Abstract
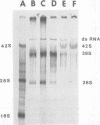
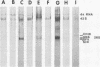
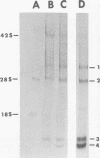
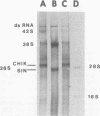
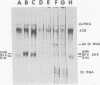
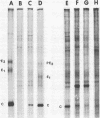
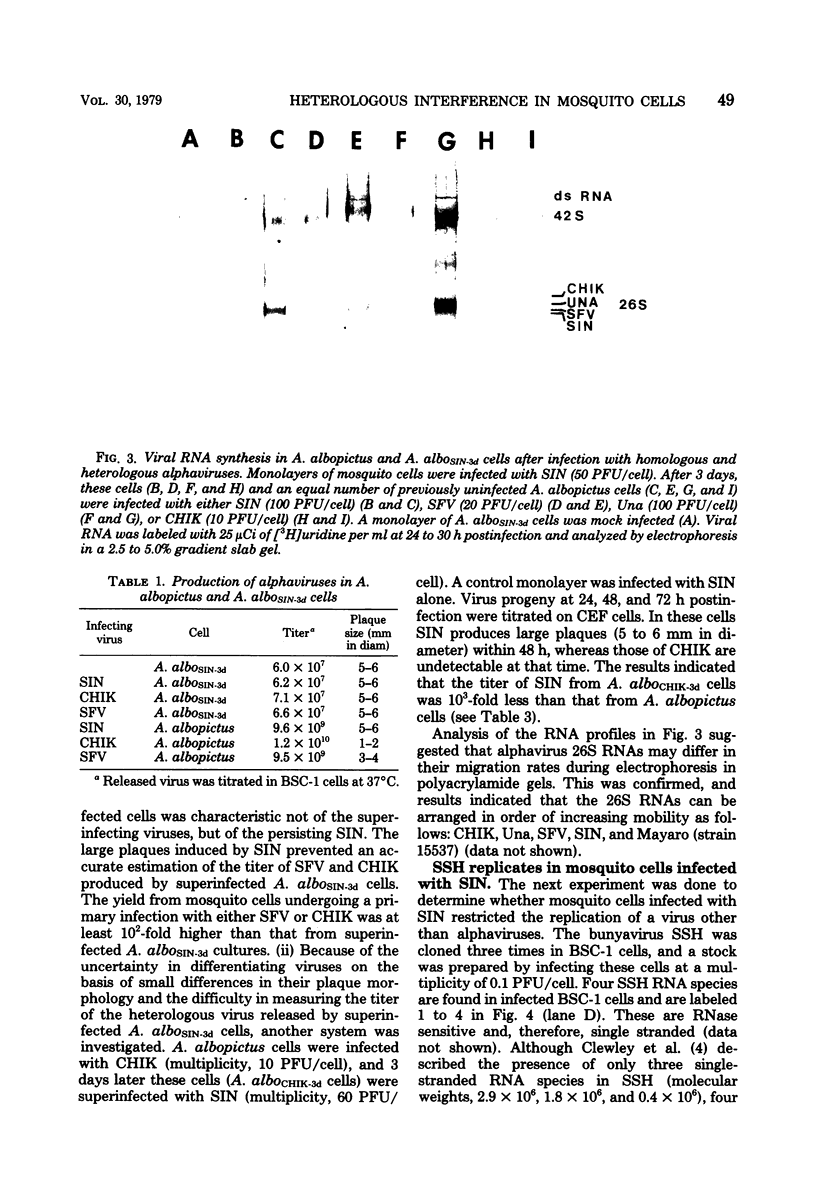
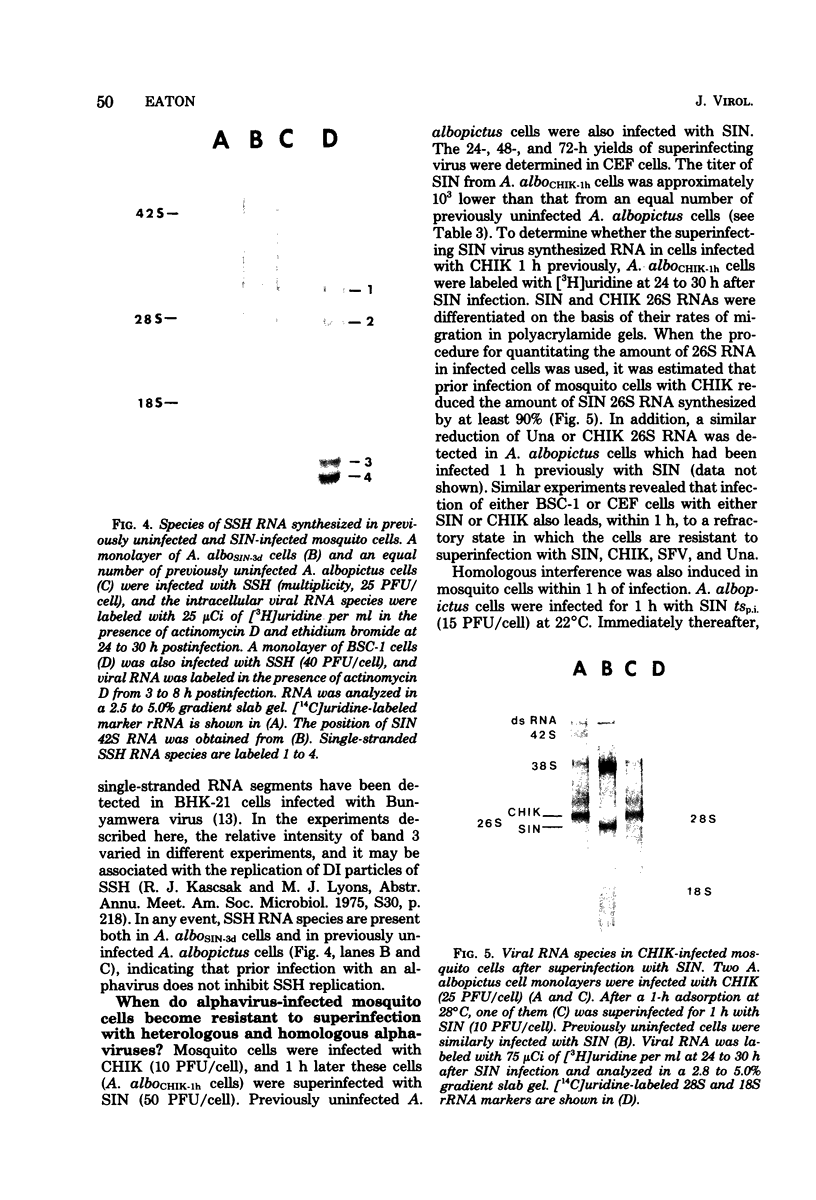
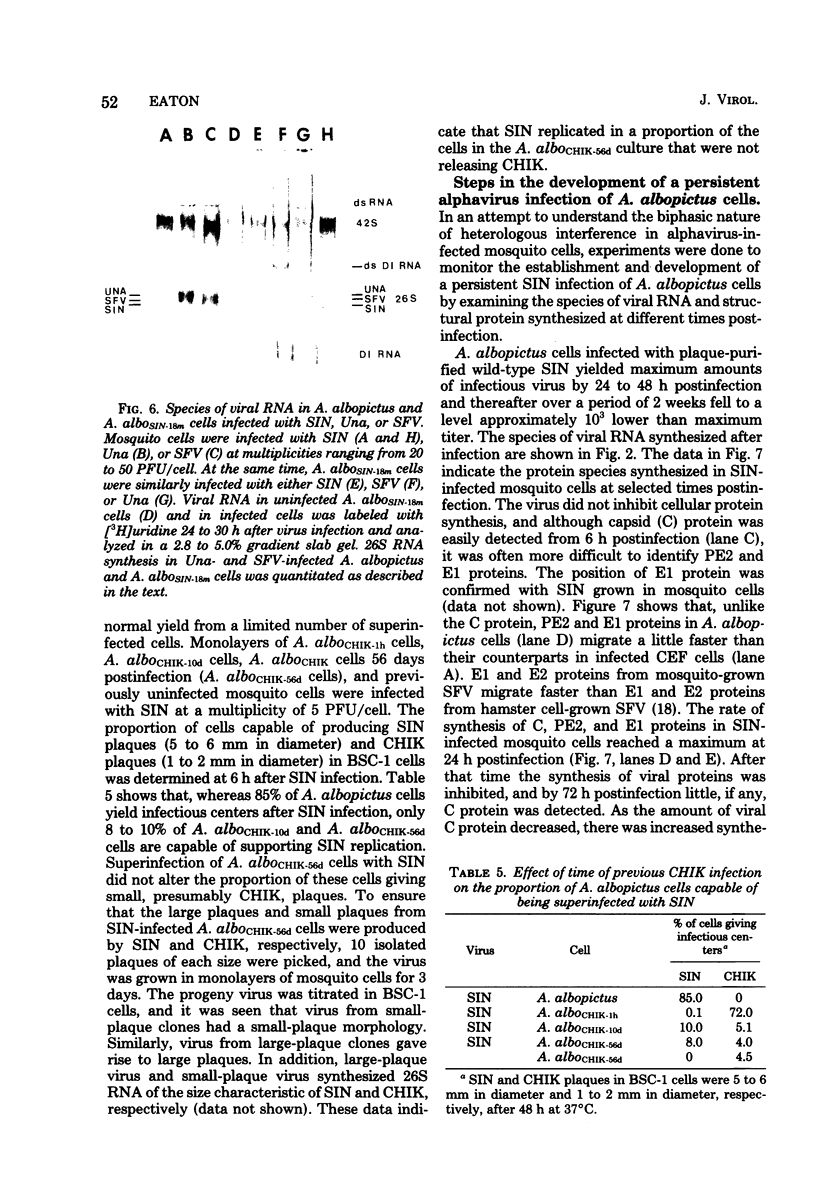
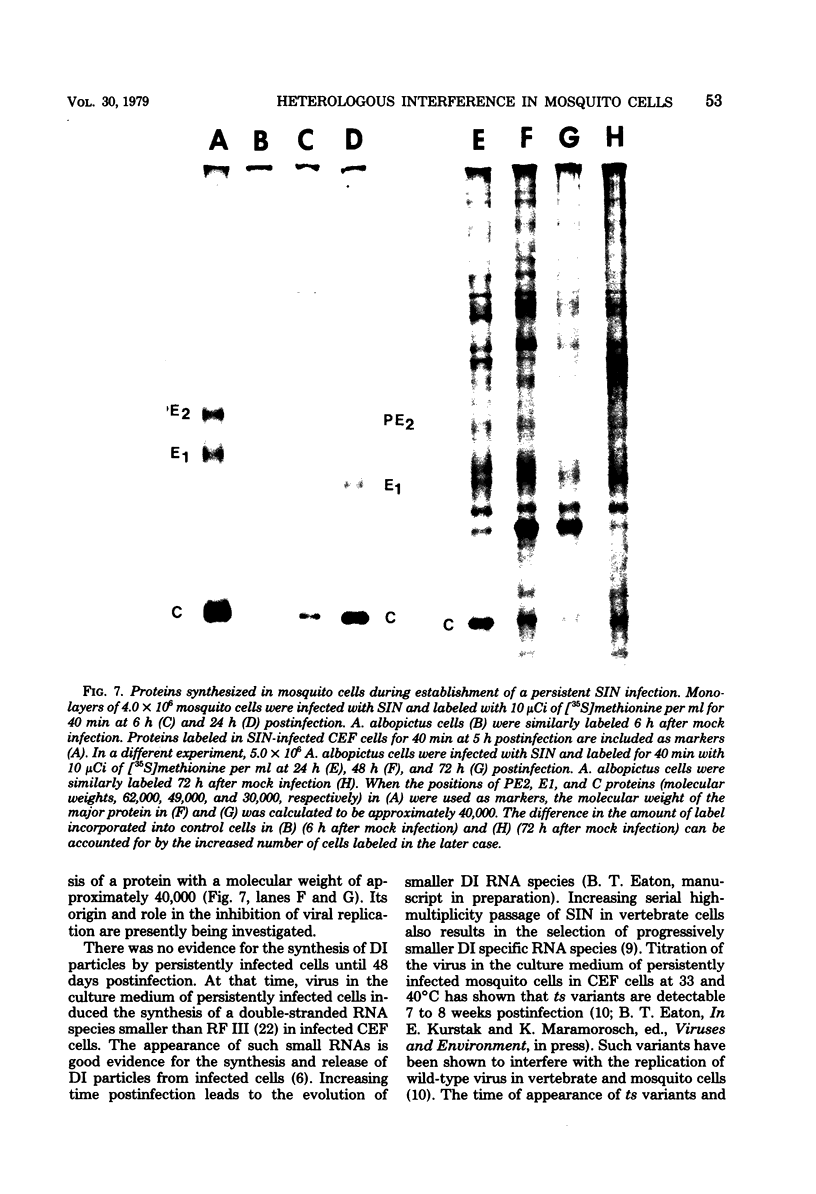
Maximum amounts of 42S and 26S single-stranded viral RNA and viral structural proteins were synthesized in Aedes albopictus cells at 24 h after Sindbis virus infection. Thereafter, viral RNA and protein syntheses were inhibited. By 3 days postinfection, only small quantities of 42S RNA and no detectable 26S RNA or structural proteins were synthesized in infected cells. Superinfection of A. albopictus cells 3 days after Sindbis virus infection with Sindbis, Semliki Forest, Una, or Chikungunya alphavirus did not lead to the synthesis of intracellular 26S viral RNA. In contrast, infection with snowshoe hare virus, a bunyavirus, induced the synthesis of snowshoe hare virus RNA in both A. Ablpictus cells 3 days after Sindbis virus infection and previously uninfected mosquito cells. These results suggested that at 3 days after infection with Sindbis virus, mosquito cells restricted the replication of both homologous and heterologous alphaviruses but remained susceptible to infection with a bunyavirus. In superinfection experiments the the alphaviruses were differentiated on