Abstract
It is epidemiologically established that obesity is frequently associated with the metabolic syndrome and poses an increased risk for the development of type 2 diabetes and cardiovascular disease. The molecular links that connect the phenomenon of obesity per se with insulin resistance and cardiovascular disease are still not fully elucidated. It is increasingly apparent that fully functional adipose tissue can be cardioprotective by reducing lipotoxic effects in other peripheral tissues and by maintaining a healthy balance of critical adipokines, thereby allowing the heart to maintain its full metabolic flexibility. The present review highlights both basic as well as clinical findings that emphasize the complex interplay of adipose tissue physiology, adipokine-mediated effects on the heart exerted by either direct effects on cardiac myocytes or indirect actions via central mechanisms through sympathetic outflow to the heart.
Keywords: obesity, diabetes mellitus, insulin-sensitivity, adipokines, adipose, heart failure
Introduction
The impact of the world-wide increase in obesity cannot be easily overstated. Current estimates suggest that ~70% of US adults are overweight or obese 1. These numbers are rapidly mirrored across the rest of the globe. Perhaps most troubling are the statistics related to the prevalence of overweight/obesity in the pediatric population. Diseases previously observed only in adulthood, e.g. hypertension and type 2 diabetes mellitus (T2DM) are now increasingly diagnosed in children. This trend threatens to translate into a deluge of cardiovascular disease (CVD), presenting decades earlier than might otherwise have been expected based on the prevalence of obesity 30 years ago.
The complex biology between adipose tissue and the cardiovascular system, in many ways, lies at the center of the pathogenesis of CVD. Although much is known vis-à-vis the relationship between adiposity and traditional CVD risk factors, there is a growing appreciation of novel markers and mediators of disease that are adipose-derived 2. Here, we summarize our current understanding of these interactions, with a particular emphasis on the association between adipose tissue biology and cardiomyopathy, as this has been an area of important recent advances in our understanding from a basic science and clinical perspective.
Constituents of Adipose Tissue
Over recent years, the notion of adipose tissue as a passive repository of lipid stored in adipocytes has been overturned. Adipose is now seen as a dynamic tissue, which is both responsive to and responsible for a wide variety of hormonal, inflammatory and metabolic interactions with other organs 3.
The prototypic cell of adipose tissue is the adipocyte, whose main, but certainly not only function is to store energy in the form of triglyceride (TG). Under the influence of insulin, the adipocyte takes up free fatty acids (FFAs) from the blood and then stores them in the form of intracellular lipid droplets. Conversely, during periods of fasting and in response to exogenous humoral signals, such as circulating catecholamines, TGs are hydrolyzed and liberated from the lipid droplet to be released into the bloodstream. FFAs can then be used at distant sites as metabolic substrates through transport into cells by interaction with fatty acid transport proteins or CD36. Alternatively, these lipids can potentially act as signals themselves. Adipocytes are also now recognized to secrete a number of more traditional hormones, referred to as adipokines, which report on the status of the adipose tissue itself. Adipokines exert effects on the central nervous system, peripheral metabolism and the immune system, as well as in a paracrine fashion on local cells in adipose tissue directly.
While adipocytes are important constituents of adipose tissue, making up the majority of tissue mass, they only account for about 50% of all cells. The remaining cells are frequently referred to as the stromal vascular fraction. Endothelial cells, pericytes and various types of immune cells make up the majority of the remaining cells. Amongst the immune cells, macrophages feature prominently in evolving and dysfunctional fat pads. These macrophages are either in a pro-inflammatory state polarized towards the M1 phenotype, or they have an alternative gene expression programs that leans more towards the M2 phenotype that is more prone to be involved in tissue remodeling. However, many additional immune cells play a vital role in maintaining adipose tissue homeostasis, including regulatory T cells, eosinophils and mast cells 4. The relative number of each of these cell types varies with the changing metabolic state and functionality of the adipose tissue 5. The macrophages play an important role in the disposal of adipocyte remnants that leave large lipid droplets behind. Lipid-phagocytosing macrophages that start to look like foam cells over time, surround lipid droplet remnants and form “crown-like structures” characteristic of dysfunctional adipose tissue 6, 7.
Adipose Tissue as an Endocrine Organ
Dysfunctional, inflamed adipose tissue in states of nutrient excess can promote systemic inflammation and insulin resistance. The ability to buffer excess nutrients is reduced, the secretion of inflammatory cytokines is increased and the secretion of the insulin-sensitizing hormone adiponectin is lowered in obese adipose tissue. Furthermore, an enhanced capacity for lipid storage in adipocytes protects against metabolic disturbances, even in the context of massive nutrient excess 8. An opposite phenotype can be observed in models of lipodystrophy, whereby the storage capacity of adipose tissue is limited 9. Therefore, it is apparent, that adipocytes, through their function as nutrient storage cells, play a key role in the pathogenesis of the metabolic syndrome and have the capability to exert profound effects on whole body metabolism. Prolonged nutrient overload results in a state of chronic, low-grade inflammation in adipose tissue that is associated with the down-regulation of critical adipokines, such as adiponectin, and an upregulation of other factors, such as leptin, resistin and the recently identified endotrophin. Adipose tissue therefore serves as a potent endocrine organ, releasing a complex set of proteinaceous factors acting on many other metabolically active tissues 10.
Leptin
Leptin is the archetypical adipokine identified in 1994 11. With more than 20,000 papers focusing on leptin action or minimally using it as a biomarker, leptin has been widely studied for its established actions in the brain affecting food intake and energy expenditure 12. Circulating levels of this hormone are directly correlated to fat mass, and as will be discussed in more detail below, it can affect metabolic processes in peripheral tissues either via direct action on the tissues or indirectly through neuronal innervation. Several splice variants of the leptin receptor are known 13, but its downstream intracellular effects are generally mediated by the JAK/STAT3 14 and PI3K pathways 15, 16.
Adiponectin
This is another widely studied adipokine first described in 1995 with approximately 10,000 publications since that time 17. Adiponectin is a member of a growing family of paralogues known as C1q/TNF-related proteins (“CTRPs”) based on its structural similarities to these proteins 18. Its effects are widely considered to be beneficial, with potent insulin-sensitizing, anti-apoptotic and anti-inflammatory properties 19. Adiponectin receptors are ubiquitously expressed leading to a host of effects on different target tissues 20. Previous work had identified an adiponectin mechanism involving downstream AMPK 21, 22 or PPARα-mediated effects 23. It has subsequently been shown that adiponectin has both an AMPK-dependent and -independent mechanism 24. More recent work, however, has identified activation of intracellular ceramidase activity as critical to adiponectin’s actions in a number of different cell types, including the cardiomyocyte 25. Hence, a potentially unifying mechanism for the diverse beneficial effects of adiponectin may be related its ability to alter cellular sphingolipid metabolism via activation of its receptors adipoR1 and adipoR2.
Resistin
Resistin has become regarded as another important adipokine 26, at least in rodents. It is generally upregulated in the obese state 27 and has been implicated as a causative factor in insulin-resistance. In distinction to rodents, resistin expression in humans is mainly limited to macrophages and circulating monocytes, which makes the relevance of animal models examining the role of this cytokine somewhat difficult to interpret. Nonetheless, as will be discussed later in this review, this cytokine has been correlated with clinical conditions in humans, namely, hypertension, atherosclerosis, and HF.
Collagen VIα3-C5/Endotrophin
Collagen VI is ubiquitously expressed, but its most abundant source is adipose tissue. It contains three chains, α1-α3, which associate further to form intracellular tetramers. Once secreted, the C5-domain of the α3 chain (also known as endotrophin) is cleaved off by a protease that has yet to be identified 28 and the tetramers subsequently aggregate into microfibrils. Local endotrophin levels within adipose tissue are high in obese mice and accumulate within areas of dysfunctional adipocytes and infiltrating macrophages (“crown-like structures”). Indeed, there is a correlation seen between total collagen VIα3 levels and macrophage infiltration in adipose tissue of obese patients 29, 30. Most recently, endotrophin has been shown to play a critical role in malignant mammary tumor progression, both in terms of primary tumor growth and distal metastasis (Park and Scherer, 2012, J Clin Inv., in press). This paracrine factor appears to be an important link in the connection between obesity and malignancy, but, more generally, ties metabolic dysregulation to a more fibrotic extracellular environment.
Other hormones
Many additional factors have been shown to be expressed in adipocytes, including many pro-inflammatory factors and acute phase reactants, omentin, chimerin and visfatin, which are merely enriched in adipocytes and found in other tissues as well 10.
Brown Adipose Tissue and Its Hormonal Control
The past several years have brought considerable excitement in the field regarding the progress made in the area of brown adipose tissue physiology. There is an increased appreciation for these cells in humans. They are not only relevant in the newborn state- as it was the general believe for many years- as they may be playing an important role in systemic metabolic homeostasis even in the adult. What makes the brown adipocytes somewhat harder to detect is that unlike in rodents, brown and white adipocytes are mixed together in the various depots. Strong indications regarding the presence of brown adipocytes came from 18F-FDG (Fluorodeoxyglucos) PET/CT scans that suggested disproportionate uptake into certain fat depots, particularly in the subclavicular region 31. The hope is that with an increased abundance of brown adipocytes in the system, an increase in overall energy expenditure can be solicited. Beyond the inducibility of brown adipocytes by exposure to cold, a number of additional mechanisms have recently been discovered that can increase the proportion of brown adipocytes, including actions by vascular endothelial growth factor (VEGF) on adipose tissue32, a newly identified, muscle-derived molecule called irisin,33 as well as the natriuretic peptides34. Future studies need to demonstrate whether the mere increased presence of brown adipocytes is sufficient to increase energy expenditure, or whether additional steps need to be taken to activate these newly acquired “brown”, “brite” or “beige” adipocytes, as several different laboratories refer to them35-37.
Distribution of Adiposity Influences Its Biological Effects
The ability of the fat mass to expand is key in maintaining the overall health of the tissue. Subcutaneous fat depots are relatively inert from an inflammatory/insulin-resistance standpoint and serve as an important energy storage depot before ectopic fat deposition. As the subcutaneous adipose tissue (SAT) mass continues to increase, fat becomes deposited in visceral adipose tissue [VAT, (e.g. intraperitoneal, retroperitoneal, and intrahepatic)]. VAT releases more inflammatory mediators (such as IL-6) than does SAT 38, 39. Furthermore, the expression of β3-adrenoreceptors is higher in VAT than SAT, 40 making this tissue more sensitive to catecholamine-induced lipolysis 41-43 and perhaps less sensitive to inhibitory α2-effects 44. VAT is also less sensitive to the anti-lipolytic effects of insulin 45, 46.
Even among those who are already obese, preferential fat expansion in SAT, rather than VAT, is associated with a generally more favorable cardiovascular risk factor profile (Neeland IJ et al, Obesity, in press; 47). Site-specific location of fat also appears to be important. Higher degrees of lower extremity adiposity relative to central deposition of fat have been associated with improved insulin sensitivity, lipid parameters and cytokine profiles 48-51. Conversely, increasing central fat deposition, even in the setting of normal BMI, is associated with worse cardiovascular risk profiles 52, insulin-sensitivity52, 53 and diastolic function 53.
Taken to the extreme, the most dramatic examples of altered fat distribution, the congenital and acquired forms of generalized lipodystrophy, are associated with loss (or lack of development) of peripheral adipose and accumulation of adipose centrally. Insulin resistance, dyslipidemia, hepatic steatosis, hypoadiponectemia and hypertension are extremely common in this population, and patients are predisposed to developing atherosclerosis at a young age. 54,55. Women are not only more easily identified from an anthropomorphic standpoint, but also are significantly more likely to develop metabolic complications than men 56. Early-onset cardiac hypertrophy is common, likely reflecting the influences of comorbid conditions; left ventricular (LV) dilation is less frequent 57.
More recently, the specific role of intrapericardial fat as a form of visceral adipose tissue has garnered increasing attention. Although pericardial and epicardial fat are biologically distinct 58,59, their differentiation and measurement from static images may be difficult, and these terms appear to be often interchanged in the reported literature. Similar to intra-abdominal VAT, pericardial fat is associated with overall adiposity and tightly associated with the presence of adverse metabolic phenotypes, including insulin-resistance 60, 61. Its accumulation has been associated with LV structure-function changes 62, 63. Pericardial fat is also associated with not only prevalent coronary artery disease 64, 65, but progression of atherosclerosis 66, ischemia on stress testing 60, and future adverse clinical events 67. The proximity of epicardial fat and the coronary arteries to one another may allow this tissue influence one another. Indeed, epicardial fat from patients with coronary atherosclerosis is associated with pro-inflammatory macrophage polarization 68 and higher levels of cytokine production 69.
