Accurate centriole duplication, controlled by Plk4, is key to proper mitosis and chromosome segregation. Holland et. al. probe the relevance of Plk4 autoregulation and find that Plk4 phosphorylates itself to promote its own degradation. This autoregulated instability controls the levels of endogenous Plk4 and limits centrosome duplication to once per cell cycle. Preventing such autoregulation results in centrosome amplification and, interestingly, an entirely p53-dependent loss of cell proliferation.
Keywords: mitosis, aneuploidy, SCF, centrosome, centriole, genome instability, p53
Abstract
Centrioles organize the centrosome, and accurate control of their number is critical for the maintenance of genomic integrity. Centriole duplication occurs once per cell cycle and is controlled by Polo-like kinase 4 (Plk4). We showed previously that Plk4 phosphorylates itself to promote its degradation by the proteasome. Here we demonstrate that this autoregulated instability controls the abundance of endogenous Plk4. Preventing Plk4 autoregulation causes centrosome amplification, stabilization of p53, and loss of cell proliferation; moreover, suppression of p53 allows growth of cells carrying amplified centrosomes. Plk4 autoregulation thus guards against genome instability by limiting centrosome duplication to once per cell cycle.
Centrosomes are microtubule-organizing centers (MTOCs) comprised of a pair of microtubule-based structures termed centrioles, surrounded by proteinaceous pericentriolar material (PCM) (Gonczy 2012). Centrioles, the organizer of the centrosome, normally duplicate once per cell cycle before the onset of mitosis to create two copies of the centrosome to instruct the formation of the bipolar mitotic spindle apparatus (Tsou and Stearns 2006). Abnormalities in centriole duplication can result in the production of extra copies of centrosomes (known as centrosome amplification), which can lead to errors in mitotic spindle formation and chromosome missegregation (Ganem et al. 2009; Silkworth et al. 2009). Centrosome amplification is frequently observed in aneuploid human tumors, where it is proposed it promotes genome instability and tumor development (Nigg and Raff 2009; Holland and Cleveland 2012). Therefore, accurate control of centrosome number is critical for the maintenance of genomic integrity.
Centriole duplication is under the control of Polo-like kinase 4 (Plk4; also known as SAK in Drosophila), a conserved upstream regulator of centriole assembly (Bettencourt-Dias et al. 2005; Habedanck et al. 2005). Loss of Plk4 results in a failure to assemble new centrioles, while, conversely, overexpression of the kinase drives the assembly of excessive new centrioles and subsequent centrosome amplification (Bettencourt-Dias et al. 2005; Habedanck et al. 2005; Kleylein-Sohn et al. 2007; Peel et al. 2007). Abnormal expression of Plk4 has been linked with genomic instability and a predisposition to tumorigenesis (Macmillan et al. 2001; Ko et al. 2005; Basto et al. 2008; Liu et al. 2012), and thus Plk4 abundance must be tightly regulated in order to correctly control centrosome number and maintain genome integrity (Holland et al. 2010a). Identifying the pathways responsible for controlling Plk4 abundance is important for our understanding of centriole duplication and the origin of centrosome amplification in cancer.
Plk4 is a low-abundance kinase whose stability is directly linked to the activity of the enzyme, with active Plk4 phosphorylating itself to promote its own destruction through the ubiquitin–proteasome pathway (Holland et al. 2010b; Brownlee et al. 2011). The SCF (Skp/Cullin/F-box) E3 ligase associates with phosphorylated Plk4 through the F-box protein β-TrCP (known as Slimb in Drosophila). Phosphorylation of two residues within the β-TrCP-binding motif of Plk4 promotes the binding of β-TrCP and subsequent ubiquitylation and destruction of the kinase (Cunha-Ferreira et al. 2009; Rogers et al. 2009; Guderian et al. 2010; Sillibourne et al. 2010). In Drosophila, the phosphatase PP2A in complex with its targeting subunit, Twins (known as B/PR55 in humans), counteracts Plk4-mediated autophosphorylation to stabilize Plk4 during mitosis (Brownlee et al. 2011).
Despite the recent progress in our understanding of centriole duplication, all of the work to date studying Plk4 regulation has relied on overexpressed Plk4 transgenes. Therefore, it is not established to what extent autocatalyzed destruction controls the stability of endogenous Plk4 and whether autoregulation plays a crucial role in controlling normal centriole duplication. In this study, we used gene targeting in human cells to determine the importance of the autoregulated destruction of Plk4 in controlling centrosome copy number.
Results and Discussion
To establish the importance of Plk4 self-regulation, we used gene targeting in nontransformed, telomerase-immortalized, human RPE-1 cells to create knock-in alleles of Plk4 that are predicted to be deficient in self-regulation. Since loss of Plk4 autoregulation is predicated to have a dominant effect that may be detrimental to the long-term viability of cells, we used homologous recombination to create heterozygous, conditional alleles of Plk4 by inserting a transcriptional/translational stop cassette into intron 5 of the endogenous Plk4 locus (Supplemental Fig. S1A). The transcriptional/translational STOP prevents transcription and truncates any translation downstream from exon 5 of the Plk4 gene, thereby creating a null allele (Holland et al. 2012). However, this cassette is flanked by LoxP sites and can be excised by Cre recombinase to restore expression of the targeted allele.
