Abstract
Objective
To investigate the prevalence, determinants and spectrum of attention-deficit hyperactivity disorder (ADHD) medication and its associations with socioeconomic status (SES), health-related behaviour and living conditions.
Design
Observational cross-sectional study.
Setting
Germany.
Participants
Representative population-based sample of non-institutionalised youth aged between 0 and 17 years (n=17 450) and examined between 2003 and 2006.
Main outcome measure
Prevalence and spectrum of ADHD medication (Anatomical Therapeutic Chemical (ATC) code N04BA) measured by standardised computer-assisted personal interview (CAPI) on drug use.
Results
The overall prevalence of ADHD medication (stimulants including atomoxetine) was 0.9% (95% CI 0.7% to 1.1%). Boys used these drugs (1.5%, 1.2% to 1.8%) five times more than girls 0.3% (0.2% to 0.5%). The highest prevalence rates were for boys aged 6–10 years (2.3%, 1.7% to 3.1%S) and 11–13 (2.7%, 2.0% to 3.7%). Boys from families with no immigration background used ADHD medication almost 6 times as frequently as boys with an immigration background (1.7% vs 0.3%). Multivariate analysis (binary logistic regression) showed boys (OR 5.16, 95% CI 3.15 to 8.47), 11-year-olds to 13-year-olds (2.24, 1.28 to 3.49), children in large cities (2.18, 1.13 to 4.22), children with no immigration background (3.06, 1.34 to 6.99), and children with only a good (vs excellent) parent-rated health status (1.91, 1.18 to 3.08) being more likely to be using ADHD medication. A visit to the doctor in the last month or last quarter was associated with a higher probability for ADHD medication (3.18, 1.29 to 7.95 and 3.59, 1.45 to 8.90, respectively).
Conclusions
Results show prevalence rates of ADHD medication use for the German child and adolescent population that are considerably lower than published prevalence rates from the USA, but comparable with those of western European and Scandinavian countries. Lower use rates in rural versus urban regions may point to differential healthcare access. The inverse association of ADHD medication use with immigration status suggests potentially restricted access to healthcare services for immigrants or may reflect culture-specific differences in attitudes towards symptoms of ADHD.
Keywords: Public Health, Paediatrics
Article summary.
Article focus
To report prevalence rates and determinants of attention-deficit hyperactivity disorder (ADHD) medication use in a nationally representative sample of German youth.
To compare these prevalence rates with published data from other developed countries.
To report indications, substance group, origin, self-rated improvement of conditions treated, tolerance, duration of use and perceived adverse drug reactions (ADRs).
Key messages
We find lower prevalence rates for German youth as they are reported for the USA, but comparably high rates as they are reported for Western European Countries and Scandinavia.
When diagnosed with ADHD, 6-year-old to 10-year-old boys were significantly more likely to being treated with ADHD medication than girls.
Lower prevalence rates in rural regions and in immigrant families may point to differential thresholds to healthcare access or may reflect culturally altered attitudes towards symptoms of ADHD, respectively.
Strengths and limitations of this study
We provide detailed information on ADHD medication use in minors in a population-based, nationally representative sample.
We rely on self-reported information on drug use in the last 7 days.
The cross-sectional study design does not allow for the assessment of causal relations.
Introduction
Alongside psychoeducation and behavioural interventions, the treatment of ADHD (attention-deficit hyperactivity disorder) with drugs such as methylphenidate or Atomoxetine is an essential part of multimodal treatment.1 2 In terms of its risk–benefit ratio, pharmacotherapy has been found to be effective and to have relatively few adverse drug reactions (ADRs). The risk that ADHD medication might lead to subsequent substance abuse is regarded as low—it has even been shown to have a protective effect.3–5 As a result, prescription statistics and epidemiological studies are reporting a growing use of these drugs. The use of stimulants by children and adolescents (<19 years) in the USA, for example, increased between 1986 and 2002 from 0.6% to 2.9%.6 Similar increases were reported in a population-based longitudinal study in Israel for the period from 1984 to 2004 (0.7% vs 2.5%).7 According to Hugtenburg et al,8 an increase in the prescription prevalence of psychotropic drugs reported in the Netherlands from 1995 to 2001 was primarily due to an increase in prescriptions of stimulants.
