Summary
Prenatal stress has been widely demonstrated to have links with behavioral problems in clinical populations and animal models, however, few investigations have examined the immediate developmental events that are affected by prenatal stress. Here, we utilize GAD67GFP transgenic mice in which GABAergic progenitors express green fluorescent protein (GFP) to examine the impact of prenatal stress on the development of these precursors to inhibitory neurons. Pregnant female mice were exposed to restraint stress three times daily from embryonic day 12 (E12) onwards. Their offspring demonstrated changes in the distribution of GFP-positive (GFP+) GABAergic progenitors in the telencephalon as early as E13 and persisting until postnatal day 0. Changes in distribution reflected alterations in tangential migration and radial integration of GFP+ cells into the developing cortical plate. Fate mapping of GAD67GFP+progenitors with bromodeoxyuridine injected at E13 demonstrated a significant increase of these cells at P0 in anterior white matter. An overall decrease in GAD67GFP+ progenitors at P0 in medial frontal cortex could not be attributed to a reduction in cell proliferation. Significant changes in dlx2, nkx2.1 and their downstream target erbb4, transcription factors which regulate interneuron migration, were found within the prenatally-stressed developing forebrain, while no differences were seen in mash1, a determinant of interneuron fate, bdnf, a maturation factor for GABAergic cells or fgf2, an early growth/differentiation factor. These results demonstrate that early disruption in GABAergic progenitor migration caused by prenatal stress may be responsible for neuronal defects in disorders with GABAergic abnormalities like schizophrenia.
Keywords: Prenatal stress, GAD67, migration, inhibitory neurons, transcription factors, erbb4
1. Introduction
Research on the effects of prenatal stress increasingly demonstrates that this adverse life event can be a significant risk factor for neuropsychiatric disorders. Stress in pregnant women has significant effects on fetal physiology (Monk et al., 2004; Wadhwa, 2005). Prenatal stress has also been linked to childhood behavioral, physiological, and emotional problems (Huot et al., 2004; Martini et al., 2010; O'Connor et al., 2003). Perinatal stressors have an association with symptom severity in Tourette syndrome (Leckman et al., 1990) and with autistic-like symptoms (Ronald et al., 2010). Psychiatric problems that develop in adolescence and young adulthood may also be related to maternal stress during pregnancy. Obstetric complications are more frequent in the maternal history of patients with bipolar disorder (Kinney et al., 1998). Furthermore, the development of schizophrenia has been shown to be correlated with stress experienced during pregnancy (Review:King et al., 2005). Changes in the brain occurring during prenatal stress must have long lasting consequences in order to explain the emergence of psychiatric illness years later. The mechanisms by which persistent cellular changes may be induced in the developing fetus by maternal stress are not understood.
Many of the neuropsychiatric disorders correlated with prenatal stress implicate the GABAergic system. Schizophrenia has been shown to be associated with a loss of GABAergic neurons in the hippocampus and neocortex and a change in their functioning, particularly those expressing parvalbumin and somatostatin (Hashimoto et al., 2008; Reynolds et al., 2004; Benes, 1999; Benes and Berretta, 2001). GABAergic abnormalities have also been implicated in the pathogenesis of autism and Tourette syndrome (Kataoka et al., 2010; Yip et al., 2008). The mechanisms by which these potentially critical changes in adult GABA neurons occur are not understood.
There are some data to suggest that prenatal stress and associated high levels of corticosteroids during embryonic development lead to changes in GABAergic populations in the adult brain which may reveal general pathological mechanisms involved in psychiatric disorders. Rat offspring of “stressed” mothers have fewer benzodiazepine binding sites in the amygdala and hippocampus, less GABA/benzodiazepine inhibitory activity and more sensitivity of GAD65 expression in these same regions in response to later corticosterone exposure (Barros et al., 2006; Stone et al., 2001; Weinstock, 1997) GABAergic synapses in the hypothalamus, as measured by vGAT immunocytochemistry, have also been shown to be increased (Viltart et al., 2006). Prenatal stress lowers the seizure threshold in the dorsal hippocampus (Edwards et al., 2002), suggesting a loss of inhibition of this region.
These results reflect changes in the adult population of GABAergic neurons following exposure to prenatal stress during critical periods of inhibitory neuron development. In rodents, GABAergic neurogenesis begins in the ganglionic eminence of the ventral telencephalon midway through embryonic development (~embryonic day 10–11) and continues for approximately a week (Fig 1A; for review see Corbin and Butt, 2011). GABaergic interneuron progenitors destined for the hippocampus and neocortex migrate away from the ventricular zone of the ventral telencephalon and tangentially move into the developing cortical plate in two migratory streams within the marginal zone and intermediate zone. After moving tangentially, GABAergic progenitors radially migrate and integrate into different cortical layers, a process that begins in lateral regions within 3 days of inhibitory neuron birth. Most migration is completed by birth. It is unknown what impact prenatal stress has on GABAergic progenitors that may result in abnormalities in the resulting mature neurons within adult brain networks. Some evidence for the impact of stress on GABAergic systems comes from studies in early postnatal life and adulthood in which acute and chronic stress leads to reductions in GABA signaling (review:Luscher et al., 2011). However, GABAergic developmental events on which stress could impinge are significantly different in prenatal vs postnatal periods.
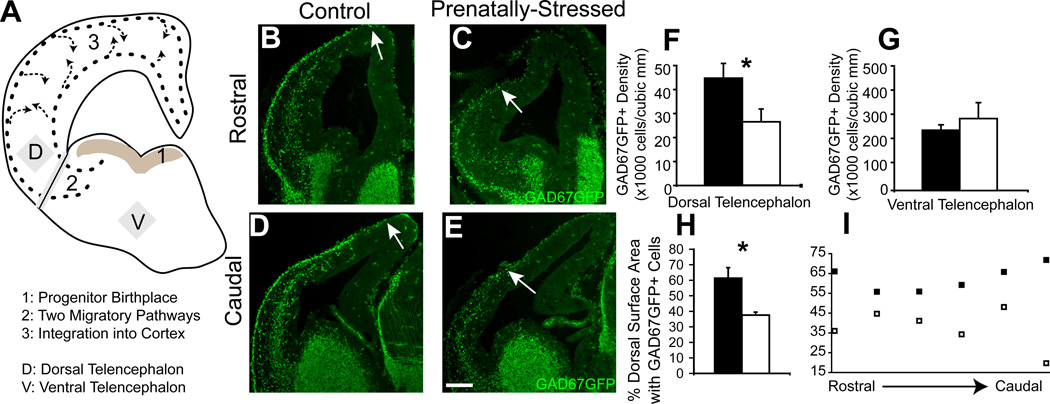
Figure 1.
Migration delay due to PS after one day. Normal events in GABAergic progenitor development are demonstrated in this diagram (A). Nonstressed offspring (B,D) and PS offspring (C,E) showed significant changes in distribution of GAD67GFP+ cells in the pathway of tangential migration across rostral (B,C) to caudal (D,E) regions of the E13 forebrain. (F,G) Stereological counts indicating that PS significantly decreased the density of GAD67GFP+ cells in the E13 dorsal telencephalon (F) but not ventral telencephalon (G). PS offspring also showed a decrease at E13 in the circumferential distribution of GAD67GFP+ cells in dorsal telencephalon (H) that was greater in caudal regions (I) (* indicates p<0.05 by two-tailed student’s t-test; arrows indicate the leading edge of migration of GAD67GFP+ cells). Scale bar=200 µm.
Prenatal stress has also been discussed as a potential major influence on neuronal migration (Schneider et al., 2002) and gestational dexamethasone treatment has been demonstrated to have an impact on migration via an actin regulatory protein, caldesmon (Fukumoto et al., 2009). Given this framework, we examined the development of precursors to inhibitory neurons in the prenatally-stressed telencephalon using GAD67GFP knock-in mice (Tamamaki et al., 2003) which consistently label GABAergic progenitors in the embryonic brain. GFP-labeled interneuron progenitor populations within the ganglionic eminence and dorsal telencephalon were evaluated at multiple embryonic time points following prenatal stress. Beginning one day after the start of prenatal stress, both the proliferation and different phases of migration of these GABAergic neuron progenitors were evaluated to assess the impact of prenatal stress.