Homologous recombination was used to create cell lines containing conditional Plk4WT (wild-type), Plk4AA, and Plk4Δ24 alleles integrated at the endogenous genomic locus (Supplemental Fig. S1A): The Plk4AA allele contains mutations in both of the phosphorylation sites required for β-TrCP binding to Plk4, while the Plk4Δ24 allele contains a deletion of a 24-amino-acid region that is rich in serine/threonine residues and heavily autophosphorylated (Supplemental Fig. S1B; Cunha-Ferreira et al. 2009; Rogers et al. 2009; Guderian et al. 2010; Holland et al. 2010b). This 24-amino-acid domain contains a conserved binding motif for SCF-β–TrCP and is required for self-catalyzed destruction of kinase-active Plk4 (Holland et al. 2010b).
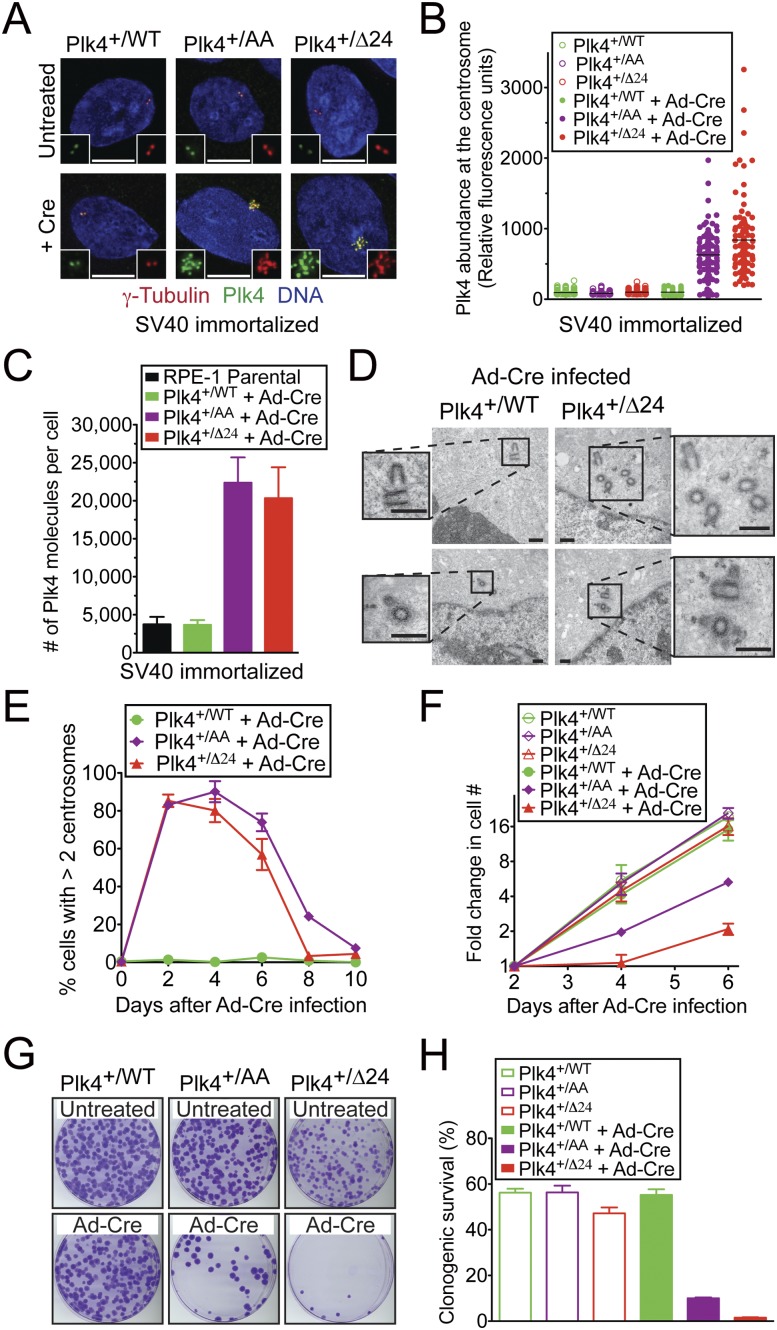
In the basal state, cells heterozygous for the conditional Plk4WT, Plk4AA, and Plk4Δ24 alleles (hereafter referred to as Plk4+/WT, Plk4+/AA, and Plk4+/Δ24 cells, respectively) displayed a 50% reduction in Plk4 mRNA (Holland et al. 2012) but have normal centrosome numbers and growth rates (Fig. 1E–H). Using quantitative immunofluorescence, we determined the level of Plk4 at the centrosome before and after activation of Plk4 conditional alleles with Cre recombinase. The Plk4WT conditional allele forms an important control for any effects derived from expression of Cre in cells. To allow for continued growth (see below), we analyzed Plk4 levels (Fig. 1A–C) in cells expressing the SV40 large T-antigen. In the basal state, Plk4+/WT, Plk4+/AA, and Plk4+/Δ24 cells accumulated similar levels of Plk4 at the centrosome, and this level was unchanged following activation of the Plk4WT allele with Cre recombinase (Fig. 1A,B). In contrast, activation of either of the Plk4 alleles that are deficient in autoregulation resulted in a sixfold to eightfold increase in centrosome-associated Plk4 (a 6.3-fold increase for Plk4+/AA cells and an 8.4-fold increase for Plk4+/Δ24 cells). Since only a portion of the total Plk4 pool is likely to be associated with the centrosome at a given time, we determined the total number of Plk4 molecules per cell using protein absolute quantification (AQUA) mass spectrometry (Gerber et al. 2003). For this, a known amount of a synthetic “heavy” labeled standard Plk4 peptide was introduced into cell lysates. Protease-digested lysates were then analyzed by tandem mass spectrometry, and the internal standard peptide was used to determine the absolute level of the corresponding native Plk4 peptide. RPE1 cells contained, on average, 3730 (±986) molecules of Plk4 per cell, a value very similar to that observed in cells expressing the Plk4WT allele (3670 [±620] molecules per cell) (Fig. 1C). On the other hand, loss of Plk4 autoregulation resulted in a fivefold to sixfold increase in the number of Plk4 molecules per cell (22,400 [±3340] molecules for Plk4+/AA cells, and 20,300 [±4080] molecules for Plk4+/Δ24 cells). While activation of the Plk4WT allele had no effect on centrosome number, the increased Plk4 level from loss of Plk4-autoregulated destruction in Plk4+/AA and Plk4+/Δ24 cells resulted in dramatic centrosome amplification, with >80% of these cells possessing more than two centrosomes within 2 d after viral transduction to express Cre (Fig. 1E). Activation of the Plk4AA and Plk4Δ24 allele caused very similar effects in cells, demonstrating that, of the 13 potential autophosphorylation sites contained within the 24-amino-acid Plk4 phosphodegron (Holland et al. 2010b; Sillibourne et al. 2010), phosphorylation of the two sites required for β-TrCP binding (S285 and T289) plays the dominant role in controlling Plk4 