Abstract
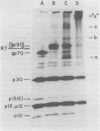
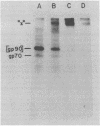
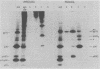
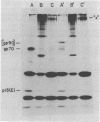
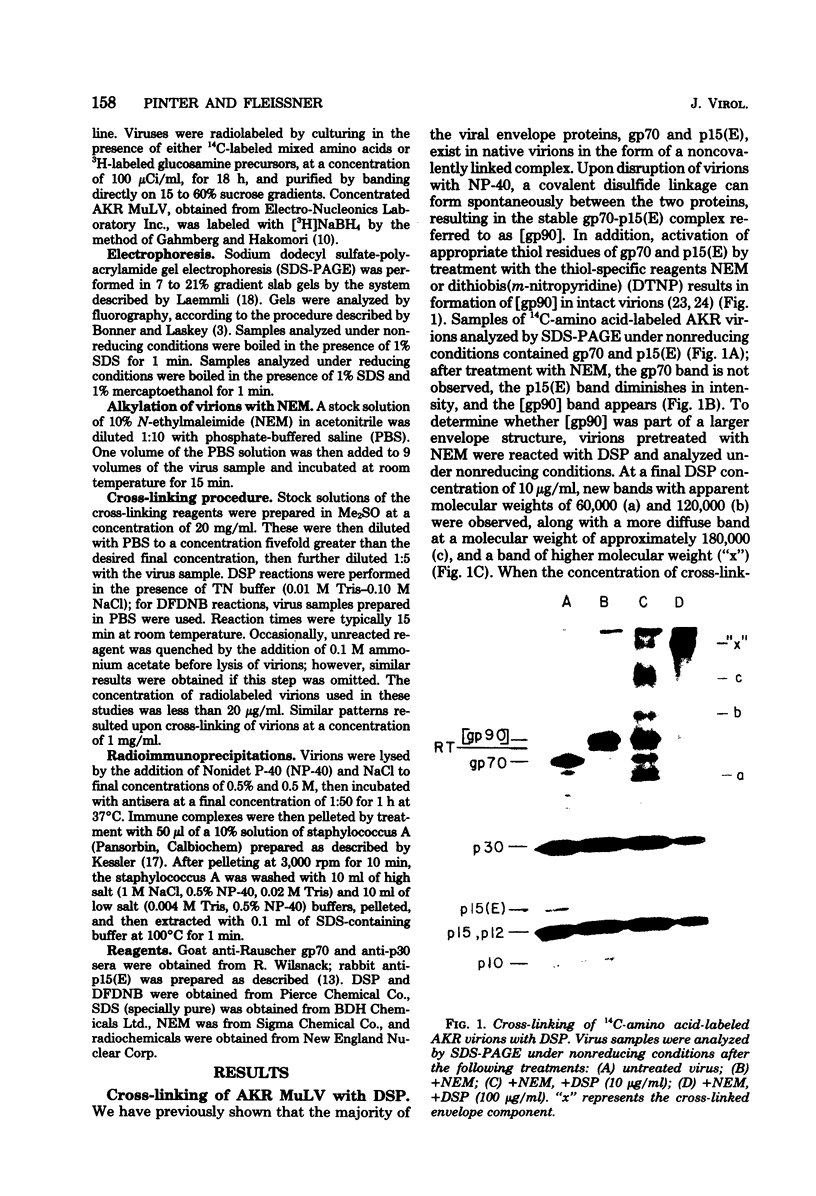
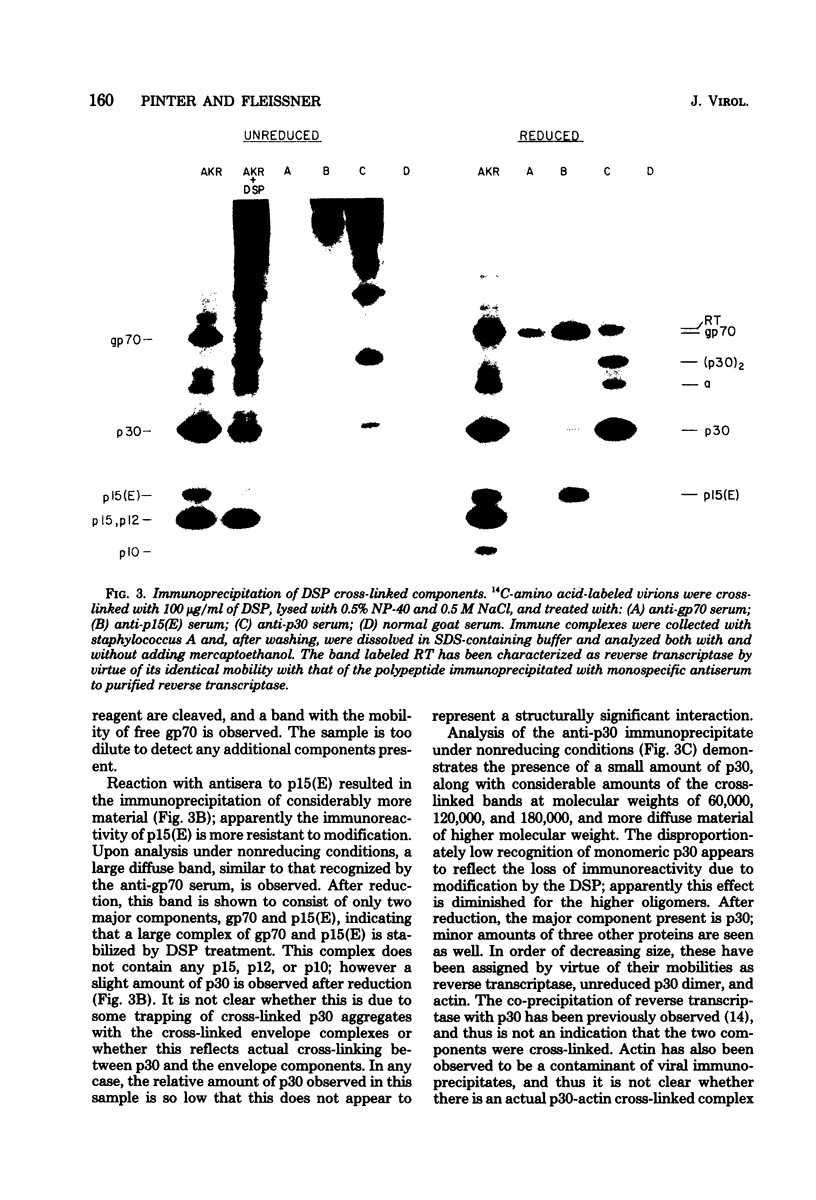
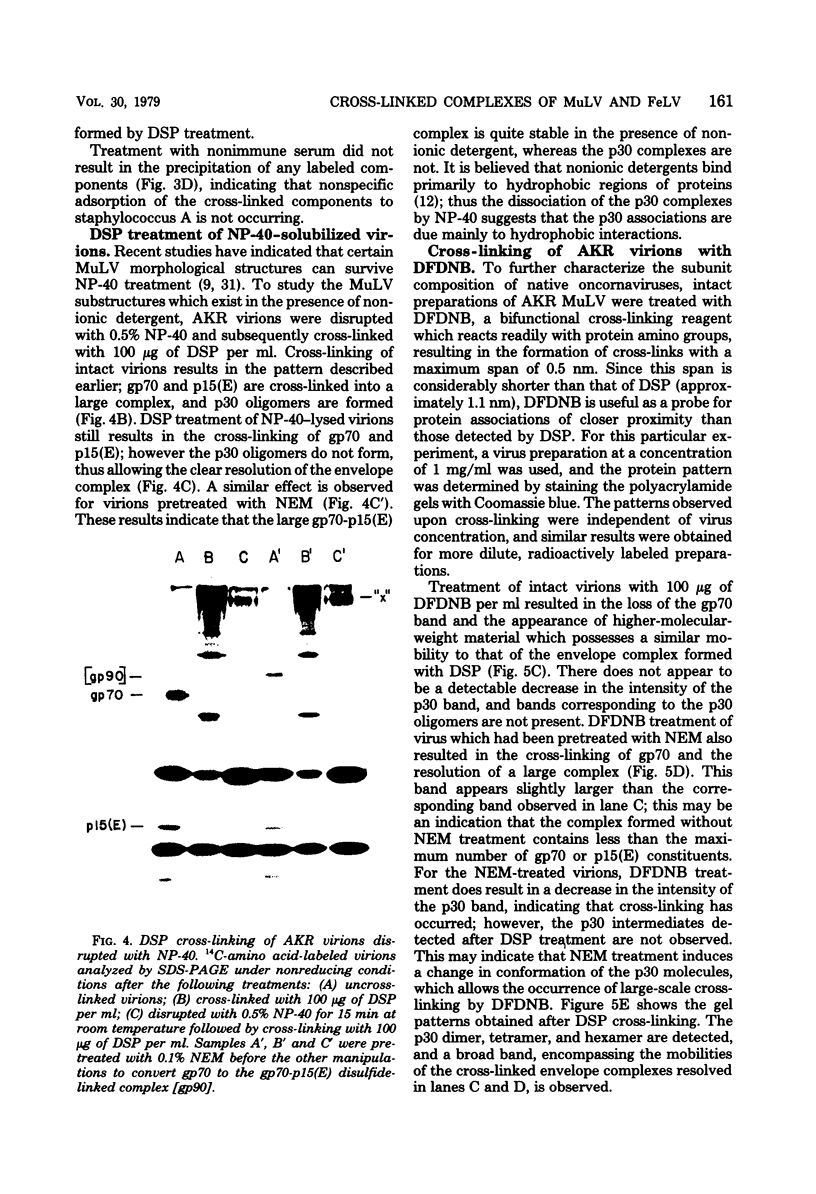
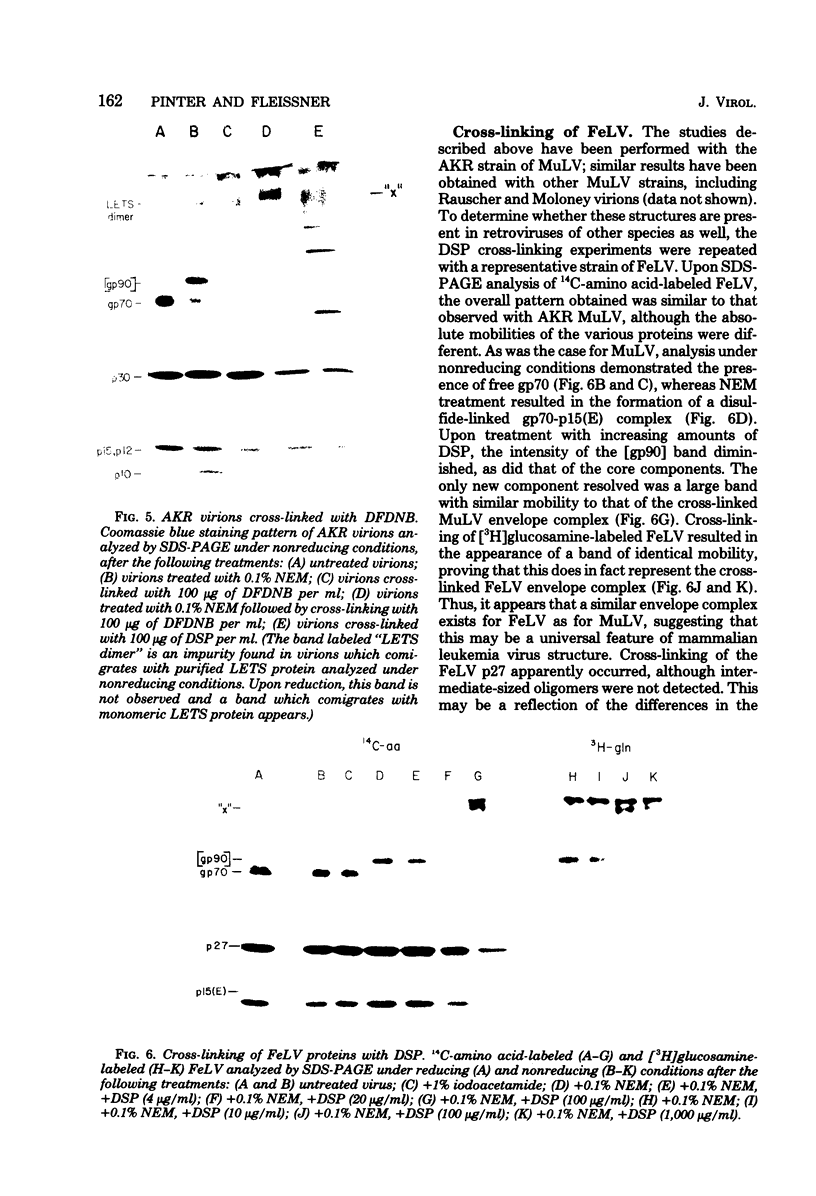
To examine the protein proximity and subunit organization of type C retroviruses, preparations of AKR murine leukemia virus were treated with bifunctional cross-linking reagents and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The cross-linked components obtained were characterized by immunoprecipitation with monospecific antisera against purified viral proteins, followed by SDS-PAGE analysis both before and after cleavage of the cross-links. With these procedures, complexes of both viral envelope and core components were identified. The major envelope subunit obtained was a large (apparent molecular weight of 450,000 to 500,000), glycosylated complex, composed of four to six gp70-p15(E) subunits. This complex was detected over a 100-fold range of cross-linker concentration and thus seems to represent a particularly stable viral substructure. The cross-linked complexes of the core proteins consisted of oligomers of p30 dimers, suggesting that the p30 dimer is a basic structural unit of the viral core. When virion preparations, which had previously been disrupted with the nonionic detergent Nonidet P-40, were cross-linked, the envelope complex was still observed, indicating that this structure is stable in the presence of Nonidet P-40. A similar envelope structure was observed for feline leukemia virus, suggesting that such a complex may be a conserved feature of oncornavirus structure.