2. Materials and Methods
2.1. Mice
GAD67-GFP(Δneo) mice (Tamamaki et al., 2003) were bred on a CD1 background. GAD67GFP+/− male mice were used for mating with CD1 females lacking the GAD67GFP knock-in gene. Timed pregnancies were monitored following detection of vaginal plug on embryonic day 0 (E0) and thirty pregnant females were singly housed from E12. All experimental procedures involving animals were performed in accordance with the Yale Animal Resources Center and Institutional Animal Care and Use Committee (IACUC) policies.
2.2. Prenatal stress
Beginning on E12, half of the pregnant female mice were subjected to acute stress within a plexiglass restraint for 45 minutes under bright lights, three times daily during the daytime light cycle (at approximately 9 am, 12:30 pm, and 4 pm). The plexiglass restraints allowed pregnant females to change positions throughout each restraint period. .
2.3. Birth-dating, tissue collection and immunocytochemistry
Offspring brain tissue for immunocytochemistry and in situ hybridization was collected on E13, E14, E15 and P0 at approximately 10 am from 3–5 litters for each time point in each condition. This permitted analysis of GABAergic cells one, two, and three days after the start, as well as during, prenatal stress, to allow an investigation of the embryonic mechanisms of this process. We also examined the day of birth to understand cumulative effects at the termination of prenatal stress. In order to birth-date GABAergic progenitors, one-time bromodeoxyuridine (BrdU) injections (100 mg/kg in a 5 mg/ml solution of 0.07N NaoH sterile filtered) were given i.p. for each pregnant female prior to the first restraint period on either E12 or E13. For offspring tissue collected at E13, BrdU was injected at E12 (25 hours earlier) and for all other experiments was injected at E13. SRY genotyping was done to ensure use of only male offspring in all analyses.
The whole head, from E13 and E14 offspring, or the brain, from E15 and P0 offspring, was first rinsed in cold RNase-free PBS and then immersion fixed and post-fixed at least 12 hours in 4% paraformaldehyde/phosphate buffered saline. Following transfer to RNase-free 20% sucrose, tissue was embedded and cryo-sectioned (Leica, CM1900, Bannockburn, Illinois) at 25 µm. Slide mounted sections were processed for staining first by blocking for 1 hour with 10% goat serum in PBS with 0.025% TritonX-100, 0.0125% Tween20 (PBS++) and then incubating for 24–48 hours at 4°C with 5% goat serum/PBS++ containing primary antibodies as follows: 2-bromodeoxyuridine (BrdU) (rat 1:500; Accurate Chemicals, OBT0030, #H8913), Ki67 (mouse monoclonal 1:200, Novacastra, NCL-L-Ki67-MM1), caspase-3 (1:500; Cell Signaling, #9661), and green fluorescent protein (GFP) (1:1000; Abcam, AB13970, #660556). Brain sections were then washed three times in PBS followed by an incubation in 5% goat serum/PBS++ containing Alexa dye-conjugated secondary antibodies (1:500-1000; Molecular Probes). Fluorescently-labeled sections were coverslipped using mounting medium with DAPI (Vector Laboratories, #H-1200).
2.4. Cell counting
Three approaches were used to evaluate the distribution of cells within the developing cortex—stereological counts, measurement of cells distributed around the external circumference of the dorsal telencephalon, and bin counting from deep to superficial layers of the cortical plate. Stereological, unbiased estimates of GAD67GFP+, GAD67GFP+/BrdU+ (E13) double-labeled cells, and Ki67+ cells within the embryonic ventral and dorsal telencephalon and the neonatal frontal cortex, hippocampal CA and hippocampal dentate gyrus were obtained with a computer running the StereoInvestigator software (Microbrightfield, Colchester, Vermont) and coupled to a Zeiss Axioskope 2 Mot Plus (Carl Zeiss, Oberkochen, Germany) equipped with a digital camera and calibrated motorized stage controller that allows precise control of y-, x and z axes. Using the optical fractionator, nuclear profiles were counted in 3-dimensional counting boxes. Every 20th coronal section was used that contained any portion of the telencephalon. Variations between counts were assessed by the Gunderson coefficients of error and data were only used when cell count error values were below 0.15.
Measurement of GAD67GFP+ cells across the external circumference of the dorsal telencephalon was done by measuring with Neurolucida (Microbrightfield) both the surface perimeter with GAD67GFP+ cells immediately underlying the pia of the cortical plate in each section and the total external circumference from most lateral to most medial extent in every coronal section. The same point in the migratory path of GAD67GFP+ cells, as they enter an organized migratory stream, was used as the most lateral extent of the cortical plate. Following the dorsal extent of the pial surface of the telencephalon, the total circumference distance was measured. The distribution of GAD67GFP+ cells across surface area was then calculated as a percent of the total circumference and this was averaged across multiple coronal sections in the brain of each offspring.
In order to evaluate distribution of GAD67GFP+ cells within the radial thickness of the embryonic cortical plate, a blind method of counting cells within standardized bins across the cortical plate was adopted. Bin counts were made using 20× Apotome Z-stack micrographs obtained on an ApoTome equipped Axiovert 200M with Axiovision 4.5 software (Carl Zeiss, Thornwood, NY, USA) for right and left sides of four to six coronal sections by an experimenter blind to the animal condition. After counts were made within equally sized bin arrays placed across the cortical plate in a lateral and dorsal sector, cell counts were grouped into a single bin at the pial surface and three other equally sized larger bins that encompassed approximately a third of the layers of the cortical plate.
To evaluate differences, results from individual offspring within a single litter were averaged and then each litter was treated as a unit for each analysis. Cell densities within embryonic ventral and dorsal telencephalon and percent of circumference containing cells were compared using a student’s t-test. Distribution of cells across the cortical plate was compared using repeated-measures ANOVA. Cell densities within neonatal cortex and hippocampus were compared using an ANOVA across gray matter regions and cell densities in the white matter were compared with a student’s t-test.
2.5. In situ hybridization and qPCR
We included tissue samples from multiple NS and PS litters for in situ hybridization and qPCR analyses, to control for litter effects of PS and the precise timing of embryonic development at tissue collection. We also included samples from embryos that both did and did not carry the GAD67GFP+ transgene. Gene expression changes were identified in non-stressed (NS) and prenatally-stressed (PS) GAD67GFP+ offspring and then substantiated in NS and PS GAD67GFP-negative offspring. We saw similar effects in both groups of animals and show here the data from GAD67GFP- embryos. Quantification of the expression of dlx1, dlx2, nkx2.1, and erbb4 was made using ventral telencephalic tissue while bdnf and fgf2 was assessed in dorsal telencephalic tissue.
Collection and sectioning of brain tissue was performed as described above for immunocytochemistry using RNAse free solutions. In situ hybridization was performed in frozen sections using the digoxigenin system as described previously (Ohkubo et al., 2004). Briefly, a riboprobe incorporating digoxygenin-labeled nucleotides was synthesized from linearized plasmids derived from the subcloned PCR fragments. Sections were fixed in 4% paraformaldehyde, washed in PBS, and dehydrated through grades of ethanol. Sections were then air dried, treated with proteinase K in buffer, fixed again, rinsed, acetylated, rinsed again in water, and air dried. Prehybridization was done with incubation in hybridization buffer followed by washes in 2X SSC and RNAase treatment. Tissue then underwent washes in 2X SSC, 50% heat-inactivated sheep serum in PBS. Sections were incubated overnight in a solution containing anti-digoxigenin Fab fragments, conjugated to alkaline phosphatase, preabsorbed with chick embryo powder to reduce background labeling. Following antibody incubation, sections were washed and incubated in developing buffer. The color reaction was carried out by incubation in developing buffer with NBT/BCIP. Finally, sections were washed in PBS and mounted in DAPI-containing mounting media.
To quantify the expression of genes involved in inhibitory neuron development, embryos were collected on E13, E14, and E15. The dorsal and ventral regions of the telencephalon were dissected out from the embryonic brains. The cerebral cortex and hippocampus were pooled in one sample from each embryo and the ganglionic eminences for a second sample, homogenized in Trizol reagent (Sigma) and frozen. After thawing, RNA was isolated using chloroform phase separation, precipitated with 75% alcohol, and re-suspended in water. RNA concentrations were determined using a Nanodrop Spectrophotometer (Thermo Scientific). cDNA was synthesized using Superscript III First Strand Synthesis Kit (Invitrogen). Quantitative PCR was carried out using Taqman Gene Assays (Applied Biosystems) for dlx1 (ID Mm00438424_m1), dlx2 (ID Mm00438427_m1), nkx2.1 (ID Mm00447558_m1), and erbb4 (ID Mm01256793_m1) using ventral telencephalic tissue and bdnf (ID Mm04230607_s1) and fgf2 (ID Mm 00433287_m1) using dorsal telencephalic tissue. Beta-actin (predeveloped) was used as an endogenous control in all cases. qPCR was run using GeneAmp PCR Mastermix (Applied Biosystems) in a StepOne™ Instrument (Applied Biosystems). The cycle number threshold for signal detection for each gene of interest for each sample were normalized to that sample’s cycle number threshold for beta-actin and the difference values in cycle number were converted to gene expression values using the formula:
[expression= 2 −(cycle number difference)].
