Abstract
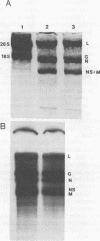
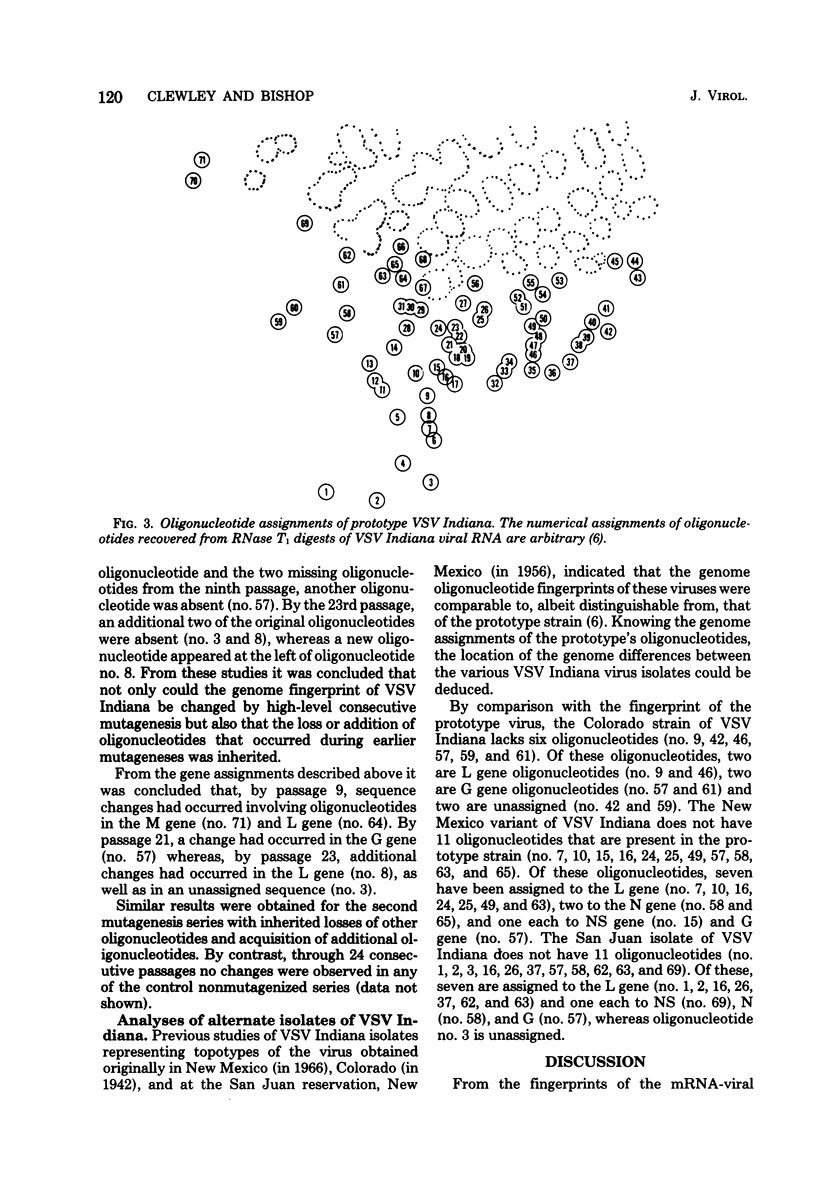
Analyses of prototype vesicular stomatitis (VSV, Indiana serotype) mRNA-32P-labeled viral RNA duplexes have established the assignments of 65 of the 72 large oligonucleotides that are recovered by two-dimensional electrophoresis of RNase T1 digests of the viral RNA. Fifty of the oligonucleotides are recovered in the L RNA duplex, four each in the N, M, and NS duplexes, and three in the G RNA duplex. Studies of three small defective-particle RNA species indicate that they have only L gene oligonucleotides in addition to three of the seven unassigned oligonucleotides. Some L gene ordering of oligonucleotides can be postulated from the defective-particle RNA sequence analyses. Analyses of naturally occurring alternate isolates of VSV Indiana have established that by comparison to the prototype virus strain, the alternate isolates minimally have genome sequence differences in L, G, N, NS and/or unassigned regions of the genome. Changes in the genome have also been induced by vitro high-level mutagenesis of the prototype virus.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham G., Banerjee A. K. Sequential transcription of the genes of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1976 May;73(5):1504–1508. doi: 10.1073/pnas.73.5.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball L. A., White C. N. Order of transcription of genes of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1976 Feb;73(2):442–446. doi: 10.1073/pnas.73.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. H., Roy P. Properties of the product synthesized by vesicular stomatitis virus particles. J Mol Biol. 1971 Jun 28;58(3):799–814. doi: 10.1016/0022-2836(71)90041-6. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Clewley J. P., Bishop D. H., Kang C. Y., Coffin J., Schnitzlein W. M., Reichmann M. E., Shope R. E. Oligonucleotide fingerprints of RNA species obtained from rhabdoviruses belonging to the vesicular stomatitis virus subgroup. J Virol. 1977 Jul;23(1):152–166. doi: 10.1128/jvi.23.1.152-166.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewley J. P., Kennedy S. I. Purification and polypeptide composition of Semliki Forest virus RNA polymerase. J Gen Virol. 1976 Sep;32(3):395–411. doi: 10.1099/0022-1317-32-3-395. [DOI] [PubMed] [Google Scholar]
