Abstract
Objective
To compare home-based cardiac rehabilitation (CR) with usual care (control group with no rehabilitation) in elderly patients who declined participation in centre-based CR.
Design
Randomised clinical trial with 12 months follow-up and mortality data after 5.5 years (mean follow-up 4½ years).
Setting
Rehabilitation unit, Department of Cardiology, Copenhagen, Denmark.
Participants
Elderly patients ≥65 years with coronary heart disease.
Intervention
A physiotherapist made home visits in order to develop an individualised exercise programme that could be performed at home and surrounding outdoor area. Risk factor intervention, medical adjustment, physical and psychological assessments were offered at baseline and after 3, 6 and 12 months.
Main outcome measurements
The primary outcome was 6 min walk test (6MWT). Secondary outcomes were blood pressure, body composition, cholesterol profile, cessation of smoking, health-related quality of life (HRQoL), anxiety and depression.
Results
40 patients participated. The study population was characterised by high age (median age 77 years, range 65–92 years) and high level of comorbidity. Patients receiving home-based CR had a significant increase in the primary outcome 6MWT of 33.5 m (95% CI: 6.2 to 60.8, p=0.02) at 3 months, whereas the usual care group did not significantly improve, but with no significant differences between the groups. At 12 months follow-up, there was a decline in 6MWT in both groups; −55.2 m (95% CI: 18.7 to 91.7, p<0.01) in the home group and −52.1 m (95% CI: −3.0 to 107.1, p=0.06) in the usual care group. There were no significant differences in blood pressure, body composition, cholesterol profile, cessation of smoking or HRQoL after 3, 6 and 12 months follow-up.
Conclusions
Participation in home-based CR improved exercise capacity among elderly patients with coronary heart disease, but there was no significant difference between the home intervention and the control group. In addition, no significant difference was found in the secondary outcomes. When intervention ceased, the initial increase in exercise capacity was rapidly lost.
Keywords: Cardiology
Article summary.
Article focus
To compare home-based cardiac rehabilitation with usual care in elderly patients with coronary heart disease who decline participation in a centre-based rehabilitation programme.
Key messages
Home-based cardiac rehabilitation improved exercise capacity among elderly patients with coronary heart disease.
This population of elderly patient had a high level of comorbidity and disability.
When the home-based intervention ceased, the effect was rapidly lost.
Strengths and limitations of the study
The randomised design provides a higher level of evidence.
This population represents the ‘real-world’ scenario of elderly cardiac patients.
The duration of the intervention may be too short to maintain changes in exercise capacity at 12 months of follow-up.
The size of the study did not allow subgroup analysis.
Introduction
Participation in cardiac rehabilitation (CR) is often the first step towards optimal secondary treatment and prevention, and is recommended to patients with coronary heart disease. The centre-based programmes are the cornerstone in the evidence of CR, with meta-analysis showing an approximately 20% reduction in all-cause and cardiac mortality and 17% reduction in re-infarction rate among patients who participated in the programmes.1 2 CR is also found to be effective among the elderly age ≥65 years.3 4 However, one of the main problems in centre-based CR is the low participation rate among patients in general and among elderly patients in particular. Participation rates are reported to be as low as 30% of eligible patients5 but, among elderly patients, participation rate is even lower.4 In addition, adherence rate to the centre-based programmes are low and drop-out rates are high.6
In order to improve access and participation rate, there has been an increasing focus on home-based CR where the entire programme, or parts hereof, is moved from the centre to the patient's home. This could be an attractive alternative to centre-based CR. Several guidelines have advocated for home-based CR7–9 and these programmes are now the main alternative to the centre-based programmes.
We have recently published a randomised clinical trial (RCT) comparing home-based CR with centre-based CR in elderly patients with coronary heart disease.10 The study showed that home-based CR was not inferior to centre-based CR, which is in accordance with a Cochrane review from 2010.11 A review from 2006,12 comparing home-based programmes with usual care (no rehabilitation) found a significantly better outcome in systolic blood pressure and in the likelihood of being a smoker. The home-based programmes had also better outcomes with regard to exercise capacity, total cholesterol, anxiety and depression score, although these data did not reach statistical significance. A limitation in the reviews and meta-analyses11–13 is that the included populations are highly selected with few elderly patients and excluding patients with comorbidity and disability. Since elderly patients with coronary heart disease is the fastest-growing subgroup of cardiac patients there is an increasing need for adjusting the CR programmes according to their requirements.
The aim of this study is, in a randomised design, to compare the effect of home-based CR with usual care (no rehabilitation) in a population of patients ≥65 years with coronary heart disease, who declined participation in a centre-based CR programme.
