Abstract
Serial undiluted passage of Semliki Forest virus in a clone of Aedes albopictus cells resulted in a marked decrease in infectious virus yields due to the generation and accumulation of defective interfering particles. Virus from the third passage had a high particle/infectivity ratio and interfered specifically with homologous but not heterologous standard virus replication. Two RNA species of molecular weights 0.78 X 10(6) and 0.61 X 10(6) were the major RNA components of purified passage 4 virus. These RNA species were also the predominant virus RNA species detected in cells infected with passage 3 virus. Synthesis of standard virus RNA and virus-specified protein was much reduced in passage 3 virus-infected cells. Interference with standard virus replication and the synthesis of large amounts of defective interfering RNA were also observed in chicken embryo cells infected with passage 3 virus from mosquito cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkins G. J., Samuels J., Kennedy S. I. Isolation and preliminary characterization of temperature-sensitive mutants of Sindbis virus strain AR339. J Gen Virol. 1974 Dec;25(3):371–380. doi: 10.1099/0022-1317-25-3-371. [DOI] [PubMed] [Google Scholar]
- Bruton C. J., Porter A., Kennedy S. I. Defective-interfering particles of Semliki Forest virus: intracellular events during interference. J Gen Virol. 1976 Jun;31(3):397–416. doi: 10.1099/0022-1317-31-3-397. [DOI] [PubMed] [Google Scholar]
- Cancedda R., Schlesinger M. J. Formation of Sindbis virus capsid protein in mammalian cell-free extracts programmed with viral messenger RNA. Proc Natl Acad Sci U S A. 1974 May;71(5):1843–1847. doi: 10.1073/pnas.71.5.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg J. C., Brzeski H., Kennedy S. I. RNA polymerase components in Semliki Forest virus-infected cells: synthesis from large precursors. J Gen Virol. 1976 Sep;32(3):413–430. doi: 10.1099/0022-1317-32-3-413. [DOI] [PubMed] [Google Scholar]
- Clegg J. C., Kennedy S. I. In vitro synthesis of structural proteins of Semliki Forest virus directed by isolated 26 S RNA from infected cells. FEBS Lett. 1974 Jun 15;42(3):327–330. doi: 10.1016/0014-5793(74)80757-x. [DOI] [PubMed] [Google Scholar]
- Clewley J. P., Kennedy S. I. Purification and polypeptide composition of Semliki Forest virus RNA polymerase. J Gen Virol. 1976 Sep;32(3):395–411. doi: 10.1099/0022-1317-32-3-395. [DOI] [PubMed] [Google Scholar]
- Davey M. W., Dennett D. P., Dalgarno L. The growth of two togaviruses in cultured mosquito and vertebrate cells. J Gen Virol. 1973 Aug;20(2):225–232. doi: 10.1099/0022-1317-20-2-225. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Eaton B. T. Defective interfering particles of Semliki Forest virus generated in BHK cells do not interfere with viral RNA synthesis in Aedes albopictus cells. Virology. 1975 Dec;68(2):534–538. doi: 10.1016/0042-6822(75)90293-7. [DOI] [PubMed] [Google Scholar]
