Abstract
Functional Electrical Stimulation (FES) may be able to augment functional arm and hand movement after stroke. However, neuroprostheses that combine voluntary effort and FES must take into account the co-contraction patterns (synergies) that are common across multiple joints. The goal of this study is to determine the principles under which voluntary effort and FES can be combined to achieve useful reach and hand opening. A reach and hand opening task is performed where different levels of voluntary effort and FES are applied to produce reach while measuring the level of hand opening that FES can produce at the hand. Initial results indicate that low levels of voluntary effort allow both greater reach and the largest hand opening response to FES.
I. Introduction
STROKE is a leading cause of disability in the US. Six months after their stroke, 50% of ischemic stroke survivors over the age of 64 still have a degree of upper limb hemiparesis [1], which limits arm and hand function, making bimanual tasks difficult if not impossible. Hemiparesis is worsened by disuse and co-contraction patterns across multiple joints (i.e. synergy patterns) [2]. These synergy patterns have been well quantified [3] and appear to be expressed in proportion to effort [4, 5]. Strong effort to abduct the arm is accompanied by strong involuntary flexor contractions that oppose reaching movements.
Functional Electrical Stimulation (FES) of paretic muscles has the potential to elicit functional limb movements, such as reaching and hand opening[6]. For example, electrical stimulation of finger extensors [7–10] can produce hand opening while the participant is relaxed. However, when the user exerts effort to reach with their arm, the hand does not open as much in response to stimulation as when the person remains relaxed, presumably because their effort to reach produces involuntary finger flexor contractions [7, 11, 12]. Therefore in order to receive maximum movement from the stimulation, the user must remain relaxed, which is counterintuitive.
The goal of this study is to determine if reducing voluntary effort exerted during reach and augmenting it with FES can increase the degree of reach and hand opening. There are two hypotheses for this study. The first hypothesis is that reducing voluntary reaching effort and augmenting it with stimulation will allow stimulation of the finger extensors to produce greater hand opening at the same position. The second hypothesis is that stimulation to augment reach can produce greater reach at an equal or greater level of hand opening produced during voluntary effort alone.
II. METHODS
A. Participants
Participants were recruited from an outpatient stroke clinic. The primary inclusion criteria included: 1) being at least 6 months post-stroke 2) the ability to follow 3-stage commands 3) the ability to reach forward at least 10 cm while the elbow and wrist were supported by the investigator 4) the inability to fully reach and open the hand while the arm is unsupported and 5) a upper extremity Fugl-Meyer score between 10 and 50. Exclusion criteria include 1) uncompensated hemineglect, 2) apraxia, or 3) severe shoulder or hand pain. Participants provided informed consent in accordance with the Declaration of Helsinki prior to participation in this study, which was approved by an Institutional Review Board. Two participants are enrolled in this study to date, and one participant with a Fugl-Meyer score of 13 has completed the study.
B. Setup
Participants performed a series of arm reach and hand opening tasks (described below) while seated with the trunk restrained. Arm position was measured by an optical tracking system (Optotrak) and a custom device measured the aperture of hand opening [13]. Partial forearm support was provided by a mobile arm support (Jaeco) in all of the trials. A participant is shown performing one of the tasks in Figure 1.
Fig 1.
Example of a participant performing the reach and open task at the ‘far’ target location.
C. Experimental Procedures
Before any reaching task sessions, a Fugl-Meyer Motor Assessment and modified Ashworth test were carried out by an occupational therapist to characterize the degree of upper limb motor impairment. Participants returned to the lab for four more sessions to learn the reaching tasks and become accustomed to the sensation of electrical stimulation. During the two final sessions, hand opening and kinematic data were collected for analysis.