Gene expression values were averaged within each litter and then averages of litters in each condition were compared. Graphs depict gene expression values for PS brain normalized to gene expression values for NS brain. Genes which have similar developmental patterns of expression were grouped and NS and PS levels were compared across embryonic ages and specific gene by ANOVA.
2. Results
2.1. Distribution of GABAergic cells
In order to assess GABAergic progenitor populations during their proliferation and migration, we examined the GAD67GFP+ cells in three ways: 1) density within the ventral telencephalon (their birthplace and initial migrating zone), , and the dorsal telencephalon (their target zone) (Fig. 1A), 2) their circumferential distribution in their migratory paths tangentially around the dorsal telencephalon (Fig. 1A), and 3) their radial distribution as they integrate into the cortical plate after the change in direction to radial migration, across a grid overlaying the thickness of the dorsal telencephalon (Fig. 2G, 3A).
Figure 2.
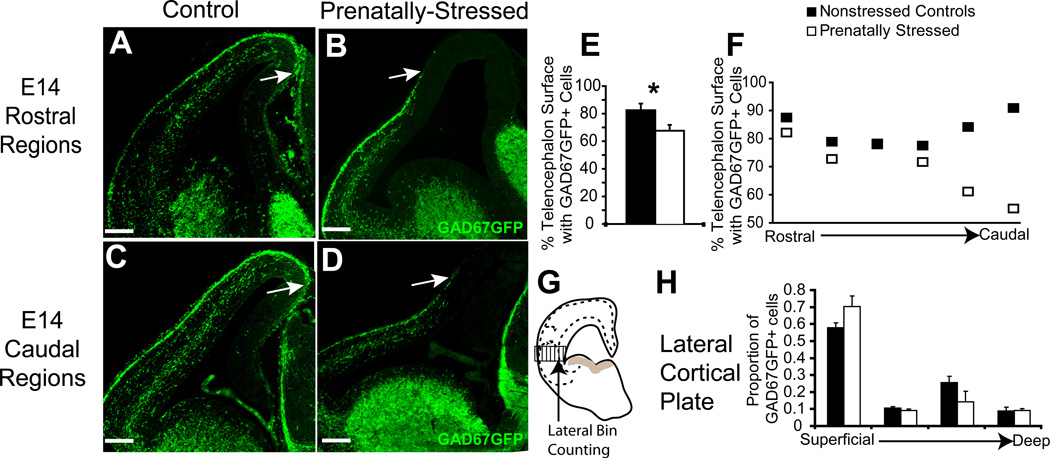
Migration delay due to PS after two days. Nonstressed offspring (A,C) and PS offspring (B,D) again showed significant changes in distribution of GAD67GFP+ cells in the pathway of tangential migration across rostral (A,B) to caudal (C,D) regions of the E14 forebrain. (E,F) Stereological counts indicating that PS offspring showed a decrease at E14 in the circumferential distribution of GAD67GFP+ cells in dorsal telencephalon (E) that was greater in caudal regions (F). (G,H) Bin-counting in lateral areas of the cortex, shown by schematic (G), demonstrated no significant difference with PS in the percentages of total GFP+ cells in different layers of cortex averaged across all coronal sections at E14 (H) (* indicates p<0.05 by two-tailed student’s t-test; arrows indicate the leading edge of migration of GAD67GFP+ cells) Scale bar=200 µm.
Figure 3.
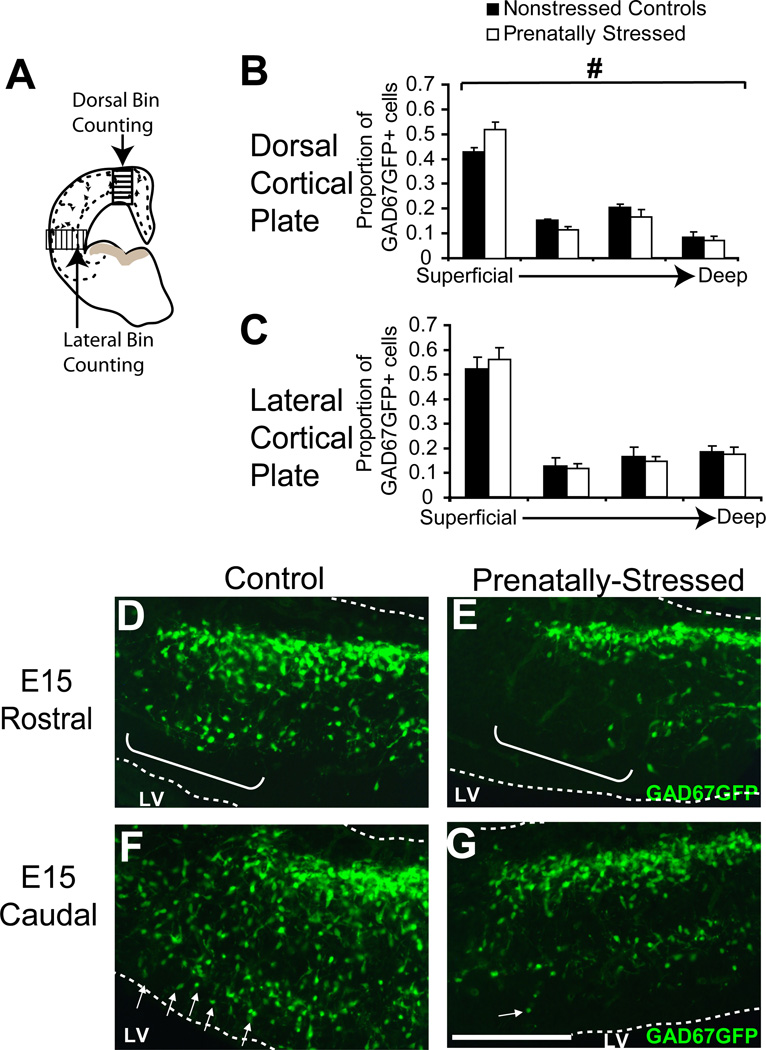
Migration delay due to PS after 3 days. (A) Schematic showing overlay of bins to count percentages of total cells in different layers in two regions, dorsal and lateral, of cortex (B,C). At E15, bin counting demonstrated that PS induced significant alterations in cortical integration of GAD67GFP+ cells in E15 dorsal cortex (B) but not in lateral (C) cortex when averages across all coronal sections were compared. (D–G) At the leading edge of migration in medial cortical sectors, nonstressed (D,F) and PS offspring (E,G) showed significant changes in radial integration of GAD67GFP+ cells in both rostral (D,E) and caudal (F,G) regions of the forebrain (brackets indicate cells at the leading edge of the deep migratory path, arrows indicate the cortically-integrating GAD67GFP+ cells in the dorsal area; LV= lateral ventricle, # indicates p<0.05 for interaction of condition by bin by ANOVA). Scale bar=200 µm.
At embryonic day 13 (E13), the distribution of GAD67GFP+ cells in the early stages of migration around the neocortex was significantly different in offspring exposed to one day of stress compared to control offspring (Fig. 1B–E). The stereological count of GAD67GFP+ cells at E13 that had entered the dorsal telencephalon of PS offspring was 48% lower (Fig. 1F; p<0.05, n=4,3). The density of GAD67GFP+ cells was unchanged in ventral telencephalon (Fig. 1G). The distribution of GABAergic progenitors within the superficial tangential migratory pathway was also altered after prenatal stress, with only 37.7% of the most superficial pathway within the cortical plate containing GAD67GFP+ cells in PS offspring compared to 56.6% in NS offspring (Fig. 1H; p<0.05, n=3;3). This distribution change was present across all levels but more prominent in more caudal regions (Fig. 1I). As a control, we found that total circumference (size of the cortex) was not significantly different between PS and NS offspring (7.6% difference, p=0.22, n=3,3).
