Abstract
Correlated light and electron microscopic (CLEM) imaging is a powerful method for dissecting cell and tissue function at high resolution. Each imaging mode provides unique information and the combination of the two can contribute to a better understanding of the spatio-temporal patterns of protein expression, trafficking and function. Critical to these methods is the use of genetically appended tags that highlight specific proteins of interest in order to be able to pick them out of their complex cellular environment. Here we review and discuss the current generation of genetic labels for direct protein identification by CLEM, addressing their relative strengths and weaknesses and in what experiments they would be most useful.
Keywords: tetracysteine, biarsenical, miniSOG, metallothionein, ferritin, photooxidation, fluorescent proteins
I. Introduction
In 2008 the Nobel Prize for chemistry was awarded to Osamu Shimomura, Martin Chalfie and Roger Y. Tsien for the discovery and development of the jellyfish protein Green Fluorescent Protein (GFP) as an imaging tool for light microscopy. The development of GFP, similarly constructed 11-stranded β barrel proteins such as the coral protein DsRed derived monomeric RFP and their spectral derivatives, enabled a revolution in live-cell light microscopic imaging as well as fluorescence microscopy of fixed preparations. Using these genetically appended tags, individual proteins could be identified and tracked within a cell as well as expressed as soluble fluorescent proteins (FPs) that can be used to delineate a particular subset of cells. Particularly in neuronal tissues, soluble FPs enable researchers to delineate and track fine neuronal processes in an otherwise highly complex milieu. Soluble FPs can also be used to denote the transfection event of a second tagged protein within the same cell. In addition, the development of photo-activated fluorescent proteins (FP) has made possible the development of a subset of super-resolution light microscopy (LM) techniques such as PALM (Henriques et al., 2011, Leung and Chou, 2011). There has been much interest within the imaging field to build or extend these kinds of genetically appended light microscopy (LM) labels to protein detection in the electron microscope (EM) with the advantage of superior resolution and a different modality of imaging based on mass density.
II. The crowded cell and spatio-temporal proteomics
The human genome contains 23,000 genes that give rise to larger numbers of active proteins. Adding to this number are different isoforms of these proteins with alternative splicing and post-translational modifications (Andersen and Mann, 2006). Organelles such as the nuclear pore complex contain many proteins (Rout et al., 2000) while the cytoplasm is a highly complex and organized matrix. The cytosol contains components mostly in a non-equilibrium system due to the multiple levels of organization of protein in it (Fulton, 1982). Viscosity measurements of the cytosol is similar to pure water, however, the diffusion of small molecules is about four-fold slower than pure water solutions (Verkman, 2002). In the axon, the protein content has been estimated to be ~ 2% of the weight of the axoplasm, while in the oocytes the cytoplasm is between 30% and 40% protein by weight, excluding the protein of the yolk. Muscle cells contain approximately 23% protein by weight; red blood cells contain about 35% protein by weight: and in general actively growing cells contain between 17% and 26% protein by weight (Fulton, 1982). These measurements are comparable to the protein content of crystals used for structure determination (Vergara et al., 2005).
The field of “spatial cell biology” (Hurtley, 2009) has been defined as the study of the spatio-temporal distribution of cellular components. In particular, the location of a cell within an organism and the location within the cell of its constituent parts affects the functions cells, tissues and organisms perform, molecular signaling partners, growth and division. The establishment and maintenance of cellular and organismal order dictates that cell components such as proteins “know their place”. In particular, the regulatory machinery of the cell must be organized in a highly sophisticated manner, both spatially as well as temporally, ensuring that, for example, signaling enzymes are able to correctly encounter their intracellular substrates. These processes involve the coordinated movement of molecules and complexes through the crowded cytoplasm to their correct positions. In order to observe this elegant molecular ballet, the development of highly specific imaging probes is crucial to identifying them from among the molecular and organellar “crowd”.
III. What EM has to offer
Microscopy has the ability to examine single cells among many and their internal molecular organization. Fluorescence imaging has proved to be very powerful because probes such as FPs, fluorescent antibodies and small probes (e.g. phalloidin staining for actin or DAPI for DNA) provide a signal from only specific proteins or nucleic acids. FPs, in particular, have been of tremendous value for live-cell imaging of dynamic events. However, interacting components and organelles must be stained as well in order to visualize multiple interactions. While the resolution of LM imaging has increased due to the development of super-resolution techniques, LM can still only provide information related to a labeled molecule or set of molecules. Even with super-resolution techniques, electron microscopy still has a minimum ~5 fold improvement in practical resolution, and transmission electron microscopy has the benefit of being able to view other cellular components when there is sufficient mass density. However, because of the limitation of putting biological samples into a vacuum, EM imaging is currently limited to biological specimens fixed or frozen at a specific time.
Techniques have been developed to combine light and electron microscopy on the same specimens in order to exploit the advantages of both imaging methods. This has been referred to as CLEM, for Correlated Light and Electron Microscopy (the same area is examined by both imaging techniques) or Correlative Light and Electron Microscopy (the same specimen, but not the same area, is imaged by LM and EM). The latter is typically less time consuming because less searching is required. The combination of LM and EM has proved to be very powerful since it combines the specificity and dynamics of the fluorescent imaging probes using LM with the increased resolution and cellular context made available by EM techniques. Specialized probes, mostly for proteins, have been developed for CLEM and have the characteristics of exhibiting fluorescence for LM observations, and either have or can be used to generate mass density contrast during the EM specimen preparation process.
IV. Immuno-markers
Antibody labeling techniques remain an extremely important tool for the subcellular localization of proteins. These probes have the advantage of high specificity and signal strength when used in combination with secondary antibodies with either fluorescent tags, colloidal gold markers, immuno-enzymatic methods or eosin-based fluorescence photooxidation (Sosinsky et al., 2007a). The primary disadvantage to pre-embedding procedures for immunolabeling is that the milder fixation conditions and detergent permeabilization incubation necessary to allow antibodies to enter a cell or tissue results in a greatly compromised cellular ultrastructure. In particular, for proteins embedded in interior membrane compartments or soluble within the cytoplasm, structures are often compromised or soluble proteins lost during incubation steps. Some of this damage can be partially mitigated by the addition of small amounts of glutaraldehyde (0.01–1%) in a standard 2–4% paraformaldehyde fixation. However, there is typically a tradeoff in the accessibility and conformation of epitopes to these immuno-probes, often resulting in a reduced LM and EM signal. CLEM using the genetic tags described below has the potential for significantly improved preservation of ultrastructure. Since no permeabilization step is necessary and because the label is intrinsically incorporated into the sample, strong fixatives such as 1–2% glutaraldehyde can be used. However, it should be noted that in certain cases, such as when examining proteins that adopt unique conformations during part of their life cycle, conformation-dependent antibodies are necessary. For example, phospho-specific antibodies are still the only way to discriminate between different phospho-forms of proteins within the cell (Sosinsky et al., 2007b).