- Clewley J., Gentsch J., Bishop D. H. Three unique viral RNA species of snowshoe hare and La Crosse bunyaviruses. J Virol. 1977 May;22(2):459–468. doi: 10.1128/jvi.22.2.459-468.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H., Vogt P. K. Gel electrophoresis of avian leukosis and sarcoma viral RNA in formamide: comparison with other viral and cellular RNA species. J Virol. 1973 Sep;12(3):594–599. doi: 10.1128/jvi.12.3.594-599.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman G. J., Rose J. K., Clinton G. M., Huang A. S. RNA synthesis of vesicular stomatitis virus. VII. Complete separation of the mRNA's of vesicular stomatitis virus by duplex formation. J Virol. 1977 Mar;21(3):1094–1104. doi: 10.1128/jvi.21.3.1094-1104.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C. Y., Glimp T., Clewley J. P., Bishop D. H. Studies on the generation of vesicular stomatitis virus (indiana serotype) defective interfering particles. Virology. 1978 Jan;84(1):142–152. doi: 10.1016/0042-6822(78)90226-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Repik P., Bishop D. H. Determination of the molecular weight of animal RNA viral genomes by nuclease digestions. I. Vesicular stomatitis virus and its defective T particle. J Virol. 1973 Nov;12(5):969–983. doi: 10.1128/jvi.12.5.969-983.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes D. P., Abraham G., Colonno R. J., Jelinek W., Banerjee A. K. Characterization of vesicular stomatitis virus mRNA species synthesized in vitro. J Virol. 1977 Mar;21(3):1105–1112. doi: 10.1128/jvi.21.3.1105-1112.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K., Knipe D. Nucleotide sequence complexities, molecular weights, and poly(A) content of the vesicular stomatitis virus mRNA species. J Virol. 1975 Apr;15(4):994–1003. doi: 10.1128/jvi.15.4.994-1003.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy P., Bishop D. H. Initiation and direction of RNA transcription by vesicular stomatitis virus virion transcriptase. J Virol. 1973 Apr;11(4):487–501. doi: 10.1128/jvi.11.4.487-501.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy P., Clewley J. P. Spring viremia of carp virus RNA and virion-associated transcriptase activity. J Virol. 1978 Mar;25(3):912–916. doi: 10.1128/jvi.25.3.912-916.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezza A. C., Clewley J. P., Gard G. P., Abraham N. Z., Compans R. W., Bishop D. H. Virion RNA species of the arenaviruses Pichinde, Tacaribe, and Tamiami. J Virol. 1978 May;26(2):485–497. doi: 10.1128/jvi.26.2.485-497.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wachter R., Fiers W. Preparative two-dimensional polyacrylamide gel electrophoresis of 32 P-labeled RNA. Anal Biochem. 1972 Sep;49(1):184–197. doi: 10.1016/0003-2697(72)90257-6. [DOI] [PubMed] [Google Scholar]