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- August J. T., Bolognesi D. P., Fleissner E., Gilden R. V., Nowinski R. C. A proposed nomenclature for the virion proteins of oncogenic RNA viruses. Virology. 1974 Aug;60(2):595–600. doi: 10.1016/0042-6822(74)90356-0. [DOI] [PubMed] [Google Scholar]
- Barbacid M., Aaronson S. A. Membrane properties of the gag gene-coded p15 protein of mouse type-C RNA tumor viruses. J Biol Chem. 1978 Mar 10;253(5):1408–1414. [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Burnette W. N., Holladay L. A., Mitchell W. M. Physical and chemical properties of Moloney murine leukemia virus p30 protein: a major core structural component exhibiting high helicity and self-association. J Mol Biol. 1976 Oct 25;107(2):131–143. doi: 10.1016/s0022-2836(76)80022-8. [DOI] [PubMed] [Google Scholar]
- Demsey A., Kawka D., Stackpole C. W. Application of freeze-drying intact cells to studies of murine oncornavirus morphogenesis. J Virol. 1977 Jan;21(1):358–365. doi: 10.1128/jvi.21.1.358-365.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubovi E. J., Wagner R. R. Spatial relationships of the proteins of vesicular stomatitis virus: induction of reversible oligomers by cleavable protein cross-linkers and oxidation. J Virol. 1977 May;22(2):500–509. doi: 10.1128/jvi.22.2.500-509.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenman R. N., Vogt V. M. The biosynthesis of oncovirus proteins. Biochim Biophys Acta. 1978 Apr 6;473(3-4):187–239. doi: 10.1016/0304-419x(78)90014-8. [DOI] [PubMed] [Google Scholar]