Methods
Trial design
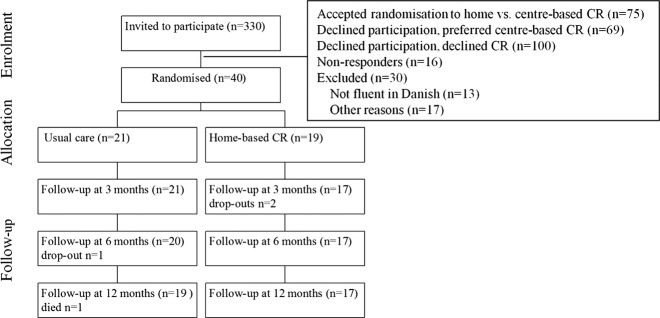
The study is a randomised clinical trial comparing home-based CR with usual care. Inclusion criteria were patients ≥65 years with a recent coronary event defined as acute myocardial infarction (MI), percutaneous transluminal coronary intervention (PCI) or coronary artery bypass graft (CABG) and who declined participation in centre-based CR. Exclusion criteria were mental disorders (dementia), social disorders (severe alcoholism and drug abuse), living in a nursing home, language barriers or use of wheelchair. Figure 1 shows the flowchart.
Figure 1.
Flowchart.
Patients were recruited from our Rehabilitation Unit which offers centre-based CR to all patients with coronary heart disease assigned to the hospital. In order to ensure that all patients receive the CR treatment offer, the referral procedure is centralised and computerised with identification of patients from a database covering diagnosis and all invasive procedures performed in the catchments area of Bispebjerg University Hospital, Copenhagen. Patients are consecutively invited by letter and non-responders are additionally contacted by telephone. At the first visit in the Rehabilitation Unit, patients were invited to participate in the previously mentioned RCT comparing home-based CR with centre-based CR,10 or as an alternative encouraged to participate in the centre-based CR programme (outside the study). Patients who declined participation in these offers were invited to participate in this study.
The recruitment period was from January 2007 to July 2008.
Inclusion of patients was not based on a sample size calculation.
Patients had to give informed consent before any trial-related procedures. Patients were randomised in alternated block sizes of 4–6 using computer-generated randomly permuted blocks. An impartial person, not related to the study, randomised the patients. Because of the nature of the intervention, concealment of randomisation was not feasible with regard to both patients and researcher. Data were collected at Bispebjerg University Hospital before randomisation and after 3, 6 and 12 months. In addition, overall mortality data were obtained in July 2012, 5.5 years after the study was initiated.
The study was approved by the local ethic committee (jr.nr.KF01327990), the Danish Data Protection Agency (j.nr. 2006-41-7212) and is registered at http://www.clinicaltrial.gov (NCT00489801).
Intervention
The home programme
Patients received two home visits by a physiotherapist in a 6-week interval with the purpose of creating a training programme that could be performed at home and outside in local surroundings. Patients were carefully instructed in the training programme and guided to optimal training effort. In between the visits, a telephone call was made by the physiotherapist to resolve any questions.
The exercise programmes were individualised but followed the international recommendations with 30 min exercise/day including 5–10 min warm up (eg, slow walking) and 10 min. cool down at a frequency of 6 days/week14 15 at an intensity of 11–13 on the Borg scale.15 For very disabled patients, the exercise programmes were of shorter duration but then repeated several times a day.
Regarding risk factor intervention and medical adjustment, the patients consulted a cardiologist at baseline and after 3, 6 and 12 months. At 4 and 5 months, a telephone call was made by the cardiologist to encourage continuous exercising and to answer any medical questions. All patients were offered dietary counselling and, if required, smoking cessation.
Usual care
This group is equivalent to a non-rehabilitation control group. Patients were not offered exercise education or dietary counselling but, as for the home group, offered risk factor intervention and medical adjustment by a cardiologist at baseline and after 3, 6 and 12 months. Telephone calls were made at 4 and 5 months. Thus, this group received solely consultation at a cardiologist which is offered to all patients in daily clinical practise who decline participation in our comprehensive centre-based CR programme.
Outcome measures
Because many patients, owing to age and comorbidity, were not able to perform a symptom-limited exercise capacity test, the primary outcome was change in exercise capacity determined by 6 min walk test (6MWT). The secondary outcomes were: sit to stand test (STS), self-reported level of physical activity, systolic and diastolic blood pressure, total-cholesterol, HDL-cholesterol and LDL-cholesterol, body mass index, waist–hip ratio, proportion of smokers, health-related quality of life (HRQoL) measured by SF-12, and anxiety and depression estimated by Hospital Anxiety and Depression Scale (HADS). Outcomes were evaluated after 3, 6 and 12 months.
In the STS-test, the patients must, as fast as possible within 30 s, change position from sitting on a chair to upright standing, without holding the handgrip, hereby measuring the strength in the lower limb. Self-reported level of physical activity was estimated by a questionnaire originally developed by Saltin and Grimby.16 It has four categories ranging from a sedentary lifestyle, to performing light activities 2–4 h/week, activity more than 4 h/week or highly vigorous physical activity more than 4 h/week. Patients in the last three categories were classified as having an active lifestyle. Medication included the use of diuretics, β-blockers, calcium antagonists, lipid-lowering drugs, antithrombotics, antidiabetic and antidepressive treatment. Sociodemographic data included level of education, main employment status, contact to children, living alone and the need of weekly assistance at home. Patients in NYHA II–IV and CCS II–IV were categorised as having dyspnoea and angina, respectively. Comorbidity was assessed by The Charlson Co-Morbidity Index (CMI),17 which measures the burden of 19 comorbid conditions through a weighted index. The CMI was categorised in 3 subgroups: 0 (no comorbid condition), 1–2 and ≥3 (high level of comorbid burden).