stability. Rosette-like arrangements of procentrioles were frequently observed 24 h after expression of Cre in Plk4+/Δ24 cells, suggesting that centrosome amplification occurs via the canonical centriole duplication pathway in cells lacking Plk4 autoregulation (Supplemental Fig. S1C). Electron microscopy revealed that the excessive centrioles generated in cells deficient in Plk4 self-regulation had a normal ultrastructure (Fig. 1D). This evidence establishes that self-catalyzed destruction of Plk4 plays not only an important role in controlling endogenous protein levels, but also a crucial role in restricting centriole duplication to allow the production of a single new centrosome per cell cycle.
Figure 1.
The autoregulated destruction of Plk4 limits centrosome duplication to once per cell cycle. To allow for continued cell growth, cells analyzed in A–C express the SV40 large T-antigen; cells in D–H did not. (A) Images show the level of Plk4 at the centrosome. (Red) γ-Tubulin; (green) Plk4; (blue) DNA. Bar, 5 μm. (B) Quantitative immunofluorescence analysis of Plk4 levels at the centrosome. Points show the fluorescence intensity of individual cells from at least two independent experiments. The horizontal line represents the mean. (C) The number of Plk4 molecules per cell was determined using AQUA mass spectrometry. Bars represent the mean of at least two replicas. Error bars represent the standard deviation (SD). (D) Thin-section transmission electron micrographs of cells 2 d after infection with adenoviral Cre (Ad-Cre). Bar, 0.5 μm. (E) Fraction of cells with more than two centrosomes at various times after infection with Ad-Cre. Points show the mean of at least three independent experiments. Error bars represent the standard error of the mean (SEM). (F) Fold increase in cell number at various times starting 2 d after infection with Ad-Cre. Points show the mean of at least three independent experiments. Error bars represent the SEM. (G) Images of crystal violet-stained colonies. (H) Percent congenic survival of the indicated cells. Bars represent the mean of at least three independent experiments carried out in triplicate. Error bars represent the SEM.
We used our gene targeted cell lines to examine the primary effect of loss of Plk4 autoregulation in nontransformed human cells. The dramatic increase in centrosome amplification in Plk4+/AA and Plk4+/Δ24 cells by 2 d post-Cre expression was followed by a progressive decline in the proportion of cells with extra centrosomes (Fig. 1E): By 10 d after Cre expression, <10% of Plk4+/AA and Plk4+/Δ24 cells exhibited centrosome amplification. Since our adenoviral transduction is only ∼90% efficient, we reasoned that the decline in cells with centrosome amplification may be due to proliferation deficits in the cells with extra centrosomes that permit them to be outcompeted by untransduced cells. Consistently, the growth rate of Plk4+/AA and Plk4+/Δ24 cells was dramatically reduced following Cre expression, while the growth properties of Plk4+/WT cells were unchanged (Fig. 1F).
The long-term growth potential of cells that have lost autoregulated control of endogenous Plk4 levels was determined in a clonogenic survival assay. Expression of Cre in Plk4+/AA and Plk4+/Δ24 cells dramatically reduced colony formation, while Cre expression in Plk4+/WT cells had no effect (Fig. 1G,H). Moreover, the few surviving colonies in Cre-infected Plk4+/Δ24 cells were analyzed by PCR and found to have escaped Cre-mediated recombination (18 of 18) (data not shown). Therefore, autoregulated control of endogenous Plk4 levels is essential for long-term cell growth.
