Abstract
Introduction
Reliable and valid tools must be used to assess spasticity in clinical practise and research settings. There is a paucity of literature regarding the validity of the Modified Modified Ashworth Scale (MMAS) and the Modified Tardieu Scale (MTS). No study, to date, has been performed to compare the validity of the MMAS and the MTS. This neurophysiological study protocol will compare the validity of the MMAS and the MTS in the assessment of poststroke wrist flexor spasticity.
Methods and analysis
Thirty-two patients with stroke from the University Rehabilitation clinics will be recruited to participate in this cross-sectional, non-interventional study. All measurements will be taken in the Physical Medicine and Rehabilitation Department of Shafa University Hospital in Tehran, Iran. First, wrist flexor spasticity will be assessed clinically using the MMAS and MTS. The tests will be applied randomly. For the MTS, the components of R1, R2, R2−R1 and quality of muscle reaction will be measured. Second, neurophysiological measures of H-reflex latency, Hmax/Mmax ratio, Hslp and Hslp/Mslp ratio will be collected from the affected side. The results will be analysed using Spearman's ρ test or Pearson's correlation test to determine the validity of the MMAS and the MTS as well as to compare the validity between the MMAS and the MTS.
Ethics and dissemination
The Research Council, School of Rehabilitation and the Ethics Committee of Tehran University of Medical Sciences (TUMS) approved the study protocol. The study results will be disseminated in peer-reviewed publications and presented at international congresses.
Keywords: Rehabilitation Medicine
Article summary.
Article focus
Comparing the validity of the Modified Modified Ashworth Scale (MMAS) and the Modified Tardieu Scale (MTS) in the assessment of wrist flexor spasticity in patients with stroke-protocol for a neurophysiological study.
Key messages
Reliable and valid tools must be used to assess spasticity accurately in clinical and research settings.
The results of this protocol will provide evidence for the validity of the MMAS and the MTS for the measurement of muscle spasticity.
Strengths and limitations of this study
This study will use conventional and new measures of motoneuron excitability for the objective evaluation of spasticity.
This study will be the first to compare the criterion validity of the MMAS and the MTS.
A weakness of the study is that the tests will be carried out by one person.
Introduction
Spasticity is a common symptom observed following upper motor neuron syndrome. Diseases such as stroke, traumatic brain injury, spinal cord injury, cerebral palsy and multiple sclerosis are associated with significant spasticity. Spasticity has been defined classically by Lance as a motor disorder characterised by a velocity-dependent increase in tonic stretch reflexes.1 There are several studies addressing the prevalence of spasticity after stroke.2 The prevalence of spasticity after a first stroke has been inconsistent, ranging between 18% and 38%.3–8 In addition, a recent study indicated that the prevalence of spasticity in patients with stroke referred to the Department of Rehabilitation Medicine was 42.4%.9 Excessive spasticity needs to be controlled, because it can interfere with functional recovery, and may lead to secondary complications such as contractures and pain.10 11 Reflex hyperexcitability and soft tissue stiffness have been reported to contribute to increased resistance to passive stretch.12
To assess spasticity accurately in clinical practise and for research purposes, reliable and valid tools must be used. The Ashworth and Tardieu Scales are common clinical measures of spasticity. The Ashworth Scale was originally developed in 1964, and modified by Bohannon and Smith in 1987.13 14 The Bohannon-Smith Modified Ashworth Scale (MAS) has been recently modified by Ansari et al15 in 2006 as the Modified Modified Ashworth Scale (MMAS; table 1). The MMAS is an ordinal level measure of spasticity, which grades the intensity of spasticity from 0 to 4. The results of several studies have demonstrated that the MMAS is a reliable measure for assessing spasticity in either upper or lower limbs of patients with spasticity.16–22
Table 1.