- Famulari N. G., Buchhagen D. L., Klenk H. D., Fleissner E. Presence of murine leukemia virus envelope proteins gp70 and p15(E) in a common polyprotein of infected cells. J Virol. 1976 Nov;20(2):501–508. doi: 10.1128/jvi.20.2.501-508.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank H., Schwarz H., Graf T., Schäfer W. Properties of mouse leukemia viruses. XV. Electron microscopic studies on the organization of Friend leukemia virus and other mammalian C-type viruses. Z Naturforsch C. 1978 Jan-Feb;33(1-2):124–138. doi: 10.1515/znc-1978-1-224. [DOI] [PubMed] [Google Scholar]
- Gahmberg C. G., Hakomori S. I. External labeling of cell surface galactose and galactosamine in glycolipid and glycoprotein of human erythrocytes. J Biol Chem. 1973 Jun 25;248(12):4311–4317. [PubMed] [Google Scholar]
- Garoff H., Simons K. Location of the spike glycoproteins in the Semliki Forest virus membrane. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3988–3992. doi: 10.1073/pnas.71.10.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A., Simons K. The binding of detergents to lipophilic and hydrophilic proteins. J Biol Chem. 1972 Jun 10;247(11):3656–3661. [PubMed] [Google Scholar]
- Ikeda H., Hardy W., Jr, Tress E., Fleissner E. Chromatographic separation and antigenic analysis of proteins of the oncornaviruses. V. Identification of a new murine viral protein, p15(E). J Virol. 1975 Jul;16(1):53–61. doi: 10.1128/jvi.16.1.53-61.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamjoom G. A., Naso R. B., Arlinghaus R. B. Further characterization of intracellular precursor polyproteins of Rauscher leukemia virus. Virology. 1977 May 1;78(1):11–34. doi: 10.1016/0042-6822(77)90075-7. [DOI] [PubMed] [Google Scholar]
