH Grant MH3760-2, ARO Collaborative Biotechnologies Grant DAAD19-03-D-0004, NICHD Grant HD-38051, and NICHD Grant HD-060563.
Footnotes
JDS conceived the USA project and was the article's principal author. FGA and BS produced stimuli and contributed to data analysis, modeling, and writing. RGC and MSM implemented and conducted the USA project and contributed to data analysis and writing. RCG and MEB conceived, implemented, and conducted the NZ project and contributed to data analysis and writing.
The authors declare no conflict of interest.
Contributor Information
J. David Smith, Department of Psychology, The University at Buffalo, State University of New York.
F. Gregory Ashby, Department of Psychology, University of California at Santa Barbara.
Mark E. Berg, Richard Stockton College of New Jersey
Matthew S. Murphy, Department of Psychology, Tufts University
Brian Spiering, Department of Psychology, University of California at Santa Barbara.
Robert G. Cook, Department of Psychology, Tufts University
Randolph C. Grace, Department of Psychology, University of Canterbury, Christchurch
References
- Ashby FG, Alfonso-Reese LA, Turken AU, Waldron EM. A neuropsychological theory of multiple systems in category learning. Psychological Review. 1998;105:442–481. doi: 10.1037/0033-295x.105.3.442. [DOI] [PubMed] [Google Scholar]
- Ashby FG, Ell SW. The neurobiology of human category learning. Trends in Cognitive Sciences. 2001;5:204–210. doi: 10.1016/s1364-6613(00)01624-7. [DOI] [PubMed] [Google Scholar]
- Ashby FG, Ell SW, Waldron EM. Procedural learning in perceptual categorization. Memory and Cognition. 2003;31:1114–1125. doi: 10.3758/bf03196132. [DOI] [PubMed] [Google Scholar]
- Ashby FG, Ennis JM, Spiering BJ. A neurobiological theory of automaticity in perceptual categorization. Psychological Review. 2007;114:632–656. doi: 10.1037/0033-295X.114.3.632. [DOI] [PubMed] [Google Scholar]
- Ashby FG, Gott RE. Decision rules in the perception and categorization of multidimensional stimuli. Journal of Experimental Psychology. 1988;14:33–53. doi: 10.1037//0278-7393.14.1.33. [DOI] [PubMed] [Google Scholar]
- Ashby FG, Maddox WT. Human category learning. Annual Review of Psychology. 2005;56:149–178. doi: 10.1146/annurev.psych.56.091103.070217. [DOI] [PubMed] [Google Scholar]
- Cook RG, Smith JD. Stages of abstraction and exemplar memorization in pigeons’ category learning. Psychological Science. 2006;17:1059–1067. doi: 10.1111/j.1467-9280.2006.01833.x. [DOI] [PubMed] [Google Scholar]
- Erickson MA, Kruschke JK. Rules and exemplars in category learning. Journal of Experimental Psychology: General. 1998;127:107–140. doi: 10.1037//0096-3445.127.2.107. [DOI] [PubMed] [Google Scholar]
- Foard CF, Kemler Nelson DG. Holistic and analytic modes of processing: The multiple determinants of perceptual analysis. Journal of Experimental Psychology: General. 1984;113:94–111. doi: 10.1037//0096-3445.113.1.94. [DOI] [PubMed] [Google Scholar]
- Hardy O, Jassik-Gerschenfeld D. Spatial frequency and temporal frequency selectivity of single cells in the pigeon optic tectum. Vision Research. 1979;19:1001–1004. doi: 10.1016/0042-6989(79)90225-6. [DOI] [PubMed] [Google Scholar]
- Herrnstein RJ, Loveland DH, Cable C. Natural concepts in pigeons. Journal of Experimental Psychology, Animal Behavior Processes. 1976;2:285–301. doi: 10.1037//0097-7403.2.4.285. [DOI] [PubMed] [Google Scholar]
- Jitsumori M. Category discrimination of artificial polymorphous stimuli based on feature learning. Journal of Experimental Psychology, Animal Behavior Processes. 1993;19:244–254. doi: 10.1037//0097-7403.22.4.405. [DOI] [PubMed] [Google Scholar]
- Knowlton BJ, Squire LR. The learning of categories: Parallel brain systems for item memory and category knowledge. Science. 1993;262:1747–1749. doi: 10.1126/science.8259522. [DOI] [PubMed] [Google Scholar]