Definitions of the Modified Tardieu Scale and the Modified Modified Ashworth Scale
| Grade | Modified Tardieu Scale (Boyd and Graham27) | Modified Modified Ashworth Scale (Ansari et al15) |
|---|---|---|
| 0 | No resistance throughout the course of the passive movement | No increase in muscle tone |
| 1 | Slight resistance throughout the course of the passive movement, with no clear catch at precise angle | Slight increase in muscle tone, manifested by a catch and release or by minimal resistance at the end of the range of motion when the affected part(s) is moved in flexion or extension |
| 2 | Clear catch at precise angle, interrupting the passive movement, followed by release | Marked increase in muscle tone, manifested by a catch in the middle range and resistance throughout the remainder of the range of motion, but affected part (s) easily moved |
| 3 | Fatigable clonus (<10 s when maintaining pressure) occurring at precise angle | Considerable increase in muscle tone, passive movement difficult |
| 4 | Infatigable clonus (>10 s when maintaining pressure) occurring at precise angle | Affected part(s) rigid in flexion or extension |
The Tardieu Scale was developed by Tardieu et al23 in 1954. Held and Pierrot-Deseilligny24 modified it in 1969, and it was further modified in 1999 by Boyd and Graham. This latest version of the Tardieu Scale is called the Modified Tardieu Scale (MTS).25 26 The MTS considers R2, R1 and R2−R1 to measure spasticity. The R2 is the passive range of motion measured during slow passive stretch. The R1 is the angle of muscle reaction measured during fast passive stretch, and occurs in a particular angle of ‘catch’ from hyperactive stretch reflex. Large and small differences between R2 and R1 indicate spasticity and muscle contracture, respectively.25 27 Quality of muscle reaction during fast passive stretch is also graded based on 0–4 scores and is defined as the MTS scores (table 1).28 29
Studies regarding the reliability of the MTS have been mostly performed in children.30–33 The reliability of the MTS has been questioned for various patient groups.32 34 35 Paulis et al36 compared the test−retest and inter-rater reliability of Tardieu Scale scores measured with inertial sensors (IS) and goniometry and found excellent reliability for IS. Ansari et al34 showed insufficient inter-rater reliability for MTS when measuring elbow flexor spasticity in adult patients with hemiplegia using goniometry. In a study by Singh et al37 it has been shown that the intrarater reliability of MTS is very good for R1, R2, R2−R1 and MTS scores across two sessions in elbow flexors and ankle plantar flexors using goniometry. A study comparing the reliability of the MTS with the Bohannon-Smith MAS in adult patients with severe brain injury found significantly higher test–retest and inter-rater reliability for MTS, however, poor-to-moderate inter-rater reliability for both scales.38
There is a paucity of literature exploring the validity of MMAS and MTS. The validity of MMAS has been evaluated neurophysiologically with the measurement of wrist flexor spasticity in patients with stroke.39 Naghdi et al39 found a significant positive correlation between the MMAS scores and Hslp/Mslp ratio (r=0.39, p=0.04), traditional index of Hmax/Mmax ratio (r=0.39, p=0.04) and Hslp (r=0.45, p=0.02) indicating the validity of the MMAS. In another study, the authors showed that the MMAS and the Brunnstrom recovery stages were highly correlated in the evaluation of motor recovery in patients with stroke.40 Recently, the content validity of the Tardieu Scale and the Ashworth Scale was assessed in independently ambulating children with cerebral palsy.41 The authors demonstrated that the Tardieu Scale was more effective than the Original Ashworth Scale in identifying the presence of spasticity, the presence of contracture and the severity of contracture. Neither scale was able to identify the severity of spasticity.41
The basic neural circuit in spasticity is the monosynaptic stretch reflex arc characterised by the sensory Ia afferent and α motor neuron.42 Various neurophysiological changes have been shown to occur in muscle spasticity, including α motor neuron hyperexcitability.12 43 The H-reflex is a simple, non-invasive method that can be used to study reflex pathway reliably.44–48 The H-reflex parameters of Hmax/Mmax ratio and latency are reliable measures of α motor neuron hyperexcitability, and have been suggested for objective quantifying of muscle spasticity.49 50 In patients with spasticity, H-reflex latency is usually decreased and Hmax/Mmax ratio is increased.49
The Hslp/Mslp ratio (the developmental slope of the H-reflex (Hslp) recruitment curve as a ratio of the developmental slope of the M-response (Mslp)) has been proposed as a better indicator for evaluating the excitability of a motor neuron pool in spasticity.51 52 The slope of the H-reflex recruitment curve (Hslp) representing the relationship between stimulation intensity and reflex recruitment of motor neurons is free from the H-reflex discharge collision and shows the intrinsic excitability of the motor neurons and the reflex arc.52 53 The Hslp is more sensitive than Hmax/Mmax, provides more information about the recruitment threshold and can estimate spinal excitability.52 54 It has been demonstrated that flexor carpi radialis (FCR) H-reflexes can be reliably evoked in poststroke paretic and non-paretic arms, and the FCR recruitment slope is a sensitive measure of spinal excitability after stroke.55 The H-reflex tests will be used for validation of the MMAS and the MTS.
Aims and objectives
No study has compared the validity of MMAS and MTS. The present neurophysiological study protocol is designed to compare the validity of the MMAS and the MTS in the assessment of poststroke wrist flexor spasticity.
Methods
Study design
This cross-sectional, non-interventional study is designed for comparing the criterion validity between the MMAS and the MTS in the assessment of wrist flexor spasticity in patients poststroke. The clinical measures of MMAS and MTS will be obtained, and will be correlated with neurophysiological measures of H-reflex latency; Hmax/Mmax ratio; Hslp and Hslp/Mslp ratio.
