Abstract
BACKGROUND
Although the incidence of cannabis abuse/dependence in Americans is rising, the neurobiology of cannabis addiction is not well understood. Imaging studies have demonstrated deficits in striatal D2/D3 receptor availability in several substance-dependent populations. However, this has not been studied in currently-using chronic cannabis users.
OBJECTIVE
The purpose of this study was to compare striatal D2/D3 receptor availability between currently-using chronic cannabis users and healthy controls.
METHODS
Eighteen right-handed males age 18–34 were studied. Ten subjects were chronic cannabis users; eight were demographically matched controls. Subjects underwent a [11C] raclopride (RAC) PET scan. Striatal RAC binding potential (BPND) was calculated on a voxel-wise basis. Prior to scanning, urine samples were obtained from cannabis users for quantification of urine Δ-9-tetrahydrocannabinol (THC) and THC metabolites (11-nor-Δ-9-THC-9-carboxylic acid; THC-COOH and 11-hydroxy-THC;OH-THC).
Results
There were no differences in D2/D3 receptor availability between cannabis users and controls. Voxel-wise analyses revealed that RAC BPND values were negatively associated with both urine levels of cannabis metabolites and self-report of recent cannabis consumption.
CONCLUSIONS
In this sample, current cannabis use was not associated with deficits in striatal D2/D3 receptor availability. There was an inverse relationship between chronic cannabis use and striatal RAC BPND. Additional studies are needed to identify the neurochemical consequences of chronic cannabis use on the dopamine system.
Keywords: dopamine, raclopride, positron emission tomography, cannabis, marijuana, D2 receptor
1 INTRODUCTION
Marijuana (Cannabis sativa) is one of the most commonly abused illicit drugs in the United States. Over 106 million people age 12 and above (42%) have reported using cannabis at least once. Although the addictive liability of cannabis is a source of debate, cannabis dependence remains a serious health concern (Clapper et al., 2009): over 1,000,000 Americans received treatment for cannabis abuse or dependence within the past year (SAMHSA, 2010). The large number of Americans at risk for cannabis abuse and dependence necessitates a better understanding of the neurobiology of cannabis use disorders.
The main psychoactive component of cannabis, Δ9-tetrahydrocannabinol (THC), exerts its effects via binding the cannabinoid type 1 (CB1) receptor (Devane et al., 1988; Herkenham et al., 1991, 1990; Mailleux and Vanderhaeghen, 1992). CB1 receptors are expressed throughout the brain, with high densities in the cortex, hippocampus, cerebellum, and striatum. This heterogeneous distribution of CB1 has been confirmed in both humans and non-human primates (Eggan and Lewis, 2007). The role of the striatum in cannabis use is of particular interest, as this structure is often involved in multiple cognitive processes that subserve addiction. The striatum is heavily innervated by midbrain dopamine (DA) neurons, and striatal dopaminergic neurotransmission is believed to mediate both the development and maintenance of addictions (for review see Robinson and Berridge, 2001, 2003).
There is a growing body of in vivo evidence that suggests striatal DA receptors may be altered in human addicts. PET and SPECT imaging studies have documented deficits in striatal D2/D3 receptor availability in several populations of abstinent and/or detoxified substance-dependent individuals, including users of cocaine (Martinez et al., 2009; Volkow et al., 1997), methamphetamine (Volkow et al., 2001), opiates (Wang et al., 1997), and alcohol [(Hietala et al., 1994; Martinez et al., 2005; Volkow et al., 1996, 2002), although see (Guardia et al., 2000; Repo et al., 1999)]. Interestingly, this phenomenon has not been demonstrated in cannabis users. Three studies investigating striatal D2/D3 receptor availability in subjects with a history of cannabis use found negligible differences between cannabis users and controls (Sevy et al., 2008; Stokes et al., 2011; Urban et al., 2012). However, these studies were conducted in subjects that had been abstinent from cannabis for an average of 15 weeks (Sevy et al., 2008), 18 months (Stokes et al., 2011), and 4 weeks (Urban et al., 2012). There is evidence to suggest that reduced D2/D3 receptor availability in addicts may recover after extended periods of abstinence (Volkow et al., 2002), although the rate of recovery is highly variable between individuals (Nader et al., 2006).
In order to better understand the role of DA in cannabis dependence, it is crucial to study individuals who are current heavy cannabis users. To date, no one has examined striatal D2/D3 binding in currently-using chronic cannabis users. Here, we used PET and [11C]raclopride (RAC), a D2/D3 antagonist, to compare striatal D2/D3 availability in currently-using chronic cannabis users and age-matched healthy controls. We hypothesized that RAC binding availability would be lower in chronic cannabis smokers relative to controls.
2. METHODS
All study procedures were approved by the Indiana University Institutional Review Board. Subjects were recruited by local advertising in the greater metropolitan Indianapolis area. All subjects signed an informed consent statement. Eighteen right-handed males completed the study. Participants in the cannabis group (CAN; n = 10) were chronic cannabis users, defined by consumption of at least one “joint” per week (or equivalent) in the last month and a positive result for THC on a urine toxicology screen (Skosnik et al., 2008a, 2006, 2008b). Control subjects (CON; n = 8) were non-cannabis smoking males with negative urine toxicology screens. Groups were matched for age and race. Subjects underwent a screening interview that included: the Structured Clinical Diagnostic Interview for DSM-IV disorders (SCID) I and II, and the Edinburgh handedness inventory (Oldfield, 1971). Patterns of alcohol and substance use were ascertained using the SCID I module E for Substance Use Disorders. Exclusion criteria were: history of any neurological disorder, current use of medications with CNS effects, consumption of > 14 alcoholic beverages per week, contraindication for magnetic resonance imaging (MRI), use of any illicit substance during the past three months (except cannabis in CAN subjects), positive urine toxicology screen (other than cannabis in CAN subjects), and DSM-IV diagnosis of an Axis I or II psychiatric disorder (other than nicotine abuse or dependence in any subject, and cannabis abuse or dependence in CAN subjects). History of illicit substance abuse or dependence (other than cannabis in CAN) was exclusionary for all subjects.