Adverse events were recorded in the study period and included admissions for MI, progressive angina, decompensated congestive heart failure, severe bleeding, new malignant disease and performance of PCI. Moreover, the number and duration of hospital admissions were recorded 1 year after randomisation. Death data were obtained from the Civil Registration System, which records the vital status of all citizens in Denmark.
Statistical analysis
To test the effect of the interventions at 3 and 12 months, a mixed model of regression analysis was used with a time×treatment interaction term. We used a mixed model in order to analyse the effect of the interventions, since this statistical model allow us to include all data into one analysis. All the models were adjusted for age and gender. We did not adjust the significance levels for multiple testing, since such an adjustment is a too conservative test to perform, when data are positively correlated, as in this study.
Data were analysed by intention to treat. All statistical analysis was performed using STATA for windows release V.10.0.
Results
A total of 40 patients participated. Baseline characteristics are listed in table 1. All patients received antithrombotics and lipid-lowering drugs and 77.4% received β-blockers.
Table 1.
Baseline characteristics according to intervention
| Characteristic | Usual care n=21 | Home n=19 |
|---|---|---|
| Age | 76.5 (7.7) | 77.3 (6.0) |
| Men (n (%)) | 11 (52.3) | 12 (63.2) |
| Risk factors | ||
| Hypertension (n (%)) | 13 (61.9) | 16 (88.9) |
| Hyperlipidaemia, n (%) | 17 (81.0) | 18 (94.7) |
| Diabetes, n (%) | 2 (9.5) | 7 (36.8) |
| BMI, kg/m2 | 26.2 (3.6) | 27.6 (4.5) |
| Current smokers, n (%) | 9 (42.9) | 8 (42.1) |
| Medical history | ||
| Previous MI, n (%) | 8 (38.1) | 6 (31.7) |
| Previous PCI, n (%) | 5 (23.8) | 4 (21.1) |
| Previous CABG, n (%) | 2 (9.5) | 0 (0) |
| Heart failure LVEF ≤45%, n (%) | 9 (42.9) | 9 (50.0) |
| Event prior to entry into the study | ||
| Post-MI without invasive procedure, n (%) | 4 (19.1) | 0 (0) |
| Post-PCI, n (%) | 14 (66.7) | 16 (84.2) |
| Post-CABG, n (%) | 3 (14.3) | 3 (15.8) |
| Clinical status | ||
| 6MWT, m | 325.9 (123.1) | 290.9 (116.5) |
| STS | 10.9 (3.7) | 8.9 (4.8) |
| Systolic blood pressure, mm Hg | 138.3 (22.2) | 153.6 (27.5) |
| Diastolic blood pressure, mm Hg | 72.2 (13.9) | 76.1 (13.0) |
| Waist–hip ratio | 0.9 (0.1) | 1.0 (0.1) |
| Dyspnoea, NYHA II–IV, n (%) | 13 (61.9) | 11 (57.9) |
| Angina, CCS II–IV, n (%) | 4 (19.1) | 4 (21.1) |
| Self-reported active lifestyle, n (%) | 10 (47.6) | 6 (31.6) |
| Comorbid conditions | ||
| CMI score 0, n (%) | 0 (0) | 1 (5.3) |
| 1–2, n (%) | 9 (42.9) | 7 (36.8) |
| ≥3, n (%) | 12 (57.1) | 11 (57.9) |
| COPD, n (%) | 7 (33.3) | 4 (21.1) |
| Peripheral arterial disease, n (%) | 3 (14.3) | 5 (26.3) |
| Laboratory values | ||
| Total cholesterol, mmol/l | 4.5 (1.1) | 4.3 (0.9) |
| HDL cholesterol, mmol/l | 1.4 (0.3) | 1.3 (0.6) |
| LDL cholesterol, mmol/l | 2.5 (2.2) | 2.4 (1.7) |
| HRQoL, anxiety and depression | ||
| HADS anxiety score | 4.7 (3.0) | 5.1 (4.9) |
| HADS depression score | 5.3 (3.8) | 4.8 (2.7) |
| SF-12 PCS | 39.0 (10.8) | 38.0 (9.9) |
| SF-12 MCS | 46.9 (10.1) | 48.9 (9.3) |
Values are mean (SD) unless stated otherwise.
BMI, body mass index; CABG, coronary artery bypass graft; CMI, Charlson comorbidity index; COPD, chronic obstructive pulmonary disease; HADS, Hospital Anxiety and Depression Scale; HDL, high density lipoprotein; HRQoL, health-related quality of life; LDL, low density lipoprotein; LVEF, left ventricular ejection fraction; MCS, mental component summary scale of SF-12; MI, myocardial infarction; PCI, percutaneous transluminal coronary intervention; PCS, physical component summary scale of SF-12; STS, sit to stand test; 6MWT, 6 min walk test.
Of eligible patients to receive CR (n=284), a total of 49% (n=140) declined to participate in the centre-based programme (figure 1). Of these, 29% accepted to participate in this study and 71% (n=100) did not receive any rehabilitation.
Exclusion rate was 10% mainly because of language barriers (n=13), social disorders (n=5), dementia (n=5) and other reasons (n=7).
