Abstract
In younger adults, recently learned episodic memories are reactivated and consolidated during slow-wave sleep (SWS). Interestingly, SWS declines across the lifespan but little research has examined whether sleep-dependent memory consolidation occurs in older adults. In the present study, younger adults and healthy older adults encoded word pairs in the morning or evening and then returned following a sleep or no-sleep interval. Sleep stage scoring was obtained using a home sleep-stage monitoring system. In the younger adult group, there was a positive correlation between word retention and amount of SWS. In contrast, the older adults demonstrated no significant positive correlations, but one significant negative correlation, between memory and SWS. These findings suggest that the link between episodic memory and SWS that is typically observed in younger adults may be weakened or otherwise changed in the healthy elderly.
Keywords: sleep, memory, consolidation, aging, slow wave sleep, cognition, learning, synaptic downscaling
Humans spend approximately one-third of their lives sleeping but scientists have yet to reach a consensus as to why sleep occurs. One likely explanation is that, like waking behavior, sleep serves multiple purposes ranging from tissue restoration (Adam & Oswald, 1977) and energy conservation (Berger & Phillips, 1995) to maintaining synaptic homeostasis (Tononi & Cirelli, 2003). In recent years scientists have discovered an additional function of sleep: sleep benefits the consolidation of memories (e.g., Wilson & McNaughton, 1994).
SWS and Memory Consolidation
One of the most intriguing recent scientific discoveries has been that slow-wave sleep (SWS) promotes memory functioning. In a seminal study, Wilson and McNaughton (1994) showed that the same hippocampal neurons that fired while a rodent was learning a maze were “replayed” while the rodent slept. Rather than simply replaying memories, single-cell recording studies have demonstrated an orchestrated pattern of firing between hippocampal and neocortical cells in which memories are theorized to be transferred from short-term hippocampal storage to long-term neocortical storage (Buzsaki, 1996; Marr, 1971). This transfer, or consolidation, process is hypothesized to promote episodic memory (i.e., explicit recall of learned information) by integrating consolidated memories into long-term storage (Takashima et al., 2006) and it may also prepare the hippocampus to encode new memories (e.g., Yoo, Hu, Gujar, Jolesz, & Walker, 2007).
The importance of SWS to human memory consolidation has been extensively demonstrated in younger adults (for reviews, see Diekelmann, Wilhelm, & Born, 2009; Walker, 2009). For example, Peigneux et al. (2004) had human participants undergo neuroimaging while learning routes in a virtual town and then again while sleeping. They found that the hippocampus was activated both during learning and during SWS and that the degree of hippocampal (re)activation during SWS correlated positively with route retrieval the following day.
Rasch, Buschel, Gais, and Born (2007) experimentally demonstrated the relationship between reactivation during SWS and memory enhancement. During the learning of an object-location pairs task, a rose scent (or an odorless control) was repeatedly delivered to younger adult participants and they were (re)exposed to that scent (or odorless control) during either SWS, rapid eye movement (REM) sleep, or wakefulness. Following the sleep (or no-sleep) retention interval, they found that performance on the object-locations task was enhanced only when the rose scent (relative to the odorless control group) was presented during learning and during SWS. No memory enhancement was observed for the REM sleep or wakefulness conditions. Compelling the conclusion that the rose scent was reactivating the object-location pairs during SWS, Rasch et al. used neuroimaging to show greater hippocampal activation during rose-scent-on periods than rose-scent-off periods in a SWS condition, relative to a wake condition. Thus, SWS is strongly linked to episodic memory consolidation in younger adults.
Sleep, Memory, and Aging
Given the importance of SWS to episodic memory (Diekelmann et al., 2009), a pertinent question is whether conditions that are associated with SWS declines, such as increasing age (Ohayon, Carskadon, Guilleminault, & Vitiello, 2004), are also associated with episodic memory impairments. In support of this idea, Backhaus et al. (2007) compared younger adults (18–25 years old) and middle-aged adults (48–55 years old) on cued recall of word pairs following sleep intervals. In addition to finding that age-related memory declines were paralleled by SWS declines, they observed that middle-aged adults who gained high levels of SWS still demonstrated evidence for consolidation (relative to those who gained little SWS). These results suggested that consolidation processes might begin to decline in middle age to the extent that SWS declines.
A related question concerns whether age-related SWS declines may account for memory declines in older adults (Buckley & Schatzberg, 2005; Hornung, Danker-Hopfe, & Heuser, 2005). Episodic memory declines are prominent in older adults and changes in SWS appear to precede or parallel these cognitive declines (compare longitudinal datasets on episodic memory: Park et al., 2002, and on sleep physiology: Van Cauter, Leproult, & Plat, 2000). Older adults get more light sleep, but less SWS, than younger adults (see Bliwise, 1993, for review; see Ohayon et al., 2004, for a meta-analysis). Not only is there nominally less SWS in older adults (even when accounting for total sleep time, i.e., examining SWS percent), but there is also an age-related decrease in the amplitude of delta waves which compose SWS (e.g., Martin, Shochat, & Ancoli-Israel, 2000). The implication of studies demonstrating changes in SWS across the lifespan is that SWS declines may underlie memory impairments in older adults directly by reducing consolidation of memories or indirectly by reducing the ability of the hippocampus to encode new memories.
The “SWS quantity” hypothesis is alluring in its ability to simply explain cognitive aging as changes in SWS quantity and is provocative in its translational implication that pharmacologically boosting SWS could augment memory in older adults. However, as detailed in the following section, both animal (e.g., Buechel et al., 2011) and human (e.g., Bonnet, 1989) studies suggest that the link between sleep, memory, and aging might not be so simple. Instead, the existing literature suggests a more complex possibility: the functional relationship between sleep physiology and cognition may weaken or change in older age.
Age-Related Changes in the Sleep—Memory Link
Two studies from the rodent literature suggest that the sleep—memory link might weaken or change with increasing age. Gerrard, Burke, McNaughton, and Barnes (2008) found that the hippocampal reactivation (“memory replay”) normally observed in rodent models (e.g., Wilson & McNaughton, 1994) is not preserved in older rats (specifically, older rodents display temporal sequence disorganization). In addition, Buechel et al. (2011) examined performance on the Morris Water Maze task in older rats. These older rats demonstrated either no significant correlation between memory performance and deep sleep (i.e., sleep that is similar to SWS in humans) or even a negative correlation whereby more deep sleep was associated with worse memory performance. Thus, the relationship between sleep and memory might differ between younger and older rodents.
Research on SWS and vigilance in humans provides further evidence for the possible erosion of the sleep—cognition link in older adults. In younger adults, increasing amounts of SWS are related to reductions in daytime fatigue, as measured by the ability to sustain attention on vigilance tasks. For example, Jurado, Luna-Villegas, and Buela-Casal (1989) observed that, in younger adults, poorer performance (slower reaction times) on a vigilance task was associated with less SWS the prior night. In contrast, Crenshaw and Edinger (1999) reported no such correlation in a group of healthy older adults. When re-evaluating these findings, Pace-Schott and Spencer (2011) suggested that “the relationship between SWS and cognitive performance may weaken as the amount of SWS diminishes with aging” (p. 82; cf. Spiegel, Koberle, & Allen, 1986).