To verify our observations, we created an additional pair of targeted RPE-1 cells carrying conditional Plk4WT and Plk4Δ24 knock-in alleles in which the transcriptional/translational stop cassette was inserted into intron 4 (as opposed to intron 5) of the endogenous Plk4 gene (Supplemental Fig. S2A). Again, activation of the Plk4Δ24 allele resulted in an increase (∼4.2-fold) in centrosome-associated Plk4 (Supplemental Fig. S2B,C), centrosome amplification (Supplemental Fig. S3A–C), impaired proliferation (Supplemental Fig. S3D–F), and a dramatically reduced clonogenic survival (Supplemental Fig. S3G,H).
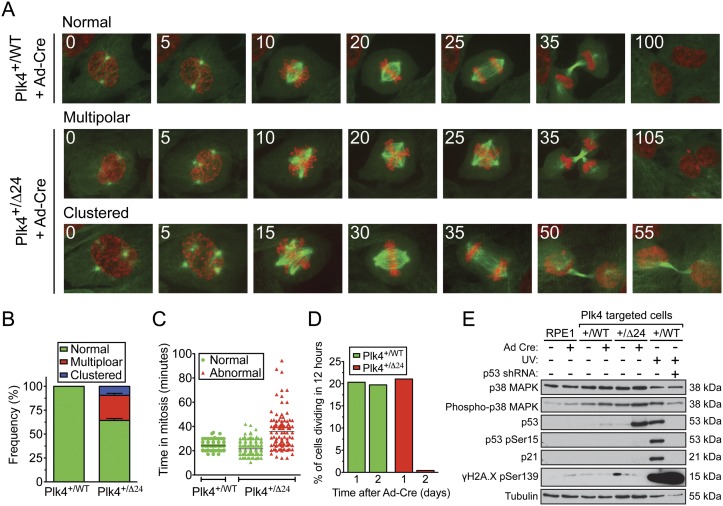
To determine the effect that activation of the Plk4Δ24 allele had on cell division, we created Plk4+/WT and Plk4+/Δ24 cells coexpressing EYFP-α-tubulin and histone H2B-mRFP and monitored cells by time-lapse microscopy. Filming began 1 d after transduction with adenoviral Cre and proceeded for 12 h. During this time, all of the Plk4+/WT cells divided normally, while 36% of the Plk4Δ24-expressing cells divided abnormally with an increased mitotic duration (average of 22 min for normal divisions compared with 35 min for abnormally dividing cells) (Fig. 2A–C; Supplemental Movie S1). Newly assembled centrioles undergo a modification during mitosis that allows them to recruit PCM and act as an MTOC in the next cell cycle (Wang et al. 2011). The low frequency of abnormally dividing Plk4+/Δ24 cells at 1 d after Cre expression is likely to reflect the additional time required for the newly assembled centrioles to mature into MTOCs.
Figure 2.
Loss of Plk4 autoregulation leads to p53/p21 stabilization and cell cycle arrest. (A) Time-lapse images of cells stably expressing histone H2B-mRFP (red) and EYFP-α-Tubulin (green). Filming began 24 h after infection with Ad-Cre. Numbers in the top left refer to the time in minutes after nuclear envelope breakdown. (B) Quantification of the proportion of cells with normal, multipolar, or clustered spindle poles at the time of division. Cells were filmed for 16 h beginning 1 d after infection with Ad-Cre. Bars show the mean of >116 cells per condition from at least two independent experiments. Error bars represent the SEM. (C) Duration of mitosis in normal or abnormally dividing cells. Cells were filmed for 16 h beginning 1 d after infection with Ad-Cre. The line shows the mean of >116 cells per condition from at least two independent experiments. (D) Fraction of cells dividing in a 12-h period beginning 1 or 2 d after Ad-Cre infection. (E) Immunoblots of protein harvested 48 h after Ad-Cre infection or 12 h after UV irradiation.
All abnormally dividing Plk4+/Δ24 cells entered mitosis with more than two MTOCs, and in most instances (73% of the time), cells failed to cluster these into two groups prior to division, dividing in a multipolar fashion (Fig. 2A,B; Supplemental Movie S2). The remaining abnormally dividing Plk4+/Δ24 cells coalesced multiple MTOCs into two groups prior to anaphase and divided in a bipolar fashion (Fig. 2A,B; Supplemental Movie S3). Remarkably, 2 d after transduction with adenoviral Cre, mitotic divisions in Plk4+/Δ24 cells ceased, while divisions in Plk4+/WT cells proceeded unabated (Fig. 2D). Consistent with the observed reduction in proliferation, Plk4+/Δ24 but not Plk4+/WT cells showed marked stabilization of p53 and the cell cycle inhibitor p21 2 d after infection with adenoviral Cre (Fig. 2E). Centrosome amplification did not create a measurable increase in γH2A.X phosphorylation in Plk4Δ24-expressing cells (Fig. 2E), suggesting that DNA damage is unlikely to be the cause of p53 and p21 stabilization in these cells.