Adipokine Action on the Heart Mediated by Central Mechanisms
Although traditionally thought of as acting through cognate receptors in peripheral target tissues, we now know that several key metabolic hormones have important central inputs, which in part mediate their peripheral effects. Insulin and leptin act in the hypothalamus to regulate glucose homeostasis independent of body weight. Central administration of insulin suppresses hepatic glucose production, and insulin-induced suppression of hepatic glucose production is attenuated in mice lacking insulin receptors in the brain. Likewise, leptin signaling in the hypothalamus is required for the control of glucose balance. It has been shown that central administration of leptin alters peripheral glucose uptake and hepatic glucose output 70. Leptin also plays a central role in the regulation of the sympathetic nervous system 71, and leptin acts within the CNS to affect heart rate and blood pressure 72. Notably, direct injections of leptin into the arcuate nucleus increased mean arterial pressure by increasing renal sympathetic nerve activity 73. More recently, Hall and colleagues produced mice with selective deletion of LEPRs in POMC neurons. They found that POMC leptin receptor knockouts had normal mean arterial pressure despite obesity. Moreover, the deletion of leptin receptors in POMC neurons blunted the effects of leptin administration to increase blood pressure, increase energy expenditure and to exert anti-diabetic effects74. Collectively, work from multiple groups has highlighted that POMC neurons are a key targets for leptin to regulate the cardiovascular system.
These central effects are clearly best described for leptin, but it is likely that additional adipocyte-derived factors exert at least some of their effects on the cardiovascular system via central action, including adiponectin 75 as well as resistin 76, 77.
Mechanisms of Insulin Resistance in the Heart
Obesity and whole-body alterations in insulin sensitivity are well-recognized to go hand-in-hand, but insulin-resistance specifically at the level of the heart has important implications for cardiac metabolism and function. Obesity and systemic insulin-resistance are marked by enhanced lipid synthesis in hepatocytes and increased lipolysis in adipocytes which together lead to increases in circulating FFA and TG. Also, elevated levels of insulin, which typify these disorders, will stimulate FFA transport into cardiomyocytes 78. Thus, hyperlipidemia and hyperinsulinemia together increase FFA delivery to myocytes, which rapidly adapt by promoting fatty acid utilization. At baseline, the myocardium is a “metabolic omnivore” capable of switching between metabolic substrates, e.g. FFA and carbohydrates, in response to changes in substrate availability and physiological conditions 79. However, if FFA delivery exceeds the oxidative capacity of the cell, these substrates accumulate, with resulting lipotoxicity as a result 80. This promotes cardiac dysfunction via several mechanisms, including the generation of reactive oxygen species (ROS) 81, ceramide production 25, 82, further impairing insulin signaling 83, depressing contractility by influencing sarcoplasmic reticular Ca2+ stores 84, and promoting mitochondrial dysfunction85,86, all while increasing oxygen demand.
In the setting myocardial insulin-resistance, metabolic flexibility is diminished, and the heart’s reliance on FFA for energy supply increases. Accumulation of FFAs leads to impaired insulin-mediated glucose uptake through inhibition of IRS and Akt. The serine protein kinases PKC-θ and IKK, which elicit serine phosphorylation of IRS1, are activated 87. In turn, this reduces signaling through PI3K and Akt, all of which has major implications for insulin-responsiveness under these conditions 88.
Several lines of evidence point to phosphorylation-dependent negative regulation of IRS1, triggering its proteolytic degradation, as a critical event in the pathogenesis of insulin-resistance. Degradation of IRS1 elicits impaired insulin signaling in a number of cell types 89, and low levels of IRS1 correlate with development of T2DM 90. This negative feedback occurs primarily through the post-translational modification of IRS1 by serine phosphorylation, an event that can both disrupt the function of this adaptor protein and target it for degradation by the ubiquitin proteasomal machinery.
Much less is known about the phosphatases that antagonize these inhibitory phosphorylation events, although protein phosphatase 2A (PP2A) has been implicated 91. Prior work has demonstrated that FoxO1 can inhibit PP2A activity. 92 This may be a relevant mechanism behind serine phosphorylation of IRS1 in T2DM, a condition under which persistent FoxO1 activation has been observed 93. Furthermore, cardiomyocytes transfected with a GFP-tagged FoxO1 show increased levels of IRS1 serine phosphorylation and decreased steady state levels of total IRS1 93. These data, along with in vivo data from FoxO1 null mice suggest that regulation of IRS1 activity and stability by FoxO1 may contribute to cardiomyocyte insulin resistance and subsequent cardiac dysfunction.
Hence, increasing adiposity may set the stage for cardiac dysfunction by promoting excessive myocardial FA utilization and the development of lipotoxicity. However, obesity also promotes global insulin resistance, which eventually leads to chronic systemic hyperglycemia. Glucotoxicity also contributes to cardiac injury through multiple mechanisms, including direct and indirect effects of glucose on cardiomyocytes, cardiac fibroblasts, and endothelial cells. Hyperglycemia promotes the over-production of ROS 94-96, which can induce apoptosis and activate poly (ADP-ribose) polymerase-1 (PARP)97. By subsequent PARP-mediated ribosylation and inhibition of glyceraldehyde phosphate dehydrogenase (GAPDH), glucose is diverted from the glycolytic pathway toward alternative biochemical cascades that participate in hyperglycemia-induced cellular injury. These pathways include the creation of advanced glycation end products (AGEs) and the activation of the hexosamine pathway, the polyol pathway, and protein kinase C 98, 99. Hyperglycemia-induced apoptosis is stimulated by these end-products, namely ROS, PARP, AGEs and aldose reductase. Hyperglycemia also contributes to altered cardiac structure and function through post-translational modification of extra-cellular matrix proteins (e.g. collagens) and altered expression/function of intramyocellular calcium channels (e.g. the ryanodine receptor and sarcoplasmic reticulum Ca2+-ATPase) which contribute to both systolic and diastolic dysfunction 99. In these ways, both glucotoxicity and lipotoxicity, each manifestations of insulin-resistance, participate in the pathogenesis of the clinical entity known commonly as the diabetic cardiomyopathy.
Obesity and Heart Failure- a Clinical Perspective
Although there is a wealth of mechanistic data linking adipose tissue biology and insulin resistance with cardiomyopathy, it can be clinically difficult to differentiate heart failure (HF) symptoms arising from cardiac limitations from other etiologies in the obese patient. The Framingham Criteria set the gold standard for the diagnosis of HF, but these criteria have not been validated in the obese population. Nevertheless, there is a robust literature supporting obesity as an independent risk factor for the development of clinical HF 100-102. This relationship persists even after controlling for the obvious confounders, such as T2DM, hypertension and coronary atherosclerosis. In a seminal study in the field, Kenchaiah et al, reporting on the Framingham cohort, estimated the 10-year age-adjusted risk of HF at ~7% in women and 10% in men with BMI≥30 103. The hazard ratio was even higher among patients with more severe degrees of obesity.
Despite the clinical data supporting the relationship between increasing adiposity and the development of HF, the structural and mechanistic underpinnings behind this association remain largely unresolved. In particular, while it is known that obesity is associated with HF events, it is unknown whether such patients have abnormal ventricular morphology [e.g. concentric left ventricular hypertrophy (LVH) or LV dilation] as an anatomic correlate at the time of their HF events are diagnosed. In general, however, cardiac hypertrophy and diastolic abnormalities are commonly seen in patients with obesity 104-107. This begs the question as to why then some obese patients develop HF in the face of these structural changes and others do not. It may be that in some patients the observed LVH is compensatory (i.e. eccentric from increased stroke volume), while in others, it is pathologic (i.e. concentric thickening); careful morphologic studies in obese patients with and without HF are needed to determine which mechanism is at work in this context.
Whether isolated obesity (i.e. the “metabolically healthy obese individual”) 108 is associated with pathologic LVH independent of T2DM and hypertension is a matter of debate 104-107. For instance, the potential association between obesity and subclinical hypertension, impaired glucose tolerance and sleep apnea, highlights the difficulties in using large databases in studying LVH in cohorts of otherwise healthy obese patients. Detailed studies to date have been relatively small in scope and cross-sectional in nature. This has limited our ability to unambiguously assign a causal role to obesity per se in promoting LVH. Indeed, many of these studies have reached contradictory conclusions.
A related question is whether it is total adiposity that is the driving force, or whether site-specific fat depots are related to HF and LV structural changes. Different depots are known to have distinct biological activities. In particular, VAT and SAT are thought to exert rather distinct physiological effects, and it is possible that the purported association between obesity and LV structure/functional is mediated primarily through the differential impact that these subtypes of fat pads exert. This hypothesis remains to be formally tested in the context of HF. We appreciate that VAT releases more inflammatory cytokines and less adiponectin than SAT, which is consistent with findings from cross-sectional studies concerning the relationship between adipokines and prevalent LVH.
Amongst all the adipokines, the association between circulating adiponectin and LVH is the best studied. Several groups have shown that lower adiponectin levels correlate with higher LV mass, even after controlling for insulin-sensitivity and BMI 109-111. A report from the Jackson Heart Study suggested that adiponectin had a more complex relationship with LVH among African Americans, i.e. the directionality of its association depended on the presence or absence of hypertension and insulin-resistance 112. Animal models are supportive of adiponectin’s influence on LVH. In mice lacking adiponectin, an exaggerated cardiac hypertrophic response to pressure-overload is seen but can be blunted with adenoviral-mediated restoration of adiponectin levels 113. This suggests that adiponectin is important in modulating cardiac hypertrophy, perhaps through an AMPK or ceramidase-mediated mechanism 25.
The relationship between both resistin 110 and leptin 114, 115 with LVH is less well explored. The existing studies, while they have methodological limitations, suggest that there is some association between elevated resistin and leptin levels with LVH. However, the mechanistic underpinnings behind these associations are still a matter of investigation. The biological effects of leptin on cardiac hypertrophy in animal models have been conflicting, with either pro- 116 or anti-hypertrophic 117 responses being observed. In the case of resistin, adenovirally-mediated cardiac-specific overexpression does result in LVH through several signaling pathways (e.g. mTOR) 118, but it is unclear how well these observations translate into the clinical setting.
To date, reports on the association between adipokines and LV structure have been cross-sectional in nature, but suggest that markers of adiposity (e.g. high leptin, low adiponectin) and inflammation (e.g. high resistin, low adiponectin) are related to the presence of hypertrophy. Since the levels of most adipokines tend to be reasonably well correlated with one another, either directly or inversely, it is not necessarily clear whether each adipokine is independently informative or simply reflective of confounding from another biomarker unadjusted for in the modeling. Potential mechanistic pathways to explain adipokines as primary drivers of LVH in animal models have been suggested. However, with prospective studies largely lacking in humans, it is perhaps still premature to assign causality between adipokines and LV structural changes. Such studies are also needed to understand whether long-term favorable changes in circulating adipokine profiles may be associated with actual regression of LV mass.
In terms of clinical HF, the role of adipokines has been explored in several large cohort studies. Although adiponectin has been associated with LVH, it has not been independently associated with the development of HF, per se 119. Studies of leptin have led to conflicting results 120, 121, perhaps because leptin is so tightly correlated with adiposity and its statistical relationship with BMI difficult to separate. Longitudinal follow-up from the MESA study group reported that the effect of obesity on HF events could be explained by CRP and IL-6 levels, suggesting that inflammatory adipokines may be mediating this clinical association 122. Similarly, resistin has been independently associated with incident HF events in the Framingham cohort 119. The mechanisms by which these inflammatory mediators may be promoting HF is unclear, but these data are suggestive that accumulation of the pro-inflammatory VAT may a key driving force for the development of obesity-related HF and pathologic LVH.
Adiposity, insulin resistance and cardiac steatosis
Intramyocardial triglyceride (mTG) accumulation is a prominent feature of several models of cardiomyopathy generated from interruption of normal cardiac FFA metabolism. Examples include, transgenic overexpression of fatty acyl-CoA synthetase 123, lipoprotein lipase 124, acyl CoA:diacylglycerol acyltransferase (DGAT) 125, and PPAR-α 126 and –γ 127. In general, these models of lipotoxic cardiomyopathy involve increased FFA flux or delivery to the heart, and highlight the potential for adipose tissue, the source of most circulating FFAs derived from lipolysis, to impact cardiac metabolism from the distance by influencing serum concentrations of these substrates.