Sleep deprivation and aging studies constitute the most developed literature suggesting that sleep may serve cognition in younger adults but not in older adults. Whereas partial or total sleep deprivation produces dramatic cognitive effects in younger adults (e.g., poor ability to maintain attention)(see Killgore, 2010, for review), older adults often show no cognitive effects of sleep deprivation (a finding that does not seem explainable by floor effects; Adam, Retey, Khatami, & Landolt, 2006; Bonnet, 1989; Duffy, Willson, Wang, & Czeisler, 2009; Philip et al., 2004; Stenuit & Kerkhofs, 2005; Webb, 1985; Webb & Levey, 1982). These studies suggest that sleep is closely related to cognition in younger adults but perhaps not in older adults, and converges with Spiegel et al.’s (1986) claim that “SWS changes its functional significance during ontogenesis…to a functionally meaningless remnant in old age” (p. 77).
Sleep and Episodic Memory Consolidation in Older Adults
The question of whether there are age-related changes in sleep-dependent episodic memory consolidation has rarely been investigated. Rauchs et al. (2008) gave younger adults, healthy older adults, and Alzheimer’s disease (AD) patients a very strong encoding task (semantic encoding strategy, frequent tests) and a less-strong encoding task (single reading of a story). Encoding took place at night and participants were tested the next morning. They found ceiling-level performance on the very strong encoding task for the younger and healthy older adults (though a reduction in the AD patients). For the less-strong encoding task, there was a significant difference between the younger adults and healthy older adults (AD patients were at floor levels). Though correlating sleep parameters with memory recall was limited by ceiling or floor effects in some cells, it is interesting to note that SWS did not correlate with memory recall in the healthy older adults (Géraldine Rauchs, personal communication, March 13, 2012). Rauchs et al.’s results suggested that sleep-dependent episodic memory consolidation might decline in healthy older adults and that SWS might not benefit episodic memory in this group.
Other studies that have tested for episodic memory consolidation across younger and older adult groups have not collected measures of sleep architecture but their behavioral results are worth discussion. Aly and Moscovitch (2010) compared recall in younger and older adults following wake versus sleep intervals. In the first session (morning or evening), the experimenter read stories to participants over the telephone and gave them an immediate test. Older adults who could not recall sufficient details of the story were read the story again. After a sleep or wake interval, participants were asked to recall the stories (over the telephone). The sleep-related benefit for story recall was similar for younger and older adults. The experimenters also assessed for “personal memories” (e.g., Who was the last person you spoke to the previous night? What was he/she wearing?). For this measure, the sleep-related benefit was significantly reduced in the older adults relative to the younger adults.
Wilson, Baran, Pace-Schott, Ivry, and Spencer (in press) behaviorally examined episodic memory recall and motor learning across sleep and wake intervals in young adults, middle-aged adults, and older adults. They found an age-related decline in sleep-dependent motor learning, but for episodic memory recall, the older adult group still demonstrated evidence for a sleep-related benefit. Because motor memory consolidation was not observed in the older adults, Wilson et al. suggested that some other variable rather than sleep processes per se (e.g., SWS) might explain the older adult episodic memory pattern.
The Present Research
The present study utilized a home sleep-stage monitor to investigate SWS-dependent episodic memory consolidation in younger and older adults. The home sleep-stage monitor distinguishes between wake, light sleep, SWS, and REM sleep. Shambroom, Fabregas, and Johnstone (2011) found that, in a sample that ranged in age from 19 to 60, stage scoring with the home device agreed highly with polysomnography-based sleep staging (according to standard definitions; Landis & Koch, 1977).
In the present study, younger and healthy older adults encoded word pairs in the morning or evening and were tested after an equal length retention interval that included nighttime sleep or daytime wake (12-hr wake versus 12-hr sleep). In addition, some participants encoded the word pairs at night and were tested 24-hrs later (cf. Ellenbogen et al., 2006). Classic interference theory (Jenkins & Dallenbach, 1924) anticipates memory performance to be worse in the 24-hr sleep group than in both the 12-hr sleep and 12-hr wake groups because the 24-hr group has spent more time awake (i.e., been subjected to greater daytime interference). Finally, because SWS-dependent memory consolidation may not only benefit memory retention, but might also indirectly benefit future learning by promoting efficient hippocampal encoding (e.g., Yoo et al., 2007), participants were also tested on brief retention of new word pairs learned post-sleep.
One possible concern when comparing younger and older adults across sleep and wake intervals is age-related circadian rhythm differences. For example, May, Hasher, and Stolzfus (1993) found that optimal time of testing (i.e., the time at which performance is best) was important in determining whether age differences were observed in cognitive tests. Though suggestive, this result is not always replicated (Brown, Goddard, Lahar, & Mosley, 1999), and furthermore, forced circadian desynchrony studies (e.g., Silva, Wang, Ronda, Wyatt, & Duffy, 2010) have found that shifting the timing of the wake-sleep cycle leads to greater cognitive impairments in younger adults than in older adults. To minimize potential age effects that might be attributed to circadian influences (caused by morning versus night testing) participants were scheduled for testing at their self-reported optimal time within a 7–10 a.m./p.m. range. In addition, participants completed the Morningness-Eveningness Questionnaire (MEQ; Horne & Ostberg, 1976), which allowed for the evaluation of optimal time of day on memory recall.
Based upon literatures suggesting a strong relationship between SWS and episodic memory in younger adults (Diekelmann et al., 2009), as well as age-related SWS declines (Bliwise, 1993; Ohayon et al., 2004), one prediction is that older adults should demonstrate less evidence for memory consolidation than younger adults. Age differences in memory consolidation might be observed in levels of retention across nighttime sleep versus daytime wake intervals as well as in the ability to learn and retain new memories during the second experimental session (e.g., post-sleep). If SWS is still functionally related to memory in older adults then there should be a positive correlation between SWS and retention (e.g., Backhaus et al., 2007); if the sleep—memory relationship is weakened or changed in older adults then SWS and retention should not correlate positively (Spiegel et al., 1986).
Method
Participants and Design
Fifty-seven younger adults (MAge = 19.73; SD = 1.09; 55.4% females) and forty-one older adults (MAge = 70.66; SD = 5.41; 70.7% females) were recruited from Washington University Psychology Department participant pools and were randomly assigned to one of three groups: 12-hour wake, 12-hour sleep, and 24-hour (night-to-night) sleep groups (Ellenbogen et al., 2006). Though participants were randomly assigned to groups, they self-selected their most optimal time during the 7–10 a.m./p.m. range to participate.
