Abstract
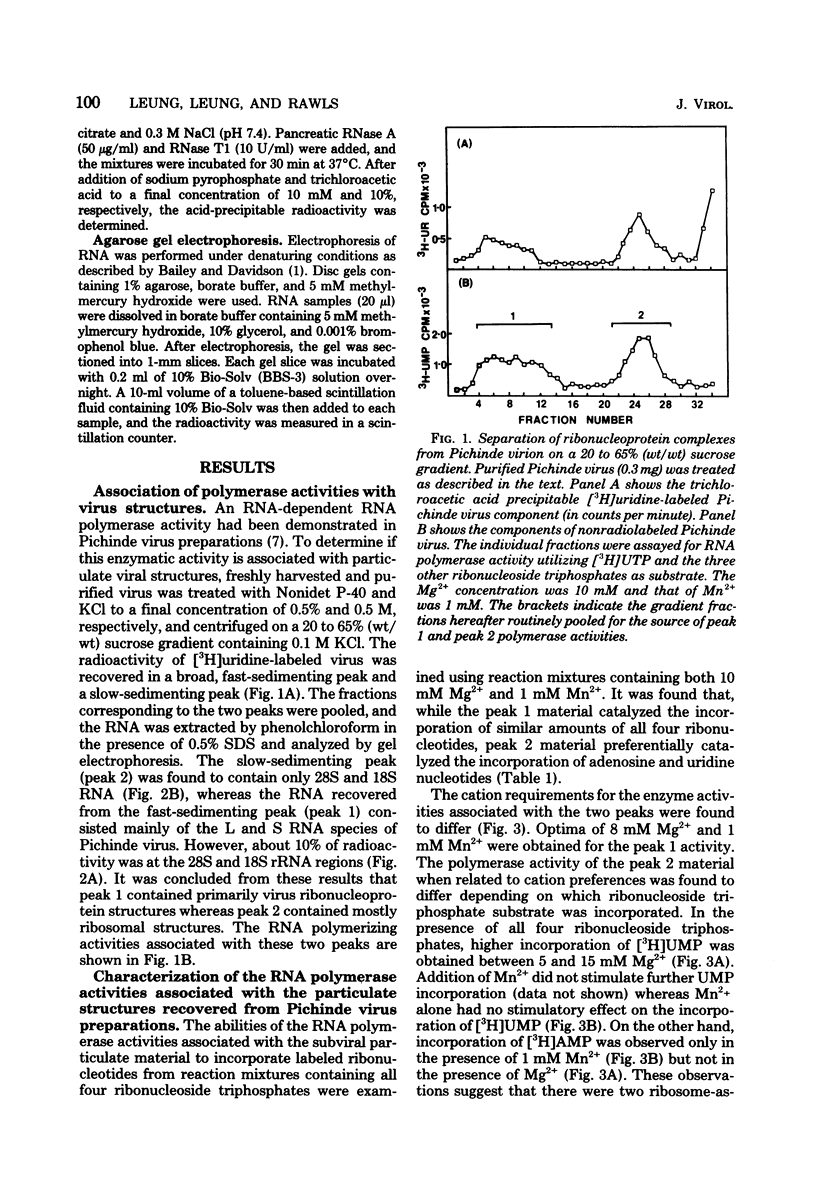
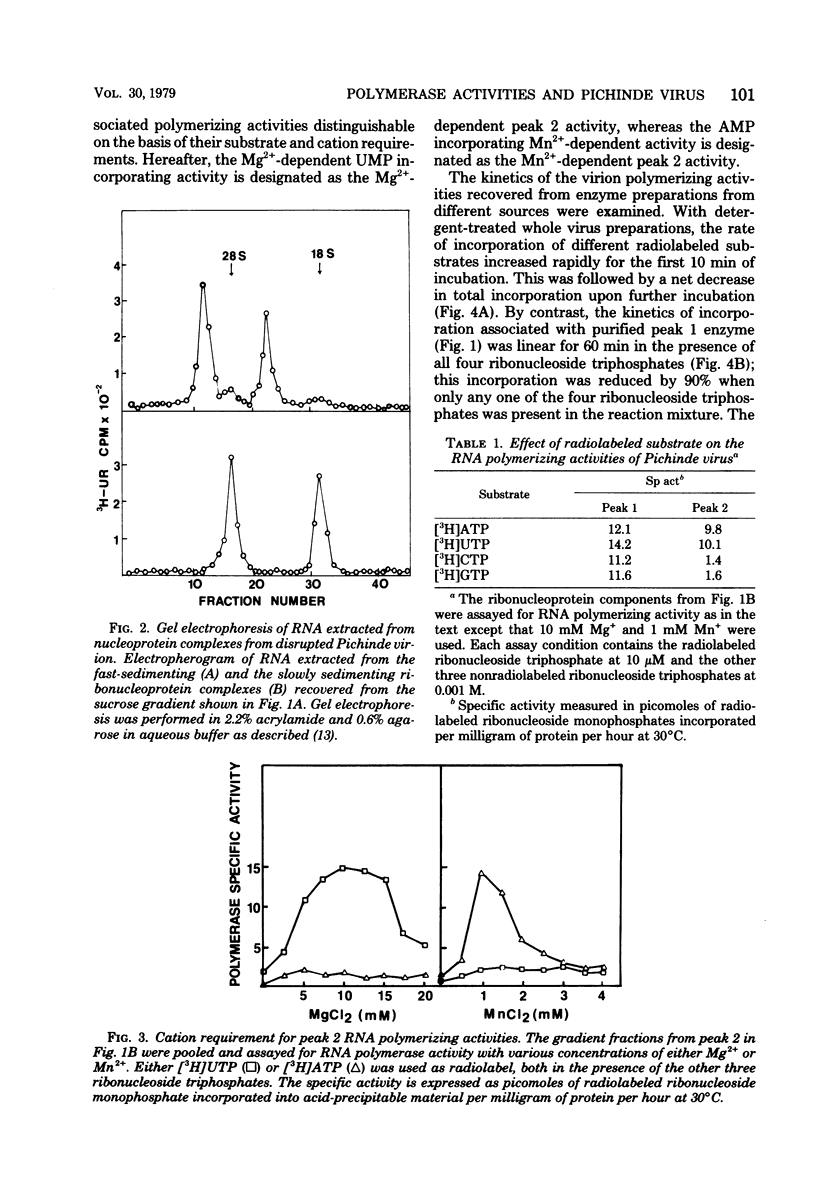
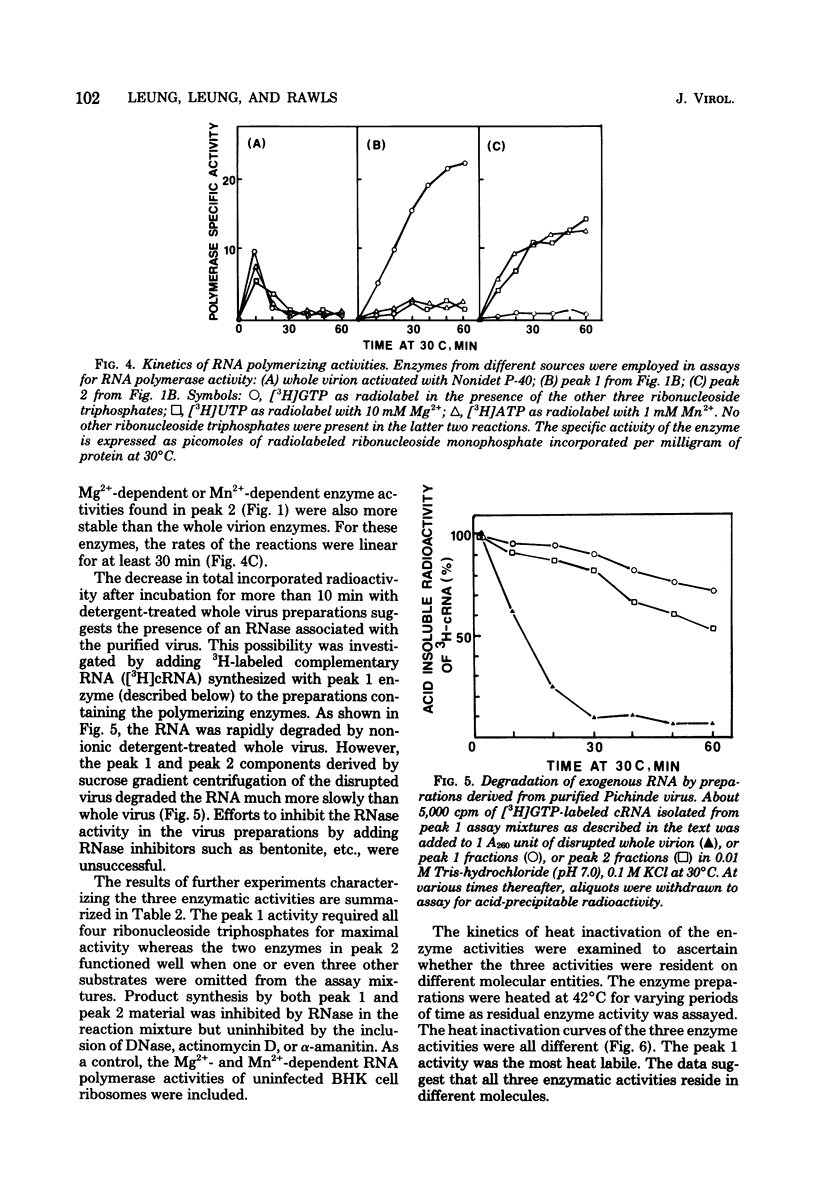
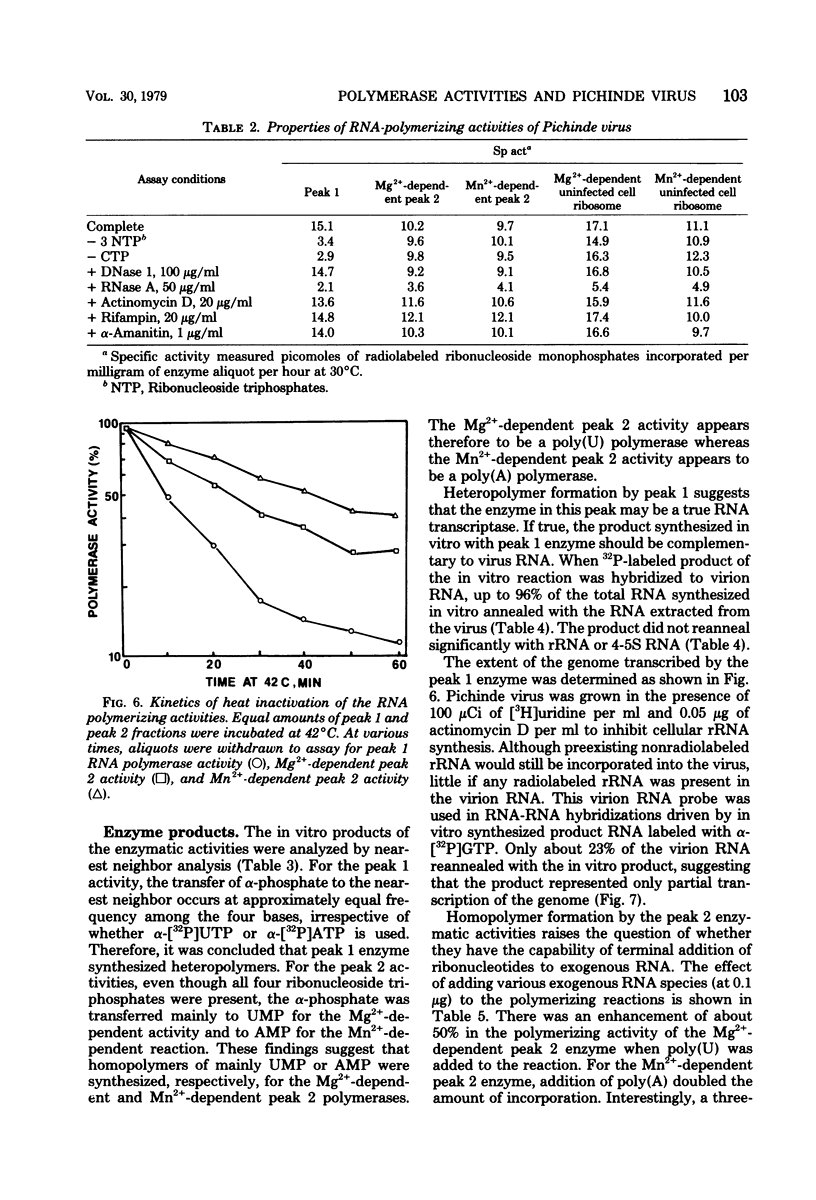
Three RNA polymerase activities were found and associated with purified Pichinde virus, a member of the Arenaviridae. A heat-labile polymerase activity which required all four ribonucleoside triphosphates for optimal activity co-sedimented on sucrose gradient centrifugation with the viral ribonucleoprotein complex from detergent-disrupted virus preparations. This enzyme synthesized heteropolymers which represented about 23% of the genome RNA as determined by nucleic acid hybridization. Two relatively heat-stable polymerase activities which differed in their cation requirement and substrate specificity were recovered with the virus-associated ribosomes. These polymerase activities synthesized homopolymers of limited chain length: in the presence of 10 mM Mg2%, polyuridylic acid was made, whereas in the presence of 1 mM Mn2%, polyadenylic acid was made. The addition of complementary RNA synthesized with the viral transcriptase in vitro to the reaction mixture containing the polyadenylic