Adiposity also promotes myocardial steatosis by promoting insulin-resistance, which occurs on a whole-body, as well as cardiac-specific level. Murine models of diabetic cardiomyopathy accumulate significant amounts of mTG 126, 128, 129. This can be reversed by cardiac-specific restoration of insulin sensitivity. Recent work has highlighted the critical role of FoxO transcription factors, specifically FoxO1, in a pathway linking insulin signaling to the development of cardiomyopathy 93. Whereas high-fat diet induces a severe cardiomyopathic phenotype with prominent myocardial steatosis in mice, this can be completely abrogated with cardiac-specific FoxO1 deletion. Without FoxO1, signaling downstream from the insulin receptor, which becomes disrupted after chronic high fat feeding, is once again restored and cardiac insulin-sensitivity is improved. This results in normalization of cardiac glucose uptake, metabolic enzyme profiles, and steatosis. These data highlight that mTG may accumulate as a consequence of local increases in FFA delivery to the heart or alterations in either global or cardiac-specific insulin-sensitivity.
In humans, mTG accumulation is also well documented and appears to occur in two major clinical contexts: (1) obesity/insulin resistance and (2) LV failure (see below). It should be recognized, however, that mTG changes observed in the diseased human heart are somewhat less dramatic than may be seen in transgenic animal models. Whereas a patient with T2DM may have 1-1.5% mTG (compared with 0-0.5% in controls), the long-chain acyl-CoA synthetase overexpressing mouse displays a dramatic 12-fold increase in mTG 123 and high-fat diet alone induces a 4-fold increase in mTG 93. Furthermore, fat accumulation in the heart is approximately an order of magnitude lower than in the liver, which is also known to accumulate fat under similar metabolic challenges.
Given the practical limitations of acquiring cardiac tissue specimens in non-transplant settings, proton magnetic resonance spectroscopy (1H-MRS) has emerged as a useful tool to non-invasively quantify the degree of myocardial steatosis. 1H-MRS has been successfully employed, on a larger scale for measuring hepatic fat 130, and TG quantification for both the liver 131, 132 and heart 133 correlate well with that obtained from biopsy. Several studies have demonstrated that mTG levels are influenced by total adiposity. However, there is a clear further increase once insulin- resistance occurs 134. Interestingly, there is no close correlation between the degree of cardiac and hepatic fat although visceral fat is an independent predictor of mTG after multivariate modeling 134. Likewise, circulating FFA levels are linked to both hepatic and cardiac TG accumulation. Adiponectin levels also correlate with cardiac steatosis (though it is unclear whether this effect is independent of insulin-sensitivity) 135. Taken together, these observations also suggest that, similar to the relationship between LVH and obesity, the accumulation of VAT may be the primary upstream culprit to accumulation of mTG by virtue of its ability to influence on circulating adipokines, lipolysis and global insulin-sensitivity.
The clinical effects of cardiac steatosis have not been well defined, particularly in the setting of normal LV function. This is largely because of the few patients studied to date, and lack of long-term follow-up. Rijewijk et al demonstrated the degree mTG to be an independent predictor of diastolic dysfunction among a cohort of patients with relatively well-controlled T2DM (HbA1c <8.5%) 136. Similarly, another recent study, again in patients with well-controlled T2DM, demonstrated an inverse relationship between mean diastolic strain rate as assessed by MRI and mTG levels 137. These observations could be clinically meaningful and may suggest a possible direct effect of mTG towards impaired diastolic function. However, it could also reflect effects of confounding factors associated with co-morbities. Importantly, the largest study reported to date on patients with T2DM (enrolled across a wide range of varying glycemic control) failed to demonstrate any relationship between the degree of mTG and diastolic function 138. Hence, the clinical implications of cardiac steatosis in the setting of insulin resistance, independent of other comorbid factors, remains ambiguous but certainly represents a relevant area for further study.
Adipose tissue function and the failing ventricle
Curiously, although obesity is an independent risk factor for the development of clinical HF, it consistently appears to be associated with improved prognosis in the setting of existing HF 139-142. This is referred to as the “obesity paradox” of HF, and we completely lack a mechanistic basis for an explanation. This implies that adipose tissue either directly or indirectly promotes survival of cardiac myocytes. Alternatively, the lack of adipose tissue (i.e. the inability to maintain a stable BMI) is associated with a deficiency of a factor, lack of which leads to a worsened clinical outcome. It is clear though that this not simply due to protection from cardiac cachexia that is frequently seen at the opposite end of the BMI spectrum. To be sure, however, BMI itself may be an inadequate measure of nutritional status, and even HF patients with high BMI may manifest hypoalbuminemia 143.
HF is well known to result in neurohormonal activation. As such, circulating levels of catecholamines and natriuretic peptides are increased. These hormones act to drive TG hydrolysis from fat and raise systemic FFAs 144, 145. Levels of FFAs are significantly elevated in HF patients 146, 147. This is, at least in part, due to the altered neurohormonal environment, as well as a consequence to the associated increase in cytokines and the accompanying insulin resistance associated with HF. Such dramatic elevations in circulating FFAs have unquestionably important implications for myocardial metabolism, given the general affinity of the heart towards FFA uptake and the well-described impairments of substrate oxidation in the failing ventricle 148.
Beyond the changes in FFAs, adipokine levels are also significantly altered in HF patients. Adiponectin is perhaps the best-studied marker in this context. Levels of adiponectin increase in a step-wise fashion with worsening scores in the New York Heart Association (NYHA) functional classification system. Higher plasma adiponectin concentrations are associated with higher mortality 149-152. This finding, that we refer to as the “adiponectin paradox” associated with HF, has been reproduced in several different populations. This observation seems to defy any easy explanations based on what we know about adiponectin action, particularly in light of the generally positive functions ascribed to adiponectin in terms of metabolism and cardioprotection. After left ventricular assist device (LVAD) support, elevated adiponectin levels have been reported to drop dramatically, in parallel with a lowering of systemic and adipose-specific markers of inflammation, as well as improvements in insulin sensitivity 153. It is possible that adiponectin is increased in this setting due to a state of “adiponectin-resistance.” Interestingly, however, LVAD support is also associated with a reduction in key mediators of lipolysis, such as natriuretic peptides 154-156. This also correlates with increases in adipocyte size (i.e. cross-sectional area) 153. While this suggests that systemic adiponectin levels may be passively coupled to the degree of TG hydrolysis and FFA release from adipose tissue, more recent data in rodent models argues against this explanation 157. Additionally, adiponectin has well-described effects on metabolism of lipotoxic intermediates 25, 158, so the dramatic increases in circulating adiponectin concentrations may be an active response to HF-induced lipolysis. Supporting this paradigm is the close correlation between BNP levels and adiponectin 152, and the finding that natriuretic peptides can directly promote adiponectin expression/release from the adipocyte 159, 160. Finally, although adipose is the major source of adiponectin, it has been reported that the heart may be a source of this cytokine, as well 143, 161, 162. Although an attractive potential explanation for the elevations seen with HF, the available human data have not demonstrated any increased adiponectin production during LV failure 163, 153.
The levels of both leptin 164, 165 and resistin 165 are also increased in HF patients, but, in distinction to adiponectin, levels of these adipokines do not change following mechanical unloading with LVAD support 153. The increase in serum leptin levels with HF appears to be independent of weight. Cardiac expression of leptin receptor mRNA and protein levels is upregulated in HF, and both levels drop with LVAD support 166. In parallel, there is an observed decrease in both STAT3 and AMPK-activation following LVAD treatment. Although the exact role and relative importance of leptin signaling in the failing heart is largely unknown, these findings suggest that there is likely some further important biology to be discovered in this area.
As seen in the insulin-resistant state (which includes HF), the failing human heart also accumulates mTG. Although it is again unclear whether the lipid accumulation is “lipotoxic” per se, intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart 167. This can be partially reversed following mechanical unloading. In particular, ceramides, which have well-described pro-apoptotic effects, but not total mTG or fatty acids, have been shown to significantly fall with LVAD 168. Thus, myocardial lipid overload in the setting of HF appears to be derived from a surplus of FFAs delivered from adipose tissue hydrolysis and a superimposed inability of the failing heart to oxidize the substrate. Improvement in HF, e.g. following LVAD, likely improves lipid-overload by decreasing the lipolytic drive at the level of adipose, although this hypothesis remains to be directly tested.
The Obesity Paradox
Increasing adiposity is unquestionably associated with the aggregation of multiple risk factors for HF and vascular disease. However, obesity is associated with an improved survival following the diagnosis of these CVD conditions. Specifically, evidence of the “obesity paradox” has been observed in chronic 163, 169-171 and acute 172, 173 coronary heart disease, stroke 172, 174, 175, and peripheral vascular disease 176, in addition to HF noted above. This paradox is more generally referred to as an example of “reverse epidemiology,” a somewhat misleading term referring to the apparent reversal of classic associations of risk factors with disease outcomes.
A variety of explanations have been offered for these observations. Some of these seek to explain the obesity paradox as an artifact- e.g. due to residual confounding at the statistical level 176, due to differences in treatments/management strategies by BMI grouping 177, survival bias, or the fact that obesity patients may become symptomatic earlier in their disease course and present earlier (e.g. lead time bias). Furthermore, it has been suggested, that at least for acute coronary syndromes, the absolute amount of additional prognostic information offered by BMI is small once statistically accounting for other more clinically relevant risk variables 178. Lead time bias, as well as the potential for misclassification of HF symptoms in the obese due to ambiguity of the Framingham criteria in this clinical context (i.e. diagnostic bias) may certainly be potential contributors to the observation of the obesity paradox of HF. However, there is a strong evidence base that “reverse epidemiology” (i.e. the counterintuitive protection observed for obesity and high cholesterol and the corresponding association with greater survival) is a real biological phenomenon. The dramatic changes in circulating adipokine profiles, lipolytic signals, insulin-sensitivity, and FFAs highlight the interplay between adipose tissue and the failing heart. How the adipose may be protective under these circumstances remains ambiguous.
Clearly, it is difficult to reconcile how obesity may on one hand promote CVD and on the other be protective once it has been diagnosed. This paradox has rightly caused some consternation among physicians, as it has become unclear whether, once controlling risk factors for disease progression, weight loss should specifically be encouraged 178, 179. Ultimately, clinical trials aimed specifically at such lifestyle interventions will be needed to address this critical question prospectively and definitively.
Outstanding Questions and Future Directions
Over the last several years, there has been an exponential increase in our understanding of adipose biology and its relevance to the CVD. Nonetheless, there are still many unanswered questions in this field. One of the most puzzling, mentioned previously, is that of the obesity paradox. How can adipose both increase the risk of CVD risk factors, and yet be protective once CVD develops? Is this phenomenon mediated through adipokines or differential SAT/VAT masses?
Much needs to be explored in the context of adipokine biology and the failing heart. Are adipokine- and insulin-resistances at the myocardial level occurring and are they a cause or consequence of HF? What is the etiology of the “adiponectin paradox” and what is the role of adiponectin in the context of HF, i.e. is it a bystander or an active counter-regulatory hormone designed to offset the insulin-resistance and lipotoxicity which accompany HF? Furthermore, we do not completely understand what the drivers of the significantly elevated adiponectin levels in HF are. PPAR-γ and C/EBPα are the two major drivers of adiponectin expression in adipose tissue but whether their activities are increased in adipose during HF is not clear. Similarly, there are additional secretagogues for adiponectin, in addition to the natriuretic peptides, e.g. FGF21; whether such circulating factors are important in driving adiponectin release from fat needs to be elucidated further.
Leptin has been extensively studied in many contexts, but our understanding of its influences on the heart is still incomplete. The direct actions of leptin in the hypothalamus can reverse hepatic insulin resistance 180, and it is fascinatingly possible that central influences of adipokines may have indirect effects on the cardiovascular system and the failing heart (i.e. mediated through actions in the brain).