Exercise capacity
Figure 2 illustrates the unadjusted means of the primary outcome measurement of 6MWT from baseline to 12 months follow-up. The figure shows a significant increase in walking distance of 33.5 m (95% CI 6.2 to 60.8, p=0.02) in the home group after the intervention followed by a significant decline of −55.2 m (95% CI 18.7 to 91.7, p<0.01) at 12 months follow-up to a level lower than the baseline value. Patients in the usual care group had a non-significant increase in walking distance of 10.1 m (95% CI: −19.3 to 39.5, P=0.5) after 3 months followed by a decline of −52.1 m (95% CI −3.0 to 107.1, p=0.06) at the end of the follow-up period. When adjusting for age and gender in a mixed model with a time×treatment interaction term, there were no significant differences between the groups at 3 months (table 2). At 12 months follow-up, a significant decline in 6MWT and STS was found in both groups with no differences between the groups (table 3).
Figure 2.
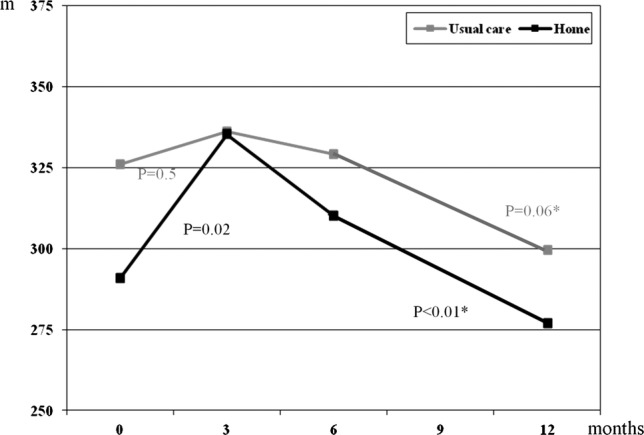
Changes in mean values of 6-min walk test. *p Value between 3 and 12 months.
Table 2.
Effect of intervention at 3 months follow-up
| Usual care |
Home |
Between groups comparison | 95% CI | |||
|---|---|---|---|---|---|---|
| Δ 0–3 months | 95% CI | Δ 0–3 months | 95% CI | |||
| Exercise capacity | ||||||
| 6MWT, m | 10.1 | −23.6, 43.9 | 36.3 | −0.9, 73.6 | 26.2 | −24.1 to 76.5 |
| STS | 0.9 | −0.8, 2.6 | 1.0 | −0.8, 2.8 | 0.1 | −2.3 to 2.6 |
| Clinical status | ||||||
| Systolic blood pressure, mm Hg | 2.0 | −8.4, 12.4 | −12.9 | −24.2, −1.6* | −14.9 | −30.2 to 0.5 |
| Diastolic blood pressure, mm Hg | 4.1 | −2.2, 10.5 | −1.5 | −8.4, 5.4 | −5.7 | −15.0 to 3.7 |
| BMI, kg/m2 | 0.1 | −1.3, 1.5 | −0.5 | −2.1, 1.1 | −0.6 | −2.7 to 1.5 |
| Waist–hip ratio | −0.01 | −0.03, 0.01 | −0.01 | −0.03, 0.01 | 0 | −0.03 to 0.03 |
| Laboratory values | ||||||
| Total cholesterol, mmol/l | −0.2 | −0.6, 0.2 | −0.1 | −0.5, 0.4 | 0.1 | −0.5, 0.7 |
| HDL cholesterol, mmol/l | 0.1 | −0.01, 0.2 | 0.1 | −0.1, 0.2 | −0.04 | −0.2, 0.1 |
| LDL cholesterol, mmol/l | −0.2 | −0.5, 0.1 | −0.1 | −0.5, 0.3 | 0.1 | −0.4 to 0.6 |
| Cholesterol/HDL ratio | −0.4 | −0.7, 0 | −0.3 | −0.7, 0.1 | 0.1 | −0.5 to 0.7 |
| HRQoL, anxiety and depression | ||||||
| HADS anxiety score | −0.9 | −2.3, 0.5 | −1.2 | −2.7, 0.6 | −0.3 | −2.4 to 1.9 |
| HADS depression score | −1.1 | −2.6, 0.4 | −1.0 | −2.7, 0.6 | 0.1 | −2.2 to 2.3 |
| SF-12 PCS | 2.7 | −1.4, 6.8 | −0.4 | 5.1, 4.3 | −3.1 | −9.4 to 3.1 |
| SF-12 MCS | 3.5 | −0.9, 7.9 | 2.4 | −2.6, 7.5 | −1.0 | −7.7 to 5.6 |
All data are adjusted for age and gender.
*p<0.05.
BMI, body mass index; HADS, hospital anxiety and depression score; HDL, high density lipoprotein; HRQoL, health-related quality of lifeLDL, low density lipoprotein; MCS, mental component summary scale of SF-12; PCS, physical component summary scale of SF-12; STS, sit to stand test; 6MWT, 6 min walk test.
Table 3.