2.1. General Study Procedures
On a day subsequent to the screening visit, qualified subjects received a structural MRI and one [11C]raclopride PET scan. Before scanning, subjects reported recent substance use-patterns using an internally developed drug-use questionnaire. All subjects submitted a urine sample for drug toxicology screening. Urine toxicology screens (Q10-1, Proxam) were administered prior to scanning to corroborate self-report and clinical interview. For quantitative cannabinoid analysis, urine samples from CAN subjects were submitted to The Center for Human Toxicology at the University of Utah for quantification of Δ9-tetrahydrocannabinol (THC), 11-nor-Δ9-tetrahydrocannabinol-9-carboxylic acid (THC-COOH), 11-hydroxy-Δ9-tetrahydrocannabinol (OH-THC), and creatinine. CAN subjects were instructed to refrain from smoking cannabis the morning before the scan to help ensure they would not be intoxicated at the time of scanning.
2.2. Image Acquisition
A magnetized prepared rapid gradient echo (MP-RAGE) magnetic resonance image (MNI) was acquired on all subjects using a Siemens 3T Trio for anatomic co-registration of PET data. RAC was synthesized as reported previously (Fei et al., 2004). RAC PET scans were acquired on an ECAT HR+ (3D mode; septa retracted). Prior to each PET scan, a 10-min transmission scan using three internal rod sources was acquired for attenuation correction. RAC PET scans were initiated with an IV infusion of 544.39 ± 38.7 MBq RAC over the course of 1.5 minutes. Injected mass was 0.17 ± 0.08 nmol/kg. Dynamic data acquisition lasted 50 minutes.
During scanning, CAN subjects responded to statements designed to assess cannabis craving. These included: “I want to smoke cannabis right now”; “I have an urge to smoke cannabis right now”; “It would be great to use cannabis right now”; “Nothing would be better than smoking cannabis right now.” Responses were given on a Likert-like scale, anchored by 1 (strongly disagree) and 7 (strongly agree). The area under the curve (AUC) for responses to each of the cannabis craving statements was calculated using the trapezoidal rule. The average AUC value across all 4 statements was used as an overall craving metric.
2.3. Image Processing
Image processing is similar to that described previously (Yoder et al., 2011, 2012). MRI DICOM and RAC PET images were converted to Neuroimaging Informatics Technology Initiative (NIfTI) format (http://nifti.nimh.nih.gov/) and processed with SPM5 (http://www.fil.ion.ucl.ac.uk/spm/). For each subject, an early-time mean PET image was co-registered to the MRI scan using the normalized mutual information algorithm in SPM5. All dynamic PET data were co-registered to the early-time mean PET image (in native MR space) to facilitate motion correction. Each subject’s MRI was spatially normalized to Montreal Neurological Institute (MNI) space, and this transformation matrix was then applied to the motion-corrected, MRI-registered PET data from each subject.
2.4. Region of Interest Analysis
Regions of interest (ROIs) were drawn on each subjects’ normalized MRI using MRIcron (http://www.cabiatl.com/mricro/mricron/). Striatal ROIs consisted of the left and right ventral striatum (VST), pre- and postcommissural dorsal caudate (pre-/post-DCA), and pre- and postcommissural dorsal (pre/post-DPU) and were drawn according to specific anatomic landmarks (Martinez et al., 2003). For the reference region (tissue that contains little to no D2/D3 receptor density), an ROI was created that contained all cerebellar gray matter except for the vermis. Cerebellar ROIs were created for each subject by tracing the cerebellum on individual gray matter maps obtained with the segmentation algorithm in SPM5. Time-activity curves for all ROIs were generated from the dynamic RAC data using the MarsBaR toolbox for SPM5 (http://marsbar.sourceforge.net/). For each striatal ROI, D2/D3 receptor availability was indexed with BPND, the binding potential of RAC calculated as bound tracer concentration relative to nondisplaceable tracer concentration (Innis et al., 2007). Estimations of BPND were conducted using the multilinear reference tissue method model (MRTM2; Ichise et al., 2003).
2.5. Voxel-wise Analysis
BPND was estimated at each brain voxel using the multilinear reference tissue method with a common reference region efflux rate to facilitate robust performance on noisy voxel data (MRTM2; Ichise et al., 2003). The resulting parametric BPND images were smoothed with an 8mm Gaussian kernel (Costes et al., 2005; Picard et al., 2006; Ziolko et al., 2006). The search area for the voxel-wise paired t-tests was restricted to the striatum, as (1) our sole focus was the striatum, and (2) the striatum has the highest density of D2/D3 receptors in the brain, and is the only brain structure with high enough signal-to-noise ratio to support quantification of D2/D3 receptor availability with RAC. A bilateral striatal restriction mask was created by tracing the anatomical boundaries of the striatum on an averaged normalized MRI across all subjects.