the basis of plaque morphology and the electrophoretic mobility of their intracellular 26S viral RNA species. Thus, it was shown that within 1 h after infection with eigher Sindbis or Chikungunya virus, A. albopictus cells were resistant to superinfection with Sindbis, Chikungunya, Una, and Semliki Forest viruses. Infected cultures were resistant to superinfection with the homologous virus indefinitely, but maximum resistance to superinfection with heterologous alphaviruses lasted for approximately 8 days. After that time, infected cultures supported the replication of heterologous alphaviruses to the same extent as did persistently infected cultures established months previously. However, the titer of heterologous alphavirus produced after superinfection of persistently infected cultures was 10- to 50-fold less than that produced by an equal number of previously uninfected A. albopictus cells. Only a small proportion (8 to 10%) of the cells in a persistently infected culture was capable of supporting the replication of a heterologous alphavirus.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bracha M., Leone A., Schlesinger M. J. Formation of a Sindbis virus nonstructural protein and its relation of 42S mRNA function. J Virol. 1976 Dec;20(3):612–620. doi: 10.1128/jvi.20.3.612-620.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg J. C., Brzeski H., Kennedy S. I. RNA polymerase components in Semliki Forest virus-infected cells: synthesis from large precursors. J Gen Virol. 1976 Sep;32(3):413–430. doi: 10.1099/0022-1317-32-3-413. [DOI] [PubMed] [Google Scholar]