Prescription data from statutory health insurance (SHI) providers in Germany show a comparable trend.9 The drug prescription report describes a more-than-fivefold increase in prescriptions from 10 million DDDs (defined daily doses) in 1999 to 53 million DDDs in 2008.10 The national and international increase in the number of prescriptions has intensified the discussion on the use of drugs for treating ADHD among both the general public and professionals. Issues discussed include the risk of providing too much, too little or the wrong kind of care.11 To answer these questions, it is not sufficient to analyse SHI data alone, since this ignores prescriptions covered by private health insurance. Private prescriptions have been found to be over-represented in an analysis of all prescriptions of methylphenidate in the city of Cologne. Twenty-eight per cent of all prescriptions fell into this category, although only about 10% of all people are privately insured.12 Moreover, statistics only allow statements to be made on their populations of insured people, and thus cannot be regarded as representative of the population. A further key deficit lies in the fact that prescription data are not necessarily identical to the actual use of a drug, as the latter depends on compliance.13 14 Beside a genetic disposition, prenatal risk factors such as mother's tobacco smoking15–17 or alcohol consumption during pregnancy18 are considered relevant for the pathogenesis of ADHD. The association of these environmental risk factors and ADHD medication use, however, has rarely been addressed. Results of a Swedish study suggest that associations might be confounded by genetic and socioeconomic factors.15 Sociodemographic characteristics, in turn, like male sex, younger age and not being an immigrant are linked with a greater likelihood of ADHD medication. In addition, education and income level, or the use of medical services have been found to be inversely related to the use of stimulants or atomoxetine in the treatment of ADHD.19–21 As this information was available from our data, they were considered as risk factors in our analyses. The aim of the present study was to describe the prevalence of the use of ADHD medication—especially stimulants—and to quantify determinants of ADHD medication on the basis of a population-based German representative sample—the German Health and Examination Survey for Children and Adolescents (KiGGS). Based on the literature reviewed above we expect that the use of ADHD medication is associated with younger age, male gender, low socioeconomic status (SES) and not being an immigrant. With regard to the current literature, we wish to explore whether smoking tobacco and drinking alcohol by the mother during pregnancy are related to a higher likelihood for ADHD medication of their offspring in childhood and adolescence. Moreover, the present study aims to assess the extent and the spectrum of drug treatment for ADHD with respect to indication, duration of use, substance group, origin of drugs and perceived ADRs.
Material and methods
Data source and study population
The KiGGS study was conducted by the Robert Koch Institute between May 2003 and May 2006. The survey's target population was made up of all non-institutionalised children and adolescents aged between 0 and 17 living in Germany. The design, sampling strategy and study protocol have been described in detail elsewhere.22 Briefly, two-stage sampling procedures were applied. In the first stage, a sample of 167 German municipalities (112 in the former West Germany, 55 in the former East Germany) was drawn which was representative of municipality sizes and structures in Germany. Stratified by sex and age, random samples of children and adolescents aged between 0 and 17 were then drawn from local population registries in proportion to the age and gender structure of Germany's child population. Children and adolescents with a foreign nationality were also included. Children who were currently in institutions such as hospitals, medical and nursing institutions were excluded. The final sample included 17 641 children and adolescents (8985 boys, 8656 girls); the response rate was 66.6%.23 Of these, 191 study participants who did not take part in the drug-use survey were excluded, resulting in a study population of 17 450 (8880 boys, 8570 girls) available for the present analysis. Parents who decided against a participation in the study were interviewed using a short non-responder questionnaire including sociodemographic and health characteristics. A comparison between responders and non-responders shows that children with mothers with higher educational levels were more likely to participate in the study. In contrast, no substantial differences were observed with regard to the health-related characteristics such as maternal smoking, maternal body mass index or the parent-rated health status of the child.23
The study was approved by the Charité/Universitätsmedizin Berlin ethics committee and the Federal Office for the Protection of Data. A written informed consent was obtained prior to each interview and examination from children's parents and the children themselves who were over 14 years of age.