- Karshin W. L., Arcement L. J., Naso R. B., Arlinghaus R. B. Common precursor for Rauscher leukemia virus gp69/71, p15(E), and p12(E). J Virol. 1977 Sep;23(3):787–798. doi: 10.1128/jvi.23.3.787-798.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keski-Oja J., Mosher D. F., Vaheri A. Dimeric character of fibronectin, a major cell surface-associated glycoprotein. Biochem Biophys Res Commun. 1977 Jan 24;74(2):699–706. doi: 10.1016/0006-291x(77)90359-x. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Lomant A. J., Fairbanks G. Chemical probes of extended biological structures: synthesis and properties of the cleavable protein cross-linking reagent [35S]dithiobis(succinimidyl propionate). J Mol Biol. 1976 Jun 14;104(1):243–261. doi: 10.1016/0022-2836(76)90011-5. [DOI] [PubMed] [Google Scholar]
- Marcus S. L., Smith S. W., Racevskis J., Sarkar N. H. The relative hydrophobicity of oncornaviral structural proteins. Virology. 1978 May 15;86(2):398–412. doi: 10.1016/0042-6822(78)90080-6. [DOI] [PubMed] [Google Scholar]
- Mudd J. A., Swanson R. E. In situ cross-linking of vesicular stomatitis virus proteins with reversible agents. Virology. 1978 Jul 15;88(2):263–280. doi: 10.1016/0042-6822(78)90284-2. [DOI] [PubMed] [Google Scholar]
- Nermut M. V., Frank H., Schäfer W. Properties of mouse leukemia viruses. 3. Electron microscopic appearance as revealed after conventional preparation techniques as well as freeze-drying and freeze-etching. Virology. 1972 Aug;49(2):345–358. doi: 10.1016/0042-6822(72)90487-4. [DOI] [PubMed] [Google Scholar]
- Pinter A., Fleissner E. The presence of disulfide-linked gp70-p15(E) complexes in AKR murine leukemia virus. Virology. 1977 Dec;83(2):417–422. doi: 10.1016/0042-6822(77)90187-8. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Vaheri A. Interaction of soluble fibroblast surface antigen with fribrinogen and fibrin. J Exp Med. 1975 Feb 1;141(2):497–501. doi: 10.1084/jem.141.