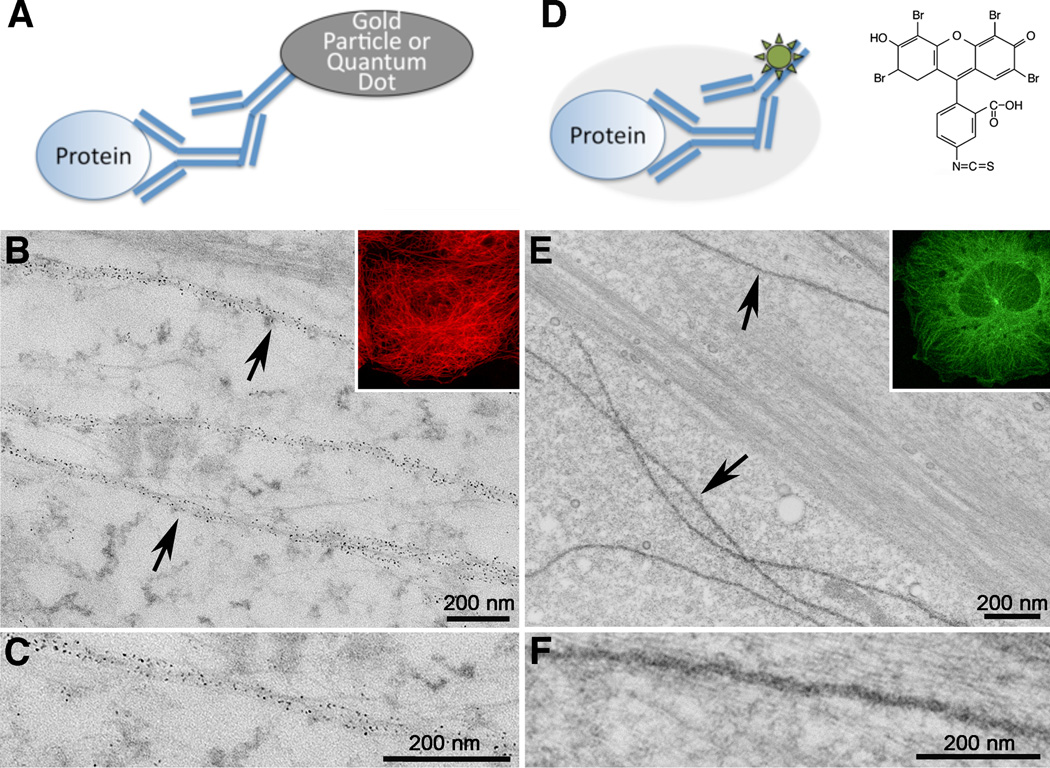
For CLEM imaging, secondary antibodies must be fluorescent and contain an electron dense label. Two types of CLEM secondary antibodies are currently commercially available. These are quantum dot conjugated secondary antibodies (Nisman et al., 2004) and FluoroNanogold™, a secondary antibody that has both a fluorescent moiety and a 1.8 nm gold cluster. Quantum dots have strong and stable fluorescence and unique geometries for multi-labeling for electron microscopy (Giepmans et al., 2005) (Fig. 1A–C), however, quantum dot conjugated secondary antibodies are about the same size as small colloidal gold beads so that epitope accessibility can still be problematic. In addition, quantum dots are less dense than gold spheres and thus, can be difficult to find within a dense cytoplasm. FluoroNanogold™ has the advantage of being fluorescent and having a smaller gold particle for better penetration (Takizawa et al., 1998, Takizawa and Robinson, 2000), but require a gold or silver enhancement step to grow the gold cluster immunolabeling in order to easily detect the particle in EM images. Fluorescence photooxidation (see next section) can also be used as a detection method using eosin conjugated to secondary antibodies or small ligands (Deerinck et al., 1994, Capani et al., 2001) (Fig. 1D–F).
Figure 1. Comparison of antibody particle labels versus antibody fluorescent photooxidation labeling.
(A) Schematic of indirect (secondary) antibody labeling for correlative light and electron microscopy where the secondary antibody is conjugated to a gold bead or quantum dot. (B) Electron micrograph of microtubules (arrows) labeled with immuno-quantum dots. The primary antibody is an α-tubulin monoclonal antibody. The fluorescence image from the quantum dots is shown in the inset. (C) Higher magnification of microtubule. (D) Schematic of photooxidized eosin-immunolabeling. The grey ellipse represents the layer of DAB precipitate after photooxidationof the eosin (green sun) on the secondary antibody. Eosin is a brominated version of the common fluorophore fluorescein, and the chemical formula of the isothiocyanate used for conjugation is shown in the upper right hand corner. (E) Electron micrograph of microtubules (arrows) labeled with anti-α tubulin primary antibodies, eosin secondary antibodies and then photooxidized in the presence of DAB and oxygen. An eosin fluorescence image is shown in the inset. (F) Higher magnification of a single microtubule. The difference in ultrastructure between B and E is primary due to the less stringent fixation methods used so the larger quantum dot secondary antibodies could penetrate into the cell.
V. Genetically appended or inserted protein tags
The key to using genetic tags for the EM portion of a CLEM label is the incorporation of diaminobenzidine (DAB) as part of the labeling protocol. DAB has a long history as a reagent for correlated light and electron microscopy specimen preparation methods. DAB is used as a substrate for enzymatic-based polymerization using peroxidase or singlet oxygen based polymerization during photooxidation (Maranto, 1982, Deerinck et al., 1994). When oxidized, DAB forms a highly insoluble reaction product that can be made electron-dense through treatment with osmium tetroxide. Although peroxidase-based systems generally have limited resolution due to diffusion of reaction product as compared to photooxidation, enzymatic systems have greater sensitivity. DAB-based reaction products are excellent for filling cells for correlated microscopy, either through enzymatic reactions or through photooxidation (Maranto, 1982, Li et al., 2010). For molecular localization using photooxidation, we have shown that fluorescence photooxidation of DAB provides superb resolution and diffusion of the reaction product is minimized by extensive chemical cross-linking by glutaraldehyde prior to the generation of the reaction product. Unlike particulate markers such as protein conjugated colloidal gold, photooxidation-based reaction products do not just decorate the targeted protein but acts almost as a negative stain, in some cases, providing exquisite structural detail (Shu et al., 2011). Staining occurs in close proximity to the molecule rather than being separated by many nanometers as occurs with antibody methods where the secondary antibody is covalently linked to a detection agent such as a colloidal gold bead, quantum dot or eosin and is separated from the target by length of the primary antibody.
To be useful as a CLEM label, there are certain requirements when using genetic tags. Genetic tags should be non-toxic when expressed in a cellular environment, have good sensitivity in the physiological range of the experiment and be active in the required physiological environment (including pH, temperature, aqueous solution, enzymatic environment and ionic concentrations), exhibit fluorescence and either independently or with the addition of additional ligands or cofactors, create an electron dense label. Genetic tags have the additional advantage that they are stoichiometric to their target protein. This quality can be important for low copy number proteins within cells. Table 1 summarizes the properties of several CLEM genetic labels. As with any exogenously expressed protein, it is critical that independent control experiments be done to ensure that recombinant proteins be expressed and trafficked in the same manner as endogenously expressed proteins. Often these controls mean a comparison to immunolabeled native systems.