Definition of health-related and sociodemographic variables
As described elsewhere in detail,22 all the children's parents/guardians and all children aged 11 years or older were asked to fill in a standard parents’ or children's questionnaire. These questionnaires were used to collect information on socioeconomic data, family backgrounds, parent-rated children's health status, health-related living conditions and behaviour patterns. A family SES score was computed including information obtained from the parents’ questionnaire on both parents’ educational level and vocational status as well as family net income.24 After computing a total score from the aforementioned items with a minimum of 3 and maximum of 21 points, study participants were assigned to one of three status groups depending on their individual score.25 Participants were thus assigned to low, middle or high SES. Family immigration status was assessed using information on nationality, country of birth and year of immigration of both parents. Study participants were classified as having an immigration background if they themselves had immigrated from another country and at least one parent was not born in Germany, or if both parents were immigrants or not of German nationality.26 Living in East or West Germany as well as living in rural or urban areas was assessed by items concerning the place of residence. Depending on the number of inhabitants, communities were distinguished as rural (<5000), small-size urban (5000−<20 000), medium-size urban (20 000−<100 000) and metropolitan (100 000 and more). Parents rated the general status of health of their children as ‘very good’, ‘good’, ‘moderate’, ‘bad’ or ‘very bad’. For multivariate analyses, the categories ‘moderate’ to ‘very bad’ were pooled due to low cell frequencies. A subject was considered an ADHD case if his or her parents confirmed a lifetime diagnosis from a physician or psychologist. In Germany, the diagnosis of ADHD is not legally restricted to child and adolescent psychiatrists or clinical child psychologists, as in other countries. It is likely, however, that clinical diagnoses are usually assigned by these professional groups. Thus, our measure reflects the clinical judgements of healthcare professionals as reported by parents. 27 Frequency of the mothers’ smoking tobacco and alcohol consumption during pregnancy (‘regularly’, ‘from time to time’, ‘never’) was assessed as self-report in the parents’ questionnaire. Because in Germany, the use of drugs with the Anatomical Therapeutic Chemical (ATC) code N06BA is legally approved from the age of 6 years on, for the bivariate and multivariate analyses a classification in the age groups 0–5, 6–10, 11–13 and 14–17 years was chosen. Parents were asked about the utilisation of healthcare services for their child including last visit with a doctor, responses options ‘< 4 weeks’ ‘1–< 3 months’, ‘3–12 months’ and ‘12+months’.28
Assessment of medication use
All survey participants and their parents were asked in advance to bring prescriptions or original medication packages to the examination site to facilitate the investigation and verification of drug use. Use of any medication, including prescribed and non-prescribed drugs, within the last 7 days before their visit in the examination site was documented using a standardised computer-assisted personal drug use interview conducted by a study physician.29 Drug use was assessed by the following question:
Has your child taken any drugs in the last seven days? Please also mention the use of any ointments, liniments, contraceptive pills, vitamin and mineral supplements, medicinal teas, herbal medicines or homoeopathic medicines.
Children aged 14 years and older were encouraged to add data on their drug use themselves. All prescribed or over-the-counter (OTC) drugs used in the 7 days before the interview were recorded in the answers to this question in the standardised computer-assisted personal interview (CAPI).
The following information on every drug mentioned by the parents or the children themselves was collected: brand name (as free text), indications (as free text), form of administration (tablets, dragees, drops, ointments, injections, liniments, etc), frequency of intake (‘several times a day’, ‘every day’, ‘regularly but not daily’ or ‘every week’), origin of the drug (‘prescribed by a doctor’, ‘prescribed by a non-medical practitioner’, ‘bought over the counter’ or ‘obtained from other sources’), duration of use (‘<1 week’, ‘1−<4 weeks’, ‘1−<12 months’ or ‘1 year or longer’) and improvement in the condition(s) treated (‘great’, ‘partial’, ‘little’, ‘none’ or ‘does not apply’). Further, parents and, respectively, the children themselves were asked whether or not the drug used was well tolerated and they were asked to describe the degree of tolerability (‘very good/good’, ‘partial’, ‘not good’ or ‘poor’). In addition, a question was asked whether any ADRs were noticed following drug intake (‘yes’ or ‘no’). If the answer was ‘yes’, the ADRs reported were documented by the study physicians. Specific ATC codes were assigned to all reported medications, and WHO ICD-10 (International Classification of Diseases-10. Revision) codes to the conditions for which the drugs were taken.