- Eaton B. T. Evidence for the synthesis of defection interfering particles by Aedes albopictus cells persistently infected with Sindbis virus. Virology. 1977 Apr;77(2):843–848. doi: 10.1016/0042-6822(77)90503-7. [DOI] [PubMed] [Google Scholar]
- Eaton B. T., Faulkner P. Altered pattern of viral RNA synthesis in cells infected with standard and defective Sindbis virus. Virology. 1973 Jan;51(1):85–93. doi: 10.1016/0042-6822(73)90368-1. [DOI] [PubMed] [Google Scholar]
- Grimley P. M., Berezesky I. K., Friedman R. M. Cytoplasmic structures associated with an arbovirus infection: loci of viral ribonucleic acid synthesis. J Virol. 1968 Nov;2(11):1326–1338. doi: 10.1128/jvi.2.11.1326-1338.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guild G. M., Stollar V. Defective interfering particles of Sindbis virus. III. Intracellular viral RNA species in chick embryo cell cultures. Virology. 1975 Sep;67(1):24–41. doi: 10.1016/0042-6822(75)90400-6. [DOI] [PubMed] [Google Scholar]
- Guild G. M., Stollar V. Defective interfering particles of Sindbis virus. V. Sequence relationships between SVSTD 42 S RNA and intracellular defective viral RNAs. Virology. 1977 Mar;77(1):175–188. doi: 10.1016/0042-6822(77)90416-0. [DOI] [PubMed] [Google Scholar]
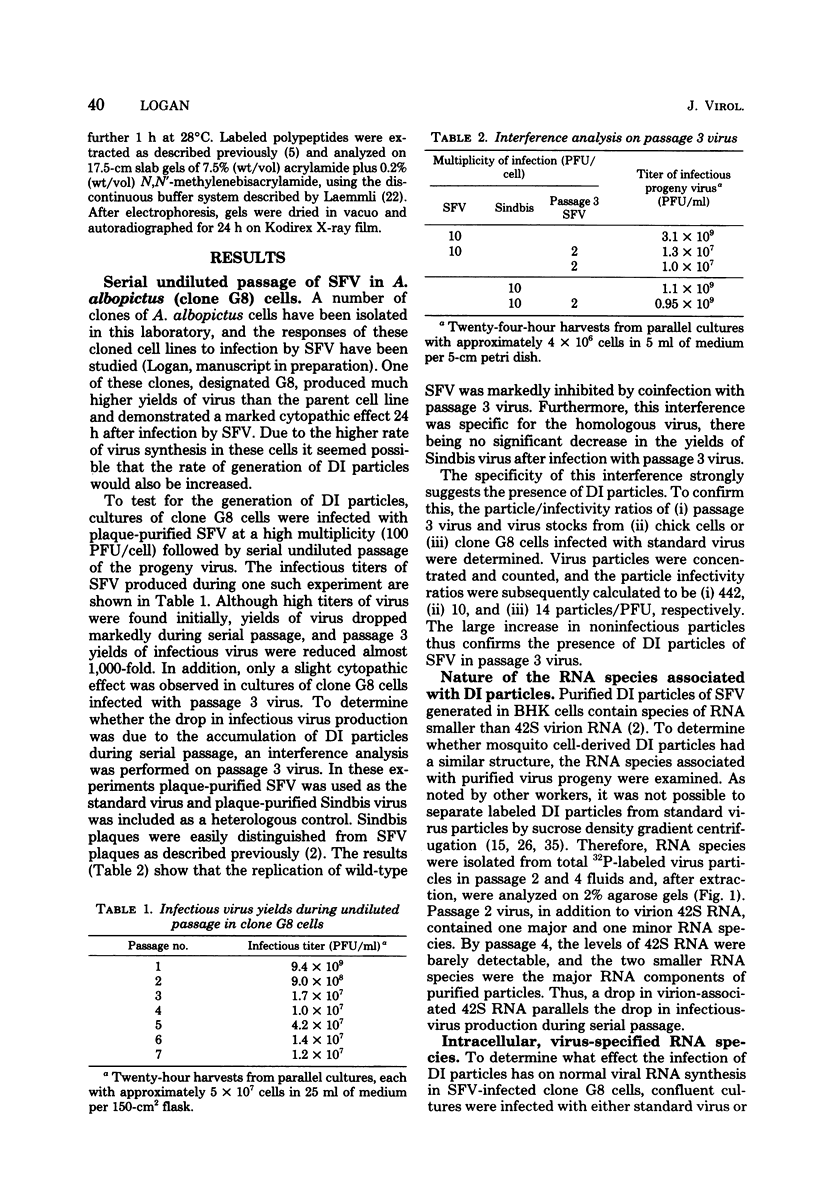
- Igarashi A. Isolation of a Singh's Aedes albopictus cell clone sensitive to Dengue and Chikungunya viruses. J Gen Virol. 1978 Sep;40(3):531–544. doi: 10.1099/0022-1317-40-3-531. [DOI] [PubMed] [Google Scholar]
- Igarashi A., Koo R., Stollar V. Evolution and properties of Aedes albopictus cell cultures persistently infected with sindbis virus. Virology. 1977 Oct 1;82(1):69–83. doi: 10.1016/0042-6822(77)90033-2. [DOI] [PubMed] [Google Scholar]
- Igarashi A., Stollar V. Failure of defective interfering particles of Sindbis virus produced in BHK or chicken cells to affect viral replication in Aedes albopictus cells. J Virol. 1976 Aug;19(2):398–408. doi: 10.1128/jvi.19.2.398-408.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C. Y., Allen R. Host function-dependent induction of defective interfering particles of vesicular stomatitis virus. J Virol. 1978 Jan;25(1):202–206. doi: 10.1128/jvi.25.1.202-206.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy S. I. Sequence relationships between the genome and the intracellular RNA species of standard and defective-interfering Semliki Forest virus. J Mol Biol. 1976 Dec;108(2):491–511. doi: 10.1016/s0022-2836(76)80132-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
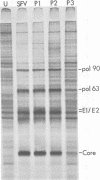
- Morser M. J., Kennedy S. I., Burke D. C. Virus-specified polypeptides in cells infected with Semliki Forest virus. J Gen Virol. 1973 Oct;21:19–29. doi: 10.1099/0022-1317-21-1-19. [DOI] [PubMed] [Google Scholar]