Table 1.
Comparison of genetic tags for CLEM
| Genetic tag | MW (kDa) |
FQY | 1O2 QY | Advantages | Disadvantages | References |
|---|---|---|---|---|---|---|
| GFP | 26.9 | 0.6 | < 0.004 | Bright fluorescence, well established technology | Poor photooxidizer, Very low reactive oxygen yield. | (Grabenbauer et al., 2005) |
| Tetracysteine/ ReAsH (present 4C or 4N generation). | 2.22 | 0.42–0.47 * | 0.024 | Small size, versatility of ligands especially for pulse chase labeling at LM and EM levels | Low reactive oxygen yield, Limited diffusion and non-specific background of ligands | (Griffin et al., 1998a, Gaietta et al., 2002, Martin et al., 2005, Gaietta et al., 2006) |
| miniSOG/ FMN | 15.4 | 0.37 | 0.47 | Medium size between GFP an tetracysteine Very good singlet oxygen generator; FMN is an endogenous ligand. Strong EM signal after photooxidation. | Weak fluorescence, bleaches rapidly | (Shu et al., 2011) |
| HRP | 34 | NA | NA | Enzymatic reaction. Labeling limited to expressing cells. No photooxidation necessary. High sensitivity. | Needs to be combined with fluorescent protein (GFP) for LM Does not work in oxidized environments, limited so far to plasma membrane or ER targeting. | (Schikorski et al., 2007, Li et al., 2010, Schikorski, 2010) |
| Metallothionein (MTH) | 6 | NA | NA | When gold atoms bind, delivers high contrast. High spatial resolution. Relatively small tag size. | Needs to be combined with fluorescent protein (GFP) for LM Difficult to get gold atoms into cells, toxic. | (Mercogliano and DeRosier, 2006, 2007, Nishino et al., 2007, Diestra et al., 2009a, Diestra et al., 2009b) |
| Ferritin | 19.4 † | NA | NA | Iron less toxic. Strong signal from iron cores. High spatial resolution. | Needs to be combined with fluorescent protein (GFP) for LM. Large size of label when oligomerized. Need to get exogenous iron atoms into cells for labeling. | (Wang et al., 2011) |
FQY = fluorescence quantum yield; 1O2 QY = singlet oxygen quantum yield; NA = not applicable
Measurements done using FRET based emission of GFP-TC proteins
monomer size.
VI. Types of genetic tags currently available
A. GFP
Historically, GFP was one of the first protein tags to be used for CLEM (Monosov et al., 1996). While the shielding of its chromophore helps to make GFP extremely bright, photostable and non-toxic, it also prevents GFP from producing enough singlet oxygen necessary for good DAB polymerization. GFP has been used as a CLEM tag in certain cases where there is high concentrations of GFP tagged expressed protein (Grabenbauer et al., 2005) that in special cases provides sufficient singlet oxygen generation, even though the level of GFP singlet oxygen quantum yield is close to zero (quantum yield < 0.004) (Jimenez-Banzo et al., 2008).
B. Tetracysteine domain/ReAsH ligand
In 1998, Albert Griffin, Stephen Adams and Roger Tsien demonstrated that recombinant proteins in intact cells can be imaged by genetically appending or inserting a small tetracysteine motif, -Cys-Cys-Xaa-Xaa-Cys-Cys- and then exposing the cells to a membrane-permeant non-fluorescent biarsenical derivative of fluorescein, termed FlAsH (Griffin et al., 1998b, Griffin et al., 2000). The uniquely designed stereochemistry of the tetracysteine domain binds small molecules containing two arsenic atoms with a specific distance that coordinate with the SH groups of the Cys residues. FlAsH binds with high affinity and specificity to the tetracysteine motif and becomes strongly green fluorescent. Toxicity from binding of the trivalent arsenic atoms to endogenous thiols is mitigated by simultaneous administration of micromolar concentrations of arsenic antidotes such as 1,2-ethanedithiol (EDT) or British anti-Lewisite (BAL; dimercaprol; 2,3-dimercaptopropanol). Upon removal of excess FlAsH, the fluorescent complexes survive for days in the absence of excessive (mM) concentrations of competing EDT. We engineered a recombinant connexin43 (Cx43) by genetically fusing a 17 amino-acid-long tetracysteine receptor domain (EAAAREACCRECCARA) to the COOH terminus of Cx43 (Gaietta et al., 2002) and showed localization to gap junctions and internal trafficking structures including vesicles, lysosomes, as well as portions of the ER and the Golgi apparatus.
One of the advantages of the tetracysteine tag/biarsenical ligand labeling approach is that one can employ multiple ligands with specialized properties for different imaging purposes, and since its introduction, multiple ligands have been successfully synthesized. One such ligand, termed ReAsH, a red fluorescent biarsenical derivative of resorufin, was found to be a modest singlet oxygen generator suitable for fluorescence photooxidation of DAB (Adams et al., 2002). Using a directed evolution approach, the tetracysteine domain sequence was optimized (Martin et al., 2005) to greatly increase the ReAsH affinity over the original peptide. This peptide has the sequence FLNCCPGCCMEP and can be appended to either the N or C terminus (referred to as a 4N or 4C tag, respectively). While an improvement, the absolute contrast value of the bound ReAsH fluorescence was still lower than that of GFP. However, this enhanced tetracysteine domain has the advantages of its small size (typically between 14 and 16 amino acids), the ability to use ligands with different spectral properties to achieve an optical pulse chase labeling of new and old proteins and its improved EM contrast due to the higher signal to noise ratio.
Because the tag size is small, tetracysteine tags can be incorporated more easily into proteins. Typically, tags are appended to the protein either at the N or C terminus (see actin example in Fig. 2), however, in many instances, tetracysteine domains have been incorporated into internal sites within a protein without any disruption of function or trafficking (Hoffmann et al., 2005, Lanman et al., 2008, Boassa et al., 2010, Ziegler et al., 2011). In particular, viral function and infectivity can be maintained when small tetracysteine tags are incorporated into internal positions (Rudner et al., 2005, Lanman et al., 2008, Whitt and Mire, 2011).