- Lea SEG, Ryan CME. In: Quantitative Analyses of Behavior. Commons ML, Herrnstein RJ, Kosslyn SM, Mumford DB, editors. Vol. 8. Erlbaum; Hillsdale NJ: 1990. pp. 165–186. [Google Scholar]
- Leith CR, Maki WS. Attention shifts during matching-to-sample performance in pigeons. Animal Learning and Behavior. 1975;3:85–89. [Google Scholar]
- Mackintosh NJ, Little L. Intradimensional and extradimensional shift learning by pigeons. Psychonomic Science. 1969;14:5–6. [Google Scholar]
- Maddox WT, Ashby FG, Bohil CJ. Delayed feedback effects on rule-based and information-integration category learning. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2003;29:650–662. doi: 10.1037/0278-7393.29.4.650. 1969. [DOI] [PubMed] [Google Scholar]
- Marcus GF, Vijayan S, Bandi Rao S, Vishton PM. Rule learning in 7-month-old infants. Science. 1999;283:77–80. doi: 10.1126/science.283.5398.77. [DOI] [PubMed] [Google Scholar]
- Murphy GL. The big book of concepts. MIT Press; Cambridge, MA: 2003. [Google Scholar]
- Nosofsky RM. Attention and learning processes in the identification and categorization of integral stimuli. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1987;13:87–108. doi: 10.1037//0278-7393.13.1.87. [DOI] [PubMed] [Google Scholar]
- Pearce JM. Similarity and discrimination: A selective review and a connectionist model. Psychological Review. 1994;101:587–607. doi: 10.1037/0033-295x.101.4.587. [DOI] [PubMed] [Google Scholar]
- Pearce JM, Esber GR, George DN, Haselgrove M. The nature of discrimination learning in pigeons. Learning & Behavior. 2008;36:18–199. doi: 10.3758/lb.36.3.188. [DOI] [PubMed] [Google Scholar]
- Posner MI, Goldsmith R, Welton KE. Perceived distance and the classification of distorted patterns. Journal of Experimental Psychology. 1967;73:28–38. doi: 10.1037/h0024135. [DOI] [PubMed] [Google Scholar]
- Rossi AF, Pessoa L, Desimone R, Ungerleider LG. The prefrontal cortex and the executive control of attention. Experimental Brain Research. 2009;192:489–497. doi: 10.1007/s00221-008-1642-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Grossman M. Multiple systems of category learning. Neuroscience and Biobehavioral Reviews. 2008;32:249–264. doi: 10.1016/j.neubiorev.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JD, Beran MJ, Crossley MJ, Boomer J, Ashby FG. Implicit and explicit category learning by macaques (Macaca mulatta) and humans (Homo sapiens). Journal of Experimental Psychology: Animal Behavior Processes. 2010;36:54–65. doi: 10.1037/a0015892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JD, Boomer J, Church BA, Beran MJ, Crossley MJ, Ashby FG. The privilege of dimensions and rules in the category learning of capuchin monkeys (Cebus apella). in preparation. [DOI] [PMC free article] [PubMed]
- Smith JD, Minda JP. Prototypes in the mist: the early epochs of category learning. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1998;24:1411–1436. [Google Scholar]
- Smith JD, Minda JP, Washburn DA. Category learning in rhesus monkeys: A study of the Shepard, Hovland, and Jenkins tasks. Journal of Experimental Psychology: General. 2004;133:398–414. doi: 10.1037/0096-3445.133.3.398. [DOI] [PubMed] [Google Scholar]
- Smith JD, Redford JS, Haas SM. Prototype abstraction by monkeys (Macaca mulatta). Journal of Experimental Psychology: General. 2008;137:390–401. doi: 10.1037/0096-3445.137.2.390. [DOI] [PubMed] [Google Scholar]
- Smith LB, Kemler DG. Levels of experienced dimensionality in children and adults. Cognitive Psychology. 1978;10:502–532. doi: 10.1016/0010-0285(78)90009-9. [DOI] [PubMed] [Google Scholar]
- Wasserman EA, Kiedinger RE, Bhatt RS. Conceptual behavior in pigeons: categories, subcategories, and pseudocategories. Journal of Experimental Psychology: Animal Behavior Processes. 1988;14:235–246. [Google Scholar]
- Wills AJ, et al. A comparative analysis of the categorization of multidimensional stimuli. Journal of Comparative Psychology. 2009;123:391–405. doi: 10.1037/a0016216. [DOI] [PubMed] [Google Scholar]