In line with the focus on episodic memory consolidation in healthy aging, participants were pre-screened for history of taking sleep-altering medications (benzodiazepines, melatonin, antidepressants, antipsychotics, nicotine, or any medication prescribed for sleep), and history of diagnosed sleep disorders (e.g., sleep apnea), neurodegenerative disorders (e.g., various dementia), or mental health disorders (e.g., depression). Of the 125 individuals initially contacted during recruitment, 21 older adults and 6 younger adults were determined to be ineligible based on the above screening criteria. This age-related difference in probability of eligibility was consistent with the increased prevalence of sleep disorders and sleep-altering medications in older age (Crowley, 2011).
Participants were excluded if they did not return for the second experimental session (nYounger = 3), if they napped extensively during the retention interval (determined by actigraphy as one hour; nYounger = 2),1 or if due to an unexpected technical glitch in the sleep-recording device no sleep stage data was recorded (nYounger = 9, nOlder = 4).2
Materials
Word lists, consisting of two-syllable nouns, were generated from lexicon databases (e.g., Balota et al., 2007; Coltheart, 1981), and were designed to be similar in average word length, imageability, frequency, and concreteness. Words were paired together randomly, with the exception that obvious semantic association between the paired words was avoided.
The possible influence of recent sleep habits was examined by administering the Pittsburg Sleep Quality Index (PSQI; Buysse, Reynolds, Monk, Berman, & Kupfer, 1989), which asks questions regarding typical wake and bedtime as well as the commonality of sleep disturbances over the past month. Similarly, the possible influence of optimal time of day was evaluated using the MEQ (Horne & Ostberg, 1976), which asks questions related to morning alertness as well as preferred time to take a test, to exercise, to work, etc.
Sleep measurement
A wireless home sleep-stage monitoring device (Zeo®), which has been validated relative to polysomnography in a sample that varied greatly in age (19–60), was used to measure sleep architecture (Shambroom et al., 2011). This system includes a clock base station and an adjustable headband that is worn on the forehead (at approximately Fp1–Fp2). The headband includes sensors that collect electrophysiological data from a single channel, pre-process the data to amplify signal and filter noise, and transmit the data wirelessly to the base station. A microprocessor in the base station then uses the signal to calculate sleep stages in accordance with standard Rechtschaffen and Kales (1968) polysomnography scoring norms. It does not provide information about sleep spindles, K-complexes, or spontaneous arousals. For full technical details see Shambroom et al. (2011), and for an additional example of the use of this device in sleep research, see Gumenyuk et al. (2011). In addition to the sleep-stage monitoring, wristband actigraphy (Actiwatch 2, Philips Respironics, Inc.) was employed to identify napping between experimental sessions.
Procedure
The first experimental session took place in the morning (7–10 a.m.) or in the evening (7–10 p.m.), and the exact time of testing depended on the participants’ self-reported most optimal performance time within the 7–10 a.m./p.m. range (to help reduce possible effects related to circadian rhythm age group differences). Participants were first asked to fill out the MEQ and the PSQI. Then participants began the word pair learning procedure. During the study phase, participants saw word pairs (e.g., Channel – Result) on the computer screen, one-at-a-time for 7 seconds per pair (presentation was randomized). After studying all 20 pairs the participants were asked to solve simple math problems (Is 7 × 3 = 23?) for two minutes, which served as a delay between study and test phases to avoid rehearsal prior to testing. Then participants were given a cued recall test in which they were provided with the cue word and had to type in the associated word (Channel – _____?). If the participant recalled less than 80% of the pairs then the program returned to the study phase. The study-math-recall cycle repeated until the participant recalled at least 80% of the pairs correctly or until 30 minutes had elapsed. After the learning phase, participants in the sleep conditions were instructed how to use the home sleep-stage monitoring device and were instructed to maintain their normal sleep (bedtime, wakeup time) schedule.
The second experimental session occurred 12 or 24 hours later. When participants returned they were seated at the same computer station and they underwent another learning phase that was identical in structure to the Session-1 learning phase, but with a different word pair set.3 After completing the Session-2 learning phase, participants were required to take a 5-min break and then they were given a final cued recall test for both Session-1 and Session-2 word pairs.4
Results
Sleep Parameters
Table 1 presents the means and inferential statistics for sleep-recording, PSQI, and MEQ scores across younger and older adults. The results were consistent with general findings in the sleep and aging literature (Bliwise, 1993; Ohayon et al., 2004): older adults demonstrated more light sleep, but less SWS, than younger adults. Other expected outcomes included worse subjective sleep quality in older than in younger adults (PSQI scores), greater morning preferences in older than younger adults (MEQ scores), greater increases in the number of awakenings from sleep in older than younger adults, and greater time spent awake during normal sleeping hours.
Table 1.
Sleep data, PSQI scores (Buysse et al., 1989), and MEQ (Horne & Ostberg, 1976) scores across younger and older adults. Sleep-recording data was obtained for the 12-hr sleep and 24-hr sleep conditions (N=54) whereas PSQI and MEQ data was available for all conditions (N=81). Inferential statistics—t and p values—refer to the age group main effect (ns indicates p > .10). Standard deviations are in parentheses. Abbreviations: SWS: slow-wave sleep; REM: rapid eye movement; WASO: wake after sleep onset; MEQ: morningness-eveningness questionnaire; PSQI: Pittsburg Sleep Quality Index.
| Younger Adults | Older Adults | t-test | p-value | |
|---|---|---|---|---|
|
|
||||
| Light Sleep (%) | .49 (.12) | .61 (.15) | 3.15 | .003 |
| SWS (%) | .26 (.12) | .15 (.09) | 3.76 | <.001 |
| REM Sleep (%) | .26 (.08) | .25 (.12) | <1 | ns |
| Light Sleep (min) | 164.43 (75.25) | 200.92 (75.49) | 1.77 | .083 |
| SWS (min) | 83.17 (33.14) | 46.54 (28.20) | 4.31 | <.001 |
| REM Sleep (min) | 90.23 (40.47) | 84.13 (50.27) | <1 | ns |
| WASO (min) | 5.40 (7.82) | 46.96 (45.27) | 4.95 | <.001 |
| Sleep Latency (min) | 19.73 (26.16) | 17.38 (18.73) | <1 | ns |
| Total Sleep Time (min) | 337.20 (112.61) | 331.13 (97.10) | <1 | ns |
| Total Awakenings | 1.43 (1.76) | 4.88 (2.71) | 5.64 | <.001 |
| MEQ Score | 43.00 (9.43) | 56.97 (9.73) | 6.55 | <.001 |
| PSQI Scores | 4.82 (2.40) | 6.19 (3.55) | 2.062 | .043 |
Memory Retention
There were no significant interval condition differences for number of items correctly recalled in younger and older adults during the Session-1 and Session-2 learning phases (all Fs < 1). Because the present study was interested in retention of items learned, all subsequent analyses control for the number of items recalled during the corresponding learning phase.