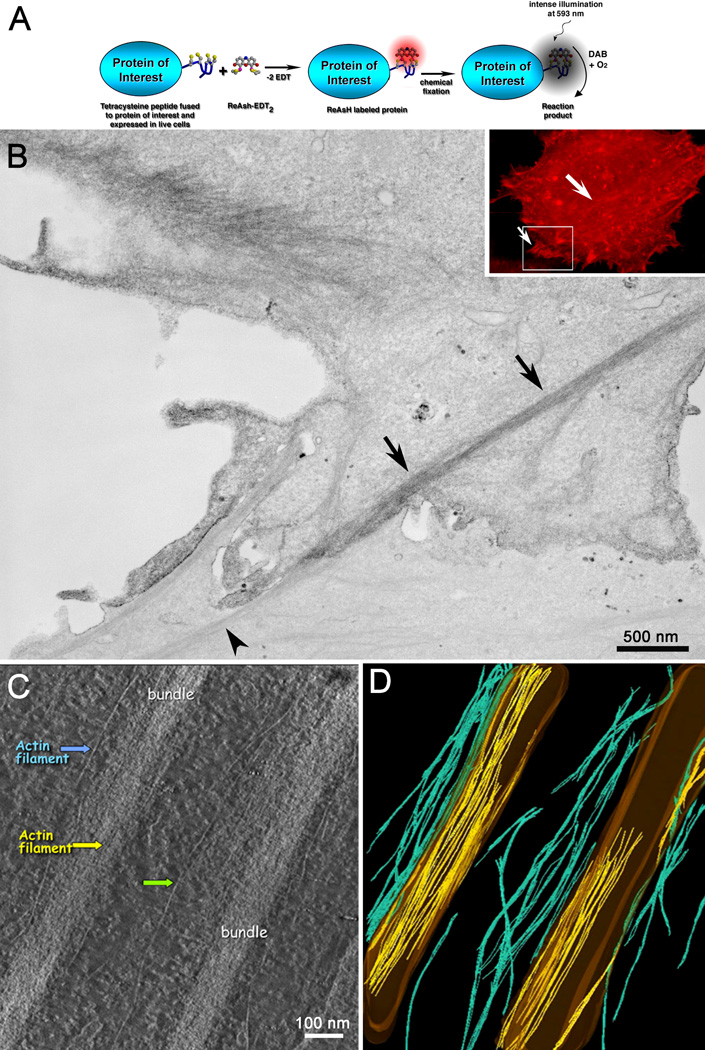
Figure 2. Genetic tetracysteine domain and ReAsH provide good labeling and ultrastructure of specific proteins.
(A) Schematic of fluorescence photooxidation for correlative LM and EM (reprinted from Sosinsky et al., (2003)). (B) Confocal fluorescence image (inset) and thin section electron microscopy of the same two adjacent cells, one transfected with an N-terminal appended tetracysteine domain. Labeling is obvious in the transfected cell (top cell) as opposed to the untransfected cell (bottom cell). Arrows denote labeled stress fiber containing actin filaments, versus an unlabeled bundle (arrowhead). (C) Slice from an EM tomogram of stress bundles containing tetracysteine/ReAsH β actin. Arrows point to individual actin filaments. (D) Tracing of actin filaments in the tomogram. The outlines of the actin bundles are shown in brown. Cyan filaments are those actin filaments separated from the actin bundle while the yellow filaments are those that were easily traced within the bundle.
Later improvements to the tetracysteine tagging system included incorporating it into GFP. The tetracysteine tag can be appended to either the N or C terminus of GFP for subsequent fusion of the complex to either the N or C terminus of the target protein. Fusing this newer tetracysteine to GFP and exciting ReAsH indirectly via fluorescence resonance energy transfer from the fluorescent protein (Gaietta et al., 2006) can increase the contrast of the 4C/ReAsH to within a factor of two of GFP. This novel combinatorial tag has several advantages for dynamic imaging, in that it combines the properties of GFP (brightness, high specificity, and ease of use) with small tetracysteine tags (pulse-chase, photooxidation, excellent preservation for EM). It is worth noting that both GFP and tetracysteines do not fluoresce well in oxidative compartments such as the Golgi or ER, but this effect can be ameliorated by carrying out the biarsenical labeling step during a short incubation that includes triethylphosphine (TEP), a membrane permeant phosphine (Gaietta et al., 2006).
C. Other genetic tags that covalently bind exogenous fluorescent ligands
A strategy similar to tetracysteine/biarsenical labeling whereby an exogenous ligand covalently binds to a genetically engineered protein domain is the SNAP-tag system. Covalent labeling with a small molecule is achieved through the mammalian O6-alkylguanine-DNA-alkyltransferase (hAGT) that irreversibly transfers the alkyl group from its substrate, O6-alkylguanine-DNA, to one of its cysteine residues. These ligands have guanine or chloropyrimidine leaving groups via a benzyl linker that bind to hAGT (Keppler et al., 2003). Dyes conjugated to benzyl linkers facilitate fluorescence labeling. A mutant version of hAGT that binds benzocysteine provides an orthogonal labeling system. The commercial versions are marketed as SNAP and CLIP (New England Biolabs). The size of the SNAP and CLIP protein tags are ~20 kDa. The commercially available HALOtag™ is 33 kDa enzyme derivitized from a modified prokaryotic dehalogenase (DhaA), that makes stable bonds can be readily made with synthetic molecules appended to a chloroalkane linker (Los et al., 2008) (Promega Corporation). While there are no reports of ligands currently available that would allow EM observation, there is a potential for having an orthogonal set of genetic labels with these reagents.
D. MiniSOG: a genetic tag that tightly binds endogenous flavin mononucleotide
Addition of an exogenous ligand is a requirement for fluorescence imaging and DAB photooxidation with the tetracysteine/biarsenical labeling system. While this is typically not a problem in tissue culture cells (Griffin et al., 1998a, Gaietta et al., 2002) or transfected primary cells in culture (Ju et al., 2004), the labeling with exogenous ligands in intact tissues can be highly problematic. In particular, diffusion of biarsenicals across the blood-brain barrier or through large expanses of tissue to their correct targets presents a challenge. In addition, tetracysteine/biarsenical labeling requires the co-administration of an antidote such as ethandithiol to prevent cellular toxicity and needs careful precautions to mitigate nonspecific background labeling. Our efforts to create other new and novel genetic tags was in part driven by the need to develop protein tags that did not require the application of an endogenous fluorescent ligand. Thus, when the tagged protein is expressed within the cellular environment, the recombinant protein would become fluorescent and capable of generating a reaction product upon light stimulation without any further treatments.
Using a completely new approach, Shu and co-workers (2011) developed a new genetically encoded tag that has the advantages of a tag size significantly smaller than conventional fluorescent proteins, exhibits intrinsic fluorescence without the need for an exogenously applied fluorescent ligand, has low cellular toxicity and exhibits a substantially higher singlet oxygen quantum yield than ReAsH (Shu et al., 2011) (Fig. 3). MiniSOG (for mini Singlet Oxygen Generator) was engineered from the LOV2 (light, oxygen and voltage) domain of the protein phototropin 2, a blue light plant photoreceptor from Arabidopsis thaliana that binds flavin mononucleotide (FMN), a highly efficient singlet oxygen photosensitizer. FMN is ubiquitous in cells and is involved in biological functions such as mitochondrial electron transport, fatty acid oxidation and vitamin metabolism. In phototropin 2, the excited state energy of FMN is consumed to form a covalent bond with a cysteine. To divert this energy into fluorescence and singlet oxygen generation, saturation mutagenesis of regions surrounding the chromophore of the LOV2 domain of phototropin 2 was performed. The current version of miniSOG, at 10.6 kDa, is less than half the size of GFP and has two emission peaks at 500 and 528 nm and a singlet oxygen quantum yield of 0.47, an almost 20x improvement over ReAsH. For comparison, eosin has a singlet oxygen quantum yield of 0.57, however, its fluorescence yield is only about 0.05 (10–20× less than fluorescein). Furthermore, in the absence of light miniSOG causes no discernable cellular toxicity.