acid polymerase activity resulted in the terminal addition of polyadenylic acid to the complementary RNA. The possible function of the ribosome-associated polymerase activities in the replication of the virus is discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey J. M., Davidson N. Methylmercury as a reversible denaturing agent for agarose gel electrophoresis. Anal Biochem. 1976 Jan;70(1):75–85. doi: 10.1016/s0003-2697(76)80049-8. [DOI] [PubMed] [Google Scholar]
- Banerjee S. N., Buchmeier M., Rawls W. E. Requirement of cell nucleus for the replication of an arenavirus. Intervirology. 1975;6(3):190–196. doi: 10.1159/000149472. [DOI] [PubMed] [Google Scholar]
- Bernard J. P., Northrop R. L. RNA polymerase in mumps virion. J Virol. 1974 Jul;14(1):183–186. doi: 10.1128/jvi.14.1.183-186.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael G. G., Weber K., Niveleau A., Wahba A. J. The host factor required for RNA phage Qbeta RNA replication in vitro. Intracellular location, quantitation, and purification by polyadenylate-cellulose chromatography. J Biol Chem. 1975 May 25;250(10):3607–3612. [PubMed] [Google Scholar]
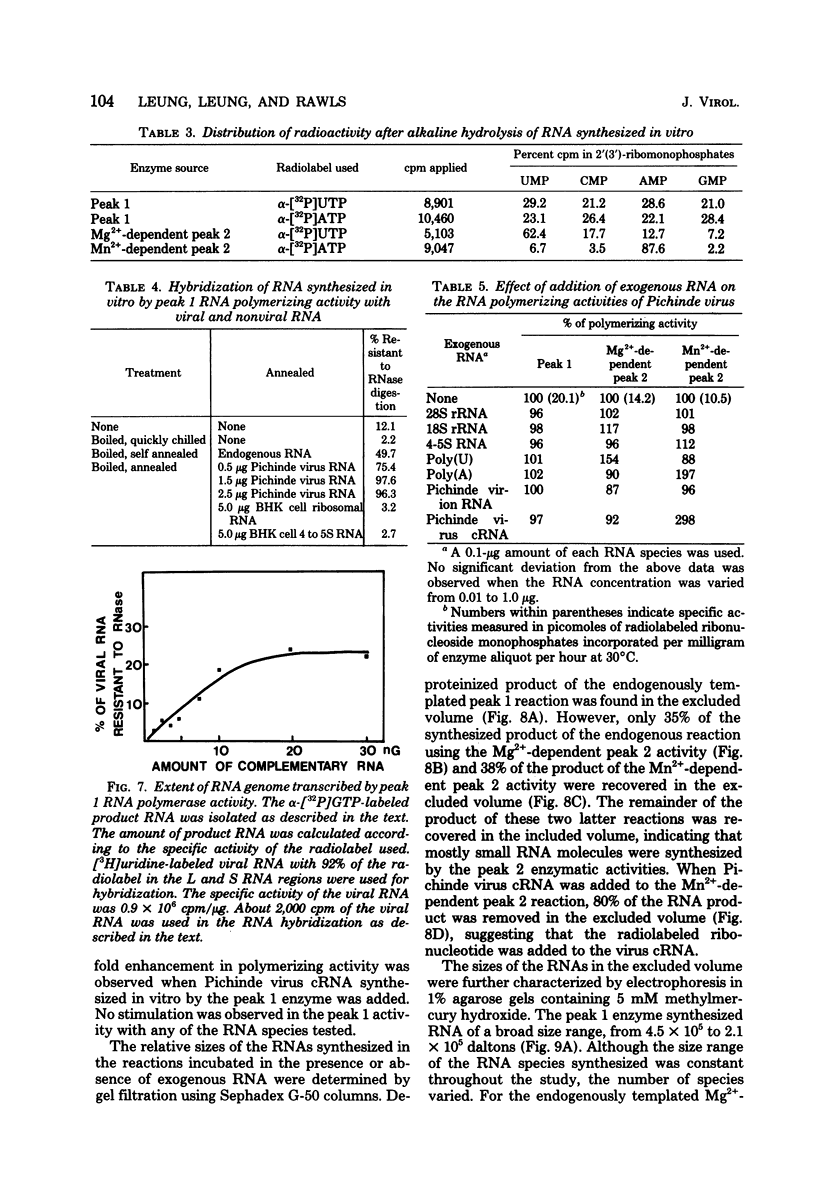