For the dependent measure of Session-1 word pair retention, which was a measure of memory consolidation, the younger adults demonstrated a significant interval condition effect, F(2, 40) = 6.17, MSE = .023, p = .005, because retention was greater in the 12-hr sleep condition (M = .658) than the 12-hr wake condition (M = .482), F(1,29) = 9.516, MSE = .023, p = .005, and 24-hr sleep condition (M = .495), F(1,30) = 8.112, MSE = .025, p = .008 (latter two conditions did not differ statistically, F < 1). Interestingly, in the older adult group, there was not a significant interval condition effect (F < 1; M12-hr Wake = .241, M12-hr Sleep = .289, M24-hr Sleep = .271).
Session-2 word pair retention, which was a measure of post-interval (e.g., post-sleep) learning, demonstrated a similar pattern. Because these pairs had been learned only a few minutes prior to testing, it is not surprising that performance in the younger adult group was very high (M = .90), and with the reduced variability, the interval condition effect was only marginally significant, F(2, 40) = 2.964, MSE = .008, p = .063 (M12-hr Wake = .854, M12-hr Sleep = .935, M24-hr Sleep = .894). The older adult group did not demonstrate a significant interval condition main effect (F < 1; M12-hr Wake = .752, M12-hr Sleep = .732, M24-hr Sleep = .740).
Sleep—Behavior Correlations
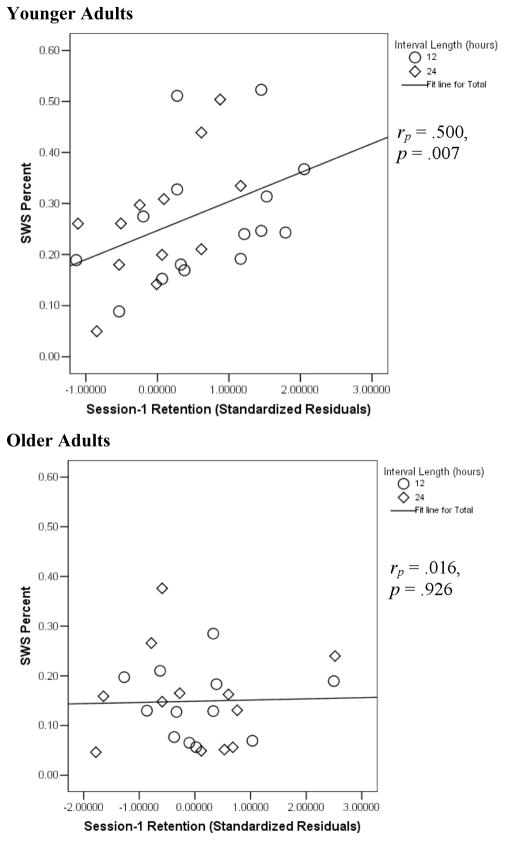
A primary interest of this research regarded whether SWS benefited episodic memory in both younger and older adults. The results thus far have demonstrated age differences in amount of SWS as well as evidence that sleep intervals benefit episodic memory retention in younger adults, but perhaps not in older adults. The next critical question is whether the positive association between SWS and episodic memory is upheld in both younger and older adults. Scatterplots illustrating these relationships are presented in Figure 1. The critical finding was that the partial correlation between SWS percent and Session-1 word pair retention (controlling for interval condition) was strong and statistically significant in the younger adults, rp(26) = .500, p = .007, but not significant and near zero in the older adults (rp(20) = .016, p = .926). Similar findings obtained for the corresponding partial correlation with SWS minutes [rp-younger(26) = .457, p = .014; rp-older(20) = .116, p = .606].
Figure 1.
Scatterplots demonstrating the relationship between SWS percent and Session-1 retention in younger and older adults. Standardized residuals were derived from regression analyses using Session-1 final recall (dependent variable) and number of items correctly recalled during the final Session-1 learning cycle.
Another potentially interesting question is whether sleep variables correlated with new learning, as measured by retention of Session-2 word pairs. Ceiling effects (i.e., no performance variability) in the younger adults limited correlating sleep variables with Session-2 retention (all correlations were therefore expectedly nonsignificant). Surprisingly, within the older adult group, there was a significant negative correlation between minutes in SWS and Session-2 retention [rp(20) = −.507, p = .016; SWS percent: rp(20) = −.416, p = .054). This finding was unexpected but it converges with Buechel et al.’s (2011) recent finding of a negative correlation with Morris Water Maze performance in older rodents, and it raises the possibility that in healthy older adults, SWS may be associated with impairments to cognitive functions (see also Seeck-Hirschner et al., in press).
No significant correlations were observed between retention and REM sleep, wake after sleep onset, number of nighttime awakenings, MEQ scores, or PSQI scores, thereby illustrating that these effects were largely isolated to SWS. Significant correlations were observed between retention and light sleep percent (Session-1, younger adults: rp(26) = −.484, p = .009; Session-2, older adults: rp(20) = .424, p = .049). Based on this correlation, it might be tempting to suggest a positive relationship between processes occurring during light sleep, such as sleep spindles, and better learning in older adults (cf. Seeck-Hirschner et al., in press). However, caution is advised because the present correlations may have arisen due to the inverse correlation between SWS percent and light sleep percent (younger adults: r(30) = −.784, p < .001; older adults: r(24) = −.595, p = .002); number of minutes in light sleep did not produce similar significant correlations (cf. memory correlations with SWS minutes).
Top Learners
Tucker, McKinley, and Stickgold (2011) suggested that memory consolidation might not occur in older adults if initial learning was insufficient. To investigate this possibility, analyses were restricted to the high-performing older adults (i.e., top half of older adults based on Session-1 learning). In these high-performing older adults, there was still no significant interval condition main effect (F(2, 18) = 2.415, MSE = .018, p = .126) and the partial correlations between Session-1 word retention and SWS (percent: rp(10) = −.020, p = .951; minutes: rp(10) = .137, p = .672) were not significant. There was still a marginally significant negative correlation between SWS minutes and Session-2 retention, rp(10) = −.560, p = .058.
Compelling the conclusion that the observed effects reflect age differences and not encoding differences, in the younger adult group memory consolidation was observed for weakly-encoded items (i.e., word pairs recalled only once during learning phase)(Drosopoulos et al., 2007; Scullin & McDaniel, 2010). There was an interval condition main effect, F(2, 41) = 3.70, MSE = .055, p = .033 (M12-hr Wake = .346, M12-hr Sleep = .584, M24-hr Sleep = .477), and recall was positively correlated with SWS percent, r(30) = .359, p = .052. Thus, even with reduced statistical power, these analyses suggested age-related changes in the SWS—memory link.
Discussion
Overview of Findings
The overarching goal of the present research was to investigate whether sleep benefits memory in older adults as it benefits younger adults (Diekelmann et al., 2009). In the younger adults, sleep benefits were observed as greater retention levels following an equal-length interval that included sleep versus wake (i.e., the 12-hr conditions) as well as a strong positive correlation between SWS and retention of word pairs encoded prior to sleep. In contrast, the older adult group demonstrated no benefits of sleep, and even a negative correlation between SWS and subsequent learning. The present results were consistent with the conceptualization that the sleep—memory link weakens or changes with increasing age.