Finally, much has been written about myocardial lipotoxicity, but the clinical effects of mTG accumulation in humans are not clear. Data from transgenic murine models make a strong case for the detrimental effects of local accumulation of lipotoxic intermediates. These models include the overexpression of DGAT181, PPAR-γ overexpressing hearts in the background of PPAR-α−/− 182, long-chain acyl-CoA synthetase overexpression 123 or the increased presence of lipoprotein lipase on cardiac myocytes 124. No systematic analysis has been performed across all these mouse models to help us understand what specific species of lipid metabolites are toxic. Detailed analysis of intramyocardial fatty acids, ceramides, diacylglycerols and acylcarnitines from human samples will be informative here but may inevitably need to be coupled with metabolic fatty acid flux data to assure proper interpretation 183. These unanswered questions are important since the accumulation of mTG alone is not sufficient181,182 and may, in fact, be protective. In other word, it may not only be absolute levels of these lipotoxic species that drive local insulin resistance, inflammation and propensity to cell death, but rather the subcellular distribution of the lipids that matters the most. Finally, whether these phenomena related to cardiomyocyte dysfunction a cause or an effect of HF also remains to be seen with refined time course studies.
Conclusions
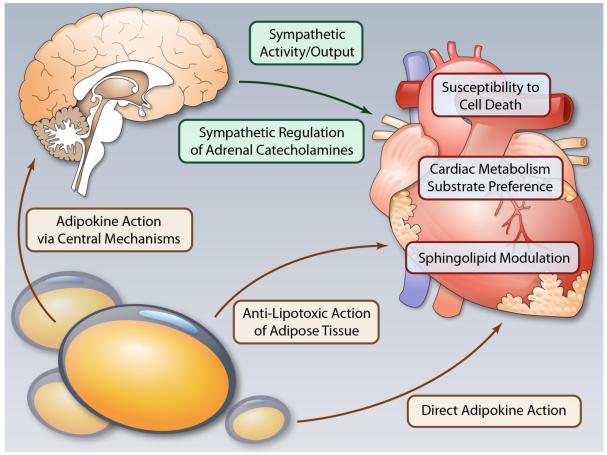
It is apparent that adipose tissue has a role beyond serving as a mere storage compartment of TGs. When dysfunctional, it exerts a negative impact on all other tissues by creating a much more lipotoxic environment in peripheral tissues, including the heart. Combined with ensuing insulin resistance and a dysregulation of key adipokines, it is at the core of systemic metabolic dysfunction, with the cardiovascular system representing the key organ system affected. This suggests that therapeutic interventions early in the progression to cardiovascular disease need to target specific metabolic and structural derangements, particularly at the level of adipose tissue. This will require an improved understanding of adipokine biology, hypothalamic control of organ homeostasis, potently influenced by adipokines, and direct effects of these adipokines on cardiovascular metabolism (Fig. 1).
Fig.1.
The cardiovascular system is influenced by adipose tissue, not just through effects on systemic insulin-sensitivity but also through direct and indirect effects of adipokines. Adipokines have effects on the central nervous system, which in turn, influence sympathetic outflow, peripheral metabolism, and (at least in the liver) organ steatosis. Better understood are the direct effects of adipokines on the heart, which may influence substrate metabolism, detoxify lipid intermediates, and promote cell-survival. (Ilustration Credit: Ben Smith).
Acknowledgments
Sources of funding
The authors are supported by NIH grants R01-DK55758 and P01DK088761 (P.E.S); HL-075173, HL-080144, and HL-090842 (J.A.H.); R01-DK53301, RL-1DK081185 and P01-DK088761 (J.K.E.)
Non-standard Abbreviations
- CVD
cardiovascular disease
- FFA
free fatty acid
- LVAD
left ventricular assist device
- LVH
left ventricular hypertrophy
- LV
left ventricle/ventricular
- mTG
myocardial triglyceride
- SAT
subcutaneous adipose tissue
- T2DM
type 2 diabetes mellitus
- TG
triglyceride
- VAT
visceral adipose tissue
Footnotes
Disclosures
None
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics--2012 update: A report from the american heart association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deng Y, Scherer PE. Adipokines as novel biomarkers and regulators of the metabolic syndrome. Ann N Y Acad Sci. 2010;1212:E1–E19. doi: 10.1111/j.1749-6632.2010.05875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scherer PE. Adipose tissue: From lipid storage compartment to endocrine organ. Diabetes. 2006;55:1537–1545. doi: 10.2337/db06-0263. [DOI] [PubMed] [Google Scholar]
- 4.Suganami T, Tanaka M, Ogawa Y. Adipose tissue inflammation and ectopic lipid accumulation [review] Endocrine journal. 2012 doi: 10.1507/endocrj.ej12-0271. [DOI] [PubMed] [Google Scholar]
- 5.Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Invest. 2011;121:2094–2101. doi: 10.1172/JCI45887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, Wang S, Fortier M, Greenberg AS, Obin MS. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 7.Murano I, Rutkowski JM, Wang QA, Cho YR, Scherer PE, Cinti S. Time course of histomorphological changes in adipose tissue upon acute lipoatrophy. Nutrition, metabolism, and cardiovascular diseases: NMCD. 2012 doi: 10.1016/j.numecd.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim JY, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, Schraw T, Durand JL, Li H, Li G, Jelicks LA, Mehler MF, Hui DY, Deshaies Y, Shulman GI, Schwartz GJ, Scherer PE. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. 2007;117:2621–2637. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asterholm IW, Halberg N, Scherer PE. Mouse models of lipodystrophy key reagents for the understanding of the metabolic syndrome. Drug Discov Today Dis Models. 2007;4:17–24. doi: 10.1016/j.ddmod.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halberg N, Wernstedt-Asterholm I, Scherer PE. The adipocyte as an endocrine cell. Endocrinol Metab Clin North Am. 2008;37:753–768. x–xi. doi: 10.1016/j.ecl.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 12.Gautron L, Elmquist JK. Sixteen years and counting: An update on leptin in energy balance. J Clin Invest. 2011;121:2087–2093. doi: 10.1172/JCI45888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards GJ, Campfield LA, Clark FT, Deeds J, Muir C, Sanker S, Moriarty A, Moore KJ, Smutko JS, Mays GG, Wool EA, Monroe CA, Tepper RI. Identification and expression cloning of a leptin receptor, ob-r. Cell. 1995;83:1263–1271. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 14.Baumann H, Morella KK, White DW, Dembski M, Bailon PS, Kim H, Lai CF, Tartaglia LA. The full-length leptin receptor has signaling capabilities of interleukin 6-type cytokine receptors. Proc Natl Acad Sci U S A. 1996;93:8374–8378. doi: 10.1073/pnas.93.16.8374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Qassab H, Smith MA, Irvine EE, Guillermet-Guibert J, Claret M, Choudhury AI, Selman C, Piipari K, Clements M, Lingard S, Chandarana K, Bell JD, Barsh GS, Smith AJ, Batterham RL, Ashford ML, Vanhaesebroeck B, Withers DJ. Dominant role of the p110beta isoform of pi3k over p110alpha in energy homeostasis regulation by pomc and agrp neurons. Cell Metab. 2009;10:343–354. doi: 10.1016/j.cmet.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niswender KD, Morton GJ, Stearns WH, Rhodes CJ, Myers MG, Jr., Schwartz MW. Intracellular signalling. Key enzyme in leptin-induced anorexia. Nature. 2001;413:794–795. doi: 10.1038/35101657. [DOI] [PubMed] [Google Scholar]
- 17.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to c1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 18.Shapiro L, Scherer PE. The crystal structure of a complement-1q family protein suggests an evolutionary link to tumor necrosis factor. Curr Biol. 1998;8:335–338. doi: 10.1016/s0960-9822(98)70133-2. [DOI] [PubMed] [Google Scholar]
- 19.Shetty S, Kusminski CM, Scherer PE. Adiponectin in health and disease: Evaluation of adiponectin-targeted drug development strategies. Trends Pharmacol Sci. 2009;30:234–239. doi: 10.1016/j.tips.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Turer AT, Scherer PE. Adiponectin: Mechanistic insights and clinical implications. Diabetologia. 2012;55:2319–2326. doi: 10.1007/s00125-012-2598-x. [DOI] [PubMed] [Google Scholar]
- 21.Tomas E, Tsao TS, Saha AK, Murrey HE, Zhang Cc C, Itani SI, Lodish HF, Ruderman NB. Enhanced muscle fat oxidation and glucose transport by acrp30 globular domain: Acetyl-coa carboxylase inhibition and amp-activated protein kinase activation. Proc Natl Acad Sci U S A. 2002;99:16309–16313. doi: 10.1073/pnas.222657499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating amp-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 23.Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, Okada-Iwabu M, Kawamoto S, Kubota N, Kubota T, Ito Y, Kamon J, Tsuchida A, Kumagai K, Kozono H, Hada Y, Ogata H, Tokuyama K, Tsunoda M, Ide T, Murakami K, Awazawa M, Takamoto I, Froguel P, Hara K, Tobe K, Nagai R, Ueki K, Kadowaki T. Targeted disruption of adipor1 and adipor2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007;13:332–339. doi: 10.1038/nm1557. [DOI] [PubMed] [Google Scholar]
- 24.Miller RA, Chu Q, Le Lay J, Scherer PE, Ahima RS, Kaestner KH, Foretz M, Viollet B, Birnbaum MJ. Adiponectin suppresses gluconeogenic gene expression in mouse hepatocytes independent of lkb1-ampk signaling. J Clin Invest. 2011;121:2518–2528. doi: 10.1172/JCI45942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holland WL, Miller RA, Wang ZV, Sun K, Barth BM, Bui HH, Davis KE, Bikman BT, Halberg N, Rutkowski JM, Wade MR, Tenorio VM, Kuo MS, Brozinick JT, Zhang BB, Birnbaum MJ, Summers SA, Scherer PE. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat Med. 2011;17:55–63. doi: 10.1038/nm.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–312. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 27.Barnes KM, Miner JL. Role of resistin in insulin sensitivity in rodents and humans. Curr Protein Pept Sci. 2009;10:96–107. doi: 10.2174/138920309787315239. [DOI] [PubMed] [Google Scholar]
- 28.Aigner T, Hambach L, Soder S, Schlotzer-Schrehardt U, Poschl E. The c5 domain of col6a3 is cleaved off from the col6 fibrils immediately after secretion. Biochem Biophys Res Commun. 2002;290:743–748. doi: 10.1006/bbrc.2001.6227. [DOI] [PubMed] [Google Scholar]
- 29.Pasarica M, Gowronska-Kozak B, Burk D, Remedios I, Hymel D, Gimble J, Ravussin E, Bray GA, Smith SR. Adipose tissue collagen vi in obesity. J Clin Endocrinol Metab. 2009;94:5155–5162. doi: 10.1210/jc.2009-0947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spencer M, Yao-Borengasser A, Unal R, Rasouli N, Gurley CM, Zhu B, Peterson CA, Kern PA. Adipose tissue macrophages in insulin-resistant subjects are associated with collagen vi and fibrosis and demonstrate alternative activation. Am J Physiol Endocrinol Metab. 2010;299:E1016–1027. doi: 10.1152/ajpendo.00329.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohade C, Mourtzikos KA, Wahl RL. “USA-fat”: Prevalence is related to ambient outdoor temperature-evaluation with 18f-fdg pet/ct. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2003;44:1267–1270. [PubMed] [Google Scholar]