- Clewley J., Gentsch J., Bishop D. H. Three unique viral RNA species of snowshoe hare and La Crosse bunyaviruses. J Virol. 1977 May;22(2):459–468. doi: 10.1128/jvi.22.2.459-468.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey M. W., Dalgarno L. Semliki Forest virus replication in cultured Aedes albopictus cells: studies on the establishment of persistence. J Gen Virol. 1974 Sep;24(3):453–463. doi: 10.1099/0022-1317-24-3-453. [DOI] [PubMed] [Google Scholar]
- Eaton B. T. Evidence for the synthesis of defection interfering particles by Aedes albopictus cells persistently infected with Sindbis virus. Virology. 1977 Apr;77(2):843–848. doi: 10.1016/0042-6822(77)90503-7. [DOI] [PubMed] [Google Scholar]
- Eaton B. T., Randlett D. J. Origin of the actinomycin D insensitive RNA species in Aedes albopictus cells. Nucleic Acids Res. 1978 Apr;5(4):1301–1314. doi: 10.1093/nar/5.4.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton B. T., Regnery R. L. Polysomal RNA in Semliki Forest virus infected Aedes albopictus cells. J Gen Virol. 1975 Oct;29(1):35–49. doi: 10.1099/0022-1317-29-1-35. [DOI] [PubMed] [Google Scholar]
- Guild G. M., Flores L., Stollar V. Defective interfering particles of Sindbis virus. IV. Virion RNA species and molecular weight determination of defective double-stranded RNA. Virology. 1977 Mar;77(1):158–174. doi: 10.1016/0042-6822(77)90415-9. [DOI] [PubMed] [Google Scholar]