2.6. Urinalysis
THC and THC-COOH
The samples were initially analyzed for THC and THC-COOH by gas chromatography-mass spectrometry (GC-MS) using extraction and GC-MS conditions described previously (Foltz et al., 1983; Huang et al., 2001). The assay had an analytical range of 0.5 to 100 ng/mL with 1.0 mL aliquots. To ensure measurement of both analytes, the urine samples were analyzed for THC on a 1-0-mL aliquot and THC-COOH on a 0.1-mL aliquot. For THC, the aliquots were pretreated with β-glucuronidase for 18 hours at 37°C. For THC-COOH, the samples were prepared under basic conditions in order to free THC-COOH from its glucuronide conjugate. Duplicate calibrators (1.0 mL with both THC-COOH and THC) were at 0.5, 1.0, 2.5, 5, 10, 25, 50 and 100 ng/mL. Duplicate 1.0-mL (with both THC-COOH and THC) quality control samples (QCs) were included at 1.5, 10 and 80 ng/mL. Triplicate 0.1 mL dilution QCs were included at 200 ng/mL. Samples were extracted by a liquid-liquid procedure, derivatized with hexafluoroisopropanol/trifluoroacetic anhydride, and analyzed by GC-MS.
Subsequently, the method was improved by using gas chromatography-tandem mass spectrometry (GC-MS/MS) with addition of 11-hydroxy-Δ9-tetrahydrocannabinol (OH-THC) to the assay. This assay had a quantitative range of 0.1 to 100 ng/mL with a 1.0-mL aliquot. All samples were reanalyzed to determine OH-THC with the β-glucuronidase pretreatment using the above methods. Samples with THC or THC-COOH results less than the lower limit of quantitation in the initial analysis were reanalyzed.
Creatinine
Creatinine was determined using a microplate colormetric test based on the Jaffe reaction where picric acid reacts with creatinine to form a colored product. Samples were diluted 10-fold (0.050 mL plus 0.450 mL water). Duplicate creatinine calibrators were run at 2, 4, 6, 8, 10, 12 and 15 mg/dL. Due to sample dilution, the calibration range was 20 to 150 mg/dL. Triplicate diluted low and high QCs were included. Samples outside the calibration range were repeated using a smaller or larger dilution as needed.
THC, THC-COOH, and OH-THC concentrations were normalized by creatinine levels to account for differing levels of urine dilution across subjects (THC/Cr, THC-COOH/Cr, and OH-THC/Cr respectively).
2.7. Statistical Analysis
Independent t-tests were used to test for differences between CAN and CON in demographic variables, substance abuse metrics, PAS, and SPQ scores, and RAC BPND. Group differences in BPND were assessed with ROI and voxel-wise analyses. Pearson’s correlation coefficient was used to screen variables for associations with striatal ROI BPND. Multiple linear regression models in SPM5 were used to test for correlations on a voxel-wise basis. SPM5 was used for voxel-wise analysis, statistical threshold was set at p < 0.05. All other statistical procedures were performed in SPSS 19.0 (SPSS, Chicago, Illinois, USA).
3. RESULTS
3.1. Subject Data
The demographic and substance abuse characteristics of subjects are shown in Table 1. CAN and CON subjects were not significantly different in any of the indices. Groups were well-matched for race, ethnicity, and use of alcohol, tobacco, and caffeine. There were no significant differences between injected radioactivity or injected mass between groups (p > 0.1).
Table 1.
Subject demographics and drug-use characteristics. THC use is defined as a one-time session of THC intoxication
| CON (n = 8) | CAN (n = 10) | p | |
|---|---|---|---|
| Age | 26.4 ± 5.6 | 25.1 ± 4.6 | n.s. |
| Race | 7C, 1AA | 6C, 3AA, 1I | n.s. |
| Ethnicity | 8 NHL | 10 NHL | n.s. |
| Education | 14.6 ± 1.3 | 14.0 ± 1.8 | n.s. |
| Recent THC use/wk | N/A | 12.7 ± 12 | |
| Recent THC use/month | N/A | 46.6 ± 42 | |
| Years of THC use | N/A | 8.8 ± 5 | |
| Hours since last THC use | N/A | 20.6 ± 8.3 | |
| Tobacco users | 2 | 5 | n.s. |
| Caffeine users | 5 | 6 | n.s. |
| Recent EtOH use | 2.94 ± 2.0 | 3.93 ± 3.7 | n.s. |
| Premorbid IQ | 112.3 ± 6.9 | 110.2 ± 4.4 | n.s. |
| Prior Drug Use: (lifetime drug use sessions) | |||
| THC | 36.1 ± 71.7 | 2571.4 ± 2490.5 | 0.01 |
| Sedatives | 0 | 0.65 ± 1.7 | n.s. |
| MDMA | 0 | 1.20 ± 3.1 | n.s. |
| Stimulants | 0 | 0.20 ± 0.4 | n.s. |
| Cocaine | 0.63 ± 1.8 | 4.20 ± 6.3 | n.s. |
| Opiates | 0 | 0.10 ± 0.3 | n.s. |
| Hallucinogens | 1.31 ± 2.7 | 1.20 ± 1.6 | n.s. |
Data are mean ± s.d.
CON: healthy controls; CAN: currently using chronic cannabis users; C: Caucasian; AA: African American; I: Asian-Indian American; NHL: Non-Hispanic Latino: N/A: not applicable. Recent EtOH use is average drinks per week in the past month.