- 32.Sun K, Wernstedt Asterholm I, Kusminski CM, Bueno AC, Wang ZV, Pollard JW, Brekken RA, Scherer PE. Dichotomous effects of vegf-a on adipose tissue dysfunction. Proc Natl Acad Sci U S A. 2012;109:5874–5879. doi: 10.1073/pnas.1200447109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Bostrom EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Hojlund K, Gygi SP, Spiegelman BM. A pgc1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bordicchia M, Liu D, Amri EZ, Ailhaud G, Dessi-Fulgheri P, Zhang C, Takahashi N, Sarzani R, Collins S. Cardiac natriuretic peptides act via p38 mapk to induce the brown fat thermogenic program in mouse and human adipocytes. J Clin Invest. 2012;122:1022–1036. doi: 10.1172/JCI59701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gburcik V, Cawthorn WP, Nedergaard J, Timmons JA, Cannon B. An essential role for tbx15 in the differentiation of brown and “brite” but not white adipocytes. Am J Physiol Endocrinol Metab. 2012 doi: 10.1152/ajpendo.00104.2012. [DOI] [PubMed] [Google Scholar]
- 36.Walden TB, Hansen IR, Timmons JA, Cannon B, Nedergaard J. Recruited vs. Nonrecruited molecular signatures of brown, “brite,” and white adipose tissues. Am J Physiol Endocrinol Metab. 2012;302:E19–31. doi: 10.1152/ajpendo.00249.2011. [DOI] [PubMed] [Google Scholar]
- 37.Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, Huang K, Tu H, van Marken Lichtenbelt WD, Hoeks J, Enerback S, Schrauwen P, Spiegelman BM. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: Depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab. 1998;83:847–850. doi: 10.1210/jcem.83.3.4660. [DOI] [PubMed] [Google Scholar]
- 39.Fain JN, Madan AK, Hiler ML, Cheema P, Bahouth SW. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology. 2004;145:2273–2282. doi: 10.1210/en.2003-1336. [DOI] [PubMed] [Google Scholar]
- 40.Krief S, Lonnqvist F, Raimbault S, Baude B, Van Spronsen A, Arner P, Strosberg AD, Ricquier D, Emorine LJ. Tissue distribution of beta 3-adrenergic receptor mrna in man. J Clin Invest. 1993;91:344–349. doi: 10.1172/JCI116191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rebuffe-Scrive M, Andersson B, Olbe L, Bjorntorp P. Metabolism of adipose tissue in intraabdominal depots of nonobese men and women. Metabolism. 1989;38:453–458. doi: 10.1016/0026-0495(89)90198-4. [DOI] [PubMed] [Google Scholar]
- 42.Ostman J, Arner P, Engfeldt P, Kager L. Regional differences in the control of lipolysis in human adipose tissue. Metabolism. 1979;28:1198–1205. doi: 10.1016/0026-0495(79)90131-8. [DOI] [PubMed] [Google Scholar]
- 43.Hellmer J, Marcus C, Sonnenfeld T, Arner P. Mechanisms for differences in lipolysis between human subcutaneous and omental fat cells. J Clin Endocrinol Metab. 1992;75:15–20. doi: 10.1210/jcem.75.1.1320047. [DOI] [PubMed] [Google Scholar]
- 44.Mauriege P, Galitzky J, Berlan M, Lafontan M. Heterogeneous distribution of beta and alpha-2 adrenoceptor binding sites in human fat cells from various fat deposits: Functional consequences. Eur J Clin Invest. 1987;17:156–165. doi: 10.1111/j.1365-2362.1987.tb02395.x. [DOI] [PubMed] [Google Scholar]
- 45.Bolinder J, Kager L, Ostman J, Arner P. Differences at the receptor and postreceptor levels between human omental and subcutaneous adipose tissue in the action of insulin on lipolysis. Diabetes. 1983;32:117–123. doi: 10.2337/diab.32.2.117. [DOI] [PubMed] [Google Scholar]
- 46.Fried SK, Russell CD, Grauso NL, Brolin RE. Lipoprotein lipase regulation by insulin and glucocorticoid in subcutaneous and omental adipose tissues of obese women and men. J Clin Invest. 1993;92:2191–2198. doi: 10.1172/JCI116821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neeland IJ, Turer AT, Ayers CR, Powell-Wiley TM, Vega GL, Farzaneh-Far R, Grundy SM, Khera A, McGuire DK, de Lemos JA. Dysfunctional adiposity and the risk of prediabetes and type 2 diabetes in obese adults. JAMA. 2012;308:1150–1159. doi: 10.1001/2012.jama.11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vega GL, Adams-Huet B, Peshock R, Willett D, Shah B, Grundy SM. Influence of body fat content and distribution on variation in metabolic risk. J Clin Endocrinol Metab. 2006;91:4459–4466. doi: 10.1210/jc.2006-0814. [DOI] [PubMed] [Google Scholar]
- 49.Turer AT, Khera A, Ayers CR, Turer CB, Grundy SM, Vega GL, Scherer PE. Adipose tissue mass and location affect circulating adiponectin levels. Diabetologia. 2011;54:2515–2524. doi: 10.1007/s00125-011-2252-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Snijder MB, Dekker JM, Visser M, Bouter LM, Stehouwer CD, Yudkin JS, Heine RJ, Nijpels G, Seidell JC. Trunk fat and leg fat have independent and opposite associations with fasting and postload glucose levels: The hoorn study. Diabetes Care. 2004;27:372–377. doi: 10.2337/diacare.27.2.372. [DOI] [PubMed] [Google Scholar]
- 51.Koster A, Stenholm S, Alley DE, Kim LJ, Simonsick EM, Kanaya AM, Visser M, Houston DK, Nicklas BJ, Tylavsky FA, Satterfield S, Goodpaster BH, Ferrucci L, Harris TB. Body fat distribution and inflammation among obese older adults with and without metabolic syndrome. Obesity (Silver Spring) 2010;18:2354–2361. doi: 10.1038/oby.2010.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Romero-Corral A, Somers VK, Sierra-Johnson J, Korenfeld Y, Boarin S, Korinek J, Jensen MD, Parati G, Lopez-Jimenez F. Normal weight obesity: A risk factor for cardiometabolic dysregulation and cardiovascular mortality. Eur Heart J. 2010;31:737–746. doi: 10.1093/eurheartj/ehp487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kosmala W, Jedrzejuk D, Derzhko R, Przewlocka-Kosmala M, Mysiak A, Bednarek-Tupikowska G. Left ventricular function impairment in patients with normal-weight obesity: Contribution of abdominal fat deposition, profibrotic state, reduced insulin sensitivity, and proinflammatory activation. Circulation. Cardiovascular imaging. 2012;5:349–356. doi: 10.1161/CIRCIMAGING.111.969956. [DOI] [PubMed] [Google Scholar]
- 54.Garg A. Clinical review#: Lipodystrophies: Genetic and acquired body fat disorders. J Clin Endocrinol Metab. 2011;96:3313–3325. doi: 10.1210/jc.2011-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Al-Shali KZ, Hegele RA. Laminopathies and atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:1591–1595. doi: 10.1161/01.ATV.0000136392.59656.8b. [DOI] [PubMed] [Google Scholar]
- 56.Garg A. Gender differences in the prevalence of metabolic complications in familial partial lipodystrophy (dunnigan variety) J Clin Endocrinol Metab. 2000;85:1776–1782. doi: 10.1210/jcem.85.5.6605. [DOI] [PubMed] [Google Scholar]
- 57.Lupsa BC, Sachdev V, Lungu AO, Rosing DR, Gorden P. Cardiomyopathy in congenital and acquired generalized lipodystrophy: A clinical assessment. Medicine (Baltimore) 2010;89:245–250. doi: 10.1097/MD.0b013e3181e9442f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Iacobellis G, Malavazos AE. Pericardial adipose tissue, atherosclerosis, and cardiovascular disease risk factors: The jackson heart study: Comment on liu et al. Diabetes Care. 2010;33:e127. doi: 10.2337/dc10-0904. author reply e128. [DOI] [PubMed] [Google Scholar]
- 59.Iacobellis G, Corradi D, Sharma AM. Epicardial adipose tissue: Anatomic, biomolecular and clinical relationships with the heart. Nature clinical practice. Cardiovascular medicine. 2005;2:536–543. doi: 10.1038/ncpcardio0319. [DOI] [PubMed] [Google Scholar]
- 60.Rosito GA, Massaro JM, Hoffmann U, Ruberg FL, Mahabadi AA, Vasan RS, O’Donnell CJ, Fox CS. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: The framingham heart study. Circulation. 2008;117:605–613. doi: 10.1161/CIRCULATIONAHA.107.743062. [DOI] [PubMed] [Google Scholar]
- 61.Iacobellis G, Ribaudo MC, Assael F, Vecci E, Tiberti C, Zappaterreno A, Di Mario U, Leonetti F. Echocardiographic epicardial adipose tissue is related to anthropometric and clinical parameters of metabolic syndrome: A new indicator of cardiovascular risk. J Clin Endocrinol Metab. 2003;88:5163–5168. doi: 10.1210/jc.2003-030698. [DOI] [PubMed] [Google Scholar]
- 62.Liu J, Fox CS, Hickson DA, May WL, Ding J, Carr JJ, Taylor HA. Pericardial fat and echocardiographic measures of cardiac abnormalities: The jackson heart study. Diabetes Care. 2011;34:341–346. doi: 10.2337/dc10-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Konishi M, Sugiyama S, Sugamura K, Nozaki T, Matsubara J, Akiyama E, Utsunomiya D, Matsuzawa Y, Yamashita Y, Kimura K, Umemura S, Ogawa H. Accumulation of pericardial fat correlates with left ventricular diastolic dysfunction in patients with normal ejection fraction. Journal of cardiology. 2012;59:344–351. doi: 10.1016/j.jjcc.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 64.Kim TH, Yu SH, Choi SH, Yoon JW, Kang SM, Chun EJ, Choi SI, Shin H, Lee HK, Park KS, Jang HC, Lim S. Pericardial fat amount is an independent risk factor of coronary artery stenosis assessed by multidetector-row computed tomography: The korean atherosclerosis study 2. Obesity (Silver Spring) 2011;19:1028–1034. doi: 10.1038/oby.2010.246. [DOI] [PubMed] [Google Scholar]
- 65.Cheng VY, Dey D, Tamarappoo B, Nakazato R, Gransar H, Miranda-Peats R, Ramesh A, Wong ND, Shaw LJ, Slomka PJ, Berman DS. Pericardial fat burden on ecg-gated noncontrast ct in asymptomatic patients who subsequently experience adverse cardiovascular events. JACC. Cardiovascular imaging. 2010;3:352–360. doi: 10.1016/j.jcmg.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nakanishi R, Rajani R, Cheng VY, Gransar H, Nakazato R, Shmilovich H, Otaki Y, Hayes SW, Thomson LE, Friedman JD, Slomka PJ, Berman DS, Dey D. Increase in epicardial fat volume is associated with greater coronary artery calcification progression in subjects at intermediate risk by coronary calcium score: A serial study using non-contrast cardiac ct. Atherosclerosis. 2011;218:363–368. doi: 10.1016/j.atherosclerosis.2011.07.093. [DOI] [PubMed] [Google Scholar]
- 67.Tamarappoo B, Dey D, Shmilovich H, Nakazato R, Gransar H, Cheng VY, Friedman JD, Hayes SW, Thomson LE, Slomka PJ, Rozanski A, Berman DS. Increased pericardial fat volume measured from noncontrast ct predicts myocardial ischemia by spect. JACC. Cardiovascular imaging. 2010;3:1104–1112. doi: 10.1016/j.jcmg.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hirata Y, Tabata M, Kurobe H, Motoki T, Akaike M, Nishio C, Higashida M, Mikasa H, Nakaya Y, Takanashi S, Igarashi T, Kitagawa T, Sata M. Coronary atherosclerosis is associated with macrophage polarization in epicardial adipose tissue. Journal of the American College of Cardiology. 2011;58:248–255. doi: 10.1016/j.jacc.2011.01.048. [DOI] [PubMed] [Google Scholar]
- 69.Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, Sarov-Blat L, O’Brien S, Keiper EA, Johnson AG, Martin J, Goldstein BJ, Shi Y. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108:2460–2466. doi: 10.1161/01.CIR.0000099542.57313.C5. [DOI] [PubMed] [Google Scholar]
- 70.Fujikawa T, Chuang JC, Sakata I, Ramadori G, Coppari R. Leptin therapy improves insulin-deficient type 1 diabetes by cns-dependent mechanisms in mice. Proc Natl Acad Sci U S A. 2010;107:17391–17396. doi: 10.1073/pnas.1008025107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rahmouni K. Obesity, sympathetic overdrive, and hypertension: The leptin connection. Hypertension. 2010;55:844–845. doi: 10.1161/HYPERTENSIONAHA.109.148932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Correia ML, Morgan DA, Sivitz WI, Mark AL, Haynes WG. Leptin acts in the central nervous system to produce dose-dependent changes in arterial pressure. Hypertension. 2001;37:936–942. doi: 10.1161/01.hyp.37.3.936. [DOI] [PubMed] [Google Scholar]