- Igarashi A., Stollar V. Failure of defective interfering particles of Sindbis virus produced in BHK or chicken cells to affect viral replication in Aedes albopictus cells. J Virol. 1976 Aug;19(2):398–408. doi: 10.1128/jvi.19.2.398-408.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. E., Wan K., Bose H. R. Homologous interference induced by Sindbis virus. J Virol. 1974 Nov;14(5):1076–1082. doi: 10.1128/jvi.14.5.1076-1082.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kascsak R. J., Lyons M. J. Bunyamwera virus. I. The molecular complexity of the virion RNA. Virology. 1977 Oct 1;82(1):37–47. doi: 10.1016/0042-6822(77)90030-7. [DOI] [PubMed] [Google Scholar]
- Kennedy S. I. Sequence relationships between the genome and the intracellular RNA species of standard and defective-interfering Semliki Forest virus. J Mol Biol. 1976 Dec;108(2):491–511. doi: 10.1016/s0022-2836(76)80132-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Levin J. G., Friedman R. M. Analysis of arbovirus ribonucleic acid forms by polyacrylamide gel electrophoresis. J Virol. 1971 Apr;7(4):504–514. doi: 10.1128/jvi.7.4.504-514.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luukkonen A., von Bonsdorff C. H., Renkonen O. Characterization of Semliki Forest virus grown in mosquito cells. Comparison with the virus from hamster cells. Virology. 1977 May 1;78(1):331–335. doi: 10.1016/0042-6822(77)90105-2. [DOI] [PubMed] [Google Scholar]