The leading edge of GAD67GFP+ cells in the tangential migratory pathway of PS animals was also consistently less advanced at E14 (Fig. 2A–D). In the E14 telencephalon of control embryos, GABAergic precursors extended along the tangential migratory pathway 83.4% of the total circumference of the dorsal telencephalon that they will eventually occupy. However, in PS embryos, GABAergic cell distribution was only 69.1% of the dorsal telencephalic circumference (Fig. 2E, p<0.05, n=4;3). Like at E13, differences in the circumferential distribution of GABAergic precursors between controls and PS animals at E14 were greater in caudal than in rostral regions (Fig. 2F).
The radial integration of GABAergic progenitors at E14 into the cortical plate was examined in the lateral region of cortex, where integration had begun at this stage of development. By superimposing a frame divided into horizontal bins over the thickness of cortex, the distribution of cells in layers of neocortex was quantified and averaged across all rostral to caudal sections (Fig. 2G). As expected, cells were most numerous in the two streams within the cortical plate, reflecting the two migratory pathways for cortical GABAergic progenitors: the most superficial region in the marginal zone (the first bin) and a deep region above the subventricular zone (the third bin) (Fig. 2G). There was no generalized effect of PS on this distribution at E14.
At E15, PS did affect radial migration of GABAergic progenitors as these cells reached the cortical plate and became incorporated into different layers (Fig 3A). In the dorsal region, GAD67GFP+ cells were redistributed in significantly greater proportions to the superficial layer after PS, with fewer cells in deeper cortical layers (all other bins) (Fig. 3B; p<0.05, n=4,4). In lateral regions, similar to E14, there was no difference in the distribution of cells in the cortical plate of PS offspring (Fig. 3C). The effect on cortical integration in the dorsal region at E15 was also seen for absolute cell number across bins reflecting true increases and decreases in cell number (data not shown). At E15, analysis also showed qualitatively that tangential GAD67GFP+ cell distribution was again delayed by PS in the most medial region of cortex (Fig. 3D–G). This delay was reflected by GAD67GFP+ cells in the deeper migratory path being clearly less advanced in PS animals (Fig. 3E,G brackets and arrows) than in NS animals (Fig. 3D,F).
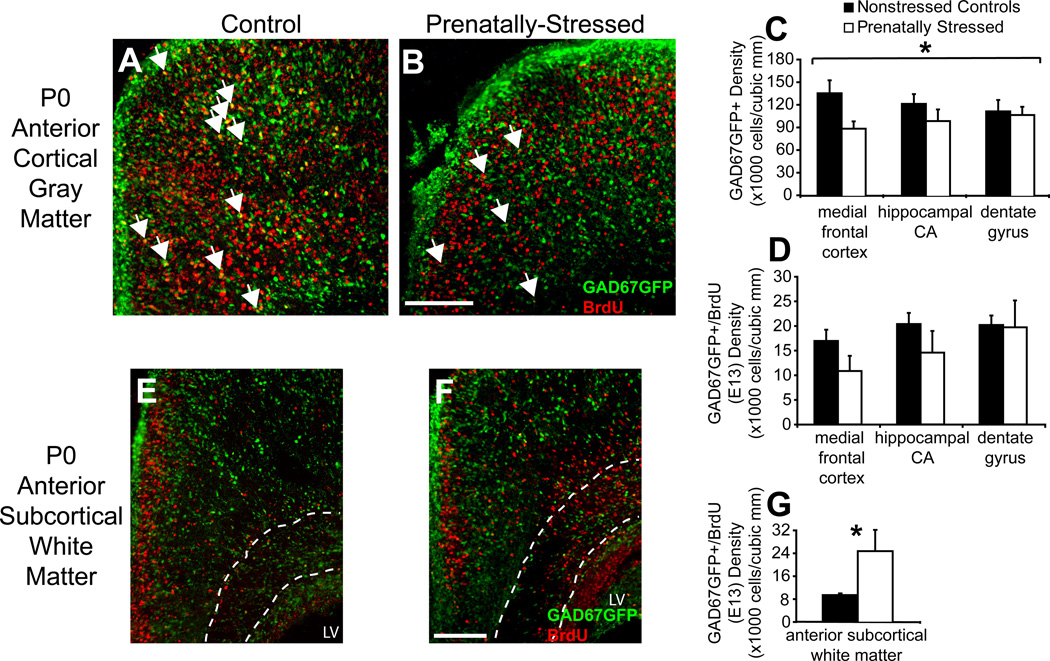
Lastly, examination of the telencephalon on the day of birth (P0) demonstrated that GAD67GFP+ density was reduced by PS in total cortical gray matter by 23% (p<0.05; n=4,4), which did not differ across rostral to caudal levels. Analysis of medial regions of cortex, hypothesized to be most affected by migration disruptions, did not show consistent, significant decreases across regions—ANOVA analysis across regions and conditions showed a significant interaction (p<0.05; n=5,4). Post-hoc analysis showed that this interaction was mainly due to a 37% reduction in medial frontal cortex GAD67GFP+ cell density by PS (Fig. 4A,B; p<0.05; n=5,4) without significant changes in hippocampal regions. Early-born cells labeled with BrdU at E13 were not decreased significantly by PS in any region, although reductions were of similar magnitude to those for total GAD67GFP+ cells (Fig. 4D). Interestingly, early born GAD67GFP+ cells appeared to be redistributed at least in part from gray matter regions to the anterior subcortical white matter, a region that serves as a migratory pathway for progenitors targeted to frontal cortical regions (Fig. 4E,F). Within this region, there was a 59% increase in GAD67-GFP+/E13 BrdU+ double-labeled cells (Fig. 4G; p<0.05, n=3,3). There was no deficit in early born cells across the total cortical volume. These findings implicate a persistent change in migration to the most medial regions of the dorsal telencephalon due to prenatal stress that is more severe for early-born GABAergic cells.
Figure 4.
Migration delay due to PS after 7 days. Nonstressed offspring (A) and PS offspring (B) showed significant changes in density of GAD67GFP+ cells and GAD67GFP+/BrdU+(E13) cells in medial frontal regions of the P0 cortex. PS significantly affected medial regions of the dorsal telencephalon at P0 with decreased density of GAD67GFP+ cells (C) but more substantial decreases GAD67GFP+/BrdU+(E13) cells (D). PS offspring (F) compared to nonstressed controls (E) also showed an increase in density of GAD67GFP+/BrdU+(E13) cells in anterior subcortical white matter, traced by dotted lines (G) (arrows indicate double-labeled GAD67GFP+/BrdU+ cells; LV=lateral ventricle, `#` indicates p<0.05 for interaction of condition by region by ANOVA, * indicates p<0.05 by two-tailed student’s t-test). Scale bar=200 µm.
2.2. GABAergic cell survival and proliferation
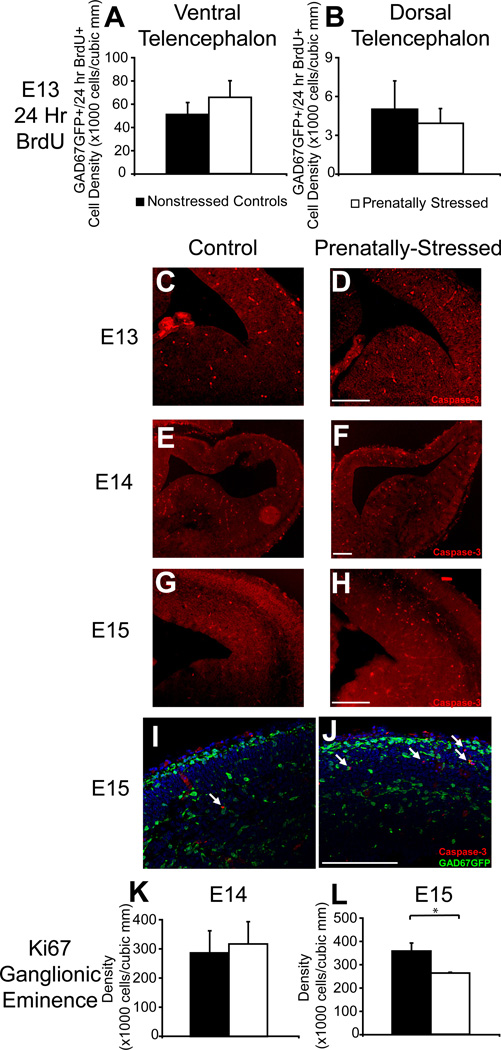
We examined whether changes in GABAergic progenitor cell numbers from E13 onwards following prenatal stress were due to changes in the survival or proliferation of these cells. Generally, these early processes were not altered by prenatal stress. Measures of BrdU labeled GAD67GFP+ cells at E13 after BrdU incorporation at E12 show that 24 hour survival of these early born cells was not deficient in PS embryos in either ventral or dorsal telencephalon (Fig. 5A,B). The overall population of 24 hour BrdU labeled cells within the ventral telencephalon at E13 was also not significantly decreased demonstrating that general cell survival in this region, regardless of expression of GAD67, was not affected by PS (data not shown). Survival was also qualitatively assessed with caspase-3 staining which showed no significant increase at E13 or E14 (Fig. 5C–F). Some increase in dorsal caspase-3 staining at E15 was found which was localized to non-GABAergic progenitors as demonstrated by a lack of co-labeling with GAD67GFP (Fig. 5G–J).