- 73.Rahmouni K, Morgan DA. Hypothalamic arcuate nucleus mediates the sympathetic and arterial pressure responses to leptin. Hypertension. 2007;49:647–652. doi: 10.1161/01.HYP.0000254827.59792.b2. [DOI] [PubMed] [Google Scholar]
- 74.do Carmo JM, da Silva AA, Cai Z, Lin S, Dubinion JH, Hall JE. Control of blood pressure, appetite, and glucose by leptin in mice lacking leptin receptors in proopiomelanocortin neurons. Hypertension. 2011;57:918–926. doi: 10.1161/HYPERTENSIONAHA.110.161349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Qi Y, Takahashi N, Hileman SM, Patel HR, Berg AH, Pajvani UB, Scherer PE, Ahima RS. Adiponectin acts in the brain to decrease body weight. Nat Med. 2004;10:524–529. doi: 10.1038/nm1029. [DOI] [PubMed] [Google Scholar]
- 76.Kosari S, Rathner JA, Chen F, Kosari S, Badoer E. Centrally administered resistin enhances sympathetic nerve activity to the hindlimb but attenuates the activity to brown adipose tissue. Endocrinology. 2011;152:2626–2633. doi: 10.1210/en.2010-1492. [DOI] [PubMed] [Google Scholar]
- 77.Ahima RS. Central actions of adipocyte hormones. Trends Endocrinol Metab. 2005;16:307–313. doi: 10.1016/j.tem.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 78.Luiken JJ, Koonen DP, Willems J, Zorzano A, Becker C, Fischer Y, Tandon NN, Van Der Vusse GJ, Bonen A, Glatz JF. Insulin stimulates long-chain fatty acid utilization by rat cardiac myocytes through cellular redistribution of fat/cd36. Diabetes. 2002;51:3113–3119. doi: 10.2337/diabetes.51.10.3113. [DOI] [PubMed] [Google Scholar]
- 79.Hue L, Taegtmeyer H. The randle cycle revisited: A new head for an old hat. Am J Physiol Endocrinol Metab. 2009;297:E578–591. doi: 10.1152/ajpendo.00093.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wende AR, Abel ED. Lipotoxicity in the heart. Biochim Biophys Acta. 2010;1801:311–319. doi: 10.1016/j.bbalip.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Khullar M, Al-Shudiefat AA, Ludke A, Binepal G, Singal PK. Oxidative stress: A key contributor to diabetic cardiomyopathy. Can J Physiol Pharmacol. 2010;88:233–240. doi: 10.1139/Y10-016. [DOI] [PubMed] [Google Scholar]
- 82.Holland WL, Summers SA. Sphingolipids, insulin resistance, and metabolic disease: New insights from in vivo manipulation of sphingolipid metabolism. Endocr Rev. 2008;29:381–402. doi: 10.1210/er.2007-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang L, Keung W, Samokhvalov V, Wang W, Lopaschuk GD. Role of fatty acid uptake and fatty acid beta-oxidation in mediating insulin resistance in heart and skeletal muscle. Biochim Biophys Acta. 2010;1801:1–22. doi: 10.1016/j.bbalip.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 84.Liu GX, Hanley PJ, Ray J, Daut J. Long-chain acyl-coenzyme a esters and fatty acids directly link metabolism to k(atp) channels in the heart. Circ Res. 2001;88:918–924. doi: 10.1161/hh0901.089881. [DOI] [PubMed] [Google Scholar]
- 85.Choo HJ, Kim JH, Kwon OB, Lee CS, Mun JY, Han SS, Yoon YS, Yoon G, Choi KM, Ko YG. Mitochondria are impaired in the adipocytes of type 2 diabetic mice. Diabetologia. 2006;49:784–791. doi: 10.1007/s00125-006-0170-2. [DOI] [PubMed] [Google Scholar]
- 86.Brookheart RT, Michel CI, Schaffer JE. As a matter of fat. Cell Metab. 2009;10:9–12. doi: 10.1016/j.cmet.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dobrin JS, Lebeche D. Diabetic cardiomyopathy: Signaling defects and therapeutic approaches. Expert review of cardiovascular therapy. 2010;8:373–391. doi: 10.1586/erc.10.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fang ZY, Prins JB, Marwick TH. Diabetic cardiomyopathy: Evidence, mechanisms, and therapeutic implications. Endocr Rev. 2004;25:543–567. doi: 10.1210/er.2003-0012. [DOI] [PubMed] [Google Scholar]
- 89.Wang Y, Nishina PM, Naggert JK. Degradation of irs1 leads to impaired glucose uptake in adipose tissue of the type 2 diabetes mouse model tallyho/jng. J Endocrinol. 2009;203:65–74. doi: 10.1677/JOE-09-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ueno M, Carvalheira JB, Tambascia RC, Bezerra RM, Amaral ME, Carneiro EM, Folli F, Franchini KG, Saad MJ. Regulation of insulin signalling by hyperinsulinaemia: Role of irs-1/2 serine phosphorylation and the mtor/p70 s6k pathway. Diabetologia. 2005;48:506–518. doi: 10.1007/s00125-004-1662-6. [DOI] [PubMed] [Google Scholar]
- 91.Hartley D, Cooper GM. Role of mtor in the degradation of irs-1: Regulation of pp2a activity. J Cell Biochem. 2002;85:304–314. doi: 10.1002/jcb.10135. [DOI] [PubMed] [Google Scholar]
- 92.Ni YG, Wang N, Cao DJ, Sachan N, Morris DJ, Gerard RD, Kuro OM, Rothermel BA, Hill JA. Foxo transcription factors activate akt and attenuate insulin signaling in heart by inhibiting protein phosphatases. Proc Natl Acad Sci U S A. 2007;104:20517–20522. doi: 10.1073/pnas.0610290104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Battiprolu PK, Hojayev B, Jiang N, Wang ZV, Luo X, Iglewski M, Shelton JM, Gerard RD, Rothermel BA, Gillette TG, Lavandero S, Hill JA. Metabolic stress-induced activation of foxo1 triggers diabetic cardiomyopathy in mice. J Clin Invest. 2012;122:1109–1118. doi: 10.1172/JCI60329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 95.Du XL, Edelstein D, Rossetti L, Fantus IG, Goldberg H, Ziyadeh F, Wu J, Brownlee M. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing sp1 glycosylation. Proc Natl Acad Sci U S A. 2000;97:12222–12226. doi: 10.1073/pnas.97.22.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cai L, Li W, Wang G, Guo L, Jiang Y, Kang YJ. Hyperglycemia-induced apoptosis in mouse myocardium: Mitochondrial cytochrome c-mediated caspase-3 activation pathway. Diabetes. 2002;51:1938–1948. doi: 10.2337/diabetes.51.6.1938. [DOI] [PubMed] [Google Scholar]
- 97.Du X, Matsumura T, Edelstein D, Rossetti L, Zsengeller Z, Szabo C, Brownlee M. Inhibition of gapdh activity by poly(adp-ribose) polymerase activates three major pathways of hyperglycemic damage in endothelial cells. J Clin Invest. 2003;112:1049–1057. doi: 10.1172/JCI18127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 99.Poornima IG, Parikh P, Shannon RP. Diabetic cardiomyopathy: The search for a unifying hypothesis. Circ Res. 2006;98:596–605. doi: 10.1161/01.RES.0000207406.94146.c2. [DOI] [PubMed] [Google Scholar]
- 100.Kenchaiah S, Sesso HD, Gaziano JM. Body mass index and vigorous physical activity and the risk of heart failure among men. Circulation. 2009;119:44–52. doi: 10.1161/CIRCULATIONAHA.108.807289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C, Whelton PK. Risk factors for congestive heart failure in us men and women: Nhanes i epidemiologic follow-up study. Archives of internal medicine. 2001;161:996–1002. doi: 10.1001/archinte.161.7.996. [DOI] [PubMed] [Google Scholar]
- 102.Chen YT, Vaccarino V, Williams CS, Butler J, Berkman LF, Krumholz HM. Risk factors for heart failure in the elderly: A prospective community-based study. Am J Med. 1999;106:605–612. doi: 10.1016/s0002-9343(99)00126-6. [DOI] [PubMed] [Google Scholar]
- 103.Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, Kannel WB, Vasan RS. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–313. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 104.Turkbey EB, McClelland RL, Kronmal RA, Burke GL, Bild DE, Tracy RP, Arai AE, Lima JA, Bluemke DA. The impact of obesity on the left ventricle: The multi-ethnic study of atherosclerosis (mesa) JACC. Cardiovascular imaging. 2010;3:266–274. doi: 10.1016/j.jcmg.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Powell BD, Redfield MM, Bybee KA, Freeman WK, Rihal CS. Association of obesity with left ventricular remodeling and diastolic dysfunction in patients without coronary artery disease. Am J Cardiol. 2006;98:116–120. doi: 10.1016/j.amjcard.2006.01.063. [DOI] [PubMed] [Google Scholar]
- 106.Lauer MS, Anderson KM, Levy D. Separate and joint influences of obesity and mild hypertension on left ventricular mass and geometry: The framingham heart study. Journal of the American College of Cardiology. 1992;19:130–134. doi: 10.1016/0735-1097(92)90063-s. [DOI] [PubMed] [Google Scholar]
- 107.Lauer MS, Anderson KM, Kannel WB, Levy D. The impact of obesity on left ventricular mass and geometry. The framingham heart study. JAMA. 1991;266:231–236. [PubMed] [Google Scholar]
- 108.Primeau V, Coderre L, Karelis AD, Brochu M, Lavoie ME, Messier V, Sladek R, Rabasa-Lhoret R. Characterizing the profile of obese patients who are metabolically healthy. Int J Obes (Lond) 2011;35:971–981. doi: 10.1038/ijo.2010.216. [DOI] [PubMed] [Google Scholar]
- 109.Mitsuhashi H, Yatsuya H, Tamakoshi K, Matsushita K, Otsuka R, Wada K, Sugiura K, Takefuji S, Hotta Y, Kondo T, Murohara T, Toyoshima H. Adiponectin level and left ventricular hypertrophy in japanese men. Hypertension. 2007;49:1448–1454. doi: 10.1161/HYPERTENSIONAHA.106.079509. [DOI] [PubMed] [Google Scholar]
- 110.McManus DD, Lyass A, Ingelsson E, Massaro JM, Meigs JB, Aragam J, Benjamin EJ, Vasan RS. Relations of circulating resistin and adiponectin and cardiac structure and function: The framingham offspring study. Obesity (Silver Spring) 2011 doi: 10.1038/oby.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gustafsson S, Lind L, Zethelius B, Venge P, Flyvbjerg A, Soderberg S, Ingelsson E. Adiponectin and cardiac geometry and function in elderly: Results from two community-based cohort studies. Eur J Endocrinol. 2010;162:543–550. doi: 10.1530/EJE-09-1006. [DOI] [PubMed] [Google Scholar]
- 112.Bidulescu A, Liu J, Musani SK, Fox ER, Samdarshi TE, Sarpong DF, Vaccarino V, Wilson PW, Arnett DK, Din-Dzietham R, Taylor HA, Gibbons GH. Association of adiponectin with left ventricular mass in blacks: The jackson heart study. Circulation. Heart failure. 2011;4:747–753. doi: 10.1161/CIRCHEARTFAILURE.110.959742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shibata R, Ouchi N, Ito M, Kihara S, Shiojima I, Pimentel DR, Kumada M, Sato K, Schiekofer S, Ohashi K, Funahashi T, Colucci WS, Walsh K. Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat Med. 2004;10:1384–1389. doi: 10.1038/nm1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Paolisso G, Tagliamonte MR, Galderisi M, Zito GA, Petrocelli A, Carella C, de Divitiis O, Varricchio M. Plasma leptin level is associated with myocardial wall thickness in hypertensive insulin-resistant men. Hypertension. 1999;34:1047–1052. doi: 10.1161/01.hyp.34.5.1047. [DOI] [PubMed] [Google Scholar]