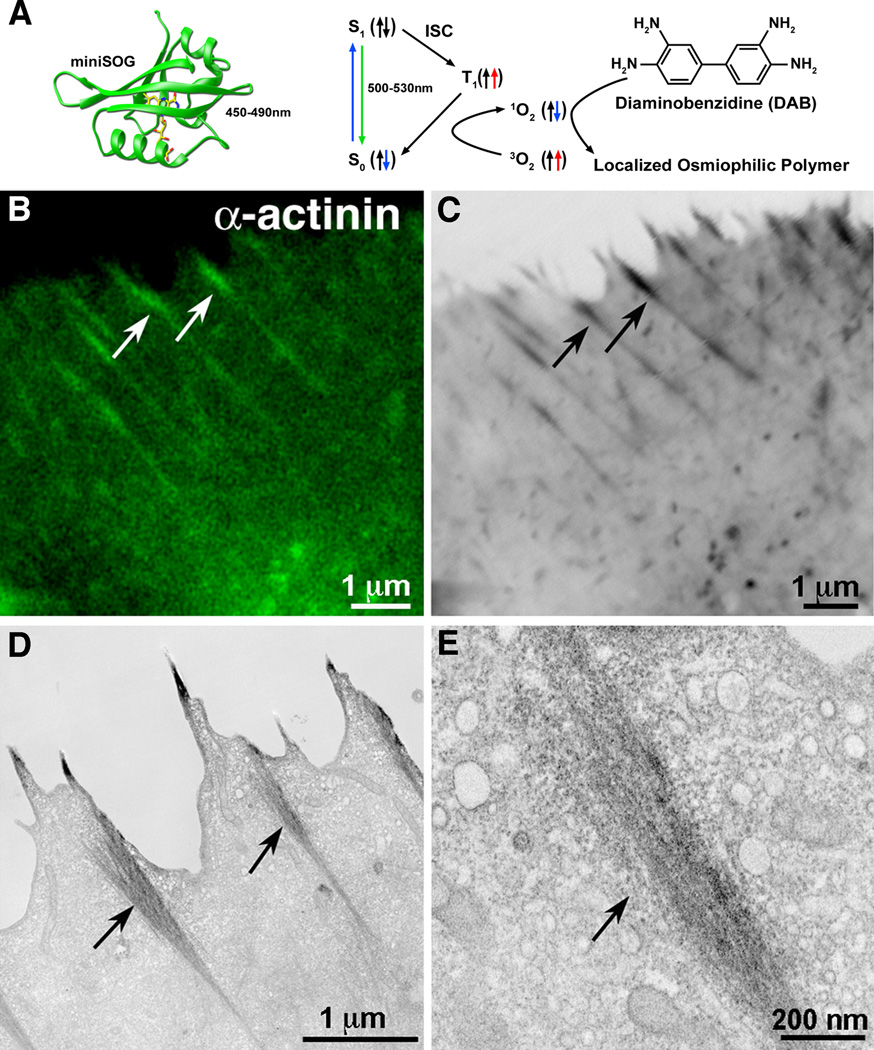
Figure 3. Fluorescence photooxidation of mini-SOG labeled α-actinin.
(A) Richardson diagram of miniSOG with FMN (left). The photooxidation process is shown in the right hand schematic. (B–E) miniSOG was genetically appended to the C-terminus of α-actinin. (B) Confocal microscope image of miniSOG fluorescence highlight actin bundles (C) Transmitted light micrograph of the same area after photooxidation. (D) Thin section EM of the same area. (E) Higher magnification of the actin bundle shows DAB precipitate surrounding labeled α-actinin. (Figure reproduced from Shu et al., (2011)).
The fluorescence from miniSOG can be used to successfully localize a wide variety of proteins and organelles in cultured mammalian cells in much the same way as can be done with GFP. Its green fluorescence, while modest compared to GFP (fluorescence quantum yield of 0.37 versus 0.6), revealed that the fusion proteins appeared to have correct localizations. These targets included the ER and Golgi apparatus (using signal sequence localizations), Rab5a, zyxin, tubulin, β-actin, and α-actinin fusions as examples of proteins tagged in cytosolic compartments (see α-actinin examples in Fig. 3). Mitochondrial targeting and nuclear histone 2B-fusions show that miniSOG expresses within those organelles. In primary neurons, SynCAM-miniSOG fusions localized to the synapse. Using the fluorescence and photo-generated singlet oxygen from miniSOG for fluorescence photooxidation of DAB, correlated confocal and EM imaging could be performed with many miniSOG fusion proteins with high sensitivity. Shu et al., (2011) also showed that miniSOG was functional in transgenic animals, showing EM localization of labeled mitochondria in the muscle walls of C. elegans transfected with miniSOG appended to a cytochrome C targeting motif as well as at synapses labeled with SynCAM-1-miniSOG or SynCAM-2-miniSOG in mice brain slices where the mice were transfected by in utero electroporation.
E. Metal ligand based labels (metallothionein and ferritin)
Theoretically, the binding of metal clusters to proteins could act as markers for EM observations because these metal clusters contain elements that sufficiently scatter electrons. In order for these tags to be useful for CLEM, these protein tags could be appended to FPs. Two protein tags that have been shown to be useful for metal deposition for EM imaging are concatenated metallothionein (MTH) (Mercogliano and DeRosier, 2006, 2007) and bacterial ferritin. Both use the principle that additions of exogenous metal atoms when bound to MTH or ferritin cluster enough atoms for EM detection.
MTH facilitates direct gold labeling of the fusion protein via a reaction with aurothiomalate to its MTH moiety. (Mercogliano and DeRosier, 2006) first demonstrated that purified MTH proteins can form gold clusters that can be imaged by EM. MTH based labels were originally developed as an electron dense label to be used in identification of protein domains in single particle reconstruction of isolated macromolecular complexes (Mercogliano and DeRosier, 2007). MTH binds ~12–20 gold atoms per copy, approximately as much as is found in the EM reagent, Nanogold® (Nanoprobes Inc., Yaphank, NY) that has a 1.4 nm diameter. One benefit is that at ~6 kDa, the present concatenated MTH is a relatively small tag and can be used as a tandem dimer to increase signal, still leaving the tag at about the same size as miniSOG (Mercogliano and DeRosier, 2007). There are a few reports of MTH as molecular labels in cells for electron microscopic imaging using either gold or cadmium ions to grow clusters to chimeric proteins expressed in E. coli or Cos7 mammalian cells (Fukunaga et al., 2007, Nishino et al., 2007, Diestra et al., 2009a, Diestra et al., 2009b). The major drawback to this tagging system is that exogenous metals must be added to and taken up by cells and these metal solutions can be toxic to the cell. As a result, most cellular studies have used bacteria that are more tolerant of higher concentrations of heavy metals. Zinc clusters, which are better tolerated, can also create an electron dense label (Bouchet-Marquis et al., 2011).