Identification of ADHD medication
In the present study, ADHD medication was defined as stimulant and non-stimulant drugs of classes with the ATC code N06BA, especially with N06BA01 (amphetamine), N06BA02 (dexamphetamine), N06BA03 (methamphetamine), N06BA04 (methylphenidate), N06BA05 (pemoline), N06BA06 (fencamfamin), N06BA07 (modafinil), N06BA08 (fenozolone) and as drugs with the ATC code N06BA09 (atomoxetine).
Statistical analysis
A weighting factor was computed and used to adjust for deviations in demographic characteristics (age, sex, residence in west or east Germany and level of urbanicity) between the survey population and official population statistics. In the tables, percentages and ORs refer to weighted data, n's are given unweighted. The prevalence rates for ADHD medication use were calculated as follows: children and adolescents with at least one application of a preparation according to our definition of ADHD medication (ATC code N06BA) were defined as users. All other children whose parents completed drug interview and did not report an application according to ATC code N06BA were defined as non-users of these drugs. Descriptive statistics (proportions and 95% CIs) were calculated to analyse characteristics of the study population and to estimate prevalence rates of stimulant and atomoxetine use and associated risk factors. ORs and 95% CIs were obtained from binary logistic regression models. The presence of statistical interactions was examined for all predictor variables in the multivariate model; however, none of the interactions reached statistical significance. Interactions including immigration background did not lead to stable model solutions due to small cell sizes. Group differences were considered statistically significant if a p value was less than 0.05 or if the 95% CIs for two rates did not overlap. All statistical analyses were performed using SPSS statistical software (release 20.0). To adjust for sample clustering effects, the SPSS complex samples module was used for all analyses.
Results
Study population
table 1 lists characteristics of the study sample by gender. The vast majority of boys and girls had a very good or good parent-rated general health status, came from families with no immigration background and resided in former West Germany, and in cities. Nearly half of boys and girls lived in a family with a medium SES, one-quarter, respectively, in families with a low or high SES. About one-third of the children and adolescents reported a visit to a doctor over the past 4 weeks. Less than 10% had not visited a doctor in the past 12 months. More than 80% of the mothers reported that they did not smoke tobacco or drink alcohol during pregnancy at all. There were no significant differences between boys and girls with regard to these characteristics. As previously published, in our sample a parent-reported ADHD diagnosis by a physician or psychologist was significantly more prevalent for boys (7.9%) than for girls (1.8%).27
Table 1.
Sociodemographic and health-related characteristics of survey participants by gender. German Health Interview and Examination Survey for Children and Adolescents 2003–2006 (KiGGS)