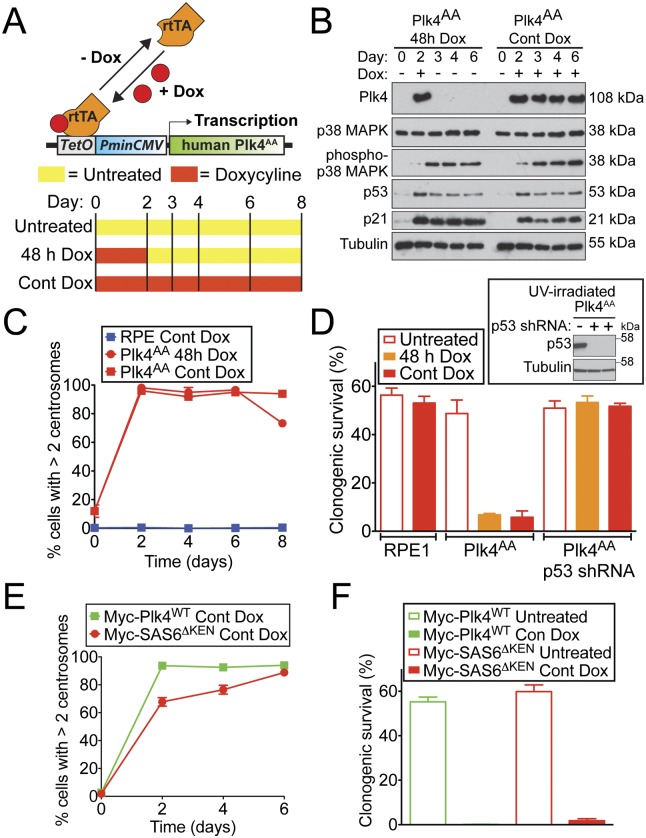
The inhibitory effect that loss of Plk4 autoregulation has on cell growth may arise as a consequence of increased levels of Plk4 kinase activity acting on what are as-yet-unidentified substrates. An alternative and nonmutually exclusive possibility is that the extra centrosomes produced as a result of excessive Plk4 levels inhibit long-term cell growth. Since centrosomes are stable structures that persist even after the signal that led to their creation has decayed, inhibition of cellular growth as a result of centrosome amplification should require only a transient elevation in Plk4 levels. To investigate this possibility, we used RPE1 cells in which reversible overexpression of Plk4AA can be achieved with a doxycycline-inducible promoter (Wang et al. 2011). Plk4AA levels were chronically elevated by continuous treatment with doxycycline or transiently elevated with a 48-h pulse of doxycycline (Fig. 3A). Elevated levels of Plk4AA declined to below the limit of detection within <24 h of removing doxycycline (Fig. 3B). Forty-eight hours of Plk4AA expression drove centrosome amplification in ∼100% of cells, an effect that was maintained even after the disappearance of Plk4AA (Fig. 3C). Increased Plk4AA levels and centrosome amplification resulted in activation of p38 MAPK signaling (as revealed by activating Thr180/Tyr182 phosphorylation), stabilization of p53, and up-regulation of the cyclin-dependent kinase inhibitor p21 (Fig. 4B). This alteration in cellular homeostasis persisted for >3 d after the transiently elevated Plk4AA was no longer detectable (Fig. 3B). Moreover, the growth rate and clonogenic survival of Plk4AA cells were severely impaired whether Plk4 levels were continually or transiently (for 48 h) elevated (Fig. 3D; Supplemental Fig. S4A). Essentially identical results were obtained in cells carrying a doxycycline-inducible Plk4WT transgene (Supplemental Fig. S4B–D). Therefore, even a transient elevation in Plk4 levels is sufficient to bring about a long-term inhibition of cell growth.
Figure 3.
Centrosome amplification inhibits proliferation of RPE1 cells. (A) Schematic of the strategy for doxycycline (Dox)-inducible expression of Plk4AA in RPE1 cells. (B) Immunoblots showing the levels of protein in Plk4AA cells at various times after doxycycline addition. Cells were left untreated, grown in the continuous presence of doxycycline, or transiently exposed to doxycycline for 48 h. (C) Fraction of RPE1 and Plk4AA cells with more than two centrosomes at various times after doxycycline addition. Points show the mean of two independent experiments, and error bars represent the SEM. (D) Percent congenic survival for the indicated cells. Immunoblots of p53 protein in UV-irradiated cells stably expressing a p53 shRNA. Bars represent the mean of two independent experiments carried out in triplicate. Error bars represent the SEM. (E) Fraction of cells with more than two centrosomes at various times after doxycycline addition. Points show the mean of two independent experiments, and error bars represent the SEM. (F) Percent congenic survival for the indicated cells. Bars represent the mean of two independent experiments carried out in triplicate. Error bars represent the SEM.