- Carter M. F., Biswal N., Rawls W. E. Characterization of nucleic acid of pichinde virus. J Virol. 1973 Jan;11(1):61–68. doi: 10.1128/jvi.11.1.61-68.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter M. F., Biswal N., Rawls W. E. Polymerase activity of Pichinde virus. J Virol. 1974 Mar;13(3):577–583. doi: 10.1128/jvi.13.3.577-583.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber F. E., Rawls W. E. Isolation of ribosome-like sturctures from Pichinde virus. J Gen Virol. 1975 Jan;26(1):21–31. doi: 10.1099/0022-1317-26-1-21. [DOI] [PubMed] [Google Scholar]
- Hozumi N., Haruna I., Watanabe I., Mikoshiba K., Tsukada Y. Poly(U) polymerase in rat brain. Nature. 1975 Jul 24;256(5515):337–339. doi: 10.1038/256337a0. [DOI] [PubMed] [Google Scholar]
- Leung W. C., Ghosh H. P., Rawls W. E. Strandedness of Pichinde virus RNA. J Virol. 1977 Apr;22(1):235–237. doi: 10.1128/jvi.22.1.235-237.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung W. C., Rawls W. E. Virion-associated ribosomes are not required for the replication of Pichinde virus. Virology. 1977 Aug;81(1):174–176. doi: 10.1016/0042-6822(77)90070-8. [DOI] [PubMed] [Google Scholar]