Setting
The measurements will be taken at the Department of Electrophysiology, University Hospital of Shafa in Tehran, Iran.
Approval of study protocol
The study protocol has been approved by the Research Council, School of Rehabilitation, Tehran University of Medical Sciences (TUMS) and Ethical approval has been granted by TUMS Ethics Committee (Reference number 1322).
Informed consent
Written informed consent from the eligible subjects will be obtained before tests are performed. We will instruct patients that they may obtain any information about the detail of the study from the investigator, and they may discontinue their participation in the study at any time.
Participants
Patients with stroke who attend the Rehabilitation clinics of TUMS will be screened for eligibility. Eligible patients will be invited to participate in the study. The study is designed to include 32 subjects with the following criteria. Inclusion criteria will be (1) first stroke, (2) history of stroke between 1 and 24 months, (3) age between 40 and 65 years and (4) ability to understand and follow instructions. Exclusion criteria will be (1) fixed contracture at wrist and elbow joints, (2) wrist pain due to degenerative changes, (3) taking antispastic drugs, (4) contraindication of passive movement at wrist joint, (5) cervical discopathy, (6) diabetes and (7) non-consent.
Procedures
The patients will be interviewed to collect demographic characteristics including age; aetiology (ie, ischaemic or vascular stroke), time elapsed from the onset of stroke and affected side. Effort will be made to provide a similar testing condition. All measurements will be taken in the morning hours between 9:00 and 12:00. Before testing commencement, all patients will be asked to rest on the bed with shoes removed for 5 min and remain comfortable and relaxed. To provide a quiet testing environment, all tests will be performed in a closed quiet room with natural light from windows. The temperature of the testing room will be set at approximately 25°C. We will also ask the patients to empty their bladder prior to testing. The room is electrically shielded and earth-grounded for H-reflex measurements.
The wrist flexors will be tested in this study, because they are usually spastic in patients poststroke, and spasticity can be reliably measured in wrist flexors.17 18 56 Clinical and neurophysiological tests will be performed in a single session. Wrist flexor spasticity will be assessed clinically on the affected side using MMAS and MTS. For MTS, the components of R1, R2, R2−R1 and quality of muscle reaction will be measured. The sequence of tests will be randomised by tossing a coin. The tester will be tossing the coin to randomise the procedure and will be observed by an impartial colleague. The neurophysiological data will also be collected from the affected side. One trained physiotherapist will perform both clinical and neurophysiological tests. The clinical tests will be applied first. The tester will be blinded to the neurophysiological data analyses. The analyst of neurophysiological data will be blinded to the clinical testing results.
Outcome measures
Muscle spasticity is primarily due to an exaggerated stretch reflex and α motor neuron excitability.1 The MMAS and MTS will be used for the assessment of muscle spasticity. The H-reflex is an objective method for the measurement of spasticity.49 The H-reflex parameters of latency; Hmax/Mmax ratio; Hslp; and Hslp/Mslp ratio will be applied to examine α motor neuron excitability.
The Hmax/Mmax ratio indicates the level of motoneuron excitability. The Hslp is a measure of the relationship between the number of motoneurons activated and a given incremental rise in stimulation intensity.51 52 The Hslp/Mslp ratio is a new method of spasticity measurement, and presumes that the developmental slope of the H-reflex (Hslp) recruitment curve as a ratio of the developmental slope of the M-response (Mslp) is a better parameter for evaluating the motoneuron excitability.
The Modified Modified Ashworth Scale
Wrist flexor spasticity will be quantified using the clinical scale MMAS, which has been shown to be reliable at the wrist joint.17 18 The procedure used and described in previous studies will be followed to measure wrist flexor spasticity. Briefly, the patient will be in supine position, head in midline, and arms alongside the trunk with shoulder in slight abduction. The rater will hold the forearm in mid-position just proximal to the wrist joint with one hand, and will grasp the patients hand with the other hand. The rater will move the wrist from maximum possible flexion to maximal possible extension counting 1001.17 18 The rater will score muscle spasticity from 0 to 4. Only one passive stretch will be applied to rate spasticity.15
The Modified Tardieu Scale
A standard goniometer will be utilised to measure R2 and R1. The patient will be in sitting position with elbow joint flexed at 90°. The stretching velocity of V1 and V3 will be applied to measure R2 and R1, respectively. The quality of muscle reaction will be graded at the stretching velocity of V3 as well.16 17 The difference between R2 and R1 will be the measure of the dynamic component of spasticity.
Measurement of H-reflex
As with the clinical test, the patient will be positioned in supine, head in midline and arms alongside the trunk. The patients will be asked to remain calm, and relax completely during testing.