3.2. Urine THC and THC Metabolite Corroborate Self-Report of Cannabis Consumption
THC/Cr, THC-COOH/Cr, and OH-THC/Cr levels were correlated with self-reported recent cannabis use. One subject was excluded from this analysis because of inconsistent self-report data. Significant positive correlations existed between: intake per day and THC-COOH/Cr (r = 0.884, p = 0.002), intake per day and THC/Cr (r = 0.738, p = 0.023), intake per week and THC-COOH/Cr (r = 0.726, p = 0.027), and intake per month and THC-COOH/Cr (r = 0.676, p = 0.045). There was a trend-level association between intake per day and OH-THC/Cr (r = 0.647, p = 0.059). There were no significant correlations between THC/Cr, THC-COOH/Cr, or OH-THC/Cr and cannabis craving during PET scanning.
3.3. Striatal D2/D3 Availability
3.3.1. CAN vs. CON
There were no significant between group differences in RAC BPND detected by voxel-wise analysis. Similarly, no group differences were found for any of the 10 striatal ROIs assessed (p > 0.4) (Table 2).
Table 2.
Region of interest analysis: comparison of striatal binding potential between chronic cannabis users (CAN) and healthy controls (CON). Groups are matched for cigarette smoking status
| [11C]RACLOPRIDE RECEPTOR AVAILABILITY (BPND)
| |||
|---|---|---|---|
| Region | CON (n = 8) | CAN (n = 10) | p |
|
| |||
| L pre-DCA | 2.37 ± 0.29 | 2.30 ± 0.32 | 0.63 |
| R pre-DCA | 2.34 ± 0.30 | 2.23 ± 0.29 | 0.44 |
|
| |||
| L post-DCA | 1.51 ± 0.27 | 1.59 ± 0.28 | 0.56 |
| R post-DCA | 1.55 ± 0.33 | 1.64 ± 0.19 | 0.52 |
|
| |||
| L pre-DPU | 3.08 ± 0.32 | 2.92 ± 0.29 | 0.28 |
| R pre-DPU | 3.04 ± 0.30 | 2.94 ± 0.22 | 0.44 |
|
| |||
| L post-DPU | 3.11 ± 0.31 | 2.98 ± 0.32 | 0.40 |
| R post-DPU | 3.00 ± 0.33 | 2.92 ± 0.32 | 0.63 |
|
| |||
| L VST | 2.52 ± 0.29 | 2.47 ± 0.33 | 0.78 |
| R VST | 2.29 ± 0.25 | 2.35 ± 0.23 | 0.58 |
Left/right = L/R, pre/post-commissural = pre/post, dorsal caudate = DCA, dorsal putamen = DPU, ventral striatum = VST.
3.3.2. Correlation with Recent Cannabis Consumption
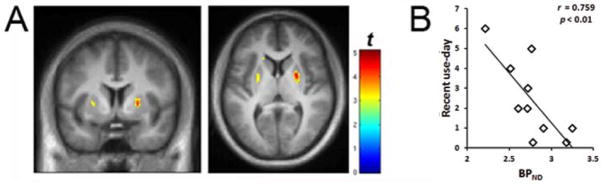
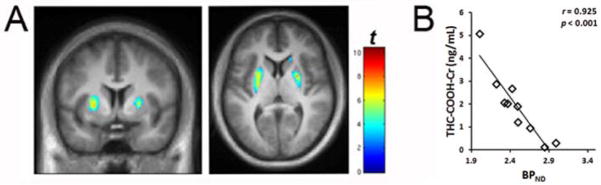
Voxel-wise analysis revealed that RAC BPND was negatively associated with both urine levels of THC-COOH (Figure 1) and self-reported recent intake per day (Figure 2). Similar correlations were found between BPND and THC/Cr, OH-THC/Cr, recent intake per week, and recent intake per month (data not shown).
Fig. 1.
A. Voxel-wise correlations between urine THC-COOH/Cr with RAC BPND in cannabis users (n = 10). The “rainbow” colorscale indicates voxels where BPND is correlated with THC-COOH/Cr. B. Linear relationship between BPND and urine THC-COOH levels. Average BPND value was determined for each subject by extracting BPND values with a region of interest defined by the significant voxels from the SPM result (shown in 1A). Display threshold is p < 0.01. MNI coordinates are: axial: 6; coronal: 24.
Fig. 2.
A. Voxel-wise correlations between self-reported average intake per day and RAC BPND in cannabis users (n = 9). The “rainbow” colorscale indicates voxels where BPND is correlated with average use per day. B. Linear relationship between BPND values and recent cannabis use per day. Average BPND value was determined for each subject by extracting BPND values with a region of interest defined by the significant voxels from the SPM result (shown in 2A). Display threshold is p < 0.01. MNI coordinates are: axial: 6; coronal: 24.
4. DISCUSSION
The present work is the first to demonstrate an association between the magnitude of recent cannabis consumption and striatal D2/D3 receptor availability. RAC BPND was strongly negatively correlated with both urine THC-COOH and self-reported recent intake per day. We did not find the expected differences in striatal D2/D3 receptor availability between cannabis users and controls, similar to what has been reported previously (Sevy et al., 2008; Stokes et al., 2011; Urban et al., 2012).