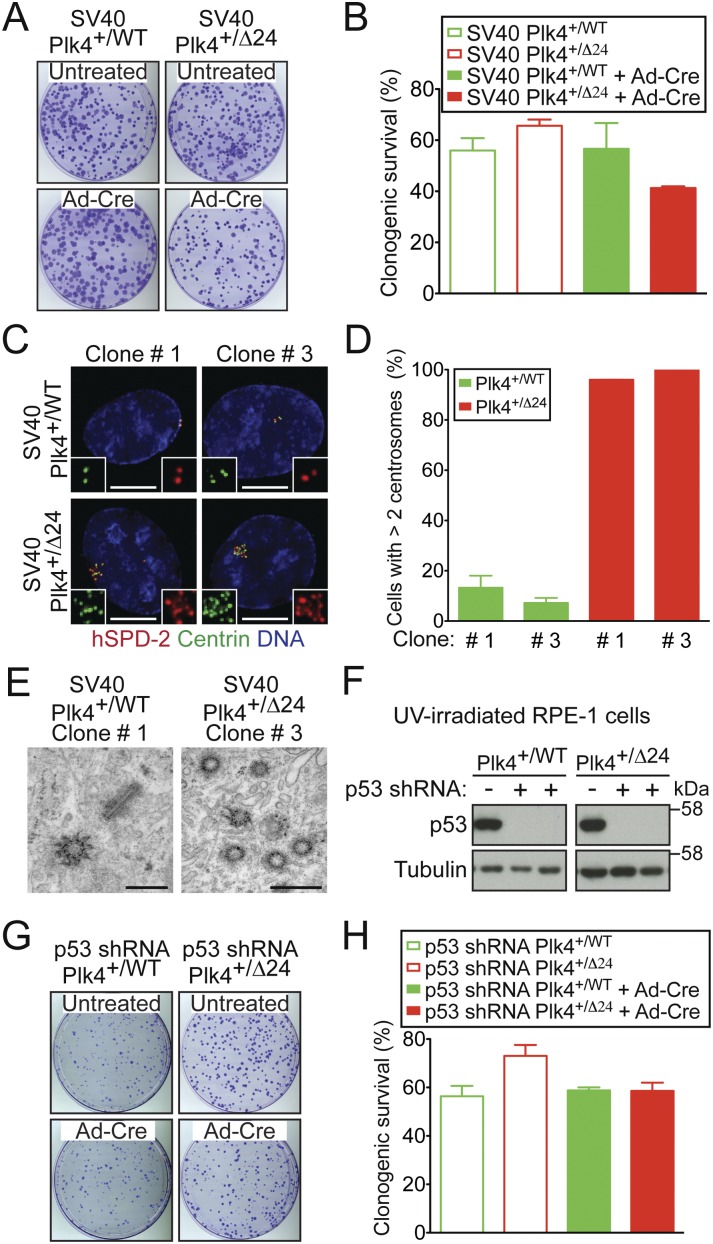
Figure 4.
p53 inhibits the proliferation of cells defective in Plk4 autoregulation. (A) Crystal violet-stained colonies of the indicated cell lines. (B) Percent congenic survival for indicated cells. Bars represent the mean of at least two independent experiments carried out in triplicate. Error bars represent the SEM. (C) Images show the level of Plk4 at the centrosome of SV40-RPE1 clones that were isolated after infection with Ad-Cre. (Red) hSPD-2; (green) Centrin; (blue) DNA. Bar, 5 μm. (D) Fraction of SV40-RPE1 clones with more than two centrosomes. Bars represent the mean of at least two independent experiments, and error bars represent the SEM. (E) Thin-section transmission electron micrographs of SV40-RPE1 clones. Bar, 0.5 μm. (F) Immunoblots of p53 protein levels in UV-irradiated cells stably expressing a p53 shRNA. (G) Crystal violet-stained colonies of the indicated cell lines. (H) Percent clonogenic survival for the indicated cells. Bars represent the mean of at least two independent experiments carried out in triplicate. Error bars represent the SEM.
As a further test of whether centrosome amplification suppresses the growth of RPE1 cells, we used an alternative means to drive the formation of excessive centrosomes that does not require overexpression of Plk4 and its kinase activity. SAS6 is a conserved structural component required for centriole assembly. Human SAS6 contains a C-terminal KEN box required to target the protein for destruction at the end of mitosis, and expression of SAS6 with a mutated KEN box (SAS6ΔKEN) has been shown to promote centrosome amplification (Strnad et al. 2007). We created monoclonal RPE1 cells containing either a Myc-SAS6ΔKEN or, as a control, a Myc-Plk4WT doxycycline-inducible transgene. As expected, overexpression of either Myc-Plk4WT or Myc-HsSAS6ΔKEN drove centrosome amplification, but the fraction of cells with excessive centrosome numbers increased more slowly in Myc-HsSAS6ΔKEN-expressing cells: More than 85% of Myc-HsSAS6ΔKEN cells had centrosome amplification at 6 d after doxycycline addition, while expression of Myc-Plk4WT for just 2 d resulted in >95% centrosome amplification (Fig. 3E). Importantly, centrosome amplification resulting from overexpression of Myc-Plk4WT or Myc-HsSAS6ΔKEN both caused a dramatic reduction in long-term clonogenic survival (Fig. 3F; Supplemental Fig. S4E). Taken together, these data are consistent with the view that centrosome amplification plays a major role in suppressing the proliferation of cells deficient in Plk4 self-regulation.
We tested whether stabilization of p53 contributes to the prolonged cell cycle delay/arrest that occurs following loss of Plk4 autoregulation and centrosome amplification. Inhibition of p53 through expression of the SV40 large T-antigen completely eliminated the growth defect and reduced clonogenic survival observed following activation of the Plk4Δ24 allele in our gene targeted cell line (Fig. 4A,B; Supplemental Fig. S5A). Clonal cell lines were isolated that stably expressed the SV40 large T-antigen and the Plk4Δ24 allele. Remarkably, these clones grew indefinitely despite the loss of the autoregulated control of Plk4 levels and dramatic centrosome amplification (Fig. 4C–E; Supplemental Fig. S5B). Preventing p53 accumulation using a stably expressed p53 shRNA (Figs. 3D, 4F) also eliminated the reduced clonogenic survival observed after (transient or chronic) doxycycline-induced expression of Plk4AA (Fig. 3D) or following activation of the Plk4Δ24 allele in the gene targeted cell line (Fig. 4G,H). Thus, increased Plk4 levels resulting from loss of Plk4 autoregulation bring about a p53-dependent cell cycle arrest.