Follow-up data at 12 months
| Usual care |
Home |
Between groups comparison | 95% CI | |||
|---|---|---|---|---|---|---|
| Δ 3–12 months | 95% CI | Δ 3–12 months | 95% CI | |||
| Exercise capacity | ||||||
| 6MWT, m | −50.9 | −86.6, −15.3** | −55.0 | −94.0, −16.1** | −4.0 | −56.8 to 48.8 |
| STS | −3.0 | −4.7, −1.3** | −2.1 | −3.9, −0.3* | 0.9 | −1.6 to 3.4 |
| Clinical status | ||||||
| Systolic blood pressure, mm Hg | 0.7 | −9.3, 10.6 | −2.5 | −13.1, 8.2 | −3.1 | −17.7 to 11.4 |
| Diastolic blood pressure, mm Hg | −0.6 | −6.4, 5.1 | 1.6 | −4.6, 7.8 | 2.2 | −6.2 to 10.7 |
| BMI, kg/m2 | 0.4 | −0.04, 0.8 | 0.6 | 0.1, 1.0* | 0.2 | −0.4 to 0.8 |
| Waist–hip ratio | 0.01 | −0.01, 0.03 | 0.0 | −0.02, 0.02 | 0.01 | −0.04 to 0.02 |
| Laboratory values | ||||||
| Total cholesterol, mmol/l | 0.1 | −0.3, 0.5 | −0.1 | −0.5, 0.3 | −0.2 | −0.8 to 0.4 |
| HDL cholesterol, mmol/l | −0.1 | −0.2, 0.01 | −0.04 | −0.1, 0.1 | 0.1 | −0.1 to 0.2 |
| LDL cholesterol, mmol/l | 0.1 | −0.3, 0.2 | −0.04 | −0.4, 0.3 | −0.1 | −0.6 to 0.4 |
| Cholesterol/HDL ratio | 0.3 | −0.1, 0.6 | 0.1 | −0.3, 0.5 | −0.2 | −0.7 to 0.3 |
| HRQoL, anxiety and depression | ||||||
| HADS anxiety score | 0.3 | −1.3, 1.9 | 0.4 | −1.3, 2.1 | 0.1 | −2.3 to 2.4 |
| HADS depression score | 0.3 | −1.2, 1.8 | 1.2 | −0.3, 2.8 | 0.9 | −1.3 to 3.1 |
| SF-12 PCS | −1.4 | −5.2, 2.3 | −1.1 | −5.3, 3.1 | 0.3 | −5.4 to 6.0 |
| SF-12 MCS | −0.3 | −4.6, 4.0 | −1.4 | −6.1, 3.3 | −1.1 | −7.5,5.3 |
All data are adjusted for age and gender.
*p<0.05.
**p<0.01.
BMI, body mass index; HADS, hospital anxiety and depression score; HDL, high density lipoprotein; HRQoL, health-related quality of life; LDL, low density lipoprotein; MCS, mental component summary scale of SF-12; PCS, physical component summary scale of SF-12; STS, sit to stand test; 6MWT, 6 min walk test.
Other outcomes
A higher proportion of patients reported a change from an inactive to an active lifestyle in the home group (27%, p<0.05) compared to the usual care group (−5%, p=0.6), after the intervention with a difference between the two groups of 33% (p<0.05). At 12 months follow-up, the proportion of patients with a self-reported active lifestyle declined again in the home group with no changes in the usual care group.
There were no significant differences in clinical status, exercise capacity, laboratory values, HRQoL or anxiety and depression score at 3 and 12 months follow-up either within or between the groups.
The number and length of acute and non-acute admissions were equally distributed at 12 months follow-up (data not shown).
A total of nine patients died during a mean follow-up of 4.5 years (usual care group n=5 and home group n=4). There was no loss to follow-up.
Discussion
To the best of our knowledge, this is the first study to investigate the effect of home-based CR compared to usual care (no rehabilitation) among elderly patients ≥65 years with coronary heart disease who declined participation in a centre-based programme. In many countries, including Denmark, centre-based programmes are often the only cardiac rehabilitation programme available, and the limited access to CR may be an important barrier for optimal secondary treatment and prevention in elderly patients with coronary heart disease.
The study found that elderly patients who decline participation in centre-based CR had a low level of exercise capacity and a high level of comorbidity. For this population, who is often found not to be eligible to centre-based CR, home-based CR was feasible. There was a trend towards clinical relevant improvement in 6MWT, but these changes were not statistically significant compared to the control group. Although the study is small and conclusions must be drawn with caution, it could identify an intervention targeting this group of patients. After having ended the home programme, the gained improvement in exercise capacity was not sustained.
Exercise capacity
The effect of our home CR programme on exercise capacity is consistent with the findings in the only other study investigating the effect of home-based CR and usual care among elderly with coronary heart disease.3 In this study, patients in the age groups 45–65 years, 66–75 years and >75 years significantly improved their exercise capacity after participating in a home programme, although the improvement was less among the very old patients (>75 years).
The meta-analysis by Jolly et al,12 which included studies of all age groups, investigated the effect of home-based CR and usual care. The meta-analysis showed an improvement in exercise capacity but could not identify any significant differences between the home and usual care group. The authors explained this by the possibility that patients in usual care groups may receive input that match the homeinterventions and thus diminish a possible difference. This could also have been the case in our study.