The inverse correlation between recent cannabis consumption (as confirmed by urine THC metabolite levels) and D2/D3 receptor availability could be interpreted as a direct effect of cannabis smoking via lower expression of striatal DA receptors, or increased basal DA concentration. There is evidence that suggests that heavy cannabis use results in inhibition of MAO activity (Schurr and Rigor, 1984; Stillman et al., 1978), and thus a higher striatal DA tone (Kaseda et al., 1999; Lakshmana et al., 1998; Lamensdorf et al., 1996). Alternatively, activation of CB1 receptors may also result in higher striatal DA concentration (Chen et al., 1990; Fadda et al., 2006; Tanda et al., 1997). We must consider the possibility that, in this study, residual THC from the most recent smoking session increased striatal DA levels; however, several lines of evidence suggest otherwise. Human imaging studies have attempted to demonstrate THC-induced DA release, with inconclusive results. One study reported a small (3%) increase in striatal DA after inhaled THC (Bossong et al., 2009), while two other groups detected no increases in striatal DA after either oral (Stokes et al., 2009) or IV-delivered THC (Barkus et al., 2011). Additionally, in the present work, it is unlikely that brain levels of THC or psychoactive metabolite were sufficient to induce measurable DA release. In a recently described pig model that closely mimics the kinetic profile of THC in humans, the concentration of a dose of IV-administered THC was greatly reduced in the brain after six hours, and completely absent after 24 hours (Brunet et al., 2006). Given that subjects in the present study had abstained from smoking an average of 20.6 hours prior to scanning, it is likely that brain levels of THC were negligible.
It is also possible that the relationship between cannabis consumption and striatal D2/D3 receptor availability is a result of lower D2/D3 receptor numbers in heavy cannabis users. Interestingly, evidence from studies of CB1 receptors supports this interpretation. CB1 receptors are co-localized with D2 receptors in the striatum (Hermann et al., 2002; Mailleux and Vanderhaeghen, 1992; Pickel et al., 2006; Wenger et al., 2003) and D2 receptors and CB1 receptors form heterodimeric receptor complexes (Kearn et al., 2005). A postmortem study showed that long-term cannabis users possess a marked reduction in the density of CB1 in human brain (Villares, 2007). Additionally, it has been demonstrated that chronic cannabis users exhibit motor learning deficits similar to those observed in CB1 knockout mice, suggesting that long-term cannabis exposure induces robust downregulation and/or desensitization of CB1 receptors (Skosnik et al., 2008a). This has recently been shown in vivo in humans using the CB1 tracer [18F]FMPEP-d2. Hirvonen et al. (2011) demonstrated CB1 downregulation in chronic cannabis users, which correlated with total years of cannabis exposure. CB1 availability returned to normal levels after four weeks of monitored abstinence. Taken together, the data from the literature indirectly suggest that chronic exposure to cannabis may lead to downregulation of striatal D2 receptors. However, in the present study, we did not find differences in D2/D3 receptor availability between controls and chronic cannabis users, suggesting that chronic cannabis exposure alone is not associated with reduced D2 receptor levels.
The present study has several limitations. The sample size is relatively small, and thus presents a risk of both Type I and Type II errors. However, our data are consistent with those from Sevy et al. (2008), Stokes et al. (2011), and Urban et al. (2012), which reported that striatal D2/D3 receptor availability is not different in individuals with a history of cannabis abuse compared to controls. Finally, although use of any illicit substance within the last three months prior to scanning was an exclusion criterion, both cannabis users and controls had previous experiences with other drugs. Thus, we cannot preclude the possibility that prior use of other illicit substances confounded our data. However, qualitative examination of the data did not indicate that subjects with previous drug experience were outliers with respect to BPND.
In conclusion, the primary finding of the current study is that current cannabis use is not associated with a reduction in striatal DA receptor availability relative to controls. We also found that recent cannabis use is negatively correlated with striatal D2/D3 availability. Future studies are needed to better understand the neurochemical basis of this finding.
Acknowledgments
Role of Funding Source
Supported by funding to KKY from the IUSM Department of Radiology and Imaging Sciences. Supplementary funding provided by NIDA 1R21DA023097-01A1 (PDS) and the Brain and Behavior Research Foundation (NARSAD; PDS). Sponsors had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
The authors would like to thank Dr. David E. Moody and Dr. David Andrenyak for performing the THC, OH-THC, and THC-COOH analysis, which was supported by NIDA contract N01DA-9-7767 to PDS.
Footnotes
Contributors
K. Yoder and P. Skosnik designed the study and wrote the protocol. K. Yoder secured all necessary regulatory approvals, and supervised all stages of the study, including subject recruitment, image data acquisition, image processing, data analysis, statistics, data interpretation, and manuscript preparation. P. Skosnik provided resources for the urine metabolite analysis, provided training and materials for subject interviews, assisted with recruitment and study day procedures, and contributed editorial input to the final draft of the manuscript. D. Albrecht managed all stages of the study (subject recruitment, image data acquisition, image processing, data analysis, data interpretation, literature search for discussion), and wrote the first draft of the manuscript. J. Vollmer and M. Brumbaugh assisted with data acquisition and subject recruitment. L. Federici, E. Patton, M. Brumbaugh, and C. Herring participated in subject recruitment. K. Perry was the study CNMT. B. Mock and Q. Zheng synthesized the [11C] raclopride used in the study. All authors contributed to and have approved the final manuscript.