An additional recently developed heavy metal binding protein probe is based on the bacterial iron binding ferritin complex that is assembled from E. coli FtnA protein (19.4 kDa) (Wang et al., 2011). Twenty-four FtnA monomers form a shell with a 7.5 nm central cavity. The center of the ferritin complex becomes loaded with iron when cells grow under iron-rich conditions and the advantage is its high imaging contrast and low toxicity. However, this is a relatively large structure of ~12 nm diameter and because the FtnA needs to oligomerize, a high concentration of chimeric protein is necessary. Test cases of the bacterial chemoreceptor sensor, CheY protein, and the septal ring protein ZapA genetically fused to GFP and FtnA showed detectable metal clusters in the correct cellular locations.
F. Genetically appended peroxidase based labels
Horseradish peroxidase has been used extensively in light and electron microscopy as a reagent for immunocytochemistry (Porstmann and Kiessig, 1992) and there is much interest in adapting it as a genetic tag because of its excellent enzymatic activity in creating an electron dense label. In order for HRP to be functional, it must be glycosylated (Veitch, 2004). Since HRP is a plant glycoprotein, the secretory signal sequence of the human growth hormone on HRP was appended onto the HRP cDNA (ssHRP) to ensure proper trafficking in mammalian cells (Connolly et al., 1994). ssHRP was then targeted to ER by adding the KDEL-retention signal (Norcott et al., 1996). Schikorski and co-workers subsequently showed that this ER marker could be made neuron-specific by modifying the construct so that it was controlled by the synapsin promoter (Schikorski et al., 2007) and can be used with transmitted light imaging as a LM probe (Schikorski, 2010). Examples of specific HRP protein labeling for EM include the HRP-Wingless chimera used to show directed Wingless trafficking in Drosophila embryos (Dubois et al., 2001) and a fusion protein of HRP and type I transmembrane protein, CD2, that labeled specific populations of gamma neurons in Drosophila (Watts et al., 2004) and HRP-synaptophysin-GFP that highlighted populations of synaptic vesicles (Ruthazer et al., 2006).
Recombinant HRP has been proposed as a potentially good target for EM cell filling that would act analogous to soluble cytosolic FPs for LM. The major application of this technique would be to identify specific cells, such as neurons, and aid segmenting their processes in three-dimensional reconstructions obtained either through EM serial section, EM tomography or serial block face scanning electron microscopy (SBEM). However, the major drawback to using the current HRP as a probe for cell filling is that it does not have enzymatic activity in the reduced environment of the cytosol (Li et al., 2010). A membrane-targeted HRP has recently been developed that allows identification of neurons because of the intense staining of their plasma membranes. This protein contains a plasma membrane signal sequence and transmembrane domain fused to the C-terminus of HRP. In combination with a bicistronic vector, soluble GFP was also expressed serving as a fluorescent, cytosolic label, thus facilitating easy identification and segmentation in 3D EM volumes.
VII. Future directions and challenges
Two challenges represent the frontiers for further development of CLEM genetic tags. The first is the development of specific nucleic acid CLEM probes analogous to the protein probes described in this review. Markers for specific DNA or RNA sequences appended to FPs are still lacking. Notably, a recent study developing spectral imaging RNA probes have generated a fluorescent toolkit similar to FPs (Paige et al., 2011) and thus, there is a potential that such imaging tools could be modified for CLEM. The second challenge is expression of EM genetic probes in animals. Several of the genetic tags discussed above require the administration of exogenous ligands for detection of the tagged protein. While ligand-based imaging tags can be very versatile, getting these exogenous reagents requires diffusion through tissues and especially across the blood brain barrier. MiniSOG and peroxidase based probes would circumvent this and in combination with strong fluorescing FPs proved an avenue for CLEM imaging of transgenic animals (Dubois et al., 2001, Shu et al., 2011). Finally, future CLEM improvements will also need to include combining these genetic labeling protocols with methods that optimally preserve the ultrastructure of cells and tissues, such as cryo-fixation methods. High pressure freezing and freeze substitution using hybrid chemical/cryo-fixation protocols show great promise for achieving this goal (Sosinsky et al., 2008).
Acknowledgements
We thank Dr. Roger Tsien and members of the Tsien laboratory for their inspiration. We also thank Dr. Ben Giepmans for preparing the tetracysteine-actin constructs shown in Fig. 2. National Institutes of Health Grants P41RR004050 (MHE), P41GM103412 (MHE), GM086197 (Roger Y. Tsien, PI) and GM065937 and GM072881 (GES) provided funding for this research
References
- Adams SR, Campbell RE, Gross LA, Martin BR, Walkup GK, Yao Y, Llopis J, Tsien RY. New biarsenical ligands and tetracysteine motifs for protein labeling in vitro and in vivo: synthesis and biological applications. J Am Chem Soc. 2002;124:6063–6076. doi: 10.1021/ja017687n. [DOI] [PubMed] [Google Scholar]
- Andersen JS, Mann M. Organellar proteomics: turning inventories into insights. EMBO Rep. 2006;7:874–879. doi: 10.1038/sj.embor.7400780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boassa D, Solan JL, Papas A, Thornton P, Lampe PD, Sosinsky GE. Trafficking and recycling of the connexin43 gap junction protein during mitosis. Traffic. 2010;11:1471–1486. doi: 10.1111/j.1600-0854.2010.01109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchet-Marquis C, Pagratis M, Kirmse R, Hoenger A. Metallothionein as a clonable highdensity marker for cryo-electron microscopy. Journal of Structural Biology. 2012;177:119–127. doi: 10.1016/j.jsb.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capani F, Deerinck TJ, Ellisman MH, Bushong E, Bobik M, Martone ME. Phalloidin-eosin followed by photo-oxidation: a novel method for localizing F-actin at the light and electron microscopic levels. J Histochem Cytochem. 2001;49:1351–1361. doi: 10.1177/002215540104901103. [DOI] [PubMed] [Google Scholar]