Sleep and Memory in Younger Adults
The first hypothesis tested in the present research was whether sleep would benefit memory retention in younger adults. The results demonstrated that Session-1 retention was better following a 12-hr sleep interval than a 12-hr wake interval. Though consistent with a memory consolidation account, the observation of better memory following sleep than wake intervals is consistent with other accounts such as protection against retroactive interference (Jenkins & Dallenbach, 1924). Interference theory predicts that greater daytime interference leads to worse memory performance, and while the theory can account for worse performance in the 24-hr sleep group than in the 12-hr sleep group in the younger adults, it cannot account for nominally greater performance in the 24-hr sleep group than in the 12-hr wake group. Interference theory also predicts a positive correlation between total sleep time and retention but the opposite pattern was observed in the younger adults (r = −.101). Instead, the results revealed a strong correlation between SWS and Session-1 memory retention, which was consistent with memory consolidation theory. Thus, the behavioral and sleep—memory correlational results converged better with memory consolidation theory than with interference theory.
Sleep and Memory in Older Adults
Another hypothesis tested concerned the more novel question of whether sleep benefited episodic memory retention in older adults. Few studies have examined episodic memory consolidation in older adults and they have produced mixed results (Aly & Moscovitch, 2010; Rauchs et al., 2008; Wilson et al., in press). In the present study, the older adult group (including the high-performing older adults) did not show a significant interval condition main effect for Session-1 or Session-2 retention. These results were consistent with prior research that has behaviorally suggested memory consolidation declines in older adults (e.g., Spencer, Gouw, & Ivry, 2007).
A related issue concerned the functional relationship between SWS and memory in older adults. One possibility is that SWS declines with increasing age but that the positive association between SWS and episodic memory is maintained with increasing age (Backhaus et al., 2007). An alternative conceptualization (Spiegel et al., 1986) that receives some support from sleep deprivation studies in older adult humans (e.g., Bonnet, 1989), is that the sleep—memory link is weakened or functionally changed in the elderly. The present results favored the functional-dissociation interpretation; whereas the younger adults demonstrated a strong correlation between Session-1 retention and SWS (measured both as percent of nighttime sleep and as total minutes; cf. light sleep percent and minutes correlations), no such relationship emerged in the older adult group. These correlations represent important findings because though some sleep, memory, and aging studies have examined sleep—memory correlations and failed to find them in older adults (e.g., Tucker et al., 2011), reporting divergent correlations in younger and older adults within the same study is a more convincing demonstration of an age-related dissociation (e.g., Peters, Ray, Smith, & Smith, 2008).
Sleep and Subsequent Learning
A final hypothesis that was considered in the present study was that sleep might benefit subsequent learning. For example, memory consolidation theory predicts that transferring memories from short-term hippocampal storage to long-term neocortical storage allows the hippocampus to efficiently encode new memories (e.g., Yoo et al., 2007). Though the Session-2 retention analyses were limited in the younger adult group due to ceiling effects, there was still a marginally significant sleep-related benefit for Session-2 retention. By contrast, in the older adult group, the wake interval group showed nominally better Session-2 retention than the sleep interval group. The most surprising finding, however, was that there was a negative correlation between the amount of SWS the older adults had gained the previous night and their Session-2 retention. Though unexpected, the negative correlation with SWS in older adults converges with Buechel et al.’s (2011) recent finding in a rodent model (see also Seeck-Hirschner et al., in press).
The negative correlation between SWS and subsequent learning in older adults is unlikely to be explained simply by memory consolidation theory, but it might be informed by understanding other functions of SWS, such as synaptic downscaling. Synaptic downscaling theory (Tonini & Cirelli, 2003) posits that during waking hours an organism learns and encodes various experiences, which causes a net increase in synaptic weights. However, a continuous net increase in synaptic weights would tax grey matter space, be energetically unsustainable (e.g., maintaining AMPA receptors), and eventually lead to saturation of synaptic networks (i.e., new learning would no longer be possible). SWS, however, is conducive to long term depression, depotentiation of synaptic transmission (e.g., internalization of AMPA receptors), and the overall decrease of synaptic weights (except for those that are being strengthened via reactivation and consolidation; Axmacher, Draguhn, Eler, & Fell, 2009). Experimental studies conducted in Drosophila (fruit flies)(Donlea, Ramanan, & Shaw, 2009; Gilestro, Tononi, & Cirelli, 2009) have supported sleep-dependent synaptic downscaling.
Assuming that synaptic downscaling is a function of SWS in humans, one possible explanation for the negative correlation between SWS and Session-2 retention in the older adult group is that synaptic downscaling is increased proportionally relative to younger adults; that is, older adults might engage in less daytime encoding than younger adults (Cirelli, in press), but “over-downscale” if they are still gaining relatively high amounts of SWS. An age-related proportional increase in downscaling would presumably become detrimental to memory functioning if synapses that could otherwise help encode new memories are pruned. Though only a preliminary hypothesis, this idea of “overactive downscaling” dovetails with Chang et al.’s (2006) finding that experimentally downscaling AMPA receptors in a rodent model contributed to Alzheimer’s disease pathology (which disrupts cognitive functioning in many older adults; Hebert et al., 2003). Another possibility, suggested by Buechel et al. (2011), is that macromolecular biosynthesis occurring during SWS (Mackiewicz et al., 2007) could possibly contribute to poor learning in older adults.
Study Limitations
Understanding the sleep, memory, and aging relationship constitutes a grand question and no single study will answer all questions regarding this issue. Therefore, it is worth describing the present study’s limitations. First, though participants were screened for sleep and neurodegenerative disorders and use of sleep-altering medications, this screening was based on self-report and not on neuropsychological testing or clinical interviews. Therefore, it is possible that the older adult group included some individuals with conditions such as mild cognitive impairment (MCI). MCI is associated with SWS and REM declines (Hita-Yañez, Atienza, Gil-Neciga, & Cantero, in press) and Crowley, Sullivan, Adalsteinsson, Pfefferbaum, and Colrain (2005) found that in AD patients electroencephalographic delta activity (used to score SWS) might actually represent pathologic activity. Critical to interpreting the present study’s results, the same SWS—memory results obtained when examining only the high-performing older adults who, based on their high cognitive ability, would not be expected to have MCI or AD.
Another limitation included the use of a home sleep-stage monitor rather than polysomnography. Polysomnography is considered the gold standard in sleep measurement because it allows for the most accurate assessment of sleep stages, the identification of sleep spindles, and the assessment of nocturnal breathing events. The home monitoring device only scores sleep stages, but its sleep staging has an epoch-by-epoch agreement to polysomnography of 75%, which makes it generally accurate but less so than polysomnography (which typically agrees at rates of 85%). Interestingly, Bruyneel et al. (2011) found that home units promote better sleep efficiency, longer sleep duration, shorter sleep latency, and greater REM sleep. It is further noteworthy that similar sleep—memory correlations to those observed in the present study have been observed with polysomnography (Rauchs et al., 2008; Seeck-Hirschner et al., in press).