The H-reflex and the M-wave will be obtained using an EMG machine (Myto П, Italy). The H-reflex will be elicited in the FCR muscle of the affected side of the participants with the arm in supination. The H-reflex in FCR muscle has been commonly employed in studies of H-reflex in the upper limb, and can be reliably evoked and measured.46 49 57 58 A digital thermometer will be used to measure the skin temperature. The bandpass filter will be set at 5 Hz–3 kHz, sweep speed at 5 ms/div and sensitivity at 200–500 µV.
The stimulator will apply rectangular electric pulses with 1 ms duration, every 5 s.39 40 Paired surface electrodes (Ag/AgCl) will be attached to the skin on the muscle belly. The median nerve will be stimulated at the elbow crease using a bipolar stimulating electrode. The stimulating electrodes will be positioned in line with the median nerve in the cubital fossa just medial to the biceps brachii tendon with the cathode proximal to the anode to prevent anodal block.59
The recording electrodes will be placed over the muscle belly of the FCR. The active electrode will be placed on the belly of the FCR at one-third of the proximal distance between the medial epicondyle of the humerous and the radial styloid. The reference electrode will be positioned 4 cm distal and lateral to the active one. The electric resistance between the two electrodes will be less than 10 kΩ. The ground electrode will be attached to the skin between stimulating and recording electrodes. Stepwise increase in stimulus intensity will be used to record the H-reflex and M-wave. The stimulus intensity will start at 0.5 mA and will be increased in steps of 0.5 mA to record Hmax and Mmax.39 40 H-reflex conditioning will not be used in this protocol, as FCR H-reflex can be easily evoked without facilitation in most cases.47
Recruitment curves
To build the recruitment curves of H-reflex and M-waves, we will follow the methods described elsewhere.39 40 51 52 60 Five pulses will be delivered sequentially at each stimulus intensity and the mean amplitude of evoked H-reflexes and the M-responses will be calculated. The amplitudes will be measured peak to peak. Mean amplitudes will be normalised according to the following formula:
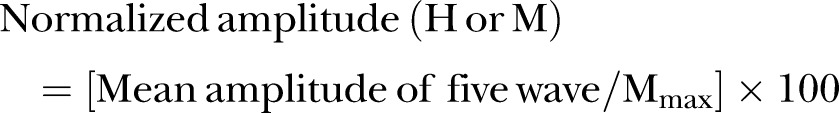 |
Stimulus intensities will be presented as the ratio of the threshold intensity of the M-wave (Mth). All data measured at stimulus intensities less than the threshold of the M-wave will be used to determine the Hslp. Data from Mth up to maximum amplitude of M-wave will determine the Mslp. Equation of simple linear regression will be determined, and the slope of this line will be considered as Hslp or Mslp. Maximum mean amplitude of the H-reflex and maximum mean amplitude of the M-wave will be regarded as Hmax and Mmax, respectively. The Hmax relative to Mmax is Hmax/Mmax ratio. The H-reflex latency will be calculated from stimulus wave to the onset of first deflection of the H-wave, and the amplitude of recorded reflexes and waves will be measured.
Sample size calculation
In the study by Naghdi et al,39 correlation of the MMAS with the Hmax/Mmax ratio or the Hslp/Mslp ratio was r=0.39. Assuming the correlation to be 0.4, and to show a similar validity with 0.95% power at 5% significance level, it will require that data are taken on 32 participants.
Statistical analysis
To determine the validity of the clinical tests, the relationships between the clinical tests and the H-reflex indices will be assessed by calculating Spearman's ρ test (ie, MMAS and the ordinal component of the MTS) or Pearson correlation test (ie, ratio components of the MTS). Comparing correlations test will be used to compare the validity of the MMAS and the MTS.61
Results
Demographic characteristics
Demographic characteristics of participants will be illustrated as shown in table 2.
Table 2.
Demographic and clinical characteristics
| Number | Mean (SD) | Minimum | Maximum | |
|---|---|---|---|---|
| Gender (M/F) | ||||
| Age | ||||
| Weight | ||||
| Height | ||||
| BMI | ||||
| Time since stroke | ||||
| Affected side (R/L) |
BMI, body mass index; R/L, right or left.
Clinical characteristics
Clinical spasticity grades with MMAS, MTS measures and quality of muscle reaction will be illustrated in table 3.
Table 3.
The results for spasticity grades and measurements of R1, R2 and R2−R1
| MTS measures | Median (IQR) | Mean (SD) | Minimum–Maximum | 95% CI |
|---|---|---|---|---|
| R1 | ||||
| R2 | ||||
| R2−R1 | ||||
| MMAS grades | ||||
| MTS grades |
MMAS, Modified Modified Ashworth Scale; MTS, Modified Tardieu Scale; R1, angle of muscle reaction; R2, passive range of motion; R2−R1, dynamic component of spasticity.