- Connolly CN, Futter CE, Gibson A, Hopkins CR, Cutler DF. Transport into and out of the Golgi complex studied by transfecting cells with cDNAs encoding horseradish peroxidase. J Cell Biol. 1994;127:641–652. doi: 10.1083/jcb.127.3.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deerinck TJ, Martone ME, Lev-Ram V, Green DP, Tsien RY, Spector DL, Huang S, Ellisman MH. Fluorescence photooxidation with eosin: a method for high resolution immunolocalization and in situ hybridization detection for light and electron microscopy. J Cell Biol. 1994;126:901–910. doi: 10.1083/jcb.126.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diestra E, Cayrol B, Arluison V, Risco C. Cellular electron microscopy imaging reveals the localization of the Hfq protein close to the bacterial membrane. PLoS One. 2009a;4:e8301. doi: 10.1371/journal.pone.0008301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diestra E, Fontana J, Guichard P, Marco S, Risco C. Visualization of proteins in intact cells with a clonable tag for electron microscopy. J Struct Biol. 2009b;165:157–168. doi: 10.1016/j.jsb.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Dubois L, Lecourtois M, Alexandre C, Hirst E, Vincent JP. Regulated endocytic routing modulates wingless signaling in Drosophila embryos. Cell. 2001;105:613–624. doi: 10.1016/s0092-8674(01)00375-0. [DOI] [PubMed] [Google Scholar]
- Fukunaga Y, Hirase A, Kim H, Wada N, Nishino Y, Miyazawa A. Electron microscopic analysis of a fusion protein of postsynaptic density-95 and metallothionein in cultured hippocampal neurons. J Electron Microsc (Tokyo) 2007;56:119–129. doi: 10.1093/jmicro/dfm027. [DOI] [PubMed] [Google Scholar]
- Fulton AB. How crowded is the cytoplasm? Cell. 1982;30:345–347. doi: 10.1016/0092-8674(82)90231-8. [DOI] [PubMed] [Google Scholar]
- Gaietta G, Deerinck TJ, Adams SR, Bouwer J, Tour O, Laird DW, Sosinsky GE, Tsien RY, Ellisman MH. Multicolor and electron microscopic imaging of connexin trafficking. Science. 2002;296:503–507. doi: 10.1126/science.1068793. [DOI] [PubMed] [Google Scholar]
- Gaietta GM, Giepmans BN, Deerinck TJ, Smith WB, Ngan L, Llopis J, Adams SR, Tsien RY, Ellisman MH. Golgi twins in late mitosis revealed by genetically encoded tags for live cell imaging and correlated electron microscopy. Proc Natl Acad Sci U S A. 2006;103:17777–17782. doi: 10.1073/pnas.0608509103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giepmans BN, Deerinck TJ, Smarr BL, Jones YZ, Ellisman MH. Correlated light and electron microscopic imaging of multiple endogenous proteins using Quantum dots. Nat Methods. 2005;2:743–749. doi: 10.1038/nmeth791. [DOI] [PubMed] [Google Scholar]
- Grabenbauer M, Geerts WJ, Fernadez-Rodriguez J, Hoenger A, Koster AJ, Nilsson T. Correlative microscopy and electron tomography of GFP through photooxidation. Nat Methods. 2005;2:857–862. doi: 10.1038/nmeth806. [DOI] [PubMed] [Google Scholar]
- Griffin BA, Adams SR, Jones J, Tsien RY. Fluorescent labeling of recombinant proteins in living cells with FlAsH. Methods Enzymol. 2000;327:565–578. doi: 10.1016/s0076-6879(00)27302-3. [DOI] [PubMed] [Google Scholar]
- Griffin BA, Adams SR, Tsien RY. Specific covalent labeling of recombinant protein molecules inside live cells. Science. 1998a;281:269–272. doi: 10.1126/science.281.5374.269. [DOI] [PubMed] [Google Scholar]
- Griffin BA, Adams SR, Tsien RY. Specific covalent labeling of recombinant protein molecules inside live cells. Science. 1998b;281:269–272. doi: 10.1126/science.281.5374.269. [DOI] [PubMed] [Google Scholar]
- Henriques R, Griffiths C, Hesper Rego E, Mhlanga MM. PALM and STORM: unlocking live-cell super-resolution. Biopolymers. 2011;95:322–331. doi: 10.1002/bip.21586. [DOI] [PubMed] [Google Scholar]
- Hoffmann C, Gaietta G, Bunemann M, Adams SR, Oberdorff-Maass S, Behr B, Vilardaga JP, Tsien RY, Ellisman MH, Lohse MJ. A FlAsH-based FRET approach to determine G protein-coupled receptor activation in living cells. Nat Methods. 2005;2:171–176. doi: 10.1038/nmeth742. [DOI] [PubMed] [Google Scholar]
- Hurtley S. Spatial cell biology. Location, location, location. Introduction. Science. 2009;326:1205. doi: 10.1126/science.326.5957.1205. [DOI] [PubMed] [Google Scholar]
- Jimenez-Banzo A, Nonell S, Hofkens J, Flors C. Singlet oxygen photosensitization by EGFP and its chromophore HBDI. Biophys J. 2008;94:168–172. doi: 10.1529/biophysj.107.107128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju W, Morishita W, Tsui J, Gaietta G, Deerinck TJ, Adams SR, Garner CC, Tsien RY, Ellisman MH, Malenka RC. Activity-dependent regulation of dendritic synthesis and trafficking of AMPA receptors. Nat Neurosci. 2004;7:244–253. doi: 10.1038/nn1189. [DOI] [PubMed] [Google Scholar]
- Keppler A, Gendreizig S, Gronemeyer T, Pick H, Vogel H, Johnsson K. A general method for the covalent labeling of fusion proteins with small molecules in vivo. Nat Biotechnol. 2003;21:86–89. doi: 10.1038/nbt765. [DOI] [PubMed] [Google Scholar]
- Lanman J, Crum J, Deerinck TJ, Gaietta GM, Schneemann A, Sosinsky GE, Ellisman MH, Johnson JE. Visualizing flock house virus infection in Drosophila cells with correlated fluorescence and electron microscopy. J Struct Biol. 2008;161:439–446. doi: 10.1016/j.jsb.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung BO, Chou KC. Review of super-resolution fluorescence microscopy for biology. Appl Spectrosc. 2011;65:967–980. doi: 10.1366/11-06398. [DOI] [PubMed] [Google Scholar]
- Li J, Wang Y, Chiu SL, Cline HT. Membrane targeted horseradish peroxidase as a marker for correlative fluorescence and electron microscopy studies. Front Neural Circuits. 2010;4:6. doi: 10.3389/neuro.04.006.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Los GV, Encell LP, McDougall MG, Hartzell DD, Karassina N, Zimprich C, Wood MG, Learish R, Ohana RF, Urh M, Simpson D, Mendez J, Zimmerman K, Otto P, Vidugiris G, Zhu J, Darzins A, Klaubert DH, Bulleit RF, Wood KV. HaloTag: a novel protein labeling technology for cell imaging and protein analysis. ACS Chem Biol. 2008;3:373–382. doi: 10.1021/cb800025k. [DOI] [PubMed] [Google Scholar]
- Maranto A. Neuronal mapping: a photooxidation reaction makes Lucifer Yellow useful for electron microscopy. Science. 1982;217:953–955. doi: 10.1126/science.7112109. [DOI] [PubMed] [Google Scholar]
- Martin BR, Giepmans BN, Adams SR, Tsien RY. Mammalian cell-based optimization of the biarsenical-binding tetracysteine motif for improved fluorescence and affinity. Nat Biotechnol. 2005;23:1308–1314. doi: 10.1038/nbt1136. [DOI] [PubMed] [Google Scholar]