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro S. Z., Strand M., August J. T. High molecular weight precursor polypeptides to structural proteins of Rauscher murine leukemia virus. J Mol Biol. 1976 Nov 15;107(4):459–477. doi: 10.1016/s0022-2836(76)80078-2. [DOI] [PubMed] [Google Scholar]
- Swanson S. K., Sulkowski E., Manly K. F. Hydrophobic binding site(s) on Moloney--murine leukemia virus P30. Virology. 1978 Mar;85(1):211–221. doi: 10.1016/0042-6822(78)90425-7. [DOI] [PubMed] [Google Scholar]
- Van Zaane D., Dekker-Michielsen J. A., Bloemers H. P. Virus-specific precursor polypeptides in cells infected with Rauscher leukemia virus: synthesis, identification, and processing. Virology. 1976 Nov;75(1):113–129. doi: 10.1016/0042-6822(76)90011-8. [DOI] [PubMed] [Google Scholar]
- Witte O. N., Baltimore D. Relationship of retrovirus polyprotein cleavages to virion maturation studied with temperature-sensitive murine leukemia virus mutants. J Virol. 1978 Jun;26(3):750–761. doi: 10.1128/jvi.26.3.750-761.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinaka Y., Luftig R. B. Murine leukemia virus morphogenesis: cleavage of P70 in vitro can be accompanied by a shift from a concentrically coiled internal strand ("immature") to a collapsed ("mature") form of the virus core. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3446–3450. doi: 10.1073/pnas.74.8.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaane D. V., Gielkens A. L., Hesselink W. G., Bloemers H. P. Identification of Rauscher murine leukemia virus-specific mRNAs for the synthesis of gag- and env-gene products. Proc Natl Acad Sci U S A. 1977 May;74(5):1855–1859. doi: 10.1073/pnas.74.5.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]