Participants performed a reach and open the hand task under different reaching conditions. During the first practice session, electrode positions and stimulation levels were found that produce reach and hand opening without eliciting pain while the participant was relaxed. The deltoids were targeted for shoulder abduction and flexion, and the triceps was targeted for elbow extension. While surface stimulation could produce hand opening and elbow extension without producing pain, it is difficult to elicit full shoulder abduction and flexion with surface stimulation. During this study we were only targeting the deltoids to produce shoulder abduction and flexion. There are additional muscles that contribute to these movements at both the glenohumeral joint and the scapula. These muscles could be used in the future to increase movement at that joint, however it is difficult to recruit these muscles with surface stimulation due to nerve depth and poor muscle selectivity with surface electrodes. For the same reasons, it can be difficult to fully stimulate the axillary nerve without causing pain or without activating nearby muscles. Due to the limits of surface stimulation in producing shoulder flexion and abduction, a mobile arm support also provided an upward force at the forearm, reducing the force that FES needed to generate at the shoulder. The same level of support was used in all of the trials.
A target was placed in front of the participant. The participant was cued to reach to the target under different combinations of voluntary effort and electrical stimulation. Once the arm reached a steady position, the participant was instructed to maintain their reaching effort level while remaining relaxed at the hand. Electrical stimulation was then applied to activate hand extensors for 4 s following a 1 s ramp. The three different reaching conditions were: 1) voluntary effort alone, 2) stimulation alone while the participant remained relaxed, and 3) partial voluntary effort and the same stimulation parameters that were used during the stimulation alone for reach trials. The participant was asked to estimate effort and try to limit reaching effort to half of their maximum reaching effort. These tasks were repeated using two different target positions. The first target position is half of the distance from the participant’s relaxed position to the furthest distance that the participant could reach. The second target was outside the voluntary range of reach at the furthest position that stimulation could reach.
D. Data Analysis
For each position, the average hand opening was calculated over the last second of each trial. A two way ANOVA was used to compare the amount of hand opening achieved using the different reaching conditions (voluntary effort alone, stimulation and half voluntary effort, stimulation alone) and positions (near and far) as factors. If the values were statistically significant, the Tukey-Kramer comparison of means was used to determine which factors were statistically different.
III. RESULTS
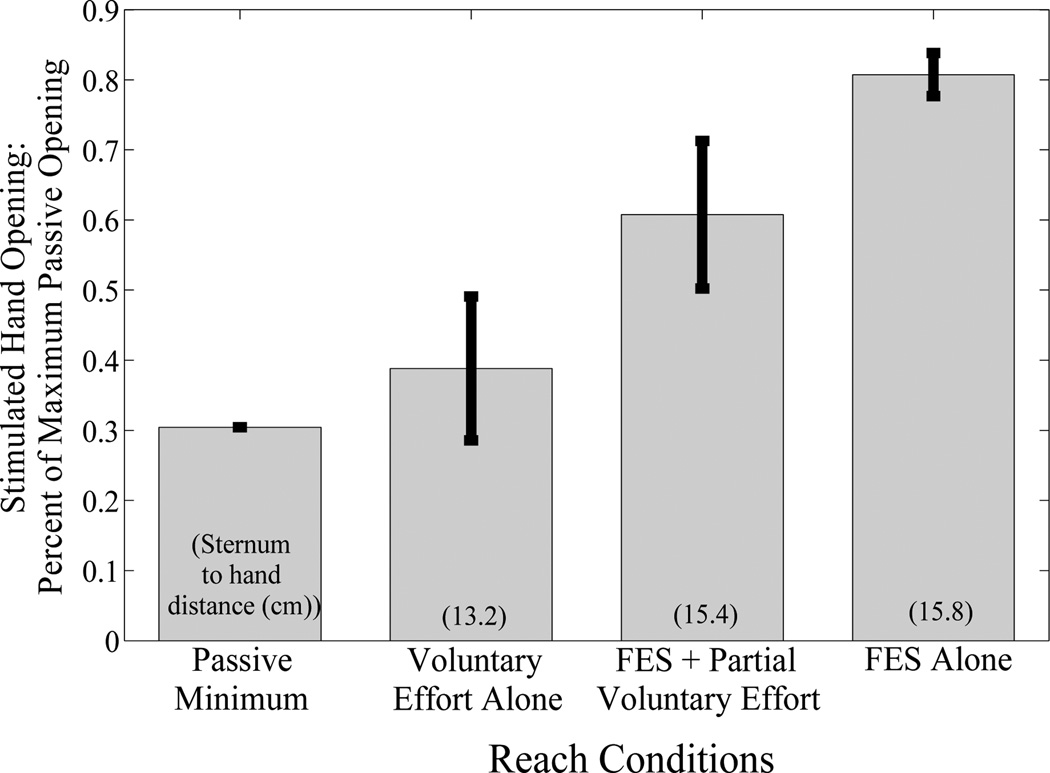
The amount of hand opening achieved with electrical stimulation increased as voluntary reaching effort was reduced and augmented with FES, i.e. supplementing voluntary effort with FES for reach made FES for hand opening more effective. Results of this comparison for the near position are shown in Figure 2. The maximum passive opening achieved for this participant was 10 cm. Due to the device used to measure hand opening, the minimum hand opening possible was 3 cm. Hand opening was significantly different based on the reaching method used (p=0.00002). Target position was not statistically significant (p=0.07). Post-hoc analyses indicate that stimulation alone to produce reach allowed greater hand opening than voluntary effort for reach in both positions (p<0.01). Similarly, the amount of hand opening achieved at the far position while voluntary reaching effort was reduced and reach was augmented with FES was greater than the hand opening achieved while reach was generated by voluntary effort alone to the near position (p<0.01). Despite appearing lower, post-hoc analyses did not indicate that stimulation for reach with partial voluntary effort was statistically significantly different from stimulation alone for reach.