- 115.Lieb W, Sullivan LM, Aragam J, Harris TB, Roubenoff R, Benjamin EJ, Vasan RS. Relation of serum leptin with cardiac mass and left atrial dimension in individuals >70 years of age. Am J Cardiol. 2009;104:602–605. doi: 10.1016/j.amjcard.2009.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rajapurohitam V, Gan XT, Kirshenbaum LA, Karmazyn M. The obesity-associated peptide leptin induces hypertrophy in neonatal rat ventricular myocytes. Circ Res. 2003;93:277–279. doi: 10.1161/01.RES.0000089255.37804.72. [DOI] [PubMed] [Google Scholar]
- 117.Barouch LA, Berkowitz DE, Harrison RW, O’Donnell CP, Hare JM. Disruption of leptin signaling contributes to cardiac hypertrophy independently of body weight in mice. Circulation. 2003;108:754–759. doi: 10.1161/01.CIR.0000083716.82622.FD. [DOI] [PubMed] [Google Scholar]
- 118.Kang S, Chemaly ER, Hajjar RJ, Lebeche D. Resistin promotes cardiac hypertrophy via the amp-activated protein kinase/mammalian target of rapamycin (ampk/mtor) and c-jun n-terminal kinase/insulin receptor substrate 1 (jnk/irs1) pathways. J Biol Chem. 2011;286:18465–18473. doi: 10.1074/jbc.M110.200022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Frankel DS, Vasan RS, D’Agostino RB, Sr., Benjamin EJ, Levy D, Wang TJ, Meigs JB. Resistin, adiponectin, and risk of heart failure the framingham offspring study. Journal of the American College of Cardiology. 2009;53:754–762. doi: 10.1016/j.jacc.2008.07.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wannamethee SG, Shaper AG, Whincup PH, Lennon L, Sattar N. Obesity and risk of incident heart failure in older men with and without pre-existing coronary heart disease: Does leptin have a role? Journal of the American College of Cardiology. 2011;58:1870–1877. doi: 10.1016/j.jacc.2011.06.057. [DOI] [PubMed] [Google Scholar]
- 121.Lieb W, Sullivan LM, Harris TB, Roubenoff R, Benjamin EJ, Levy D, Fox CS, Wang TJ, Wilson PW, Kannel WB, Vasan RS. Plasma leptin levels and incidence of heart failure, cardiovascular disease, and total mortality in elderly individuals. Diabetes Care. 2009;32:612–616. doi: 10.2337/dc08-1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bahrami H, Bluemke DA, Kronmal R, Bertoni AG, Lloyd-Jones DM, Shahar E, Szklo M, Lima JA. Novel metabolic risk factors for incident heart failure and their relationship with obesity: The mesa (multi-ethnic study of atherosclerosis) study. Journal of the American College of Cardiology. 2008;51:1775–1783. doi: 10.1016/j.jacc.2007.12.048. [DOI] [PubMed] [Google Scholar]
- 123.Chiu HC, Kovacs A, Ford DA, Hsu FF, Garcia R, Herrero P, Saffitz JE, Schaffer JE. A novel mouse model of lipotoxic cardiomyopathy. J Clin Invest. 2001;107:813–822. doi: 10.1172/JCI10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yagyu H, Chen G, Yokoyama M, Hirata K, Augustus A, Kako Y, Seo T, Hu Y, Lutz EP, Merkel M, Bensadoun A, Homma S, Goldberg IJ. Lipoprotein lipase (lpl) on the surface of cardiomyocytes increases lipid uptake and produces a cardiomyopathy. J Clin Invest. 2003;111:419–426. doi: 10.1172/JCI16751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Glenn DJ, Wang F, Nishimoto M, Cruz MC, Uchida Y, Holleran WM, Zhang Y, Yeghiazarians Y, Gardner DG. A murine model of isolated cardiac steatosis leads to cardiomyopathy. Hypertension. 2011;57:216–222. doi: 10.1161/HYPERTENSIONAHA.110.160655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Finck BN, Lehman JJ, Leone TC, Welch MJ, Bennett MJ, Kovacs A, Han X, Gross RW, Kozak R, Lopaschuk GD, Kelly DP. The cardiac phenotype induced by pparalpha overexpression mimics that caused by diabetes mellitus. J Clin Invest. 2002;109:121–130. doi: 10.1172/JCI14080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Son NH, Park TS, Yamashita H, Yokoyama M, Huggins LA, Okajima K, Homma S, Szabolcs MJ, Huang LS, Goldberg IJ. Cardiomyocyte expression of ppargamma leads to cardiac dysfunction in mice. J Clin Invest. 2007;117:2791–2801. doi: 10.1172/JCI30335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhou YT, Grayburn P, Karim A, Shimabukuro M, Higa M, Baetens D, Orci L, Unger RH. Lipotoxic heart disease in obese rats: Implications for human obesity. Proc Natl Acad Sci U S A. 2000;97:1784–1789. doi: 10.1073/pnas.97.4.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Inoguchi T, Battan R, Handler E, Sportsman JR, Heath W, King GL. Preferential elevation of protein kinase c isoform beta ii and diacylglycerol levels in the aorta and heart of diabetic rats: Differential reversibility to glycemic control by islet cell transplantation. Proc Natl Acad Sci U S A. 1992;89:11059–11063. doi: 10.1073/pnas.89.22.11059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Szczepaniak LS, Nurenberg P, Leonard D, Browning JD, Reingold JS, Grundy S, Hobbs HH, Dobbins RL. Magnetic resonance spectroscopy to measure hepatic triglyceride content: Prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288:E462–468. doi: 10.1152/ajpendo.00064.2004. [DOI] [PubMed] [Google Scholar]
- 131.Thomsen C, Becker U, Winkler K, Christoffersen P, Jensen M, Henriksen O. Quantification of liver fat using magnetic resonance spectroscopy. Magn Reson Imaging. 1994;12:487–495. doi: 10.1016/0730-725x(94)92543-7. [DOI] [PubMed] [Google Scholar]
- 132.Longo R, Pollesello P, Ricci C, Masutti F, Kvam BJ, Bercich L, Croce LS, Grigolato P, Paoletti S, de Bernard B, et al. Proton mr spectroscopy in quantitative in vivo determination of fat content in human liver steatosis. Journal of magnetic resonance imaging: JMRI. 1995;5:281–285. doi: 10.1002/jmri.1880050311. [DOI] [PubMed] [Google Scholar]
- 133.Szczepaniak LS, Dobbins RL, Metzger GJ, Sartoni-D’Ambrosia G, Arbique D, Vongpatanasin W, Unger R, Victor RG. Myocardial triglycerides and systolic function in humans: In vivo evaluation by localized proton spectroscopy and cardiac imaging. Magnetic resonance in medicine: official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2003;49:417–423. doi: 10.1002/mrm.10372. [DOI] [PubMed] [Google Scholar]
- 134.McGavock JM, Lingvay I, Zib I, Tillery T, Salas N, Unger R, Levine BD, Raskin P, Victor RG, Szczepaniak LS. Cardiac steatosis in diabetes mellitus: A 1h-magnetic resonance spectroscopy study. Circulation. 2007;116:1170–1175. doi: 10.1161/CIRCULATIONAHA.106.645614. [DOI] [PubMed] [Google Scholar]
- 135.Iozzo P, Lautamaki R, Borra R, Lehto HR, Bucci M, Viljanen A, Parkka J, Lepomaki V, Maggio R, Parkkola R, Knuuti J, Nuutila P. Contribution of glucose tolerance and gender to cardiac adiposity. J Clin Endocrinol Metab. 2009;94:4472–4482. doi: 10.1210/jc.2009-0436. [DOI] [PubMed] [Google Scholar]
- 136.Rijzewijk LJ, van der Meer RW, Smit JW, Diamant M, Bax JJ, Hammer S, Romijn JA, de Roos A, Lamb HJ. Myocardial steatosis is an independent predictor of diastolic dysfunction in type 2 diabetes mellitus. Journal of the American College of Cardiology. 2008;52:1793–1799. doi: 10.1016/j.jacc.2008.07.062. [DOI] [PubMed] [Google Scholar]
- 137.Korosoglou G, Humpert PM, Ahrens J, Oikonomou D, Osman NF, Gitsioudis G, Buss SJ, Steen H, Schnackenburg B, Bierhaus A, Nawroth PP, Katus HA. Left ventricular diastolic function in type 2 diabetes mellitus is associated with myocardial triglyceride content but not with impaired myocardial perfusion reserve. Journal of magnetic resonance imaging: JMRI. 2012;35:804–811. doi: 10.1002/jmri.22879. [DOI] [PubMed] [Google Scholar]
- 138.McGavock J, Szczepaniak LS, Ayers CR, Abdullah SM, See R, Gore MO, Drazner MH, de Lemos JA, McGuire DK. The effects of rosiglitazone on myocardial triglyceride content in patients with type 2 diabetes: A randomised, placebo-controlled trial. Diab Vasc Dis Res. 2012;9:131–137. doi: 10.1177/1479164111428628. [DOI] [PubMed] [Google Scholar]
- 139.Horwich TB, Fonarow GC, Hamilton MA, MacLellan WR, Woo MA, Tillisch JH. The relationship between obesity and mortality in patients with heart failure. Journal of the American College of Cardiology. 2001;38:789–795. doi: 10.1016/s0735-1097(01)01448-6. [DOI] [PubMed] [Google Scholar]
- 140.Fonarow GC, Srikanthan P, Costanzo MR, Cintron GB, Lopatin M. An obesity paradox in acute heart failure: Analysis of body mass index and inhospital mortality for 108,927 patients in the acute decompensated heart failure national registry. American heart journal. 2007;153:74–81. doi: 10.1016/j.ahj.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 141.Curtis JP, Selter JG, Wang Y, Rathore SS, Jovin IS, Jadbabaie F, Kosiborod M, Portnay EL, Sokol SI, Bader F, Krumholz HM. The obesity paradox: Body mass index and outcomes in patients with heart failure. Archives of internal medicine. 2005;165:55–61. doi: 10.1001/archinte.165.1.55. [DOI] [PubMed] [Google Scholar]
- 142.Clark AL, Fonarow GC, Horwich TB. Waist circumference, body mass index, and survival in systolic heart failure: The obesity paradox revisited. Journal of cardiac failure. 2011;17:374–380. doi: 10.1016/j.cardfail.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 143.Guder G, Frantz S, Bauersachs J, Allolio B, Wanner C, Koller MT, Ertl G, Angermann CE, Stork S. Reverse epidemiology in systolic and nonsystolic heart failure: Cumulative prognostic benefit of classical cardiovascular risk factors. Circulation. Heart failure. 2009;2:563–571. doi: 10.1161/CIRCHEARTFAILURE.108.825059. [DOI] [PubMed] [Google Scholar]
- 144.Lafontan M, Moro C, Sengenes C, Galitzky J, Crampes F, Berlan M. An unsuspected metabolic role for atrial natriuretic peptides: The control of lipolysis, lipid mobilization, and systemic nonesterified fatty acids levels in humans. Arterioscler Thromb Vasc Biol. 2005;25:2032–2042. doi: 10.1161/01.ATV.0000183728.14712.d8. [DOI] [PubMed] [Google Scholar]
- 145.Lafontan M, Langin D. Lipolysis and lipid mobilization in human adipose tissue. Prog Lipid Res. 2009;48:275–297. doi: 10.1016/j.plipres.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 146.Norrelund H, Wiggers H, Halbirk M, Frystyk J, Flyvbjerg A, Botker HE, Schmitz O, Jorgensen JO, Christiansen JS, Moller N. Abnormalities of whole body protein turnover, muscle metabolism and levels of metabolic hormones in patients with chronic heart failure. J Intern Med. 2006;260:11–21. doi: 10.1111/j.1365-2796.2006.01663.x. [DOI] [PubMed] [Google Scholar]
- 147.Lommi J, Kupari M, Yki-Jarvinen H. Free fatty acid kinetics and oxidation in congestive heart failure. Am J Cardiol. 1998;81:45–50. doi: 10.1016/s0002-9149(97)00804-7. [DOI] [PubMed] [Google Scholar]
- 148.Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev. 2005;85:1093–1129. doi: 10.1152/physrev.00006.2004. [DOI] [PubMed] [Google Scholar]
- 149.Tsutamoto T, Tanaka T, Sakai H, Ishikawa C, Fujii M, Yamamoto T, Horie M. Total and high molecular weight adiponectin, haemodynamics, and mortality in patients with chronic heart failure. Eur Heart J. 2007;28:1723–1730. doi: 10.1093/eurheartj/ehm154. [DOI] [PubMed] [Google Scholar]
- 150.McEntegart MB, Awede B, Petrie MC, Sattar N, Dunn FG, MacFarlane NG, McMurray JJ. Increase in serum adiponectin concentration in patients with heart failure and cachexia: Relationship with leptin, other cytokines, and b-type natriuretic peptide. Eur Heart J. 2007;28:829–835. doi: 10.1093/eurheartj/ehm033. [DOI] [PubMed] [Google Scholar]