Neurophysiological data
Objective neurophysiological assessment results using the H-reflex are shown in table 4.
Table 4.
The results for H-reflex indices
| Mean (SD) | Minimum–Maximum | 95% CI | |
|---|---|---|---|
| Hmax/Mmax | |||
| Latency | |||
| Hslp/Mslp | |||
| Hslp |
Criterion validity
The criterion validity will be analysed by correlations between the clinical measures and neurophysiological tests. Criterion validity will be established when significant moderate correlations between the MMAS/the MTS and the neurophysiological measures are found. The results of correlation analyses will be presented in table 5.
Table 5.
Correlation coefficients between spasticity clinical measures and neurophysiological tests
| Hmax/Mmax | Latency | Hslp/Mslp | Hslp | ||
|---|---|---|---|---|---|
| MMAS | Spearman's ρ sig. | ||||
| MTS | Spearman's ρ sig. | ||||
| R2–R1 | Pearson correlation sig. | ||||
MMAS, Modified Modified Ashworth Scale; MTS, Modified Tardieu Scale; sig., significance.
Discussion
This article describes a neurophysiological study protocol for comparative evaluation of the validity of two important clinical measures in the field of neurological rehabilitation. To our knowledge, this investigation will be the first to compare the criterion validity of the MMAS and the MTS. This protocol utilises conventional and new indicators of motoneuron excitability in spasticity for comparative validity evaluation. Further, the protocol utilises standard methodology for spasticity assessment to indicate the excitability of the α motoneuron pool.49
The results of the present protocol will be important. Owing to the limitations of the Ashworth Scale and questioned reliability and validity,62 the new MMAS has emerged to improve the metric characteristics of the scales. The results of the metrics for the MMAS have been encouraging up to this point. The MMAS needs to be further examined for additional psychometric properties and be utilised during intervention-based studies.63 Since the original and MAS have been reported to have poor reliability and validity the MTS has been suggested as an alternative and suitable measure for use in assessment of spasticity.29 64 However, the results of reliability for the MTS have been mixed, with a dearth of studies exploring the validity of the MTS. This study will be the first report investigating the validity of the MTS through establishing correlation between the MTS and the α motor neuron excitability indicators. While the MTS has been explained theoretically as a suitable spasticity measure, there is no evidence to compare this scale with existing clinical measures such as the MMAS. The results of this protocol will provide neurophysiological evidence for the validity of the MMAS and the MTS as clinical scales for the measurement of muscle spasticity.
Limitations
A limitation of the study is that the tests will be performed by only one person, which is the most significant limitation of the study.
Supplementary Material
Acknowledgments
We are grateful to the Research Deputy, Tehran University of Medical Sciences (TUMS) for their support.
Footnotes
Contributors: HA, NNA, SN, KM, NG and SH contributed to the conception of the study. All authors contributed to the study design. HA contributed to drafting the protocol. All authors read and revised the manuscript for important intellectual content. All authors reviewed the final manuscript and gave approval of the version to be published.
Funding: Support for this study was provided by the Research Deputy of TUMS.
Competing interests: None.
Ethics approval: The Ethics Committee of Tehran University of Medical Sciences (TUMS) approved the study protocol.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Lance JW. The control of muscle tone, reflexes, and movement: Robert Wartenberg Lecture. Neurology 1980;30:1303–13 [DOI] [PubMed] [Google Scholar]
- 2.Schinwelski M, Stawek J. Prevalence of spasticity following stroke and its impact on quality of life with emphasis on disability in activities of daily living. Systematic review. Neurol Neurochir Pol 2010;44:1–8 [DOI] [PubMed] [Google Scholar]