- Mercogliano CP, DeRosier DJ. Gold nanocluster formation using metallothionein: mass spectrometry and electron microscopy. J Mol Biol. 2006;355:211–223. doi: 10.1016/j.jmb.2005.10.026. [DOI] [PubMed] [Google Scholar]
- Mercogliano CP, DeRosier DJ. Concatenated metallothionein as a clonable gold label for electron microscopy. J Struct Biol. 2007;160:70–82. doi: 10.1016/j.jsb.2007.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monosov EZ, Wenzel TJ, Luers GH, Heyman JA, Subramani S. Labeling of peroxisomes with green fluorescent protein in living P. pastoris cells. J Histochem Cytochem. 1996;44:581–589. doi: 10.1177/44.6.8666743. [DOI] [PubMed] [Google Scholar]
- Nishino Y, Yasunaga T, Miyazawa A. A genetically encoded metallothionein tag enabling efficient protein detection by electron microscopy. J Electron Microsc (Tokyo) 2007;56:93–101. doi: 10.1093/jmicro/dfm008. [DOI] [PubMed] [Google Scholar]
- Nisman R, Dellaire G, Ren Y, Li R, Bazett-Jones DP. Application of quantum dots as probes for correlative fluorescence, conventional, and energy-filtered transmission electron microscopy. J Histochem Cytochem. 2004;52:13–18. doi: 10.1177/002215540405200102. [DOI] [PubMed] [Google Scholar]
- Norcott JP, Solari R, Cutler DF. Targeting of P-selectin to two regulated secretory organelles in PC12 cells. J Cell Biol. 1996;134:1229–1240. doi: 10.1083/jcb.134.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paige JS, Wu KY, Jaffrey SR. RNA mimics of green fluorescent protein. Science. 2011;333:642–646. doi: 10.1126/science.1207339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porstmann T, Kiessig ST. Enzyme immunoassay techniques. An overview. J Immunol Methods. 1992;150:5–21. doi: 10.1016/0022-1759(92)90061-w. [DOI] [PubMed] [Google Scholar]
- Rout MP, Aitchison JD, Suprapto A, Hjertaas K, Zhao Y, Chait BT. The yeast nuclear pore complex: composition, architecture, and transport mechanism. J Cell Biol. 2000;148:635–651. doi: 10.1083/jcb.148.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudner L, Nydegger S, Coren LV, Nagashima K, Thali M, Ott DE. Dynamic fluorescent imaging of human immunodeficiency virus type 1 gag in live cells by biarsenical labeling. J Virol. 2005;79:4055–4065. doi: 10.1128/JVI.79.7.4055-4065.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruthazer ES, Li J, Cline HT. Stabilization of axon branch dynamics by synaptic maturation. J Neurosci. 2006;26:3594–3603. doi: 10.1523/JNEUROSCI.0069-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schikorski T. Horseradish peroxidase as a reporter gene and as a cell-organelle-specific marker in correlative light-electron microscopy. Methods Mol Biol. 2010;657:315–327. doi: 10.1007/978-1-60761-783-9_25. [DOI] [PubMed] [Google Scholar]
- Schikorski T, Young SM, Jr, Hu Y. Horseradish peroxidase cDNA as a marker for electron microscopy in neurons. J Neurosci Methods. 2007;165:210–215. doi: 10.1016/j.jneumeth.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Shu X, Lev-Ram V, Deerinck TJ, Qi Y, Ramko EB, Davidson MW, Jin Y, Ellisman MH, Tsien RY. A genetically encoded tag for correlated light and electron microscopy of intact cells, tissues, and organisms. PLoS Biol. 2011;9:e1001041. doi: 10.1371/journal.pbio.1001041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosinsky GE, Crum J, Jones YZ, Lanman J, Smarr B, Terada M, Martone ME, Deerinck TJ, Johnson JE, Ellisman MH. The combination of chemical fixation procedures with high pressure freezing and freeze substitution preserves highly labile tissue ultrastructure for electron tomography applications. J Struct Biol. 2008;161:359–371. doi: 10.1016/j.jsb.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosinsky GE, Gaietta GM, Hand G, Deerinck TJ, Han A, Mackey M, Adams SR, Bouwer J, Tsien RY, Ellisman MH. Tetracysteine genetic tags complexed with biarsenical ligands as a tool for investigating gap junction structure and dynamics. Cell Commun Adhes. 2003;10:181–186. doi: 10.1080/cac.10.4-6.181.186. [DOI] [PubMed] [Google Scholar]
- Sosinsky GE, Giepmans BN, Deerinck TJ, Gaietta GM, Ellisman MH. Markers for correlated light and electron microscopy. Methods in cell biology. 2007a;79:575–591. doi: 10.1016/S0091-679X(06)79023-9. [DOI] [PubMed] [Google Scholar]
- Sosinsky GE, Solan JL, Gaietta GM, Ngan L, Lee GJ, Mackey MR, Lampe PD. The C-terminus of connexin43 adopts different conformations in the Golgi and gap junction as detected with structure-specific antibodies. Biochem J. 2007b;408:375–385. doi: 10.1042/BJ20070550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takizawa T, Robinson JM. FluoroNanogold is a bifunctional immunoprobe for correlative fluorescence and electron microscopy. J Histochem Cytochem. 2000;48:481–486. doi: 10.1177/002215540004800405. [DOI] [PubMed] [Google Scholar]
- Takizawa T, Suzuki K, Robinson JM. Correlative microscopy using FluoroNanogold on ultrathin cryosections. Proof of principle. J Histochem Cytochem. 1998;46:1097–1102. doi: 10.1177/002215549804601001. [DOI] [PubMed] [Google Scholar]
- Veitch NC. Horseradish peroxidase: a modern view of a classic enzyme. Phytochemistry. 2004;65:249–259. doi: 10.1016/j.phytochem.2003.10.022. [DOI] [PubMed] [Google Scholar]
- Vergara A, Lorber B, Sauter C, Giege R, Zagari A. Lessons from crystals grown in the Advanced Protein Crystallisation Facility for conventional crystallisation applied to structural biology. Biophys Chem. 2005;118:102–112. doi: 10.1016/j.bpc.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Verkman AS. Solute and macromolecule diffusion in cellular aqueous compartments. Trends Biochem Sci. 2002;27:27–33. doi: 10.1016/s0968-0004(01)02003-5. [DOI] [PubMed] [Google Scholar]
- Wang Q, Mercogliano CP, Lowe J. A ferritin-based label for cellular electron cryotomography. Structure. 2011;19:147–154. doi: 10.1016/j.str.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Watts RJ, Schuldiner O, Perrino J, Larsen C, Luo L. Glia engulf degenerating axons during developmental axon pruning. Curr Biol. 2004;14:678–684. doi: 10.1016/j.cub.2004.03.035. [DOI] [PubMed] [Google Scholar]
- Whitt MA, Mire CE. Utilization of fluorescently-labeled tetracysteine-tagged proteins to study virus entry by live cell microscopy. Methods. 2011;55:127–136. doi: 10.1016/j.ymeth.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Ziegler N, Batz J, Zabel U, Lohse MJ, Hoffmann C. FRET-based sensors for the human M1-, M3-, and M5-acetylcholine receptors. Bioorg Med Chem. 2011;19:1048–1054. doi: 10.1016/j.bmc.2010.07.060. [DOI] [PubMed] [Google Scholar]