Figure 5.
No changes in GABAergic survival or proliferation due to PS. At E13, NS and PS offspring showed no significant differences in survival of GAD67GFP+ cells born at E12 in the ventral (A) or dorsal (B) telencephalon. NS (C,E,G,I) and PS (D,F,H,J) offspring showed no difference in apoptotic cells at E13 (C,D) or E14 (E,F) but an increase in apoptosis at E15 (G,H) that was specific to non-GABAergic cells (I,J). Proliferative markers in the ganglionic eminence of NS and PS offspring were similar at E13 (K) and showed a trend difference at E14 (L) that was greater in caudal regions (H) (* indicates p<0.05 by two-tailed student’s t-test; arrows indicate caspase-3+ cells that show no labeling with GAD67GFP). Scale bar=200 µm.
To examine whether proliferation of GABAergic progenitors was affected by PS, we assessed the cell cycle marker, Ki67, within the ganglionic eminences of E14 and E15 mice. The density of Ki67+ cells at E14 was not decreased in the ventral telencephalon of prenatally stressed offspring (Fig. 5K). At E15, there was a significant reduction in density of Ki67+ cells (Fig. 5L, p<0.05, n=3;3) suggesting a possible late-onset effect of prenatal stress on cell proliferation in the ganglionic eminence.
2.3. Mechanisms of GABAergic cell development
In order to understand the molecular mechanism by which prenatal stress altered inhibitory neuron migration and integration in the cortex, we examined by in situ hybridization a selected group of transcription factors known to play a role in neuronal differentiation and GABAergic cell migration, namely, ngn2, mash1, dlx1, dlx2, and nkx2.1.
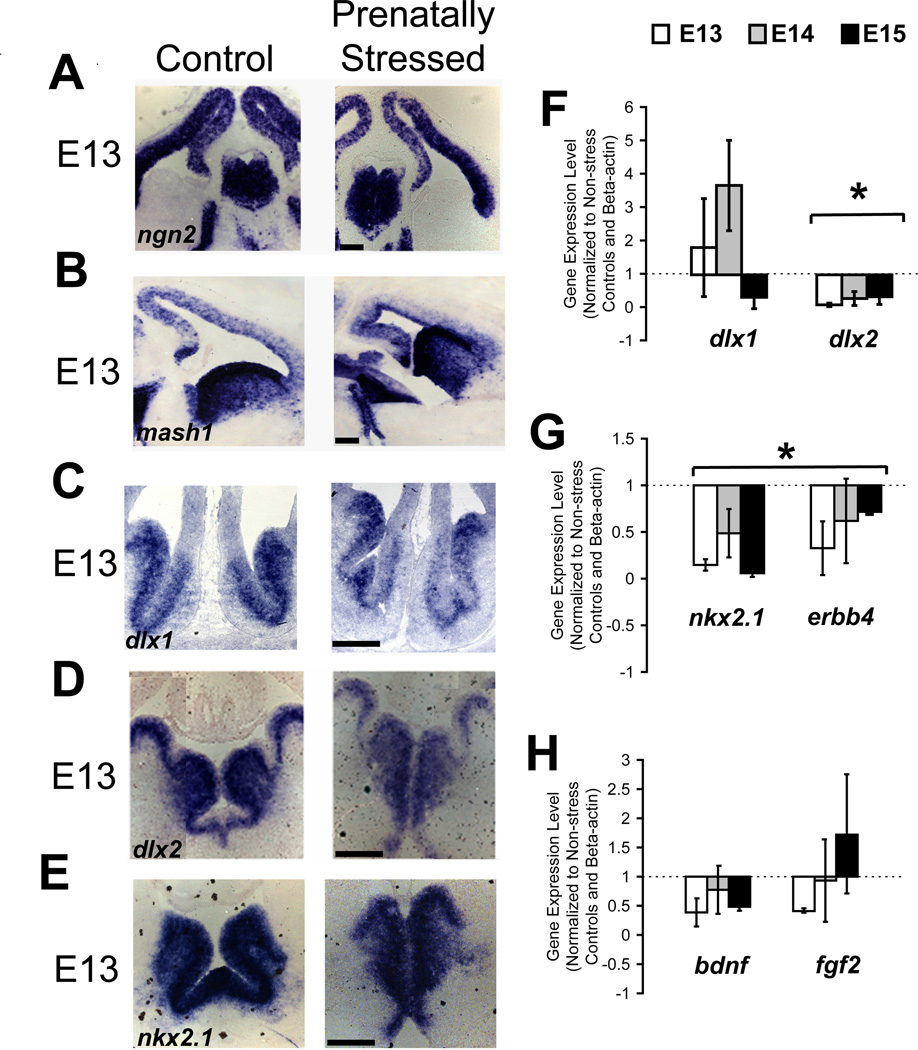
PS exposure caused no apparent difference in expression at E13–E16 of mash1 and ngn2, two transcription factors that are critical for neural determination and specification of ventral and dorsal telencephalic phenotypes, respectively, by in situ hybridization (Fig. 6A,B show representative findings from E13). These data suggest that neither the differentiation of inhibitory nor excitatory progenitors were affected by prenatal stress. In contrast, In situ hybridization for dlx1 and dlx2, two factors that promote cortical GABAergic phenotype and interneuron migration, demonstrated that both had altered expression in the ventral telencephalon of PS embryos (Fig. 6C,D). The changes in dlx2 were more apparent than in dlx1 by qualitative assessment. Quantification and comparison of gene expression levels to normalized controls by qPCR showed no significant alteration in dlx1 expression by prenatal stress, but consistently decreased dlx2 at all ages (Fig. 6F; p<0.05; n=4,4 at E13; 4,3 at E14; 4,3 at E15).
Figure 6.
Decreased transcription factors (TFs) for GABAergic migration due to PS. NS and PS offspring did not differ in TFs determining dorsal-ventral fate (A, B) at E13. Variable decreases in expression with PS without changes in localization was shown at E13 for TFs involved in GABAergic progenitor migration, dlx1 (C), dlx2 (D), and nkx2.1 (E). Quantification of gene expression changes with PS in the ventral telencephalon by qPCR in basal ganglia for these TFs demonstrated that dlx1 showed no consistent alteration at E13 (F, left) but dlx2 (F, right), nkx2.1 (G, left), and their downstream target erbb4 (G, right) were decreased at all embryonic time points. Quantification of gene expression in dorsal telencephalon for bdnf and fgf2 showed no significant changes with PS (H) (* indicates p<0.05 for main effect of condition by one-way ANOVA, *^ indicates p<0.05 for interaction of condition by age by two-way ANOVA). Scale bar=200 µm.
Expression of nkx2.1 was decreased qualitatively at E13 (Fig. 6E). Downstream of nkx2.1 via other transcription factors is erbb4, a receptor for the chemoattractant neuregulin-1. These two factors were consistently downregulated following prenatal stress (Fig. 6G; p<0.05; n=3,4 at E13; 3,3 at E14; 3,3 at E15 for nkx2.1 and n=4,4 at E13; 3,3 at E14; 3,3 at E15 for erbb4) demonstrating possible mechanisms by which GABAergic progenitor migration may be affected. In contrast, we found no significant systematic change in either brain derived neurotropic factor (bdnf), which affects the migration of GABAergic progenitors by stimulation of cell motility, or fibroblast growth factor (fgf2) which has a role in the developing brain not known to be specific to inhibitory progenitors (Fig. 6H). However, the variability in the data from the use of embryonic brain across multiple litters may conceal more subtle alterations.