| Boys |
Girls |
|||||
|---|---|---|---|---|---|---|
| N** | %* | 95% CI | N** | %* | 95% CI | |
| Total | 8880 | 8570 | ||||
| Age group (years) | ||||||
| 0–5 | 2816 | 29.2 | (28.6 to 29.8) | 2794 | 29.2 | (28.5 to 29.9) |
| 6–10 | 2609 | 27.1 | (26.6 to 27.7) | 2490 | 27.2 | (26.6 to 27.4) |
| 11–13 | 1572 | 17.3 | (17.0 to 17.6) | 1468 | 17.3 | (17.0 to 17.7) |
| 14–17 | 1883 | 26.4 | (25.8 to 27.0) | 1818 | 26.3 | (25.7 to 26.9) |
| Region | ||||||
| East | 2889 | 16.5 | (12.3 to 21.9) | 2847 | 16.5 | (12.3 to 21.9) |
| West | 5991 | 83.5 | (78.1 to 87.7) | 5723 | 83.5 | (78.1 to 87.7) |
| Urbanicity | ||||||
| Rural | 1958 | 17.9 | (12.6 to 24.8) | 1939 | 17.9 | (12.6 to 24.7) |
| Small-size urban | 2337 | 27.6 | (20.9 to 35.6) | 2229 | 27.2 | (20.5 to 35.1) |
| Medium-size urban | 2498 | 29.0 | (22.2 to 37.0) | 2475 | 29.3 | (22.4 to 37.2) |
| Metropolitan | 2087 | 25.5 | (19.0 to 33.3) | 1927 | 25.6 | (19.1 to 33.5) |
| Migrant background | ||||||
| Yes | 1350 | 17.4 | (15.4 to 19.6) | 1230 | 16.9 | (14.9 to 19.1) |
| No | 7498 | 82.6 | (80.4 to 84.6) | 7292 | 83.1 | (80.9 to 85.1) |
| Missing | 32 | 48 | ||||
| SES | ||||||
| Low | 2454 | 27.7 | (26.1 to 29.4) | 2306 | 27.3 | (25.9 to 28.8) |
| Middle | 4011 | 45.2 | (43.7 to 46.8) | 3890 | 45.7 | (44.1 to 47.2) |
| High | 2185 | 27.0 | (25.2 to 29.0) | 2181 | 27.1 | (25.2 to 29.0) |
| Missing | 230 | 193 | ||||
| Parent-rated subjective health status | ||||||
| Excellent | 3407 | 38.2 | (36.8 to 39.6) | 3466 | 40.1 | (38.7 to 41.6) |
| Good | 4759 | 54.7 | (53.2 to 56.1) | 4491 | 53.6 | (52.2 to 55.0) |
| Moderate | 567 | 6.9 | (6.2 to 7.6) | 486 | 5.9 | (5.3 to 6.6) |
| Bad | 19 | 0.2 | (0.1 to 0.4) | 18 | 0.3 | (0.2 to 0.5) |
| Very bad | 7 | 0.1 | (0.0 to 0.2) | 5 | 0.1 | (0.0 to 0.1) |
| Missing | 121 | 104 | ||||
| Tobacco smoking of the mother during pregnancy | ||||||
| Regular | 386 | 4.8 | (4.2 to 5.4) | 402 | 5.1 | (4.5 to 5.9) |
| From time to time | 1080 | 12.4 | (11.6 to 13.3) | 1009 | 12.8 | (11.9 to 13.9) |
| Never | 7123 | 82.8 | (81.7 to 83.9) | 6901 | 82.0 | (80.8 to 83.2) |
| Missing | 291 | 258 | ||||
| Alcohol consumption by the mother during pregnancy | ||||||
| Regular | 10 | 0.2 | (0.1 to 0.3) | 15 | 0.1 | (0.1 to 0.2) |
| From time to time | 1122 | 13.6 | (12.2 to 14.3) | 1146 | 14.0 | (13.0 to 15.2) |
| Never | 7482 | 86.6 | (85.5 to 87.6) | 7183 | 85.8 | (84.7 to 86.9) |
| Missing | 266 | 226 | ||||
| Last visit to a doctor | ||||||
| <4 weeks | 2949 | 32.8 | (31.5 to 34.0) | 2985 | 34.7 | (33.5 to 35.9) |
| 1−<3 months | 2596 | 29.6 | (28.5 to 30.7) | 2414 | 28.5 | (27.5 to 29.6) |
| 3–12 months | 2448 | 28.8 | (27.6 to 30.0) | 2438 | 29.6 | (28.5 to 30.7) |
| 12+months | 688 | 8.8 | (8.2 to 9.5) | 547 | 7.2 | (6.4 to 8.0) |
| Missing | 199 | 186 | ||||
| ADHD diagnosis (physician or psychologist) | ||||||
| Yes | 521 | 7.9 | (7.1 to 8.8) | 133 | 1.8 | (1.4 to 2.2) |
| No | 6147 | 92.1 | (91.1 to 92.8) | 6538 | 98.2 | (97.8 to 98.6) |
| Not apply (age) | 1397 | 1373 | ||||
| Missing | 815 | 526 | ||||
%*, weighted; N**, unweighted; ADHD, attention deficit/hyperactivity disorder; SES, socioeconomic status.