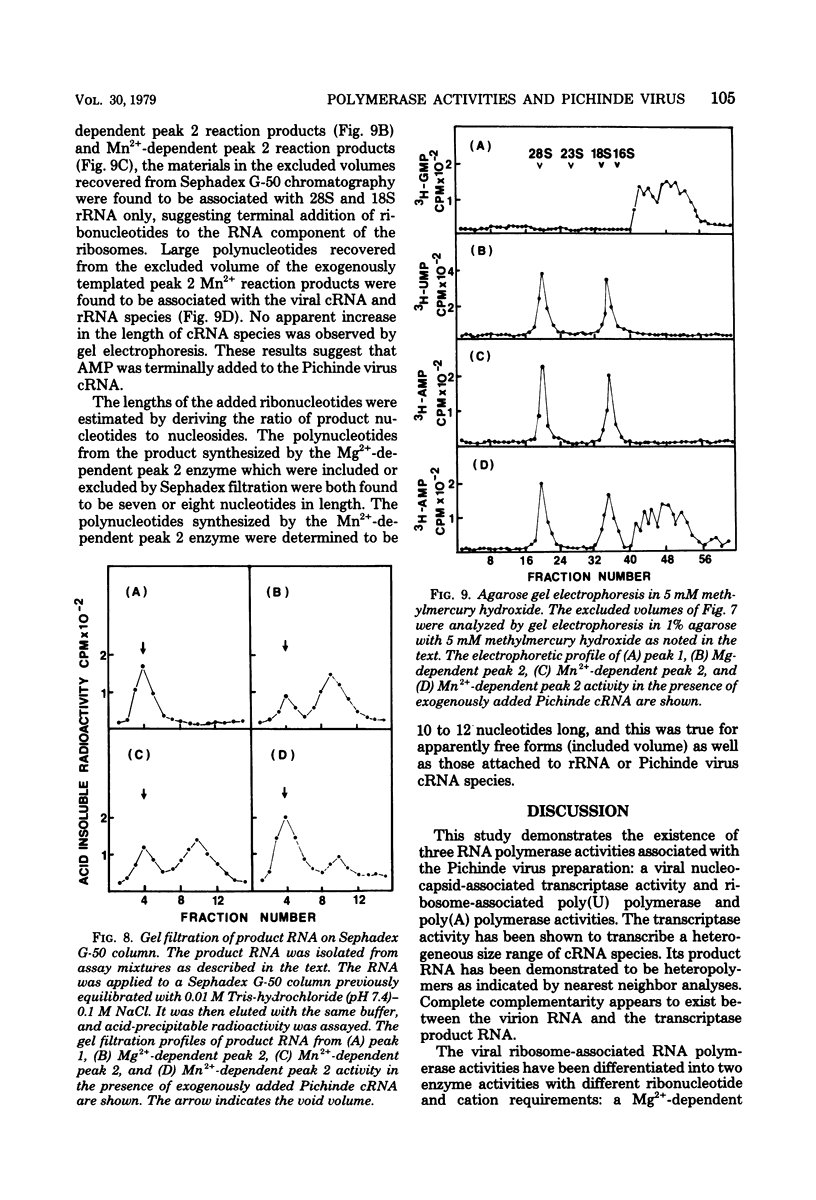
- Marx P. A., Portner A., Kingsbury D. W. Sendai virion transcriptase complex: polyeptide composition and inhibition by virion envelope proteins. J Virol. 1974 Jan;13(1):107–112. doi: 10.1128/jvi.13.1.107-112.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mifune K., Carter M., Rawls W. Characterization studies of the Pichinde virus-a member of the arenavirus group. Proc Soc Exp Biol Med. 1971 Feb;136(2):637–644. doi: 10.3181/00379727-136-35330. [DOI] [PubMed] [Google Scholar]
- Milchev G. I., Hadjiolov A. A. Association of poly(A) and poly(U) polymerases with cytoplasmic ribosomes. Eur J Biochem. 1978 Mar;84(1):113–121. doi: 10.1111/j.1432-1033.1978.tb12147.x. [DOI] [PubMed] [Google Scholar]
- Murphy F. A., Webb P. A., Johnson K. M., Whitfield S. G., Chappell W. A. Arenoviruses in Vero cells: ultrastructural studies. J Virol. 1970 Oct;6(4):507–518. doi: 10.1128/jvi.6.4.507-518.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito S., Ishihama A. Function and structure of RNA polymerase from vesicular stomatitis virus. J Biol Chem. 1976 Jul 25;251(14):4307–4314. [PubMed] [Google Scholar]