Fig 2.
Percent of stimulated hand opening under different reach conditions for the ‘near’ target location. Hand opening achieved increases as voluntary effort decreases.
IV. DISCUSSION
Stimulation is capable of producing effective hand opening and reach similar to how FES has been used to increase torque in the presence of voluntary effort[14]. As seen in Figure 2, decreasing effort to reach increases the amount of hand opening that FES can produce for this participant. These results indicate that by reducing voluntary reaching effort and augmenting it with FES, greater hand opening can be achieved during reach, which supports Hypothesis 1 (FES and reduced reaching effort allows greater hand opening at same position). Stimulation to augment reach also enabled greater hand opening at a further reaching position. These results support Hypothesis 2 (FES and reduced reaching effort allows equal or greater hand opening at a farther position). In addition to being statistically different it is important to note that the hand opening achieved at both positions was almost the participant’s full range of hand opening and is large enough to place the hand around typical objects. Similarly, the elbow extension achieved by stimulation was sufficient to fully extend the elbow.
Reducing voluntary effort likely limits the expression of synergy patterns, which would have overpowered the effect of electrical stimulation. It is also important to note that while partial effort combined with FES did not produce as much hand opening as FES alone, the partial reaching effort did not completely overpower the effect of FES at the hand. It is expected that there would be a decrease in the amount of stimulated hand opening during FES and partial voluntary effort for reach as compared to FES alone for reach because the effort exerted during reach could produce the involuntary flexor synergy pattern resulting in hand flexor muscles being activated. The large range of hand opening produced during combined FES and partial voluntary effort is partially due to the fact that the level of partial effort was not carefully constrained in this preliminary study. The participant was asked to exert roughly half effort reaching, but there was no training done to elicit a specific amount of effort. For future studies it is important to control for the amount of effort being exerted during partial effort in order to determine the acceptable levels of voluntary effort that can be used as a command signal for an upper extremity stroke FES system. Similarly, future experiments will attempt to produce shoulder movement with percutaneous stimulation to determine if sufficient movement can be produced at the shoulder by FES without any mechanical support. The end goal would be an implanted system, which would allow greater muscle selectivity and larger levels of activation.
Acknowledgment
The authors thank Terri Hisel and Margaret Maloney for administering the impairment assessments and scheduling the visits respectively.
This work was supported in part by the U.S. National Institutes of Health National Institute for Child Health and Development under Grant R21HD05256
Contributor Information
Nathaniel S. Makowski, Cleveland Functional Electrical Stimulation Center and the Department of Biomedical Engineering at Case Western Reserve University, Cleveland, OH 44106 USA (phone: 216-368-8675; nhm6@cwru.edu)
Jayme S. Knutson, Cleveland Functional Electrical Stimulation Center and the Department of Biomedical Engineering at Case Western Reserve University, Cleveland OH 44106 USA (jsk12@cwru.edu)
John Chae, Cleveland Functional Electrical Stimulation Center, the Department of Physical Medicine and Rehabilitation at MetroHealth Medical Center, and the School of Medicine at Case Western Reserve University, Cleveland OH 44106 USA (jchae@metrohealth.org)
Patrick Crago, Cleveland Functional Electrical Stimulation Center and the Department of Biomedical Engineering at Case Western Reserve University, Cleveland, OH 44106 USA (pec3@cwru.edu).