A third limitation regards the minimal information available on sleep fragmentation. The present study has measures of total nighttime awakenings and wake after sleep onset—neither of which significantly correlated with memory retention—but polysomnography would have allowed for identification of very brief arousals (e.g., those lasting 3 seconds). Future studies using polysomnography should examine SWS fragmentation in relation to memory retention across younger and older adults (but see Hita-Yañez et al., in press, for null effects when correlating SWS fragmentation with recall in healthy older adults and MCI patients).
Conclusions and Future Directions
The assumption most often expressed in sleep, memory, and aging papers is that if older adults gained more SWS then age-related memory deficits would be minimized. However, this hypothesis assumes that the sleep—memory relationship that is prevalent in younger adults is relatively maintained in older adults. The present research suggests that this assumption might be wrong, and even that SWS might possibly be associated with some negative consequences to memory functions in older adults.
The best sleep-based intervention for cognitive declines in aging is likely to be derived by better knowledge of the mechanism(s) driving the weakening of the sleep—cognition link, but one tentative possibility is to experimentally prime older adults to reactivate memories during sleep. Rasch et al. (2007) found that when they forged an association between a memory and a rose odor, later re-presenting that odor during SWS led to better retention of the associated memory (in younger adults). This control over consolidation might be employed repeatedly in older adults in an attempt to prime them to consolidate memories during sleep. If effective, then that would suggest that older adults maintain the neural circuits and cognitive abilities to consolidate memories, and the next question would concern why they may not normally consolidate memories during sleep. If a consolidation “training” procedure were not effective in older adults then that would suggest that they lack the ability to consolidate memories, perhaps due to functional connectivity changes (e.g., Grady, 2006), dopamine depletion (e.g., Backman, Nyberg, Lindenberger, Li, & Farde, 2006), increased incidence of nocturnal hypoxia (Yamout, Goldstein, Lah, Levey, & Bliwise, 2012), or some other mechanism. Pinpointing why the sleep—cognition link begins to weaken in older age and how such changes might be reversed or prevented could be one of the next great research questions for science.
Acknowledgments
The National Institute on Aging (Grants T32AG00030 and F32AG041543), APF/COGDOP, and Psi Chi supported this research. ZeoR provided for the home sleep-stage monitoring devices and Philips Respironics, Inc., loaned the Actiwatches for this project. Mark McDaniel, Don Bliwise, Roddy Roediger, Larry Jacoby, Sandy Hale, Paul Shaw, and James Wertsch provided helpful guidance and comments for this dissertation project. Sophie Goloff provided excellent assistance with analyses.
Footnotes
The major results are unchanged if participants who showed any actigraphy-determined daytime napping are removed. The main effect of interval condition on Session-1 memory retention is still significant in the younger adults, F(2, 35) = 5.004, MSE =.024, but not in the older adults (F < 1). The partial correlation between nocturnal SWS percent and Session-1 memory retention was also still significant in the younger adults (rp(23) = .491, p = .013). No older adults in the sleep groups (n=1 in the 12-hr wake group) demonstrated daytime napping so those results were unchanged.
The major behavioral results are unchanged when including all participants.
An original intention was to examine experimentally-induced retroactive interference during Session 2 (cf. Ellenbogen et al., 2006). Therefore, some Session-2 pairs contained a Session-1 cue word, but with a different associated word (A–B/A–C paradigm; Barnes & Underwood, 1959). Surprisingly, this manipulation proved ineffective (i.e., no experimentally induced interference was observed) in both age groups and each interval condition so word pair type is collapsed for all analyses. The null effect may have resulted from manipulating word pair type within-subjects (but see Delprato, 1971; Kuhl, Shah, DuBrow, & Wagner, 2010), from using general feedback rather than specific feedback during learning (but see Barnes & Underwood), or from using a filler-task delay between study and test sessions thereby requiring words to be recalled from secondary memory rather than primary memory.
Recognition tests typically demonstrate smaller sleep benefits in younger adults (Diekelmann et al., 2009), but a different pattern could emerge for older adults. Following the cued recall test, participants took a cued multiple-choice recognition test. Cued recognition was at ceiling in the younger adults (M = .98) and thus not interpretable. Performance was off of ceiling in the older adults, but they did not show a significant interval condition main effect (F < 1; M12-hr Sleep = .75, M12-hr Wake = .79, M24-hr Sleep = .86).
References
- Adam K, Oswald I. Sleep is for tissue restoration. Journal of the Royal College of Physicians of London. 1977;11:376–388. [PMC free article] [PubMed] [Google Scholar]
- Adam M, Retey JV, Khatami R, Landolt HP. Age-related changes in the time course of vigilant attention during 40 hours without sleep in men. Sleep. 2006;29:55–57. doi: 10.1093/sleep/29.1.55. [DOI] [PubMed] [Google Scholar]
- Aly M, Moscovitch M. The effects of sleep on episodic memory in older and younger adults. Memory. 2010;18:327–334. doi: 10.1080/09658211003601548. [DOI] [PubMed] [Google Scholar]
- Axmacher N, Draguhn A, Eler CE, Fell J. Memory processes during sleep: Beyond the standard consolidation theory. Cellular and Molecular Life Sciences. 2009;66:2285–2297. doi: 10.1007/s00018-009-0019-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backhaus J, Born J, Hoeckesfeld R, Fokuhl S, Hohagen F, Junghanns K. Midlife decline in declarative memory consolidation is correlated with a decline in slow wave sleep. Learning and Memory. 2007;14:336–341. doi: 10.1101/lm.470507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backman L, Nyberg L, Lindenberger U, Li SC, Farde L. The correlative triad among aging, dopamine, and cognition: Current status and future prospects. Neuroscience and Biobehavioral Reviews. 2006;30:791–807. doi: 10.1016/j.neubiorev.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Balota DA, Yap MJ, Cortese MJ, Hutchison KA, Kessler B, Loftis B, et al. The english lexicon project. Behavior Research Methods. 2007;39:445–459. doi: 10.3758/bf03193014. [DOI] [PubMed] [Google Scholar]