- 3.Moura Rde C, Fukujima MM, Aguiar AS, et al. Predictive factors for spasticity among ischemic stroke patients. Arq Neuropsiquiatr 2009;67:1029–36 [DOI] [PubMed] [Google Scholar]
- 4.Lundstrom E, Terent A, Borg J. Prevalence of disabling spasticity 1 year after first-ever stroke. Eur J Neurol 2008;15:533–9 [DOI] [PubMed] [Google Scholar]
- 5.Welmer AK, von Arbin M, Widen HL, et al. Spasticity and its association with functioning and health-related quality of life 18 months after stroke. Cerebrovasc Dis 2006;21:247–53 [DOI] [PubMed] [Google Scholar]
- 6.Sommerfeld DK, Eek EU, Svensson AK, et al. Spasticity after stroke: its occurrence and association with motor impairments and activity limitations. Stroke 2004;35:134–9 [DOI] [PubMed] [Google Scholar]
- 7.Leathley MJ, Gregson JM, Moore AP, et al. Predicting spasticity after stroke in those surviving to 12 months. Clin Rehabil 2004;18:438–43 [DOI] [PubMed] [Google Scholar]
- 8.Watkins CL, Leathley MJ, Gregson JM, et al. Prevalence of spasticity post stroke. Clin Rehabil 2002;16:515–22 [DOI] [PubMed] [Google Scholar]
- 9.Ryu JS, Lee JW, Lee SI, et al. Factors predictive of spasticity and their effects on motor recovery and functional outcomes in stroke patients. Top Stroke Rehabil 2010;17:380–8 [DOI] [PubMed] [Google Scholar]
- 10.Gracies JM. Pathophysiology of spastic paresis. I: Paresis and soft tissue changes. Muscle Nerve 2005;31:535–51 [DOI] [PubMed] [Google Scholar]
- 11.Saulino M, Jacobs BW. The pharmacological management of spasticity. J Neurosci Nurs 2006;38:456–9 [PubMed] [Google Scholar]
- 12.Sheean G, McGuire JR. Spastic hypertonia and movement disorders: pathophysiology, clinical presentation, and quantification. PMR 2009;1:827–33 [DOI] [PubMed] [Google Scholar]
- 13.Ashworth B. Preliminary trial of carisoprodal in multiple sclerosis. Practitioner 1964;192:540–2 [PubMed] [Google Scholar]
- 14.Bohannon RW, Smith MBO. Inter-rater reliability of a Modified Ashworth Scale of muscle spasticity. Phys Ther 1987;67:206–7 [DOI] [PubMed] [Google Scholar]
- 15.Ansari NN, Naghdi S, Moammeri H, et al. Ashworth scales are unreliable for the assessment of muscle spasticity. Physiother Theory Pract 2006;22:119–25 [DOI] [PubMed] [Google Scholar]
- 16.Ansari NN, Naghdi S, Younesian P, et al. Inter and intrarater reliability of the Modified Modified Ashworth Scale in patients with knee extensor poststroke spasticity. Physiother Theory Pract 2008;24:205–13 [DOI] [PubMed] [Google Scholar]
- 17.Naghdi S, Ansari NN, Azarnia S, et al. Interrater reliability of the Modified Modified Ashworth Scale (MMAS) for patients with wrist flexor muscle spasticity. Physiother Theory Pract 2008;24:372–9 [DOI] [PubMed] [Google Scholar]
- 18.Ansari NN, Naghdi S, Hasson S, et al. Assessing the reliability of the Modified Modified Ashworth Scale between two physiotherapists in adult patients with hemiplegia. NeuroRehabilitation 2009;25:235–40 [DOI] [PubMed] [Google Scholar]
- 19.Ansari NN, Naghdi S, Hasson S, et al. Inter-rater reliability of the modified modified Ashworth scale as a clinical tool in measurements of post stroke elbow flexor spasticity. NeuroRehabilitation 2009;24:225–9 [DOI] [PubMed] [Google Scholar]
- 20.Ghotbi N, Ansari NN, Naghdi S, et al. Inter-rater reliability of the Modified Modified Ashworth Scale in assessing lower limb muscle spasticity. Brain Inj 2009;23:815–9 [DOI] [PubMed] [Google Scholar]
- 21.Ghotbi N, Ansari NN, Naghdi S, et al. Measurement of lower-limb muscle spasticity: intrarater reliability of Modified Modified Ashworth Scale. J Rehabil Res Dev 2011;48:83–8 [DOI] [PubMed] [Google Scholar]
- 22.Kaya T, Goksel KA, Gunaydin R, et al. Inter-rater reliability of the Modified Ashworth Scale and Modified Modified Ashworth Scale in assessing poststroke elbow flexor spasticity. Int J Rehabil Res 2011;34:59–64 [DOI] [PubMed] [Google Scholar]
- 23.Tardieu G, Shentoub S, Delarue R. A la recherche d'une technique de measure de la spasticite. Rev Neurol (Paris) 1954;91:143–4 [PubMed] [Google Scholar]
- 24.Held J, Peierrot-Deseilligny E. Reeducation motrice des avections neurologiques. Paris: Bailliere, 1969 [Google Scholar]