- Niessing J. Three distinct forms of nuclear poly(A) polymerase. Eur J Biochem. 1975 Nov 1;59(1):127–135. doi: 10.1111/j.1432-1033.1975.tb02433.x. [DOI] [PubMed] [Google Scholar]
- Palmer E. L., Obijeski J. F., Webb P. A., Johnson K. M. The circular, segmented nucleocapsid of an arenavirus-Tacaribe virus. J Gen Virol. 1977 Sep;36(3):541–545. doi: 10.1099/0022-1317-36-3-541. [DOI] [PubMed] [Google Scholar]
- Randerath E., Randerath K. Ion-exchange thin-layer chromatography. XII. Quantitative elution and microdetermination of nucleoside monophosphates, ATP, and other nucleotide coenzymes. Anal Biochem. 1965 Jul;12(1):83–93. doi: 10.1016/0003-2697(65)90145-4. [DOI] [PubMed] [Google Scholar]
- Robinson W. S. Ribonucleic acid polymerase activity in Sendai virions and nucleocapsid. J Virol. 1971 Jul;8(1):81–86. doi: 10.1128/jvi.8.1.81-86.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone H. O., Portner A., Kingsbury D. W. Ribonucleic acid transcriptases in Sendai Virions and infected cells. J Virol. 1971 Aug;8(2):174–180. doi: 10.1128/jvi.8.2.174-180.