At 12 months, a significant decline in exercise capacity was found in both the home and usual care group reaching a level lower than at entrance to the study. We identified two other studies with long-term follow-up.3 18 In contrast to our study, they both found a sustained improvement in exercise capacity after 12 months, if the exercise programme was initiated at home. The discrepancy could be caused by the duration of our home intervention that may have been too short to maintain changes in lifestyle at 12 months follow-up, but our home intervention is in line with other home-based programmes.12 13 The majority of programmes have a duration of 6–12 weeks.7 9 11–13 It has been suggested that more intensive programmes with prolonged duration beyond 12 weeks have a more successful long-term outcome.19 20 However, in a previous study of heart failure patients,21 even a prolonged centre-based maintenance programme with supervised sessions every 2 weeks, in addition to home exercise training, could not maintain the improvements achieved during initial CR.21 Furthermore, in the very large HF-ACTION trial,22 patients participated in an initial centre-based exercise programme of 36 sessions in 3 months followed by a home-based exercise programme with intensive follow-up and equipment for home training was provided. In the HF-ACTION trial22 there were no changes in exercise capacity at 12 months follow-up. This was explained partly by insufficient adherence to training that was below the target set at all time points. The HF-ACTION trial mainly included middle-aged men with no major comorbidities or limitations that could interfere with training. Thus, in spite of intensive exercise programmes with close follow-up in patients with no significant concomitant comorbidities, it is difficult to motivate patients to adhere to training. Feasible solutions to overcome this have not yet been identified.
The discrepancy between studies may also be due to the differences in the enrolled populations. Our population was significantly older (mean age 77.3±6.0 vs 69.0±9.0 years3 and 64.3±0.5 years18) and had a high degree of comorbidity and low level of exercise capacity. Age, comorbidity and disability are all found to be negatively correlated with physical activity15 23 and adherence to training6 24 25 and thus may have contributed significantly to the lack of sustained effect at 12 months. Moreover, in the only other study targeting the elderly,3 the population was highly selected with exclusion rate of 72% among the very old patients (>75 years) due to comorbidity, disability and congestive heart failure, leading to a much ‘healthier’ population, compared to our population in which only 10% were excluded.
Coronary heart disease is one of the leading causes of disability and, with increasing age, other chronic non-cardiac conditions further limit function.26 Our population of elderly had a very high frequency of comorbid conditions (57% had CMI≥3). For comparison, a recent very large nationwide study, including 234 000 patients (median age 68 years in men and 75 years in women) with first time acute MI, found that only 6% of that population had CMI≥3.27 In addition to the high frequency of comorbidity, we found a low level of exercise capacity at baseline, with mean 6MWT=308.4 m±120. In healthy elderly subjects, mean 6MWT is found to be approximately 659 m±74 m28 and, in a recent RCT study from our group comparing home-based CR with centre-based CR10 a baseline mean 6MWT of 340 m±122 m in the centre group was found.10 These characteristics indicate that the group of elderly patients who decline participation in centre-based rehabilitation is very vulnerable and not necessarily comparable with the population who accept centre-based CR. Our finding is in concordance with previous studies who found that older age, high burden of comorbidity and low level of exercise capacity was negatively correlated with participation rate in centre-based CR programmes.6 24
The high burden of comorbidity in this population is most likely explained by the computerised identification of patients which eliminated the selection and referral bias often seen in rehabilitation units, which is not in favour of the elderly and patients with comorbidity.24 29–31
Other outcomes
Self-reported active lifestyle and systolic blood pressure changed favourably in the home group after the intervention but there were no significant differences in diastolic blood pressure, body composition, cessation of smoking, cholesterol profile and HRQoL between the groups. Our population had a favorable cardiovascular risk factor control and low anxiety and depression score (HADS score <8 is within normal rage)32 33 at entrance to the study why a further improvement could not be expected.
We did not find any significant changes in HRQoL measured by SF-12. This is partly due to lack of statistical power and the limited duration of our home intervention but is in concordance with the meta-analysis by Jolly et al12 and with a recent published review concerning CR and HRQoL.34 We did not have any specific psychological intervention but the type of intervention (comprehensive programmes, exercise only or mainly psychological interventions) do not seem to affect these results.12 34
In central Europe, centre-based CR is the traditional choice of CR services. However, establishing of home-based CR programmes as an alternative for elderly patients could improve CR attendance rate. In English-speaking countries and in countries where health services are not free, home-base CR programmes are more commonly used, primarily through the adoption of The Heart Manual.35 36 This is currently not an option in non-English-speaking countries, in many of which there is a stronger tradition of centre-based CR.
In the everyday scenario at the rehabilitation units, there is only one CR programme available, and this is often a centre-based programme. Patients who decline enrolment in these programmes do not have alternatives. A total of 29% of patients, who initially declined centre-based CR, did accept to participate in this study and the proportion could have been even higher if the home-based CR programme was not part of an RCT study. Thus, with alternative concomitant CR programmes, accessibility increases and participation rate will be expected to rise.
The main limitation of this study is the number of patients included. With the additionally large variation in the effect of intervention as reflected in the wide CIs, there is a risk of type II error. However, wide variations in the effect of intervention are often seen in exercise trials and our results are in concordance with other much larger exercise trials.22 35 The strength of our study is the randomised design and the unselected population of elderly patients with high comorbidity, which probably makes our population more representative of the elderly population in daily clinical practice.