Prevalence and determinants of current ADHD medication
Parents of 158 (0.9%, 95% CI 0.7% to 1.1%) children and adolescents from 0 to 17 years reported ADHD medication throughout the past 7 days, 132 (1.5%; 95% CI 1.2% to 1.8%) boys and 26 girls (0.3%; 0.2% to 0.5%; see table 2). Given a total child and adolescent population aged between 0 and 17 years of 14 828 835 on 31 December 2004 in Germany, these were about 133 460 youth with ADHD medication use by the mean time of our study. Boys thus used these drugs five times more frequently. There were significant differences in the prevalence of use as a function of age, too. Here, the highest prevalence rates were for boys aged 6–13 and girls aged 11–13 years. In each age group, the rates for girls were well below those of boys. Among boys, there was a significant inverse association between an immigration background and the prevalence of stimulant and non-stimulant use. Boys from families with no immigration background were affected more than five times as frequently as boys with an immigrant background (1.7% vs 0.3%). However, there was no evidence of any correlation with SES. A good general health status was more frequently associated with stimulant and non-stimulant use than a very good or moderate to very bad one. Children of mothers who reported regular smoking tobacco displayed significantly higher prevalence rates of ADHD diagnoses than children of mothers who never smoked tobacco (7.5%, 5.3% to 10.7% vs 4.4%, 3.9% to 4.9%). Similarly, regularly drinking alcohol during pregnancy was significantly associated with higher rates of diagnosed ADHD in the offspring (27.5%, 11.1% to 53.4% vs 4.8%, 4.3% to 5.3%, data not shown in table) A similar pattern was observed for ADHD medication use, however, the differences were not statistically significant. It has to be mentioned though that the cell frequency of mothers who reported regularly drinking alcohol during pregnancy and ADHD medication of their child was only two. A visit to the doctor within the last 3 months was associated with a higher prevalence of ADHD medication. Generally, a shorter distance in time to the last visit to the doctor was associated with a higher prevalence of ADHD medication. The differences, however, were not statistically significant. Nevertheless, when using χ2 test instead of CI analysis there were significant differences with respect to the last visit to the doctor in the total sample as well as in boys (data not shown). This discrepancy in the significance tests is probably due to the small cell sizes. Because of this uncertainty we cannot reliably rule out that there actually are differences. Boys with a diagnosis of ADHD were more likely to being treated with ADHD medication as compared with girls diagnosed with ADHD (21.8% vs 14.8%). The gender differences were statistically significant in the 6-year-old to 10-year-old group (figure 1). The great majority of the boys and girls only used one preparation; a combination of two drugs of the ATC group N06BA was rarely used (table 2). We did not observe significant differences in the prevalence of ADHD medication between the single years of the survey period (2003–2006) (data not shown in table).
Table 2.
Prevalence rates of ADHD medication use (ATC N06BA) by gender. German Health Interview and Examination Survey for Children and Adolescents 2003–2006 (KiGGS)
| Total |