3. Discussion
Here we demonstrate that a well-validated model of prenatal stress in mice resulted in changes in the distribution of GAD67GFP+ GABAergic cells within the dorsal telencephalon of the offspring that support a model in which stress impairs the migration of GABAergic progenitors and their subsequent integration in the cerebral cortex. This change in the migration of GABAergic progenitors began after only one day of stress and was still observable after the last day of stress. In addition, there were no changes in proliferation or survival of these early born cells during the first three days of prenatal stress. Lastly, we demonstrated that concurrent with this change in GABAergic progenitor distribution, the expression of the transcription factors dlx2 and nkx2.1 and one of their downstream targets, erbb4, was significantly and consistently decreased during prenatal stress suggesting a mechanism by which prenatal stress impacts GABAergic neuron development.
In the developing cortical plate of prenatally stressed animals, the density of GABAergic progenitors was reduced and the leading edge of migration in the most superficial migratory pathway was less advanced. Both of these findings suggest that tangential movement of GABAergic progenitors was delayed by prenatal stress. We concluded that a delay in migration was the most likely process to account for these changes, by ruling out any decrease in the survival of GABAergic progenitors and by demonstrating no alteration in proliferation within the ganglionic eminences after prenatal stress, both of which could have contributed to the finding that fewer GAD67GFP+ cells were entering the dorsal telencephalon. Previous work has demonstrated that prenatal corticosteroids do alter the migration of cortical excitatory progenitors (Fukumoto et al., 2009), providing converging evidence that migration of embryonic progenitors is susceptible to this type of environmental impact. The timing of neuronal migration coincides with periods during which rodents and nonhuman and human primates have demonstrated susceptibility to prenatal stress—the last gestational week for rodents and the late first and early second trimester for humans (Davis and Sandman, 2010; Schneider et al., 2002; Stone et al., 2001). Because of consistent effects seen on migration from E13–E15, these findings also suggest that GABAergic progenitor migration was influenced by mechanisms occurring at the earliest time point of prenatal stress (on E12).
The alterations seen in GABAergic progenitors from E13 to E15 were more significant in more caudal regions of the embryonic telencephalon. This could reflect an amplification of the effects on migration of early GABAergic progenitors which must travel greater distances from medial ganglionic eminence to caudal regions (Miyoshi et al., 2010). However, there may also be region-specific components of GABAertic progenitor migration that have not been identified here. Cell-autonomous components of MGE- and CGE-derived progenitors may be differentially affected by prenatal stress and/or signaling molecules such as TBR2, NRG1, or CXCL12 (Neddens and Buonanno, 2010; Sessa et al., 2010; Tiveron et al., 2006) expressed in rostral and caudal regions of the dorsal telencephalon that are targets for GABAergic progenitors may be affected by prenatal stress in a regionally-specific manner.
We found that in the most dorsal regions of the cortical plate, prenatal stress resulted in significantly less integration of GAD67GFP+ cells radially into all layers. This suggests that the processes induced by PS influencing GABAergic progenitors in the tangential migratory processes were generally continued in the next stage of these cells’ development. The greater effect seen in dorsal regions at E15 than in lateral regions at either E14 or E15 also suggests that the effects of prenatal stress are not more prominent in regions that have earlier arriving precursors; if this was the most critical aspect of the effects of stress, deficits in the E14 lateral cortical plate would have been comparable to those seen in the E15 dorsal cortical plate, as both evaluated early arriving precursors. Instead, cell migration over longer distances and integration into more dorsal regions may be processes more affected by PS.
GABAergic cells were persistently deficient in neonatal (P0) dorsal telencephalon, a result which was disproportionately greater in medial frontal regions. Medial frontal cortex showed a greater deficiency of GAD67GFP+ cells than in overall cortex and more posterior hippocampal regions. Thus, despite findings at E13–E14 that posterior regions were more affected by PS, by P0 the more anterior regions were more significantly affected. An increase in early-born progenitors in the deep migratory white matter pathway after PS converges with other evidence from our study suggesting a delayed migration into medial cortical regions. The sensitivity of these early-born cells to the effects of prenatal stress most likely reflects the importance of timing of when these cells were born and the distance over which they traveled to reach their targets.
Altered migration of GABAergic progenitors could be a source of significant changes in inhibitory functioning in the mature telencephalon following prenatal stress (Barros et al., 2006; Edwards et al., 2002; Stone et al., 2001; Viltart et al., 2006; Weinstock, 1997). Alterations in the functioning or location of inhibitory cells during development even temporarily, however, could independently influence the development of other neural circuits, with persistent consequences into adulthood.
Our findings that proliferation within the ganglionic eminence was not significantly affected until prenatal stress had been continued more chronically (i.e. a significant change at E15 after 3 days) broadens our understanding of how stress or exogenous corticosteroids affect dividing cells. While prenatal stress has been shown to influence later postnatal proliferation and neurogenesis (Lemaire et al., 2006; Ulupinar et al., 2006), no previous studies have addressed how early embryonic neurogenesis is affected. The initial resistance of progenitor proliferation to stress may be due to compensatory mechanisms within the ventral telencephalon or the lack of the specific glucocorticoid responsive elements expressed at early time points that influence proliferation. Similarly, our findings that apoptosis of GABAergic progenitors was not a significant effect of prenatal stress suggest that these cells are not sensitive to the pro-apoptotic effects of stress seen in other forebrain regions (Fujioka et al., 1999).
Transcription factors involved in GABAergic cell migration were altered by prenatal stress in expression level but not in spatial distribution. As early as 24 hours after stress was induced and persisting at least into the 4th day of prenatal stress, expression of dlx2, nkx2.1 and its downstream target, erbb4, were reduced. Dlx1 and Dlx2 both promote migration of inhibitory precursors through transcriptional regulation of other transcription factors such as lhx6 and arx (Anderson et al., 1997; Cobos et al., 2007; Colasante et al., 2008) but also through independent regulation of other genes involved in migration (Fulp et al., 2008). Nkx2.1 also regulates inhibitory precursor migration (Butt et al., 2008; Nobrega-Pereira et al., 2008). The persistent downregulation of dlx2 and nkx2.1 may be the mechanism by which migration is delayed in prenatally stressed offspring, although the promoter elements in these genes that are directly responding to glucocorticoids or other components of prenatal stress response in the ventral telencephalon have not been identified. Possible increases in expression of dlx1 after stress may represent a compensatory response in inhibitory progenitors. All of these transcription factors are also important for the differentiation and fate of inhibitory precursors suggesting that migration changes may occur simultaneously with other alterations of GABAergic progenitors, i.e., a change in cell phenotype. For example, the ability of these cells to make GABA may be reduced due to the reduction of dlx2, which could, in turn, alter activation of GABAA receptors, significantly affecting the migration of GABAergic progenitors (Cuzon et al., 2006). The similarity of mash1 expression in non-stressed and prenatally-stressed mice did suggest that the general ventral fate of these cells was not disrupted (Fode et al., 2000). While other prenatal environmental exposures with relevance for later behavioral disorders have been shown to alter transcription factors of the ganglionic eminence (Godin et al., 2011; Oskvig et al., 2012), this is the first data showing that prenatal stress affects early regulators of cortical inhibitory neurons.
Our finding does not rule out the possibility that prenatal stress may also influence the migration of GABAergic progenitors through other mechanisms, such as excess expression of the cell motility protein caldesmon involved in radial migration of cortical excitatory progenitors (Fukumoto et al., 2009). Growth factors are also a potential mediator of the effects of prenatal stress on GABAergic progenitors. We demonstrated no significant change in bdnf, a growth factor that acts as a permissive cue for interneuron migration (Polleux et al., 2002) and has been shown to be altered postnatally by prenatal stress (Van den Hove et al., 2006). However, further examinations of the contribution of growth factors to the developmental mechanisms occurring during prenatal stress are warranted.
Conclusions
The effects of prenatal stress on inhibitory neuron precursor migration may demonstrate one component of the developmental pathophysiology of stress-related behavioral problems. Alterations in the migration of embryonic neuronal precursors may arise from environmental influences, genetic variation or the interaction of these two. One gene implicated in trait anxiety, MAMDC1 or MDGA1, is involved in neuronal migration (van den Oord et al., 2008; Takeuchi and O'Leary, 2006) suggesting that a subset of anxiety problems, which have links to prenatal stress (Bergman et al., 2007; O'Connor et al., 2002; Vallee et al., 1997) may result from altered neuronal migration within the brain. ErbB4, which we report decreased by prenatal stress, and its ligand Nrg-1 affect parvalbumin+ cell migration (Flames et al., 2004; Martini et al., 2009; Neddens and Buonanno, 2010) and the genes for these proteins are also associated with schizophrenia (Flames et al., 2004). Mouse models of schizophrenia, including the 22q11 chromosomal deletion and the DISC-1 mutation, have been shown to have a significant impairment in neuron migration (Meechan et al., 2009; Meyer and Morris, 2009). Together with data from our prenatal stress model, these findings suggest that the etiology of psychiatric disorders may occur during early phases of development with specific implication for the migration of neurons that occurs during this period.