Here we provide the first evidence that the self-catalyzed destruction of endogenous Plk4 plays a critical role in suppressing Plk4 protein levels to limit centriole duplication to the production of one new centrosome per cell cycle. Preventing Plk4 autoregulation elicits rapid and highly penetrant centrosome amplification followed by a p53-dependent proliferative block. While we cannot formally exclude the possibility that transiently increased Plk4 levels directly suppress cellular proliferation, this would be hard to reconcile with continued growth inhibition after removal of excessive Plk4 and with the block to proliferation caused by increases in the level of SAS6, a structural component of the centrosome. Taken together, our data argue that it is centrosome amplification itself that severely impairs the proliferation of nontransformed RPE1 cells deficient in Plk4 autoregulation. Importantly, removal of p53 overcomes this proliferative block, providing a mechanism for how loss of p53 could lay the foundation for centrosome amplification and further genomic instability.
Materials and methods
Cell culture and creation of stable cell lines
hTERT RPE-1 cells were used in all experiments and maintained at 37°C in a 5% CO2 atmosphere with 21% oxygen. Cells were grown in DMEM:F12 medium containing 10% tetracycline-free fetal bovine serum (Clontech), 0.348% sodium bicarbonate, 100 U/mL penicillin, 100 U/mL streptomycin, and 2 mM L-glutamine. To carry out Cre recombination, cells were incubated for 4 h with adenoviral-Cre in DMEM:F12 medium containing 2% tetracycline-free fetal bovine serum (Clontech), 0.348% sodium bicarbonate, 100 U/mL penicillin, 100 U/mL streptomycin, and 2 mM L-glutamine. Two days after transduction, cells were counted, and the same number of cells in the untreated or Cre-infected population was seeded for growth assays and clonogenic assays.
Stable cell lines expressing RFP-tagged histone H2B, EYFP-α-Tubulin, SV40 large T-antigen, or p53 shRNA were created as described (Holland et al. 2012). Doxycycline-inducible Myc-Plk4 and Myc-SAS6ΔKEN hTERT RPE1 cells were created using lentiviral delivery, and single clones were isolated using fluorescence-activated cell sorting. Expression of transgenes was induced with 1 μg/mL doxycycline (Sigma).
Gene targeting
Gene targeting was performed using adeno-associated virus as previously described (Holland et al. 2012). Taq polymerase (Invitrogen) was used to amplify fragments of the human Plk4 locus from genomic RPE1 DNA for use in construction of the targeting construct. Plk4 AA and Δ24 mutations were created by QuikChange site-directed mutagenesis (Agilent Technologies). The targeting construct and targeted Plk4 allele were sequenced to verify their integrity.
Detailed Materials and Methods can be found in the Supplemental Material.
Acknowledgments
We thank Jeffrey Salisbury (Mayo Clinic Foundation, Minnesota) for providing the anti-Centrin antibody, Karen Oegema and Arshad Desai (Ludwig Institute for Cancer Research, California) for providing the human anti-SPD2 and anti-SAS6 antibody, Prasad Jallepalli (Memorial Sloan-Kettering Cancer Center, New York) for providing reagents for AAV-mediated gene targeting, Pierre Gönczy (Swiss Federal Institute of Technology, Switzerland) for providing the human SAS6 clone, and Bryan Tsou (Memorial Sloan-Kettering Cancer Center, New York) for providing the doxycycline-inducible Plk4WT and Plk4AA RPE1 cell lines. We thank Timo Meerloo and Ying Jones of the University of California at San Diego Cellular and Molecular Medicine Electron Microscopy Core Facility for their generous help with electron microscopy, and the University of California at San Diego Neuroscience Microscopy Shared Facility (P30 NS047101). This work was supported by a grant (GM29513) from the National Institutes of Health to D.W.C., who receives salary support from the Ludwig Institute for Cancer Research. A.J.H. is supported by a Leukemia and Lymphoma Society special fellowship. E.A.N. and M.B. gratefully acknowledge support by the Swiss National Science Foundation (31003A_132428/1). I.M.V. is an American Cancer Society Professor of Molecular Biology and holds the Irwin and Joan Jacobs Chair in Exemplary Life Science. This work was supported in part by a Cancer Center Core Grant (P30 CA014195-38). Q.Z. was supported by a Department of Defense Idea Expansion Award (W81XWH-10-1-0963). D.F. was supported by a European Molecular Biology Organization (EMBO) long-term fellowship.
Footnotes
Supplemental material is available for this article.
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.207027.112.