- Barnes JM, Underwood BJ. Fate of first-list association in transfer theory. Journal of Experimental Psychology. 1959;58:97–105. doi: 10.1037/h0047507. [DOI] [PubMed] [Google Scholar]
- Berger RJ, Phillips NH. Energy conservation and sleep. Behavioral Brain Research. 1995;69:65–73. doi: 10.1016/0166-4328(95)00002-b. [DOI] [PubMed] [Google Scholar]
- Bliwise DL. Sleep in normal aging and dementia. Sleep. 1993;16:40–81. doi: 10.1093/sleep/16.1.40. [DOI] [PubMed] [Google Scholar]
- Bonnet MH. The effect of sleep fragmentation on sleep and performance in younger and older subjects. Neurobiology of Aging. 1989;10:21–25. doi: 10.1016/s0197-4580(89)80006-5. [DOI] [PubMed] [Google Scholar]
- Brown LN, Goddard KM, Lahar CJ, Mosley JL. Age-related deficits in cognitive functioning are not mediated by time of day. Experimental Aging Research. 1999;25:81–93. doi: 10.1080/036107399244156. [DOI] [PubMed] [Google Scholar]
- Bruyneel M, Sanida C, Art G, Libert W, Cuvelier L, Paesmans M, Sergysels R, et al. Sleep efficiency during sleep studies: Results of a prospective study comparing home-based and in-hospital polysomnograpy. Journal of Sleep Research. 2011;20:201–206. doi: 10.1111/j.1365-2869.2010.00859.x. [DOI] [PubMed] [Google Scholar]
- Buckley TM, Schatzberg AF. Aging and the role of the HPA axis and rhythm in sleep and memory-consolidation. American Journal of Geriatric Psychiatry. 2005;12:344–352. doi: 10.1176/appi.ajgp.13.5.344. [DOI] [PubMed] [Google Scholar]
- Buechel HM, Popovic J, Searcy JL, Porter NM, Thibault O, Blalock EM. Deep sleep and parietal cortex gene expression changes are related to cognitive deficits with age. PLos One. 2011;6:e18387. doi: 10.1371/journal.pone.0018387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. The hippocampo-neocortical dialogue. Cerebral Cortex. 1996;6:81–92. doi: 10.1093/cercor/6.2.81. [DOI] [PubMed] [Google Scholar]
- Chang EH, Savage MJ, Flood DG, Thomas JM, Levy RB, Mahadomrongkul V, et al. AMPA receptor downscaling at the onset of Alzheimer’s disease pathology in double knockin mice. Proceedings of the National Academy of Sciences, USA. 2006;103:3410–3415. doi: 10.1073/pnas.0507313103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirelli C. Brain plasticity, sleep and aging. Gerontology. doi: 10.1159/000336149. in press. [DOI] [PubMed] [Google Scholar]
- Coltheart M. The MRC Psycholinguistic Database. Quarterly Journal of Experimental Psychology. 1981;33:497–505. [Google Scholar]
- Crenshaw MC, Edinger JD. Slow-wave sleep and waking cognitive performance among older adults with and without insomnia complaints. Physiology & Behavior. 1999;66:485–492. doi: 10.1016/s0031-9384(98)00316-3. [DOI] [PubMed] [Google Scholar]
- Crowley K. Sleep and sleep disorders in older adults. Neuropsychological Review. 2011;21:41–53. doi: 10.1007/s11065-010-9154-6. [DOI] [PubMed] [Google Scholar]
- Crowley K, Sullivan EV, Adalsteinsson E, Pfefferbaum A, Colrain IM. Differentiating pathologic delta from healthy physiologic delta in patients with Alzheimer disease. Sleep. 2005;28:865–870. doi: 10.1093/sleep/28.7.865. [DOI] [PubMed] [Google Scholar]
- Delprato DJ. Specific-pair interference on recall and associative-matching retention tests. American Journal of Psychology. 1971;84:185–193. [Google Scholar]
- Diekelmann S, Wilhelm I, Born J. The whats and whens of sleep-dependent memory consolidation. Sleep Medicine Reviews. 2009;13:309–321. doi: 10.1016/j.smrv.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Donlea JM, Ramanan N, Shaw PJ. Use-dependent plasticity in clock neurons regulates sleep need in drosophila. Science. 2009;324:105–108. doi: 10.1126/science.1166657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drosopoulos S, Schulze C, Fischer S, Born J. Sleep’s function in the spontaneous recovery and consolidation of memories. Journal of Experimental Psychology: General. 2007;136:169–183. doi: 10.1037/0096-3445.136.2.169. [DOI] [PubMed] [Google Scholar]
- Duffy JF, Willson HJ, Wang W, Czeisler CA. Healthy older adults better tolerate sleep deprivation than young adults. Journal of the American Geriatrics Society. 2009;57:1245–1251. doi: 10.1111/j.1532-5415.2009.02303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenbogen JM, Hulbert JC, Stickgold R, Dinges DF, Thompson-Schill SL. Interfering with theories of sleep and memory: Sleep, declarative memory, and associative interference. Current Biology. 2006;16:1290–1294. doi: 10.1016/j.cub.2006.05.024. [DOI] [PubMed] [Google Scholar]
- Gerrard JL, Burke SN, McNaughton BL, Barnes CA. Sequence reactivation in the hippocampus is impaired in aged rats. Journal of Neuroscience. 2008;28:7883–7890. doi: 10.1523/JNEUROSCI.1265-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilestro GF, Tononi G, Cirelli C. Widespread changes in synaptic markers as a function of sleep and wakefulness in drosophila. Science. 2009;324:109–112. doi: 10.1126/science.1166673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL. Functional connectivity during memory tasks in healthy aging and dementia. In: Cabeza R, Nyberg L, Park D, editors. Cognitive Neuroscience of Aging: Linking Cognitive and Cerebral Aging. Oxford University Press; 2006. pp. 286–308. [Google Scholar]
- Gumenyuk V, Roth T, Korzyukov O, Jefferson C, Bowyer S, Drake CL. Habitual short sleep impacts frontal switch mechanism in attention to novelty. Sleep. 2011;34:1659–1670. doi: 10.5665/sleep.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Archives of Neurology. 2003;60:1119–22. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- Hita-Yañez E, Atienza M, Gil-Neciga E, Cantero JL. Disturbed sleep patterns in elders with mild cognitive impairment: The role of memory decline and ApoE E4 genotype. Current Alzheimer Research. doi: 10.2174/156720512800107609. in press. [DOI] [PubMed] [Google Scholar]
- Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. International Journal of Chrono-biology. 1976;4:97–110. [PubMed] [Google Scholar]
- Hornung OP, Danker-Hopfe H, Heuser I. Age-related changes in sleep and memory: Commonalities and interrelationships. Experimental Gerontology. 2005;40:279–285. doi: 10.1016/j.exger.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Jenkins JG, Dallenbach KM. Oblivescence during sleep and waking. American Journal Psychology. 1924;35:605–612. [Google Scholar]
- Jurado JL, Luna Villegas G, Buela–Casal G. Normal human subjects with slow reaction times and larger time estimations after waking have diminished delta sleep. Electroencephalography and Clinical Neurophysiology. 1989;73:124–128. doi: 10.1016/0013-4694(89)90191-0. [DOI] [PubMed] [Google Scholar]
- Killgore WD. Effects of sleep deprivation on cognition. Progress in Brain Research. 2010;185:105–29. doi: 10.1016/B978-0-444-53702-7.00007-5. [DOI] [PubMed] [Google Scholar]