Conflict of Interest
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barkus E, Morrison PD, Vuletic D, Dickson JC, Ell PJ, Pilowsky LS, Brenneisen R, Holt DW, Powell J, Kapur S, Murray RM. Does intravenous {Delta}9-tetrahydrocannabinol increase dopamine release? A SPET study. J Psychopharmacol. 2011;25:1462–1468. doi: 10.1177/0269881110382465. [DOI] [PubMed] [Google Scholar]
- Bossong MG, van Berckel BN, Boellaard R, Zuurman L, Schuit RC, Windhorst AD, van Gerven JM, Ramsey NF, Lammertsma AA, Kahn RS. Delta 9-tetrahydrocannabinol induces dopamine release in the human striatum. Neuropsychopharmacology. 2009;34:759–766. doi: 10.1038/npp.2008.138. [DOI] [PubMed] [Google Scholar]
- Brunet B, Doucet C, Venisse N, Hauet T, Hebrard W, Papet Y, Mauco G, Mura P. Validation of large white pig as an animal model for the study of cannabinoids metabolism: application to the study of THC distribution in tissues. Forensic Sci Int. 2006;161:169–174. doi: 10.1016/j.forsciint.2006.04.018. [DOI] [PubMed] [Google Scholar]
- Chen JP, Paredes W, Li J, Smith D, Lowinson J, Gardner EL. Delta 9-tetrahydrocannabinol produces naloxone-blockable enhancement of presynaptic basal dopamine efflux in nucleus accumbens of conscious, freely-moving rats as measured by intracerebral microdialysis. Psychopharmacology (Berl) 1990;102:156–162. doi: 10.1007/BF02245916. [DOI] [PubMed] [Google Scholar]
- Clapper JR, Mangieri RA, Piomelli D. The endocannabinoid system as a target for the treatment of cannabis dependence. Neuropharmacology. 2009;56(Suppl 1):235–243. doi: 10.1016/j.neuropharm.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costes N, Merlet I, Ostrowsky K, Faillenot I, Lavenne F, Zimmer L, Ryvlin P, Le Bars D. A 18F-MPPF PET normative database of 5-HT1A receptor binding in men and women over aging. J Nucl Med. 2005;46:1980–1989. [PubMed] [Google Scholar]
- Devane WA, Dysarz FA, 3rd, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol. 1988;34:605–613. [PubMed] [Google Scholar]
- Eggan SM, Lewis DA. Immunocytochemical distribution of the cannabinoid CB1 receptor in the primate neocortex: a regional and laminar analysis. Cereb Cortex. 2007;17:175–191. doi: 10.1093/cercor/bhj136. [DOI] [PubMed] [Google Scholar]
- Fadda P, Scherma M, Spano MS, Salis P, Melis V, Fattore L, Fratta W. Cannabinoid self-administration increases dopamine release in the nucleus accumbens. Neuroreport. 2006;17:1629–1632. doi: 10.1097/01.wnr.0000236853.40221.8e. [DOI] [PubMed] [Google Scholar]
- Fei X, Mock BH, DeGrado TR, Wang JQ, Glick-Wilson BE, Sullivan ML, Hutchins GD, Zheng QH. An improved synthesis of PET dopamine D2 receptors radioligand [11C]raclopride. Synthetic Commun. 2004;34:1897–1907. [Google Scholar]
- Foltz RL, McGinnis KM, Chinn DM. Quantitative measurement of delta 9-tetrahydrocannabinol and two major metabolites in physiological specimens using capillary column gas chromatography negative ion chemical ionization mass spectrometry. Biomed Mass Spectrom. 1983;10:316–323. doi: 10.1002/bms.1200100503. [DOI] [PubMed] [Google Scholar]
- Guardia J, Catafau AM, Batlle F, Martin JC, Segura L, Gonzalvo B, Prat G, Carrio I, Casas M. Striatal dopaminergic D (2) receptor density measured by [(123)I] iodobenzamide SPECT in the prediction of treatment outcome of alcohol-dependent patients. Am J Psychiatry. 2000;157:127–129. doi: 10.1176/ajp.157.1.127. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, de Costa BR, Richfield EK. Neuronal localization of cannabinoid receptors in the basal ganglia of the rat. Brain Res. 1991;547:267–274. doi: 10.1016/0006-8993(91)90970-7. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, Rice KC. Cannabinoid receptor localization in brain. Proc Natl Acad Sci USA. 1990;87:1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann H, Marsicano G, Lutz B. Coexpression of the cannabinoid receptor type 1 with dopamine and serotonin receptors in distinct neuronal subpopulations of the adult mouse forebrain. Neuroscience. 2002;109:451–460. doi: 10.1016/s0306-4522(01)00509-7. [DOI] [PubMed] [Google Scholar]
- Hietala J, West C, Syvalahti E, Nagren K, Lehikoinen P, Sonninen P, Ruotsalainen U. Striatal D2 dopamine receptor binding characteristics in vivo in patients with alcohol dependence. Psychopharmacology (Berl) 1994;116:285–290. doi: 10.1007/BF02245330. [DOI] [PubMed] [Google Scholar]
- Hirvonen J, Goodwin RS, Li CT, Terry GE, Zoghbi SS, Morse C, Pike VW, Volkow ND, Huestis MA, Innis RB. Reversible and regionally selective downregulation of brain cannabinoid CB(1) receptors in chronic daily cannabis smokers. Mol Psychiatry. 2011;17:642–649. doi: 10.1038/mp.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Moody DE, Andrenyak DM, Smith EK, Foltz RL, Huestis MA, Newton JF. Simultaneous determination of delta9-tetrahydrocannabinol and 11-nor-9-carboxy-delta9-tetrahydrocannabinol in human plasma by solid-phase extraction and gas chromatography-negative ion chemical ionization-mass spectrometry. J Anal Toxicol. 2001;25:531–537. doi: 10.1093/jat/25.7.531. [DOI] [PubMed] [Google Scholar]