References
- Basto R, Brunk K, Vinadogrova T, Peel N, Franz A, Khodjakov A, Raff JW 2008. Centrosome amplification can initiate tumorigenesis in flies. Cell 133: 1032–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettencourt-Dias M, Rodrigues-Martins A, Carpenter L, Riparbelli M, Lehmann L, Gatt MK, Carmo N, Balloux F, Callaini G, Glover DM 2005. SAK/PLK4 is required for centriole duplication and flagella development. Curr Biol 15: 2199–2207 [DOI] [PubMed] [Google Scholar]
- Brownlee CW, Klebba JE, Buster DW, Rogers GC 2011. The protein phosphatase 2A regulatory subunit Twins stabilizes Plk4 to induce centriole amplification. J Cell Biol 195: 231–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha-Ferreira I, Rodrigues-Martins A, Bento I, Riparbelli M, Zhang W, Laue E, Callaini G, Glover DM, Bettencourt-Dias M 2009. The SCF/Slimb ubiquitin ligase limits centrosome amplification through degradation of SAK/PLK4. Curr Biol 19: 43–49 [DOI] [PubMed] [Google Scholar]
- Ganem NJ, Godinho SA, Pellman D 2009. A mechanism linking extra centrosomes to chromosomal instability. Nature 460: 278–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber SA, Rush J, Stemman O, Kirschner MW, Gygi SP 2003. Absolute quantification of proteins and phosphoproteins from cell lysates by tandem MS. Proc Natl Acad Sci 100: 6940–6945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonczy P 2012. Towards a molecular architecture of centriole assembly. Nat Rev Mol Cell Biol 13: 425–435 [DOI] [PubMed] [Google Scholar]
- Guderian G, Westendorf J, Uldschmid A, Nigg EA 2010. Plk4 trans-autophosphorylation regulates centriole number by controlling βTrCP-mediated degradation. J Cell Sci 123: 2163–2169 [DOI] [PubMed] [Google Scholar]
- Habedanck R, Stierhof YD, Wilkinson CJ, Nigg EA 2005. The Polo kinase Plk4 functions in centriole duplication. Nat Cell Biol 7: 1140–1146 [DOI] [PubMed] [Google Scholar]
- Holland AJ, Cleveland DW 2012. Losing balance: The origin and impact of aneuploidy in cancer. EMBO Rep 13: 501–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland AJ, Lan W, Cleveland DW 2010a. Centriole duplication: A lesson in self-control. Cell Cycle 9: 2731–2736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland AJ, Lan W, Niessen S, Hoover H, Cleveland DW 2010b. Polo-like kinase 4 kinase activity limits centrosome overduplication by autoregulating its own stability. J Cell Biol 188: 191–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland AJ, Fachinetti D, Da Cruz S, Zhu Q, Vitre B, Lince-Faria M, Chen D, Parish N, Verma IM, Bettencourt-Dias M, et al. 2012. Polo-like kinase 4 controls centriole duplication but does not directly regulate cytokinesis. Mol Biol Cell 23: 1838–1845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleylein-Sohn J, Westendorf J, Le Clech M, Habedanck R, Stierhof YD, Nigg EA 2007. Plk4-induced centriole biogenesis in human cells. Dev Cell 13: 190–202 [DOI] [PubMed] [Google Scholar]
- Ko MA, Rosario CO, Hudson JW, Kulkarni S, Pollett A, Dennis JW, Swallow CJ 2005. Plk4 haploinsufficiency causes mitotic infidelity and carcinogenesis. Nat Genet 37: 883–888 [DOI] [PubMed] [Google Scholar]
- Liu L, Zhang CZ, Cai M, Fu J, Chen GG, Yun J 2012. Downregulation of polo-like kinase 4 in hepatocellular carcinoma associates with poor prognosis. PLoS ONE 7: e41293 doi: 10.1371/journal.pone.0041293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macmillan JC, Hudson JW, Bull S, Dennis JW, Swallow CJ 2001. Comparative expression of the mitotic regulators SAK and PLK in colorectal cancer. Ann Surg Oncol 8: 729–740 [DOI] [PubMed] [Google Scholar]
- Nigg EA, Raff JW 2009. Centrioles, centrosomes, and cilia in health and disease. Cell 139: 663–678 [DOI] [PubMed] [Google Scholar]
- Peel N, Stevens NR, Basto R, Raff JW 2007. Overexpressing centriole-replication proteins in vivo induces centriole overduplication and de novo formation. Curr Biol 17: 834–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers GC, Rusan NM, Roberts DM, Peifer M, Rogers SL 2009. The SCF Slimb ubiquitin ligase regulates Plk4/Sak levels to block centriole reduplication. J Cell Biol 184: 225–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silkworth WT, Nardi IK, Scholl LM, Cimini D 2009. Multipolar spindle pole coalescence is a major source of kinetochore mis-attachment and chromosome mis-segregation in cancer cells. PLoS ONE 4: e6564 doi: 10.1371/journal.pone.0006564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillibourne JE, Tack F, Vloemans N, Boeckx A, Thambirajah S, Bonnet P, Ramaekers FC, Bornens M, Grand-Perret T 2010. Autophosphorylation of polo-like kinase 4 and its role in centriole duplication. Mol Biol Cell 21: 547–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strnad P, Leidel S, Vinogradova T, Euteneuer U, Khodjakov A, Gonczy P 2007. Regulated HsSAS-6 levels ensure formation of a single procentriole per centriole during the centrosome duplication cycle. Dev Cell 13: 203–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsou MF, Stearns T 2006. Controlling centrosome number: Licenses and blocks. Curr Opin Cell Biol 18: 74–78 [DOI] [PubMed] [Google Scholar]
- Wang WJ, Soni RK, Uryu K, Tsou MF 2011. The conversion of centrioles to centrosomes: Essential coupling of duplication with segregation. J Cell Biol 193: 727–739 [DOI] [PMC free article] [PubMed] [Google Scholar]