- 25.Morris S. Ashworth and Tardieu scales: their clinical relevance for measuring spasticity in adult and pediatric neurological populations. Phys Ther Rev 2002;7:53–62 [Google Scholar]
- 26.Boyd RN, Ada L. Physiotherapy management of spasticity. In: Barnes MP, Johnson GR, editors. Upper motor neurone syndrome and spasticity. Cambridge: Cambridge University Press, 2001;96–121 [Google Scholar]
- 27.Boyd RN, Graham HK. Objective measurement of clinical findings in the use of botulinum toxin type A for the management of children with cerebral palsy. Eur J Neurol 1999;6(Suppl 4):S23–35 [Google Scholar]
- 28.Gracies JM, Marosszeky JE, Renton R, et al. Short-term effects of dynamic lycra splints on upper limb in hemiplegic patients. Arch Phys Med Rehabil 2000;81:547–55 [DOI] [PubMed] [Google Scholar]
- 29.Haugh AB, Pandyan AD, Johnson GR. A systematic review of the Tardieu scale for the measurement of spasticity. Disabil Rehabil 2006;28:899–907 [DOI] [PubMed] [Google Scholar]
- 30.Gracies JM, Burk K, Clegg NJ, et al. Reliability of the Tardieu Scale for assessing spasticity in children with cerebral palsy. Arch Phys Med Rehabil 2010;91:421–8 [DOI] [PubMed] [Google Scholar]
- 31.Yam WK, Leung MS. Interrater reliability of Modified Ashworth Scale and Modified Tardieu Scale in children with spastic cerebral palsy. J Child Neurol 2006;21:1031–5 [DOI] [PubMed] [Google Scholar]
- 32.Mackey AH, Walt SE, Lobb G, et al. Intraobserver reliability of the Modified Tardieu Scale in the upper limb of children with hemiplegia. Dev Med Child Neurol 2004;46:267–72 [DOI] [PubMed] [Google Scholar]
- 33.Fosang AL, Galea MP, McCoy AT, et al. Measures of muscle and joint performance in the lower limb of children with cerebral palsy. Dev Med Child Neurol 2003;45:664–70 [DOI] [PubMed] [Google Scholar]
- 34.Ansari NN, Naghdi S, Hasson S, et al. The Modified Tardieu Scale for the measurement of elbow flexor spasticity in adult patients with hemiplegia. Brain Inj 2008;22:1007–12 [DOI] [PubMed] [Google Scholar]
- 35.Waninge A, Rook RA, Dijkhuizen A, et al. Feasibility, test−retest reliability, and interrater reliability of the Modified Ashworth Scale and Modified Tardieu Scale in persons with profound intellectual and multiple disabilities. Res Dev Disabil 2011;32:613–20 [DOI] [PubMed] [Google Scholar]
- 36.Paulis WD, Horemans HL, Brouwer BS, et al. Excellent test−retest and inter-rater reliability for Tardieu Scale measurements with inertial sensors in elbow flexors of stroke patients. Gait Posture 2011;33:185–9 [DOI] [PubMed] [Google Scholar]
- 37.Singh P, Joshua AM, Ganeshan S, et al. Intra-rater reliability of the Modified Tardieu Scale to quantify spasticity in elbow flexors and ankle plantar flexors in adult stroke subjects. Ann Indian Acad Neurol 2011;14:23–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mehrholz J, Wagner K, Meissner D, et al. Reliability of the Modified Tardieu Scale and the Modified Ashworth Scale in adult patients with severe brain injury: a comparison study. Clin Rehabil 2005;19:751–9 [DOI] [PubMed] [Google Scholar]
- 39.Naghdi S, Ansari NN, Mansouri K, et al. Neurophysiological examination of the Modified Modified Ashworth Scale (MMAS) in patients with wrist flexor spasticity after stroke. Electromyogr Clin Neurophysiol 2008;48:35–41 [PubMed] [Google Scholar]
- 40.Naghdi S, Ansari NN, Mansouri K, et al. A neurophysiological and clinical study of Brunnstrom recovery stages in the upper limb following stroke. Brain Inj 2010;24:1372–8 [DOI] [PubMed] [Google Scholar]
- 41.Alhusaini AA, Dean CM, Crosbie J, et al. Evaluation of spasticity in children with cerebral palsy using Ashworth and Tardieu Scales compared with laboratory measures. J Child Neurol 2010;25:1242–7 [DOI] [PubMed] [Google Scholar]
- 42.Satkunam LE. Rehabilitation medicine: 3. Management of adult spasticity. CMAJ 2003;169:1173–9 [PMC free article] [PubMed] [Google Scholar]
- 43.Nielsen JB, Crone C, Hultborn H. The spinal pathophysiology of spasticity—from a basic science point of view. Acta Physiol (Oxf) 2007;189:171–80 [DOI] [PubMed] [Google Scholar]
- 44.Knikou M. The H-reflex as a probe: pathways and pitfalls. J Neurosci Methods 2008;171:1–12 [DOI] [PubMed] [Google Scholar]