Conclusion
In this study of patients ≥65 years with coronary heart disease, home-based CR improved exercise capacity, but there was no significant difference between the home intervention and the control group. In addition, no significant difference was found in the secondary outcomes. The study found that elderly cardiac patients who declined participation in centre-based CR had high level of comorbidity and low exercise capacity. These characteristics indicate that results from exercise trials excluding this group of patients should be cautiously applied to the elderly population. After cessation of the home intervention, the gained improvement in exercise capacity was rapidly lost. This emphasises that close follow-up with continuous guidance beyond the initial rehabilitation period is important. This study could contribute to the scientific gap on how to manage the large population of elderly cardiac patients who are not interested in (or capable of) participating in a centre-based CR programme. Larger trials of unselected older patients are needed in order to confirm our findings and ways to overcome the barriers for adherence to exercise training has to be established.
Supplementary Material
Acknowledgments
The authors would like to thank the physiotherapists, nurses and dieticians involved in the study.
Footnotes
Contributors: BO designed and initiated the study, collected the data, wrote the statistical analysis plan, analysed the data and drafted and revised the paper. She is guarantor. EP contributed with design, wrote the statistical analysis plan, analysed the data and revised the draft paper. MF designed the study and collected some of the data and revised the paper. JFH designed the study and revised the draft paper.
Funding: This work was supported by VELUX FOUNDATION.
Competing interests: None.
Patient consent: Obtained.
Ethics approval: The study was approved by the Local ethics committee in Copenhagen, Denmark, (jr.nr.KF01327990) and the Danish Data Protection Agency (j.nr. 2006-41-7212).
Provenance and peer review: Not commissioned, externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Clark AM, Hartling L, Vandermeer B, et al. Meta-analysis: secondary prevention programs for patients with coronary artery disease. Ann Intern Med 2005;143:659–72 [DOI] [PubMed] [Google Scholar]
- 2.Taylor RS, Brown A, Ebrahim S, et al. Exercise-based rehabilitation for patients with coronary heart disease: systematic review and meta-analysis of randomized controlled trials. Am J Med 2004;116:682–92 [DOI] [PubMed] [Google Scholar]
- 3.Marchionni N, Fattirolli F, Fumagalli S, et al. Improved exercise tolerance and quality of life with cardiac rehabilitation of older patients after myocardial infarction: results of a randomized, controlled trial. Circulation 2003;107:2201–6 [DOI] [PubMed] [Google Scholar]
- 4.Pasquali SK, Alexander KP, Peterson ED. Cardiac rehabilitation in the elderly. Am Heart J 2001;142:748–55 [DOI] [PubMed] [Google Scholar]
- 5.Jackson L, Leclerc J, Erskine Y, et al. Getting the most out of cardiac rehabilitation: a review of referral and adherence predictors. Heart 2005;91:10–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Worcester MU, Murphy BM, Mee VK, et al. Cardiac rehabilitation programmes: predictors of non-attendance and drop-out. Eur J Cardiovasc Prev Rehabil 2004;11:328–35 [DOI] [PubMed] [Google Scholar]
- 7.Giannuzzi P, Saner H, Bjornstad H, et al. Secondary prevention through cardiac rehabilitation: position paper of the Working Group on Cardiac Rehabilitation and Exercise Physiology of the European Society of Cardiology. Eur Heart J 2003;24:1273–8 [DOI] [PubMed] [Google Scholar]
- 8.Graham I, Atar D, Borch-Johnsen K, et al. European guidelines on cardiovascular disease prevention in clinical practice: full text. Fourth Joint Task Force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of nine societies and by invited experts). Eur J Cardiovasc Prev Rehabil 2007;14(Suppl 2):S1–113 [DOI] [PubMed] [Google Scholar]
- 9.Thomas RJ, King M, Lui K, et al. AACVPR/ACC/AHA 2007 performance measures on cardiac rehabilitation for referral to and delivery of cardiac rehabilitation/secondary prevention services endorsed by the American College of Chest Physicians, American College of Sports Medicine, American Physical Therapy Association, Canadian Association of Cardiac Rehabilitation, European Association for Cardiovascular Prevention and Rehabilitation, Inter-American Heart Foundation, National Association of Clinical Nurse Specialists, Preventive Cardiovascular Nurses Association, and the Society of Thoracic Surgeons. J Am Coll Cardiol 2007;50:1400–33 [DOI] [PubMed] [Google Scholar]
- 10.Oerkild B, Frederiksen M, Hansen JF, et al. Home-based cardiac rehabilitation is as effective as centre-based cardiac rehabilitation among elderly with coronary heart disease: results from a randomised clinical trial. Age Ageing 2011;40:78–85 [DOI] [PubMed] [Google Scholar]