- Sarver N., Stollar V. Sindbis virus-induced cytopathic effect in clones of Aedes albopictus (Singh) cells. Virology. 1977 Jul 15;80(2):390–400. doi: 10.1016/s0042-6822(77)80014-7. [DOI] [PubMed] [Google Scholar]
- Schlesinger S., Schlesinger M., Burge B. W. Defective virus particles from Sindbis virus. Virology. 1972 May;48(2):615–617. doi: 10.1016/0042-6822(72)90076-1. [DOI] [PubMed] [Google Scholar]
- Shenk T. E., Stollar V. Defective-interfering particles of Sindbis virus. I. Isolation and some chemical and biological properties. Virology. 1973 May;53(1):162–173. doi: 10.1016/0042-6822(73)90475-3. [DOI] [PubMed] [Google Scholar]
- Shenk T. E., Stollar V. Defective-interfering particles of Sindbis virus. II. Homologous interference. Virology. 1973 Oct;55(2):530–534. doi: 10.1016/0042-6822(73)90197-9. [DOI] [PubMed] [Google Scholar]
- Simmons D. T., Strauss J. H. Translation of Sindbis virus 26 S RNA and 49 S RNA in lysates of rabbit reticulocytes. J Mol Biol. 1974 Jun 25;86(2):397–409. doi: 10.1016/0022-2836(74)90027-8. [DOI] [PubMed] [Google Scholar]
- Stark C., Kennedy S. I. The generation and propagation of defective-interfering particles of Semliki Forest virus in different cell types. Virology. 1978 Aug;89(1):285–299. doi: 10.1016/0042-6822(78)90060-0. [DOI] [PubMed] [Google Scholar]
- Stevens T. M. Arbovirus replication in mosquito cell lines (Singh) grown in monolayer or suspension culture. Proc Soc Exp Biol Med. 1970 May;134(1):356–361. doi: 10.3181/00379727-134-34793. [DOI] [PubMed] [Google Scholar]
- Stollar V., Shenk T. E. Homologous viral interference in Aedes albopictus cultures chronically infected with Sindbis virus. J Virol. 1973 Apr;11(4):592–595. doi: 10.1128/jvi.11.4.592-595.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stollar V., Shenk T. E., Koo R., Igarashi A., Schlesinger R. W. Observations of Aedes albopictus cell cultures persistently infected with Sindbis virus. Ann N Y Acad Sci. 1975;266:214–231. doi: 10.1111/j.1749-6632.1975.tb35103.x. [DOI] [PubMed] [Google Scholar]
- Weiss B., Schlesinger S. Defective interfering passages of Sindbis virus: chemical composition, biological activity, and mode of interference. J Virol. 1973 Oct;12(4):862–871. doi: 10.1128/jvi.12.4.862-871.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wengler G., Beato M., Hackemack B. A. Translation of 26 S virus-specific RNA from Semliki Forest virus-infected cells in vitro. Virology. 1974 Sep;61(1):120–128. doi: 10.1016/0042-6822(74)90247-5. [DOI] [PubMed] [Google Scholar]
- Wengler G., Wengler G. Localization of the 26-S RNA sequence on the viral genome type 42-S RNA isolated from SFV-infected cells. Virology. 1976 Aug;73(1):190–199. doi: 10.1016/0042-6822(76)90073-8. [DOI] [PubMed] [Google Scholar]