Acknowledgments
We would like to acknowledge Rachael Couture and Shawna Ellis for technical assistance and members of the Vaccarino lab for helpful discussion. This work was supported by National Institutes Health Grants K08 MH086812-01 (HES), T32 MH018268 (HES), R25 MH071584 (HES), R25 MH077823 (HES), and R01 MH067715 (FV), the Harris Professorship fund (FV), Brain and Behavior Research Foundation NARSAD Young Investigator Award from the Mortimer D. Sackler Psychobiology Program (HES), American Academy of Child and Adolescent Psychiatry Pilot Research Award (HES), and an American Psychiatric Institute for Research and Education/Wyeth Pharmaceuticals Fellowship (HES).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson SA, Eisenstat DD, Shi L, Rubenstein JL. Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science. 1997;278:474–476. doi: 10.1126/science.278.5337.474. [DOI] [PubMed] [Google Scholar]
- Barros VG, Rodriguez P, Martijena ID, Perez A, Molina VA, Antonelli MC. Prenatal stress and early adoption effects on benzodiazepine receptors and anxiogenic behavior in the adult rat brain. Synapse. 2006;60:609–618. doi: 10.1002/syn.20336. [DOI] [PubMed] [Google Scholar]
- Benes FM. Evidence for altered trisynaptic circuitry in schizophrenic hippocampus. Biol Psychiatry. 1999;46:589–599. doi: 10.1016/s0006-3223(99)00136-5. [DOI] [PubMed] [Google Scholar]
- Benes FM, Berretta S. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology. 2001;25:1–27. doi: 10.1016/S0893-133X(01)00225-1. [DOI] [PubMed] [Google Scholar]
- Bergman K, Sarkar P, O'Connor TG, Modi N, Glover V. Maternal stress during pregnancy predicts cognitive ability and fearfulness in infancy. J Am Acad Child Adolesc Psychiatry. 2007;46:1454–1463. doi: 10.1097/chi.0b013e31814a62f6. [DOI] [PubMed] [Google Scholar]
- Butt SJ, Sousa VH, Fuccillo MV, Hjerling-Leffler J, Miyoshi G, Kimura S, Fishell G. The requirement of Nkx2-1 in the temporal specification of cortical interneuron subtypes. Neuron. 2008;59:722–732. doi: 10.1016/j.neuron.2008.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobos I, Borello U, Rubenstein JL. Dlx transcription factors promote migration through repression of axon and dendrite growth. Neuron. 2007;54:873–888. doi: 10.1016/j.neuron.2007.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colasante G, Collombat P, Raimondi V, Bonanomi D, Ferrai C, Maira M, Yoshikawa K, Mansouri A, Valtorta F, Rubenstein JL, Broccoli V. Arx is a direct target of Dlx2 and thereby contributes to the tangential migration of GABAergic interneurons. J Neurosci. 2008;28:10674–10686. doi: 10.1523/JNEUROSCI.1283-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin JG, Butt SJ. Developmental mechanisms for the generation of telencephalic interneurons. Dev Neurobiol. 2011;71:710–732. doi: 10.1002/dneu.20890. [DOI] [PubMed] [Google Scholar]
- Cuzon VC, Yeh PW, Cheng Q, Yeh HH. Ambient GABA promotes cortical entry of tangentially migrating cells derived from the medial ganglionic eminence. Cereb Cortex. 2006;16:1377–1388. doi: 10.1093/cercor/bhj084. [DOI] [PubMed] [Google Scholar]
- Davis EP, Sandman CA. The timing of prenatal exposure to maternal cortisol and psychosocial stress is associated with human infant cognitive development. Child Dev. 2010;81:131–148. doi: 10.1111/j.1467-8624.2009.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards HE, Dortok D, Tam J, Won D, Burnham WM. Prenatal stress alters seizure thresholds and the development of kindled seizures in infant and adult rats. Horm Behav. 2002;42:437–447. doi: 10.1006/hbeh.2002.1839. [DOI] [PubMed] [Google Scholar]
- Flames N, Long JE, Garratt AN, Fischer TM, Gassmann M, Birchmeier C, Lai C, Rubenstein JL, Marin O. Short- and long-range attraction of cortical GABAergic interneurons by neuregulin-1. Neuron. 2004;44:251–261. doi: 10.1016/j.neuron.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Fode C, Ma Q, Casarosa S, Ang SL, Anderson DJ, Guillemot F. A role for neural determination genes in specifying the dorsoventral identity of telencephalic neurons. Genes & Development. 2000;14:67–80. [PMC free article] [PubMed] [Google Scholar]
- Fujioka T, Sakata Y, Yamaguchi K, Shibasaki T, Kato H, Nakamura S. The effects of prenatal stress on the development of hypothalamic paraventricular neurons in fetal rats. Neuroscience. 1999;92:1079–1088. doi: 10.1016/s0306-4522(99)00073-1. [DOI] [PubMed] [Google Scholar]
- Fukumoto K, Morita T, Mayanagi T, Tanokashira D, Yoshida T, Sakai A, Sobue K. Detrimental effects of glucocorticoids on neuronal migration during brain development. Mol Psychiatry. 2009;14:1119–1131. doi: 10.1038/mp.2009.60. [DOI] [PubMed] [Google Scholar]
- Fulp CT, Cho G, Marsh ED, Nasrallah IM, Labosky PA, Golden JA. Identification of Arx transcriptional targets in the developing basal forebrain. Hum Mol Genet. 2008;17:3740–3760. doi: 10.1093/hmg/ddn271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godin EA, Dehart DB, Parnell SE, O'Leary-Moore SK, Sulik KK. Ventromedian forebrain dysgenesis follows early prenatal ethanol exposure in mice. Neurotoxicol Teratol. 2011;33:231–239. doi: 10.1016/j.ntt.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Arion D, Unger T, Maldonado-Aviles JG, Morris HM, Volk DW, Mirnics K, Lewis DA. Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2008;13:147–161. doi: 10.1038/sj.mp.4002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huot RL, Brennan PA, Stowe ZN, Plotsky PM, Walker EF. Negative affect in offspring of depressed mothers is predicted by infant cortisol levels at 6 months and maternal depression during pregnancy, but not postpartum. Ann N Y Acad Sci. 2004;1032:234–236. doi: 10.1196/annals.1314.028. [DOI] [PubMed] [Google Scholar]
- Kataoka Y, Kalanithi PS, Grantz H, Schwartz ML, Saper C, Leckman JF, Vaccarino FM. Decreased number of parvalbumin and cholinergic interneurons in the striatum of individuals with Tourette syndrome. J Comp Neurol. 2010;518:277–291. doi: 10.1002/cne.22206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King S, Laplante D, Joober R. Understanding putative risk factors for schizophrenia: retrospective and prospective studies. J Psychiatry Neurosci. 2005;30:342–348. [PMC free article] [PubMed] [Google Scholar]
- Kinney DK, Yurgelun-Todd DA, Tohen M, Tramer S. Pre- and perinatal complications and risk for bipolar disorder: a retrospective study. J Affect Disord. 1998;50:117–124. doi: 10.1016/s0165-0327(98)00015-9. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Dolnansky ES, Hardin MT, Clubb M, Walkup JT, Stevenson J, Pauls DL. Perinatal factors in the expression of Tourette's syndrome: an exploratory study. J Am Acad Child Adolesc Psychiatry. 1990;29:220–226. doi: 10.1097/00004583-199003000-00010. [DOI] [PubMed] [Google Scholar]
- Lemaire V, Lamarque S, Le Moal M, Piazza PV, Abrous DN. Postnatal stimulation of the pups counteracts prenatal stress-induced deficits in hippocampal neurogenesis. Biol Psychiatry. 2006;59:786–792. doi: 10.1016/j.biopsych.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Luscher B, Shen Q, Sahir N. The GABAergic deficit hypothesis of major depressive disorder. Mol Psychiatry. 2011;16:383–406. doi: 10.1038/mp.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini FJ, Valiente M, Lopez Bendito G, Szabo G, Moya F, Valdeolmillos M, Marin O. Biased selection of leading process branches mediates chemotaxis during tangential neuronal migration. Development. 2009;136:41–50. doi: 10.1242/dev.025502. [DOI] [PubMed] [Google Scholar]