- 11.Taylor RS, Dalal H, Jolly K, et al. Home-based versus centre-based cardiac rehabilitation. Cochrane Database Syst Rev 2010;(1):CD007130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jolly K, Taylor RS, Lip GY, et al. Home-based cardiac rehabilitation compared with centre-based rehabilitation and usual care: a systematic review and meta-analysis. Int J Cardiol 2006; 111:343–51 [DOI] [PubMed] [Google Scholar]
- 13.Dalal HM, Zawada A, Jolly K, et al. Home based versus centre based cardiac rehabilitation: Cochrane systematic review and meta-analysis. BMJ 2010;340:b5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith SC, Jr, Allen J, Blair SN, et al. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update: endorsed by the National Heart, Lung, and Blood Institute. Circulation 2006;113:2363–72 [DOI] [PubMed] [Google Scholar]
- 15.Fletcher GF, Balady GJ, Amsterdam EA, et al. Exercise standards for testing and training: a statement for healthcare professionals from the American Heart Association. Circulation 2001;104:1694–740 [DOI] [PubMed] [Google Scholar]
- 16.Saltin B, Grimby G. Physiological analysis of middle-aged and old former athletes. Comparison with still active athletes of the same ages. Circulation 1968;38:1104–15 [DOI] [PubMed] [Google Scholar]
- 17.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83 [DOI] [PubMed] [Google Scholar]
- 18.Smith KM, Arthur HM, McKelvie RS, et al. Differences in sustainability of exercise and health-related quality of life outcomes following home or hospital-based cardiac rehabilitation. Eur J Cardiovasc Prev Rehabil 2004;11:313–19 [DOI] [PubMed] [Google Scholar]
- 19.Perk J, De BG, Gohlke H, et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 2012;33:1635–701 [DOI] [PubMed] [Google Scholar]
- 20.Clark AM, Catto S, Bowman G, et al. Design matters in secondary prevention: individualization and supervised exercise improves the effectiveness of cardiac rehabilitation. Eur J Cardiovasc Prev Rehabil 2011;18:761–9 [DOI] [PubMed] [Google Scholar]
- 21.Prescott E, Hjardem-Hansen R, Dela F, et al. Effects of a 14-month low-cost maintenance training program in patients with chronic systolic heart failure: a randomized study. Eur J Cardiovasc Prev Rehabil 2009;16:430–7 [DOI] [PubMed] [Google Scholar]
- 22.O'Connor CM, Whellan DJ, Lee KL, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 2009;301:1439–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaplan MS, Newsom JT, McFarland BH, et al. Demographic and psychosocial correlates of physical activity in late life. Am J Prev Med 2001;21:306–12 [DOI] [PubMed] [Google Scholar]
- 24.Cooper AF, Jackson G, Weinman J, et al. Factors associated with cardiac rehabilitation attendance: a systematic review of the literature. Clin Rehabil 2002;16:541–52 [DOI] [PubMed] [Google Scholar]
- 25.Witt BJ, Jacobsen SJ, Weston SA, et al. Cardiac rehabilitation after myocardial infarction in the community. J Am Coll Cardiol 2004;44:988–96 [DOI] [PubMed] [Google Scholar]
- 26.Pinsky JL, Jette AM, Branch LG, et al. The framingham disability study: relationship of various coronary heart disease manifestations to disability in older persons living in the community. Am J Public Health 1990;80:1363–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmidt M, Jacobsen JB, Lash TL, et al. 25 year trends in first time hospitalisation for acute myocardial infarction, subsequent short and long term mortality, and the prognostic impact of sex and comorbidity: a Danish nationwide cohort study. BMJ 2012;344:e356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jenkins S, Cecins N, Camarri B, et al. Regression equations to predict 6-minute walk distance in middle-aged and elderly adults. Physiother Theory Pract 2009;25:516–22 [DOI] [PubMed] [Google Scholar]
- 29.Nielsen KM, Faergeman O, Foldspang A, et al. Cardiac rehabilitation: health characteristics and socio-economic status among those who do not attend. Eur J Public Health 2008;18:479–83 [DOI] [PubMed] [Google Scholar]
- 30.Cortes O, Arthur HM. Determinants of referral to cardiac rehabilitation programs in patients with coronary artery disease: a systematic review. Am Heart J 2006;151:249–56 [DOI] [PubMed] [Google Scholar]
- 31.Cottin Y, Cambou JP, Casillas JM, et al. Specific profile and referral bias of rehabilitated patients after an acute coronary syndrome. J Cardiopulm Rehabil 2004;24:38–44 [DOI] [PubMed] [Google Scholar]
- 32.Bjelland I, Dahl AA, Haug TT, et al. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res 2002;52:69–77 [DOI] [PubMed] [Google Scholar]
- 33.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361–70 [DOI] [PubMed] [Google Scholar]
- 34.Shepherd CW, While AE. Cardiac rehabilitation and quality of life: a systematic review. Int J Nurs Stud 2012;49:755–71 [DOI] [PubMed] [Google Scholar]
- 35.Jolly K, Lip GY, Taylor RS, et al. The Birmingham Rehabilitation Uptake Maximisation study (BRUM): a randomised controlled trial comparing home-based with centre-based cardiac rehabilitation. Heart 2009;95:36–42 [DOI] [PubMed] [Google Scholar]
- 36.Dalal HM, Evans PH, Campbell JL, et al. Home-based versus hospital-based rehabilitation after myocardial infarction: a randomized trial with preference arms—Cornwall Heart Attack Rehabilitation Management Study (CHARMS). Int J Cardiol 2007;119:202–11 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.