- Martin B. A., Burke D. C. The replication of Semliki Forest virus. J Gen Virol. 1974 Jul;24(1):45–66. doi: 10.1099/0022-1317-24-1-45. [DOI] [PubMed] [Google Scholar]
- Renz D., Brown D. T. Characteristics of Sindbis virus temperature-sensitive mutants in cultured BHK-21 and Aedes albopictus (Mosquito) cells. J Virol. 1976 Sep;19(3):775–781. doi: 10.1128/jvi.19.3.775-781.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel B., Brown D. T. Role of extracellular virus on the maintenance of the persistent infection induced in Aedes albopictus (mosquito) cells by Sindbis virus. J Virol. 1977 Sep;23(3):554–561. doi: 10.1128/jvi.23.3.554-561.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D. T., Strauss J. H. Replication of Sindbis virus. II. Multiple forms of double-stranded RNA isolated from infected cells. J Mol Biol. 1972 Nov 28;71(3):615–631. doi: 10.1016/s0022-2836(72)80027-5. [DOI] [PubMed] [Google Scholar]
- Simmons D. T., Strauss J. H. Replication of Sindbis virus. V. Polyribosomes and mRNA in infected cells. J Virol. 1974 Sep;14(3):552–559. doi: 10.1128/jvi.14.3.552-559.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stollar V., Shenk T. E. Homologous viral interference in Aedes albopictus cultures chronically infected with Sindbis virus. J Virol. 1973 Apr;11(4):592–595. doi: 10.1128/jvi.11.4.592-595.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zebovitz E., Brown A. Interference among group A arboviruses. J Virol. 1968 Nov;2(11):1283–1289. doi: 10.21236/ad0844170. [DOI] [PMC free article] [PubMed] [Google Scholar]