- Martini J, Knappe S, Beesdo-Baum K, Lieb R, Wittchen HU. Anxiety disorders before birth and self-perceived distress during pregnancy: associations with maternal depression and obstetric, neonatal and early childhood outcomes. Early Hum Dev. 2010;86:305–310. doi: 10.1016/j.earlhumdev.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Meechan DW, Tucker ES, Maynard TM, LaMantia AS. Diminished dosage of 22q11 genes disrupts neurogenesis and cortical development in a mouse model of 22q11 deletion/DiGeorge syndrome. Proc Natl Acad Sci U S A. 2009;106:16434–16445. doi: 10.1073/pnas.0905696106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer KD, Morris JA. Disc1 regulates granule cell migration in the developing hippocampus. Hum Mol Genet. 2009;18:3286–3297. doi: 10.1093/hmg/ddp266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi G, Hjerling-Leffler J, Karayannis T, Sousa VH, Butt SJ, Battiste J, Johnson JE, Machold RP, Fishell G. Genetic fate mapping reveals that the caudal ganglionic eminence produces a large and diverse population of superficial cortical interneurons. J Neurosci. 2010;30:1582–1594. doi: 10.1523/JNEUROSCI.4515-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk C, Sloan RP, Myers MM, Ellman L, Werner E, Jeon J, Tager F, Fifer WP. Fetal heart rate reactivity differs by women's psychiatric status: an early marker for developmental risk? J Am Acad Child Adolesc Psychiatry. 2004;43:283–290. doi: 10.1097/00004583-200403000-00009. [DOI] [PubMed] [Google Scholar]
- Neddens J, Buonanno A. Selective populations of hippocampal interneurons express ErbB4 and their number and distribution is altered in ErbB4 knockout mice. Hippocampus. 2010;20:724–744. doi: 10.1002/hipo.20675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobrega-Pereira S, Kessaris N, Du T, Kimura S, Anderson SA, Marin O. Postmitotic Nkx2-1 controls the migration of telencephalic interneurons by direct repression of guidance receptors. Neuron. 2008;59:733–745. doi: 10.1016/j.neuron.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor TG, Heron J, Golding J, Beveridge M, Glover V. Maternal antenatal anxiety and children's behavioural/emotional problems at 4 years. Report from the Avon Longitudinal Study of Parents and Children. Br J Psychiatry. 2002;180:502–508. doi: 10.1192/bjp.180.6.502. [DOI] [PubMed] [Google Scholar]
- O'Connor TG, Heron J, Golding J, Glover V. Maternal antenatal anxiety and behavioural/emotional problems in children: a test of a programming hypothesis. J Child Psychol Psychiatry. 2003;44:1025–1036. doi: 10.1111/1469-7610.00187. [DOI] [PubMed] [Google Scholar]
- Oskvig DB, Elkahloun AG, Johnson KR, Phillips TM, Herkenham M. Maternal immune activation by LPS selectively alters specific gene expression profiles of interneuron migration and oxidative stress in the fetus without triggering a fetal immune response. Brain Behav Immun. 2012;26:623–634. doi: 10.1016/j.bbi.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polleux F, Whitford KL, Dijkhuizen PA, Vitalis T, Ghosh A. Control of cortical interneuron migration by neurotrophins and PI3-kinase signaling. Development. 2002;129:3147–3160. doi: 10.1242/dev.129.13.3147. [DOI] [PubMed] [Google Scholar]
- Reynolds GP, Abdul-Monim Z, Neill JC, Zhang ZJ. Calcium binding protein markers of GABA deficits in schizophrenia--postmortem studies and animal models. Neurotox Res. 2004;6:57–61. doi: 10.1007/BF03033297. [DOI] [PubMed] [Google Scholar]
- Ronald A, Butcher LM, Docherty S, Davis OS, Schalkwyk LC, Craig IW, Plomin R. A genome-wide association study of social and non-social autistic-like traits in the general population using pooled DNA, 500 K SNP microarrays and both community and diagnosed autism replication samples. Behav Genet. 2010;40:31–45. doi: 10.1007/s10519-009-9308-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider ML, Moore CF, Kraemer GW, Roberts AD, DeJesus OT. The impact of prenatal stress, fetal alcohol exposure, or both on development: perspectives from a primate model. Psychoneuroendocrinology. 2002;27:285–298. doi: 10.1016/s0306-4530(01)00050-6. [DOI] [PubMed] [Google Scholar]
- Sessa A, Mao CA, Colasante G, Nini A, Klein WH, Broccoli V. Tbr2-positive intermediate (basal) neuronal progenitors safeguard cerebral cortex expansion by controlling amplification of pallial glutamatergic neurons and attraction of subpallial GABAergic interneurons. Genes Dev. 2010;24:1816–1826. doi: 10.1101/gad.575410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone DJ, Walsh JP, Sebro R, Stevens R, Pantazopolous H, Benes FM. Effects of pre- and postnatal corticosterone exposure on the rat hippocampal GABA system. Hippocampus. 2001;11:492–507. doi: 10.1002/hipo.1066. [DOI] [PubMed] [Google Scholar]
- Takeuchi A, O'Leary DD. Radial migration of superficial layer cortical neurons controlled by novel Ig cell adhesion molecule MDGA1. J Neurosci. 2006;26:4460–4464. doi: 10.1523/JNEUROSCI.4935-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamamaki N, Yanagawa Y, Tomioka R, Miyazaki J, Obata K, Kaneko T. Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. J Comp Neurol. 2003;467:60–79. doi: 10.1002/cne.10905. [DOI] [PubMed] [Google Scholar]
- Tiveron MC, Rossel M, Moepps B, Zhang YL, Seidenfaden R, Favor J, Konig N, Cremer H. Molecular interaction between projection neuron precursors and invading interneurons via stromal-derived factor 1 (CXCL12)/CXCR4 signaling in the cortical subventricular zone/intermediate zone. J Neurosci. 2006;26:13273–13278. doi: 10.1523/JNEUROSCI.4162-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulupinar E, Yucel F, Ortug G. The effects of prenatal stress on the Purkinje cell neurogenesis. Neurotoxicol Teratol. 2006;28:86–94. doi: 10.1016/j.ntt.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Vallee M, Mayo W, Dellu F, Le Moal M, Simon H, Maccari S. Prenatal stress induces high anxiety and postnatal handling induces low anxiety in adult offspring: correlation with stress-induced corticosterone secretion. J Neurosci. 1997;17:2626–2636. doi: 10.1523/JNEUROSCI.17-07-02626.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Hove DL, Steinbusch HW, Scheepens A, Van de Berg WD, Kooiman LA, Boosten BJ, Prickaerts J, Blanco CE. Prenatal stress and neonatal rat brain development. Neuroscience. 2006;137:145–155. doi: 10.1016/j.neuroscience.2005.08.060. [DOI] [PubMed] [Google Scholar]
- van den Oord EJ, Kuo PH, Hartmann AM, Webb BT, Moller HJ, Hettema JM, Giegling I, Bukszar J, Rujescu D. Genomewide association analysis followed by a replication study implicates a novel candidate gene for neuroticism. Arch Gen Psychiatry. 2008;65:1062–1071. doi: 10.1001/archpsyc.65.9.1062. [DOI] [PubMed] [Google Scholar]
- Viltart O, Mairesse J, Darnaudery M, Louvart H, Vanbesien-Mailliot C, Catalani A, Maccari S. Prenatal stress alters Fos protein expression in hippocampus and locus coeruleus stress-related brain structures. Psychoneuroendocrinology. 2006;31:769–780. doi: 10.1016/j.psyneuen.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Wadhwa PD. Psychoneuroendocrine processes in human pregnancy influence fetal development and health. Psychoneuroendocrinology. 2005;30:724–743. doi: 10.1016/j.psyneuen.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Weinstock M. Does prenatal stress impair coping and regulation of hypothalamic-pituitary-adrenal axis? Neurosci Biobehav Rev. 1997;21:1–10. doi: 10.1016/s0149-7634(96)00014-0. [DOI] [PubMed] [Google Scholar]
- Yip J, Soghomonian JJ, Blatt GJ. Increased GAD67 mRNA expression in cerebellar interneurons in autism: implications for Purkinje cell dysfunction. J Neurosci Res. 2008;86:525–530. doi: 10.1002/jnr.21520. [DOI] [PubMed] [Google Scholar]