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wongr ND, Wylie-Rosett J. Heart Disease and Stroke Statistics--2011 Update: A Report From the American Heart Association. Circulation. Feb 1;vol. 123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Twitchell TE. Sensory factors in purposive movement. J Neurophysiol. 1954 May;vol. 17:239–252. doi: 10.1152/jn.1954.17.3.239. [DOI] [PubMed] [Google Scholar]
- 3.Dewald JP, Beer RF. Abnormal joint torque patterns in the paretic upper limb of subjects with hemiparesis. Muscle Nerve. 2001 Feb;vol. 24:273–283. doi: 10.1002/1097-4598(200102)24:2<273::aid-mus130>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 4.Sukal TM, Ellisr MD, Dewald JP. Shoulder abduction-induced reductions in reaching work area following hemiparetic stroke: neuroscientific implications. Exp Brain Res. 2007 Nov;vol. 183:215–223. doi: 10.1007/s00221-007-1029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beer RF, Ellis MD, Holubarr BG, Dewald JP. Impact of gravity loading on post-stroke reaching and its relationship to weakness. Muscle Nerve. 2007 Aug;vol. 36:242–250. doi: 10.1002/mus.20817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kilgore KL, Peckhamr P, Keith MW. Twenty year experience with implanted neuroprostheses. Conf Proc IEEE Eng Med Biol Soc. 2009;vol. 2009:7212–7215. doi: 10.1109/IEMBS.2009.5335272. [DOI] [PubMed] [Google Scholar]
- 7.Chae J, Hart R. Intramuscular hand neuroprosthesis for chronic stroke survivors. Neurorehabil Neural Repair. 2003 Jun;vol. 17:109–117. doi: 10.1177/0888439003017002005. [DOI] [PubMed] [Google Scholar]
- 8.Alon G, McBrider K, Ring H. Improving selected hand functions using a noninvasive neuroprosthesis in persons with chronic stroke. J Stroke Cerebrovasc Dis. 2002 Mar-Apr;vol. 11:99–106. doi: 10.1053/jscd.2002.127107. [DOI] [PubMed] [Google Scholar]
- 9.Ring H, Rosenthal N. Controlled study of neuroprosthetic functional electrical stimulation in sub-acute post-stroke rehabilitation. J Rehabil Med. 2005 Jan;vol. 37:32–36. doi: 10.1080/16501970410035387. [DOI] [PubMed] [Google Scholar]
- 10.Chiou YH, Luh JJ, Chen SC, Chen YL, Lair JS, Kuo TS. Patient-driven loop control for hand function restoration in a non-invasive functional electrical stimulation system. Disabil Rehabil. 2008;vol. 30:1499–1505. doi: 10.1080/09638280701615246. [DOI] [PubMed] [Google Scholar]
- 11.Lin C. The effects of ipsilateral forearm movement and contralateral hand grasp on the spastic hand opened by electrical stimulation. Neurorehabil Neural Repair. 2000;vol. 14:199–205. doi: 10.1177/154596830001400305. [DOI] [PubMed] [Google Scholar]
- 12.Kamper DG, Schmitr BD, Rymer WZ. Effect of muscle biomechanics on the quantification of spasticity. Ann Biomed Eng. 2001 Dec;vol. 29:1122–1134. doi: 10.1114/1.1424918. [DOI] [PubMed] [Google Scholar]
- 13.Memberg WD, Crago PE. An analysis of the input-output properties of neuroprosthetic hand grasps. J Rehabil Res Dev. 2000 Jan-Feb;vol. 37:11–21. [PubMed] [Google Scholar]
- 14.Keller T, Ellisr MD, Dewald JP. Overcoming abnormal joint torque patterns in paretic upper extremities using triceps stimulation. Artif Organs. 2005 Mar;vol. 29:229–232. doi: 10.1111/j.1525-1594.2005.29041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]