- Kuhl BA, Shah AT, DuBrow S, Wagner AD. Resistance to forgetting associated with hippocampus mediated reactivation during new learning. Nature Neuroscience. 2010;13:501–508. doi: 10.1038/nn.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- Mackiewicz M, Shockley KR, Romer MA, Galante RJ, Zimmerman JE, Naidoo N, et al. Macromolecule biosynthesis: a key function of sleep. Physiological Genomics. 2007;31:441–457. doi: 10.1152/physiolgenomics.00275.2006. [DOI] [PubMed] [Google Scholar]
- Marr D. Simple memory: A theory for archicortex. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 1971;262:23–81. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- Martin J, Shochat T, Ancoli-Israel S. Assessment and treatment of sleep disturbances in older adults. Clinical Psychology Review. 2000;20:783–805. doi: 10.1016/s0272-7358(99)00063-x. [DOI] [PubMed] [Google Scholar]
- May CP, Hasher L, Stoltzfus ER. Optimal time of day and the magnitude of age differences in memory. Psychological Science. 1993;4:326–330. [Google Scholar]
- Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: Developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–1273. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- Pace-Schott EF, Spencer RMC. Age-related changes in the cognitive function of sleep. Progress in Brain Research. 2011;191:75–89. doi: 10.1016/B978-0-444-53752-2.00012-6. [DOI] [PubMed] [Google Scholar]
- Park DC, Lautenschlager G, Hedden T, Davidson NS, Smith AD, Smith PK. Models of visualspatial and verbal memory across the adult life span. Psychology and Aging. 2002;17:299–320. [PubMed] [Google Scholar]
- Peigneux P, Laureys S, Fuchs S, Collette F, Perrin F, Reggers J, et al. Are spatial memories strengthened in the human hippocampus during slow wave sleep? Neuron. 2004;44:535–545. doi: 10.1016/j.neuron.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Peters KR, Ray L, Smith V, Smith C. Changes in the density of stage 2 sleep spindles following motor learning in young and older adults. Journal of Sleep Research. 2008;17:23–33. doi: 10.1111/j.1365-2869.2008.00634.x. [DOI] [PubMed] [Google Scholar]
- Philip P, Taillard J, Sagaspe P, Valtat C, Sanchez-Ortuno M, Moore N, Charles A, et al. Age, performance and sleep deprivation. Journal of Sleep Research. 2004;13:105–110. doi: 10.1111/j.1365-2869.2004.00399.x. [DOI] [PubMed] [Google Scholar]
- Rasch B, Buchel C, Gais S, Born J. Odor cues during slow-wave sleep prompt declarative memory consolidation. Science. 2007;315:1426–1429. doi: 10.1126/science.1138581. [DOI] [PubMed] [Google Scholar]
- Rauchs G, Schabus M, Parapatics S, Bertran F, Clochon P, Hot P, Denise P, et al. Is there a link between sleep changes and memory in Alzheimer’s disease? NeuroReport. 2008;19:1159–1162. doi: 10.1097/WNR.0b013e32830867c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Brain Information Service/Brain Research Institute; Los Angeles: University of California; Los Angeles: 1968. [Google Scholar]
- Scullin MK, McDaniel MA. Remembering to execute a goal: Sleep on it! Psychological Science. 2010;21:1028–1035. doi: 10.1177/0956797610373373. [DOI] [PubMed] [Google Scholar]
- Seeck-Hirschner M, Baier PC, Weinhold SL, Dittmar M, Heiermann S, Aldenhoff JB, Goder R. American Journal of Geriatric Psychiatry. Declarative memory performance is associated with the number of sleep spindles in elderly women. in press. [DOI] [PubMed] [Google Scholar]
- Silva EJ, Wang W, Ronda JM, Wyatt JK, Duffy JF. Circadian and wake-dependent influences on subjective sleepiness, cognitive throughput, and reaction time performance in older and young adults. Sleep. 2010;33:481–490. doi: 10.1093/sleep/33.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shambroom JR, Fabregas SE, Johnstone J. Validation of an automated wireless system to monitor sleep in healthy adults. Journal of Sleep Research. doi: 10.1111/j.1365-2869.2011.00944.x. in press. [DOI] [PubMed] [Google Scholar]
- Spencer RMC, Gouw AM, Ivry RB. Age-related decline of sleep-dependent consolidation. Learning and Memory. 2007;14:480–484. doi: 10.1101/lm.569407. [DOI] [PubMed] [Google Scholar]
- Spiegel R, Koberle S, Allen SR. Significance of slow wave sleep: Considerations from a clinical viewpoint. Sleep. 1986;9:66–79. doi: 10.1093/sleep/9.1.66. [DOI] [PubMed] [Google Scholar]
- Stenuit P, Kerkhofs M. Age modulates the effects of sleep restriction in women. Sleep. 2005;28:1283–1288. doi: 10.1093/sleep/28.10.1283. [DOI] [PubMed] [Google Scholar]
- Takashima A, Petersson KM, Rutters F, Tendolkar I, Jensen O, Zwarts MJ, et al. A prospective functional magnetic resonance imaging study. Proceedings of the National Academy of Sciences, USA. 2006;103:756–761. doi: 10.1073/pnas.0507774103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi G, Cirelli C. Sleep and synaptic homeostasis: A hypothesis. Brain Research Bulletin. 2003;62:143–150. doi: 10.1016/j.brainresbull.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Tucker M, McKinley S, Stickgold R. Sleep optimizes motor skill in older adults. Journal of the American Geriatric Society. 2011;59:603–609. doi: 10.1111/j.1532-5415.2011.03324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cauter E, Leproult R, Plat L. Age-related changes in slow wave sleep and REM sleep and relationship with growth hormone and cortisol levels in healthy men. Journal of the American Medical Association. 2000;284:861–868. doi: 10.1001/jama.284.7.861. [DOI] [PubMed] [Google Scholar]
- Walker MP. The role of sleep in cognition and emotion. Annals of the New York Academy of Sciences. 2009;1156:168–197. doi: 10.1111/j.1749-6632.2009.04416.x. [DOI] [PubMed] [Google Scholar]
- Webb WB. A further analysis of age and sleep deprivation effects. Psychophysiology. 1985;22:156–161. doi: 10.1111/j.1469-8986.1985.tb01579.x. [DOI] [PubMed] [Google Scholar]
- Webb WB, Levy CM. Age, sleep deprivation, and performance. Psychophysiology. 1982;19:272–276. doi: 10.1111/j.1469-8986.1982.tb02561.x. [DOI] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- Wilson JK, Baran B, Pace-Schott EF, Ivry RB, Spencer RMC. Sleep modulates word-pair learning but not motor sequence learning in healthy older adults. Neurobiology of Aging. doi: 10.1016/j.neurobiolaging.2011.06.029. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamout K, Goldstein FC, Lah JJ, Levey AI, Bliwise DL. Neurocognitive correlates of nocturnal oxygen desaturation in a memory clinic population. Journal of Clinical Experimental Neuropsychology. doi: 10.1080/13803395.2011.642849. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SS, Hu PT, Gujar N, Jolesz FA, Walker MP. A deficit in the ability to form new human memories without sleep. Nature Neuroscience. 2007;10:385–392. doi: 10.1038/nn1851. [DOI] [PubMed] [Google Scholar]