- 45.Misiaszek JE. The H-reflex as a tool in neurophysiology: its limitations and uses in understanding nervous system function. Muscle Nerve 2003;28:144–60 [DOI] [PubMed] [Google Scholar]
- 46.Zehr EP. Considerations for use of the Hoffmann reflex in exercise studies. Eur J Appl Physiol 2002;86:455–68 [DOI] [PubMed] [Google Scholar]
- 47.Christie AD, Inglis JG, Boucher JP, et al. Reliability of the FCR H-reflex. J Clin Neurophysiol 2005;22:204–9 [PubMed] [Google Scholar]
- 48.Hopkins JT, Ingersoll CD, Cordova ML, et al. Intrasession and intersession reliability of the soleus H-reflex in supine and standing positions. Electromyogr Clin Neurophysiol 2000;40:89–94 [PubMed] [Google Scholar]
- 49.Voerman GE, Gregoric M, Hermens HJ. Neurophysiological methods for the assessment of spasticity: the Hoffmann reflex, the tendon reflex, and the stretch reflex. Disabil Rehabil 2005;27:33–68 [DOI] [PubMed] [Google Scholar]
- 50.Sehgal N, McGuire JR. Beyond Ashworth, electrophysiologic quantification of spasticity. Phys Med Rehabil Clin N Am 1998;9:949–79 [PubMed] [Google Scholar]
- 51.Funase K, Imanaka K, Nishihira Y. Excitability of the soleus motoneuron pool revealed by the developmental slope of H-reflex as reflex gain. Electromyogr Clin Neurophysiol 1994;34:477–89 [PubMed] [Google Scholar]
- 52.Higashi T, Funase K, Kusano K, et al. Motorneuron pool excitability of hemiplegic patients: assessing recovery stages by using H-reflex and M-response. Arch Phys Med Rehabil 2001;82:1604–10 [DOI] [PubMed] [Google Scholar]
- 53.Funase K, Higashi T, Yashimura T, et al. Evident difference in the excitability of the motoneuron pool between normal subjects and patients with spasticity assessed by a new method using H-reflex and M-response. Neurosci Lett 1996;203:127–30 [DOI] [PubMed] [Google Scholar]
- 54.Bradnam L, Rochester L, Vujnovich A. Manual cervical traction reduces alpha-motoneuron excitability in normal subjects. Electromyogr Clin Neurophysiol 2000;40:259–66 [PubMed] [Google Scholar]
- 55.Phadke CP, Robertson CT, Condliffe EG, et al. Upper-extremity H-reflex measurement post-stroke: Reliability and inter-limb differences. Clin Neurophysiol 2012;123:606–15 [DOI] [PubMed] [Google Scholar]
- 56.Ansari NN, Naghdi S, Arab TK, et al. The interrater and intrarater reliability of the Modified Ashworth Scale in the assessment of muscle spasticity: Limb and muscle group effect. NeuroRehabilitation 2008;23:231–7 [PubMed] [Google Scholar]
- 57.Jaberzadeh S, Scutter S, Warden-Flood A, et al. Between-days reliability of H-reflexes in human flexor carpi radialis. Arch Phys Med Rehabil 2004;85:168–73 [DOI] [PubMed] [Google Scholar]
- 58.Pierrot-Deseilligny E, Mazevet D. The monosynaptic reflex: a tool to investigate motor control in humans. Interest and limits . Clin Neurophysiol 2000;30:67–80 [DOI] [PubMed] [Google Scholar]
- 59.Capaday C, Stein RB. Amplitude modulation of the soleus H-reflex in human during walking and standing. J Neurosci 1986;6:1308–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Naghdi S, Ebrahimi I, Asgari A, et al. A preliminary study into the criterion validity of the Modified Modified Ashworth Scale using the new measure of the alpha motoneuron excitability in spastic hemiplegia. Electromyogr Clin Neurophysiol 2007;47:187–92 [PubMed] [Google Scholar]
- 61.Meng XL, Rosenthal R, Rubin DB. Comparing correlated correlation coefficients. Psychol Bull 1992;111:172–5 [Google Scholar]
- 62.Fleuren JF, Voerman GE, Erren-Wolters CV, et al. Stop using the Ashworth Scale for the assessment of spasticity. J Neurol Neurosurg Psychiatry 2010;81:46–52 [DOI] [PubMed] [Google Scholar]
- 63.Gomez-Medina O, Elovic E. Measurement tools and treatment outcomes in patients with spasticity. In: Brashear A, Elovic E, eds. Spasticity, diagnosis and management. New York: Demos Medical Publishing, 2011;51–70 [Google Scholar]
- 64.Scholtes VAB, Becher JG, Beelen A, et al. Clinical assessment of spasticity in children with spasticity: A critical review of available instruments. Dev Med Child Neurol 2006;48:64–73 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