- Ichise M, Liow JS, Lu JQ, Takano A, Model K, Toyama H, Suhara T, Suzuki K, Innis RB, Carson RE. Linearized reference tissue parametric imaging methods: application to [11C]DASB positron emission tomography studies of the serotonin transporter in human brain. J Cereb Blood Flow Metab. 2003;23:1096–1112. doi: 10.1097/01.WCB.0000085441.37552.CA. [DOI] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Holden J, Houle S, Huang SC, Ichise M, Iida H, Ito H, Kimura Y, Koeppe RA, Knudsen GM, Knuuti J, Lammertsma AA, Laruelle M, Logan J, Maguire RP, Mintun MA, Morris ED, Parsey R, Price JC, Slifstein M, Sossi V, Suhara T, Votaw JR, Wong DF, Carson RE. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Kaseda S, Nomoto M, Iwata S. Effect of selegiline on dopamine concentration in the striatum of a primate. Brain Res. 1999;815:44–50. doi: 10.1016/s0006-8993(98)01089-0. [DOI] [PubMed] [Google Scholar]
- Kearn CS, Blake-Palmer K, Daniel E, Mackie K, Glass M. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors enhances heterodimer formation: a mechanism for receptor cross-talk? Mol Pharmacol. 2005;67:1697–1704. doi: 10.1124/mol.104.006882. [DOI] [PubMed] [Google Scholar]
- Lakshmana MK, Rao BS, Dhingra NK, Ravikumar R, Govindaiah Sudha S, Meti BL, Raju TR. Role of monoamine oxidase type A and B on the dopamine metabolism in discrete regions of the primate brain. Neurochem Res. 1998;23:1031–1037. doi: 10.1023/a:1020799700885. [DOI] [PubMed] [Google Scholar]
- Lamensdorf I, Youdim MB, Finberg JP. Effect of long-term treatment with selective monoamine oxidase A and B inhibitors on dopamine release from rat striatum in vivo. J Neurochem. 1996;67:1532–1539. doi: 10.1046/j.1471-4159.1996.67041532.x. [DOI] [PubMed] [Google Scholar]
- Mailleux P, Vanderhaeghen JJ. Distribution of neuronal cannabinoid receptor in the adult rat brain: a comparative receptor binding radioautography and in situ hybridization histochemistry. Neuroscience. 1992;48:655–668. doi: 10.1016/0306-4522(92)90409-u. [DOI] [PubMed] [Google Scholar]
- Martinez D, Gil R, Slifstein M, Hwang DR, Huang Y, Perez A, Kegeles L, Talbot P, Evans S, Krystal J, Laruelle M, Abi-Dargham A. Alcohol dependence is associated with blunted dopamine transmission in the ventral striatum. Biol Psychiatry. 2005;58:779–786. doi: 10.1016/j.biopsych.2005.04.044. [DOI] [PubMed] [Google Scholar]
- Martinez D, Greene K, Broft A, Kumar D, Liu F, Narendran R, Slifstein M, Van Heertum R, Kleber HD. Lower level of endogenous dopamine in patients with cocaine dependence: findings from PET imaging of D(2)/D(3) receptors following acute dopamine depletion. Am J Psychiat. 2009;166:1170–1177. doi: 10.1176/appi.ajp.2009.08121801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D, Slifstein M, Broft A, Mawlawi O, Hwang DR, Huang Y, Cooper T, Kegeles L, Zarahn E, Abi-Dargham A, Haber SN, Laruelle M. Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. J Cereb Blood Flow Metab. 2003;23:285–300. doi: 10.1097/01.WCB.0000048520.34839.1A. [DOI] [PubMed] [Google Scholar]
- Nader MA, Morgan D, Gage HD, Nader SH, Calhoun TL, Buchheimer N, Ehrenkaufer R, Mach RH. PET imaging of dopamine D2 receptors during chronic cocaine self-administration in monkeys. Nat Neurosci. 2006;9:1050–1056. doi: 10.1038/nn1737. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Picard F, Bruel D, Servent D, Saba W, Fruchart-Gaillard C, Schollhorn-Peyronneau MA, Roumenov D, Brodtkorb E, Zuberi S, Gambardella A, Steinborn B, Hufnagel A, Valette H, Bottlaender M. Alteration of the in vivo nicotinic receptor density in ADNFLE patients: a PET study. Brain. 2006;129:2047–2060. doi: 10.1093/brain/awl156. [DOI] [PubMed] [Google Scholar]
- Pickel VM, Chan J, Kearn CS, Mackie K. Targeting dopamine D2 and cannabinoid-1 (CB1) receptors in rat nucleus accumbens. J Comp Neurol. 2006;495:299–313. doi: 10.1002/cne.20881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repo E, Kuikka JT, Bergstrom KA, Karhu J, Hiltunen J, Tiihonen J. Dopamine transporter and D2-receptor density in late-onset alcoholism. Psychopharmacology (Berl) 1999;147:314–318. doi: 10.1007/s002130051173. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96:103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- SAMHSA. Results from the 2010 National Survey on Drug Use and Health: National Findings. Office of Applied Studies; Rockville, MD: 2010. [Google Scholar]
- Schurr A, Rigor BM. Cannabis extract, but not delta 1-tetrahydrocannabinol, inhibits human brain and liver monoamine oxidase. Gen Pharmacol. 1984;15:171–174. doi: 10.1016/0306-3623(84)90104-6. [DOI] [PubMed] [Google Scholar]