- 151.Masson S, Gori F, Latini R, Milani V, Flyvbjerg A, Frystyk J, Crociati L, Pietri S, Vago T, Barlera S, Maggioni AP, Tognoni G, Tavazzi L, Omland T, Franzosi MG. Adiponectin in chronic heart failure: Influence of diabetes and genetic variants. Eur J Clin Invest. 2011;41:1330–1338. doi: 10.1111/j.1365-2362.2011.02548.x. [DOI] [PubMed] [Google Scholar]
- 152.Kistorp C, Faber J, Galatius S, Gustafsson F, Frystyk J, Flyvbjerg A, Hildebrandt P. Plasma adiponectin, body mass index, and mortality in patients with chronic heart failure. Circulation. 2005;112:1756–1762. doi: 10.1161/CIRCULATIONAHA.104.530972. [DOI] [PubMed] [Google Scholar]
- 153.Khan RS, Kato TS, Chokshi A, Chew M, Yu S, Wu C, Singh P, Cheema FH, Takayama H, Harris C, Reyes-Soffer G, Knoll R, Milting H, Naka Y, Mancini D, Schulze PC. Adipose tissue inflammation and adiponectin resistance in patients with advanced heart failure: Correction after ventricular assist device implantation. Circulation. Heart failure. 2012;5:340–348. doi: 10.1161/CIRCHEARTFAILURE.111.964031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Xydas S, Rosen RS, Ng C, Mercando M, Cohen J, DiTullio M, Magnano A, Marboe CC, Mancini DM, Naka Y, Oz MC, Maybaum S. Mechanical unloading leads to echocardiographic, electrocardiographic, neurohormonal, and histologic recovery. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2006;25:7–15. doi: 10.1016/j.healun.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 155.Thompson LO, Skrabal CA, Loebe M, Lafuente JA, Roberts RR, Akgul A, Jones V, Bruckner BA, Thohan V, Noon GP, Youker KA. Plasma neurohormone levels correlate with left ventricular functional and morphological improvement in lvad patients. J Surg Res. 2005;123:25–32. doi: 10.1016/j.jss.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 156.Milting H, A ELB, Kassner A, Fey O, Sarnowski P, Arusoglu L, Thieleczek R, Brinkmann T, Kleesiek K, Korfer R. The time course of natriuretic hormones as plasma markers of myocardial recovery in heart transplant candidates during ventricular assist device support reveals differences among device types. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2001;20:949–955. doi: 10.1016/s1053-2498(01)00289-3. [DOI] [PubMed] [Google Scholar]
- 157.Kusminski CM, Holland WL, Sun K, Park JY, Spurgin SB, Lin Y, Askew GR, Simcox JA, McClain DA, Li C, Scherer PE. Mitoneet-driven alterations in adipocyte mitochondrial activity during obesity reveal a critical adaptive process that preserves insulin sensitivity. Nature Medicine. 2012 doi: 10.1038/nm.2899. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mullen KL, Pritchard J, Ritchie I, Snook LA, Chabowski A, Bonen A, Wright D, Dyck DJ. Adiponectin resistance precedes the accumulation of skeletal muscle lipids and insulin resistance in high-fat-fed rats. Am J Physiol Regul Integr Comp Physiol. 2009;296:R243–251. doi: 10.1152/ajpregu.90774.2008. [DOI] [PubMed] [Google Scholar]
- 159.Yamaji M, Tsutamoto T, Tanaka T, Kawahara C, Nishiyama K, Yamamoto T, Fujii M, Horie M. Effect of carperitide on plasma adiponectin levels in acute decompensated heart failure patients with diabetes mellitus. Circulation journal : official journal of the Japanese Circulation Society. 2009;73:2264–2269. doi: 10.1253/circj.cj-09-0371. [DOI] [PubMed] [Google Scholar]
- 160.Tsukamoto O, Fujita M, Kato M, Yamazaki S, Asano Y, Ogai A, Okazaki H, Asai M, Nagamachi Y, Maeda N, Shintani Y, Minamino T, Asakura M, Kishimoto I, Funahashi T, Tomoike H, Kitakaze M. Natriuretic peptides enhance the production of adiponectin in human adipocytes and in patients with chronic heart failure. Journal of the American College of Cardiology. 2009;53:2070–2077. doi: 10.1016/j.jacc.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 161.Wang Y, Lau WB, Gao E, Tao L, Yuan Y, Li R, Wang X, Koch WJ, Ma XL. Cardiomyocyte-derived adiponectin is biologically active in protecting against myocardial ischemia-reperfusion injury. Am J Physiol Endocrinol Metab. 2010;298:E663–670. doi: 10.1152/ajpendo.00663.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ding G, Qin Q, He N, Francis-David SC, Hou J, Liu J, Ricks E, Yang Q. Adiponectin and its receptors are expressed in adult ventricular cardiomyocytes and upregulated by activation of peroxisome proliferator-activated receptor gamma. J Mol Cell Cardiol. 2007;43:73–84. doi: 10.1016/j.yjmcc.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Skurk C, Wittchen F, Suckau L, Witt H, Noutsias M, Fechner H, Schultheiss HP, Poller W. Description of a local cardiac adiponectin system and its deregulation in dilated cardiomyopathy. Eur Heart J. 2008;29:1168–1180. doi: 10.1093/eurheartj/ehn136. [DOI] [PubMed] [Google Scholar]
- 164.Schulze PC, Kratzsch J, Linke A, Schoene N, Adams V, Gielen S, Erbs S, Moebius-Winkler S, Schuler G. Elevated serum levels of leptin and soluble leptin receptor in patients with advanced chronic heart failure. European journal of heart failure. 2003;5:33–40. doi: 10.1016/s1388-9842(02)00177-0. [DOI] [PubMed] [Google Scholar]
- 165.Bobbert P, Jenke A, Bobbert T, Kuhl U, Rauch U, Lassner D, Scheibenbogen C, Poller W, Schultheiss HP, Skurk C. High leptin and resistin expression in chronic heart failure: Adverse outcome in patients with dilated and inflammatory cardiomyopathy. European journal of heart failure. 2012 doi: 10.1093/eurjhf/hfs111. [DOI] [PubMed] [Google Scholar]
- 166.McGaffin KR, Moravec CS, McTiernan CF. Leptin signaling in the failing and mechanically unloaded human heart. Circulation. Heart failure. 2009;2:676–683. doi: 10.1161/CIRCHEARTFAILURE.109.869909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sharma S, Adrogue JV, Golfman L, Uray I, Lemm J, Youker K, Noon GP, Frazier OH, Taegtmeyer H. Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. FASEB J. 2004;18:1692–1700. doi: 10.1096/fj.04-2263com. [DOI] [PubMed] [Google Scholar]
- 168.Chokshi A, Drosatos K, Cheema FH, Ji R, Khawaja T, Yu S, Kato T, Khan R, Takayama H, Knoll R, Milting H, Chung CS, Jorde U, Naka Y, Mancini DM, Goldberg IJ, Schulze PC. Ventricular assist device implantation corrects myocardial lipotoxicity, reverses insulin resistance, and normalizes cardiac metabolism in patients with advanced heart failure. Circulation. 2012;125:2844–2853. doi: 10.1161/CIRCULATIONAHA.111.060889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Stamou SC, Nussbaum M, Stiegel RM, Reames MK, Skipper ER, Robicsek F, Lobdell KW. Effect of body mass index on outcomes after cardiac surgery: Is there an obesity paradox? The Annals of thoracic surgery. 2011;91:42–47. doi: 10.1016/j.athoracsur.2010.08.047. [DOI] [PubMed] [Google Scholar]
- 170.Lancefield T, Clark DJ, Andrianopoulos N, Brennan AL, Reid CM, Johns J, Freeman M, Charter K, Duffy SJ, Ajani AE, Proietto J, Farouque O. Is there an obesity paradox after percutaneous coronary intervention in the contemporary era? An analysis from a multicenter australian registry. JACC. Cardiovascular interventions. 2010;3:660–668. doi: 10.1016/j.jcin.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 171.Hastie CE, Padmanabhan S, Slack R, Pell AC, Oldroyd KG, Flapan AD, Jennings KP, Irving J, Eteiba H, Dominiczak AF, Pell JP. Obesity paradox in a cohort of 4880 consecutive patients undergoing percutaneous coronary intervention. Eur Heart J. 2010;31:222–226. doi: 10.1093/eurheartj/ehp317. [DOI] [PubMed] [Google Scholar]
- 172.Bucholz EM, Rathore SS, Reid KJ, Jones PG, Chan PS, Rich MW, Spertus JA, Krumholz HM. Body mass index and mortality in acute myocardial infarction patients. Am J Med. 2012;125:796–803. doi: 10.1016/j.amjmed.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Angeras O, Albertsson P, Karason K, Ramunddal T, Matejka G, James S, Lagerqvist B, Rosengren A, Omerovic E. Evidence for obesity paradox in patients with acute coronary syndromes: A report from the swedish coronary angiography and angioplasty registry. Eur Heart J. 2012 doi: 10.1093/eurheartj/ehs217. [DOI] [PubMed] [Google Scholar]
- 174.Vemmos K, Ntaios G, Spengos K, Savvari P, Vemmou A, Pappa T, Manios E, Georgiopoulos G, Alevizaki M. Association between obesity and mortality after acute first-ever stroke: The obesity-stroke paradox. Stroke. 2011;42:30–36. doi: 10.1161/STROKEAHA.110.593434. [DOI] [PubMed] [Google Scholar]
- 175.Ovbiagele B, Bath PM, Cotton D, Vinisko R, Diener HC. Obesity and recurrent vascular risk after a recent ischemic stroke. Stroke. 2011;42:3397–3402. doi: 10.1161/STROKEAHA.111.624957. [DOI] [PubMed] [Google Scholar]
- 176.Sohn MW, Budiman-Mak E, Oh EH, Park MS, Stuck RM, Stone NJ, Pearce WB. Obesity paradox in amputation risk among nonelderly diabetic men. Obesity (Silver Spring) 2012;20:460–462. doi: 10.1038/oby.2011.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Diercks DB, Roe MT, Mulgund J, Pollack CV, Jr., Kirk JD, Gibler WB, Ohman EM, Smith SC, Jr., Boden WE, Peterson ED. The obesity paradox in non-st-segment elevation acute coronary syndromes: Results from the can rapid risk stratification of unstable angina patients suppress adverse outcomes with early implementation of the american college of cardiology/american heart association guidelines quality improvement initiative. American heart journal. 2006;152:140–148. doi: 10.1016/j.ahj.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 178.Mehta RH, Califf RM, Garg J, White HD, Van de Werf F, Armstrong PW, Pieper KS, Topol EJ, Granger CB. The impact of anthropomorphic indices on clinical outcomes in patients with acute st-elevation myocardial infarction. Eur Heart J. 2007;28:415–424. doi: 10.1093/eurheartj/ehl329. [DOI] [PubMed] [Google Scholar]
- 179.Lavie CJ, Milani RV, Ventura HO. Obesity and the “obesity paradox” in cardiovascular diseases. Clin Pharmacol Ther. 2011;90:23–25. doi: 10.1038/clpt.2011.87. [DOI] [PubMed] [Google Scholar]
- 180.Berglund ED, Vianna CR, Donato J, Jr., Kim MH, Chuang JC, Lee CE, Lauzon DA, Lin P, Brule LJ, Scott MM, Coppari R, Elmquist JK. Direct leptin action on pomc neurons regulates glucose homeostasis and hepatic insulin sensitivity in mice. J Clin Invest. 2012;122:1000–1009. doi: 10.1172/JCI59816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Liu L, Shi X, Bharadwaj KG, Ikeda S, Yamashita H, Yagyu H, Schaffer JE, Yu YH, Goldberg IJ. Dgat1 expression increases heart triglyceride content but ameliorates lipotoxicity. J Biol Chem. 2009;284:36312–36323. doi: 10.1074/jbc.M109.049817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Son NH, Yu S, Tuinei J, Arai K, Hamai H, Homma S, Shulman GI, Abel ED, Goldberg IJ. Ppargamma-induced cardiolipotoxicity in mice is ameliorated by pparalpha deficiency despite increases in fatty acid oxidation. J Clin Invest. 2010;120:3443–3454. doi: 10.1172/JCI40905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Turer AT. Using metabolomics to assess myocardial metabolism and energetics in heart failure. J Mol Cell Cardiol. 2012 doi: 10.1016/j.yjmcc.2012.08.025. [DOI] [PubMed] [Google Scholar]