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezza A. C., Clewley J. P., Gard G. P., Abraham N. Z., Compans R. W., Bishop D. H. Virion RNA species of the arenaviruses Pichinde, Tacaribe, and Tamiami. J Virol. 1978 May;26(2):485–497. doi: 10.1128/jvi.26.2.485-497.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezza A. C., Gard G. P., Compans R. W., Bishop D. H. Structural components of the arenavirus Pichinde. J Virol. 1977 Sep;23(3):776–786. doi: 10.1128/jvi.23.3.776-786.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh R. M., Burner P. A., Holland J. J., Oldstone M. B., Thompson H. A., Villarreal L. P. A comparison of biochemical and biological properties of standard and defective lymphocytic choriomeningitis virus. Bull World Health Organ. 1975;52(4-6):403–408. [PMC free article] [PubMed] [Google Scholar]
- Wilkie N. M., Smellie R. M. Chain extension of ribonucleic acid by enzymes from rat liver cytoplasm. Biochem J. 1968 Oct;109(4):485–494. doi: 10.1042/bj1090485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkie N. M., Smellie R. M. Polyribonucleotide synthesis by subfractions of microsomes from rat liver. Biochem J. 1968 Sep;109(2):229–238. doi: 10.1042/bj1090229. [DOI] [PMC free article] [PubMed] [Google Scholar]