- Sevy S, Smith GS, Ma Y, Dhawan V, Chaly T, Kingsley PB, Kumra S, Abdelmessih S, Eidelberg D. Cerebral glucose metabolism and D2/D3 receptor availability in young adults with cannabis dependence measured with positron emission tomography. Psychopharmacology (Berl) 2008;197:549–556. doi: 10.1007/s00213-008-1075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skosnik PD, Edwards CR, O’Donnell BF, Steffen A, Steinmetz JE, Hetrick WP. Cannabis use disrupts eyeblink conditioning: evidence for cannabinoid modulation of cerebellar-dependent learning. Neuropsychopharmacology. 2008a;33:1432–1440. doi: 10.1038/sj.npp.1301506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skosnik PD, Krishnan GP, Aydt EE, Kuhlenshmidt HA, O’Donnell BF. Psychophysiological evidence of altered neural synchronization in cannabis use: relationship to schizotypy. Am J Psychiat. 2006;163:1798–1805. doi: 10.1176/ajp.2006.163.10.1798. [DOI] [PubMed] [Google Scholar]
- Skosnik PD, Park S, Dobbs L, Gardner WL. Affect processing and positive syndrome schizotypy in cannabis users. Psychiatry Res. 2008b;157:279–282. doi: 10.1016/j.psychres.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Stillman RC, Wyatt RJ, Murphy DL, Rauscher FP. Low platelet monoamine oxidase activity and chronic marijuana use. Life Sci. 1978;23:1577–1581. doi: 10.1016/0024-3205(78)90585-4. [DOI] [PubMed] [Google Scholar]
- Stokes PR, Egerton A, Watson B, Reid A, Lappin J, Howes OD, Nutt DJ, Lingford-Hughes AR. History of cannabis use is not associated with alterations in striatal dopamine D2/D3 receptor availability. J Psychopharmacol. 2011;26:144–149. doi: 10.1177/0269881111414090. [DOI] [PubMed] [Google Scholar]
- Stokes PR, Mehta MA, Curran HV, Breen G, Grasby PM. Can recreational doses of THC produce significant dopamine release in the human striatum? Neuroimage. 2009;48:186–190. doi: 10.1016/j.neuroimage.2009.06.029. [DOI] [PubMed] [Google Scholar]
- Tanda G, Pontieri FE, Di Chiara G. Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. Science. 1997;276:2048–2050. doi: 10.1126/science.276.5321.2048. [DOI] [PubMed] [Google Scholar]
- Urban NB, Slifstein M, Thompson JL, Xu X, Girgis RR, Raheja S, Haney M, Abi-Dargham A. Dopamine release in chronic cannabis users: a [(11)C]raclopride positron emission tomography study. Biol Psychiatry. 2012;71:677–683. doi: 10.1016/j.biopsych.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villares J. Chronic use of marijuana decreases cannabinoid receptor binding and mRNA expression in the human brain. Neuroscience. 2007;145:323–334. doi: 10.1016/j.neuroscience.2006.11.012. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, Logan J, Franceschi D, Gatley J, Hitzemann R, Gifford A, Wong C, Pappas N. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001;158:2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R, Chen AD, Dewey SL, Pappas N. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature. 1997;386:830–833. doi: 10.1038/386830a0. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Hitzemann R, Ding YS, Pappas N, Shea C, Piscani K. Decreases in dopamine receptors but not in dopamine transporters in alcoholics. Alcohol Clin Exp Res. 1996;20:1594–1598. doi: 10.1111/j.1530-0277.1996.tb05936.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Maynard L, Fowler JS, Jayne B, Telang F, Logan J, Ding YS, Gatley SJ, Hitzemann R, Wong C, Pappas N. Effects of alcohol detoxification on dopamine D2 receptors in alcoholics: a preliminary study. Psychiatry Res. 2002;116:163–172. doi: 10.1016/s0925-4927(02)00087-2. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Fowler JS, Logan J, Abumrad NN, Hitzemann RJ, Pappas NS, Pascani K. Dopamine D2 receptor availability in opiate-dependent subjects before and after naloxone-precipitated withdrawal. Neuropsychopharmacology. 1997;16:174–182. doi: 10.1016/S0893-133X(96)00184-4. [DOI] [PubMed] [Google Scholar]
- Wenger T, Moldrich G, Furst S. Neuromorphological background of cannabis addiction. Brain Res Bull. 2003;61:125–128. doi: 10.1016/s0361-9230(03)00081-9. [DOI] [PubMed] [Google Scholar]
- Yoder KK, Albrecht DS, Kareken DA, Federici LM, Perry KM, Patton EA, Zheng QH, Mock BH, O’Connor S, Herring CM. Test-retest variability of [11C]raclopride-binding potential in nontreatment-seeking alcoholics. Synapse. 2011;65:553–561. doi: 10.1002/syn.20874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder KK, Albrecht DS, Kareken DA, Federici LM, Perry KM, Patton EA, Zheng QH, Mock BH, O’Connor SJ, Herring CM. Reliability of striatal [11C]raclopride binding in smokers wearing transdermal nicotine patches. Eur J Nucl Med Mol Imaging. 2012;39:220–225. doi: 10.1007/s00259-011-1965-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziolko SK, Weissfeld LA, Klunk WE, Mathis CA, Hoge JA, Lopresti BJ, DeKosky ST, Price JC. Evaluation of voxel-based methods for the statistical analysis of PIB PET amyloid imaging studies in Alzheimer’s disease. Neuroimage. 2006;33:94–102. doi: 10.1016/j.neuroimage.2006.05.063. [DOI] [PubMed] [Google Scholar]