Boys |
Girls |
|||||
|---|---|---|---|---|---|---|---|
| N** | %* | 95% CI | %* | 95% CI | %* | 95% CI | |
| 158 | 0.9 | (0.7 to 1.1) | 1.5 | (1.2 to 1.8) | 0.3 | (0.2 to 0.5) | |
| Age (years) | |||||||
| 0–5 | 0 | ||||||
| 6–10 | 70 | 1.3 | (1.2 to 2.1) | 2.3 | (1.7 to 3.1) | 0.3 | (0.1 to 0.7) |
| 11–13 | 54 | 1.7 | (1.3 to 2.3) | 2.7 | (2.0 to 3.7) | 0.7 | (0.3 to 1.4) |
| 14–17 | 34 | 0.9 | (0.6 to 1.3) | 1.4 | (0.9 to 2.1) | 0.4 | (0.1 to 0.9) |
| Region | |||||||
| East | 54 | 0.8 | (0.5 to 1.2) | 1.3 | (0.8 to 2.0) | 0.2 | (0.1 to 0.5) |
| West | 104 | 0.9 | (0.7 to 1.1) | 1.5 | (1.2 to 1.8) | 0.3 | (0.2 to 0.5) |
| Urbanicity | |||||||
| Rural | 30 | 0.7 | (0.4 to 1.1) | 1.2 | (0.7 to 2.0) | 0.1 | (0.0 to 0.2) |
| Small-size urban | 41 | 0.9 | (0.6 to 1.3) | 1.6 | (1.1 to 2.4) | 0.1 | (0.0 to 0.4) |
| Medium-size urban | 50 | 0.9 | (0.7 to 1.3) | 1.4 | (1.0 to 1.9) | 0.5 | (0.2 to 1.0) |
| Metropolitan | 37 | 1,0 | (0.7 to 1.4) | 1.6 | (1.1 to 2.2) | 0.4 | (0.2 to 0.9) |
| Migrant background | |||||||
| Yes | 7 | 0.4 | (0.2 to 0.8) | 0.3 | (0.1 to 0.8) | 0.4 | (0.1 to 0.4) |
| No | 151 | 1.0 | (0.8 to 1.2) | 1.7 | (1.4 to 2.1) | 0.3 | (0.1 to 0.4) |
| SES | |||||||
| Low | 42 | 0.9 | (0.6 to 1.2) | 1.3 | (0.9 to 1.8) | 0.5 | (0.2 to 1.0) |
| Middle | 81 | 1.0 | (0.8 to 1.3) | 1.8 | (1.4 to 2.4) | 0.2 | (0.1 to 0.5) |
| High | 33 | 0.7 | (0.5 to 1.1) | 1.2 | (0.8 to 1.8) | 0.2 | (0.1 to 0.6) |
| Parent-rated subjective health status | |||||||
| Excellent | 29 | 0.5 | (0.3 to 0.7) | 0.9 | (0.6 to 1.4) | 0.1 | (0.0 to 0.3) |
| Good | 119 | 1.2 | (1.0 to 1.5) | 1.9 | (1.5 to 2.4) | 0.5 | (0.3 to 0.8) |
| Moderate/Bad/Very Bad† | 9 | 0.8 | (0.4 to 1.7) | 1.4 | (0.6 to 2.9) | 0.2 | (0.0 to 1.3) |
| Tobacco smoking by the mother during pregnancy | |||||||
| Regular | 16 | 1.7 | (0.9 to 3.0) | 2.4 | (1.2 to 4.6) | 1.0 | (0.4 to 2.7) |
| From time to time | 15 | 0.8 | (0.4 to 1.4) | 1.3 | (0.7 to 2.4) | 0.2 | (0.1 to 0.9) |
| Never | 123 | 0.9 | (0.7 to 1.1) | 1.5 | (1.2 to 1.8) | 0.3 | (0.1 to 0.5) |
| Alcohol consumption by the mother during pregnancy | |||||||
| Regular | 2 | 7.7 | (1.9 to 26.5) | 14.2 | (3.2 to 45.0) | ||
| From time to time | 19 | 0.7 | (0.4 to 1.3) | 1.0 | (0.5 to 1.8) | 0.5 | (0.2 to 1.4) |
| Never | 134 | 0.9 | (0.8 to 1.9) | 1.6 | (1.3 to 1.9) | 0.3 | (0.1 to 0.5) |
| Last visit to a doctor | |||||||
| <4 weeks | 56 | 0.9 | (0.7 to 1.3) | 1.6 | (1.1 to 2.3) | 0.3 | (0.1 to 0.6) |
| 1-<3 months | 62 | 1.3 | (1.0 to 1.7) | 2.2 | (1.6 to 2.9) | 0.3 | (0.1 to 0.8) |
| 3–12 months | 32 | 0.6 | (0.4 to 0.9) | 0.9 | (0.5 to 1.4) | 0.3 | (0.2 to 0.7) |
| 12+months | 6 | 0.5 | (0.2 to 1.1) | 0.8 | (0.3 to 1.9) | 0.1 | (0.0 to 0.6) |
| ADHD diagnosis (physician or psychologist) | |||||||
| Yes | 142 | 0.8 | (0.6 to 0.9) | 1.3 | (1.1 to 1.6) | 0.2 | (0.1 to 0.4) |
| No | 3 | 0.0 | (0.0 to 0.1) | 0.1 | (0.0 to 0.2) | ||
| Number of medicines (N06BA) | |||||||
| 1 | 146 | 0.8 | (0.7 to 1.0) | 1.3 | (1.1 to 1.6) | 0.3 | (0.2 to 0.4) |
| 2+ | 12 | 0.1 | (0.0 to 0.1) | 0.1 | (0.1 to 0.2) | 0.0 | (0.0 to 0.1) |
*%, weighted; N**, unweighted. The sum of each category may not be the total number because of data missing.
†The categories ‘Moderate’, ‘Bad’ and ‘Very Bad’ were collapsed due to low cell frequencies.
ADHD, attention-deficit hyperactivity disorder; ATC, Anatomical Therapeutic Chemical; SES, socioeconomic status.
Figure 1.
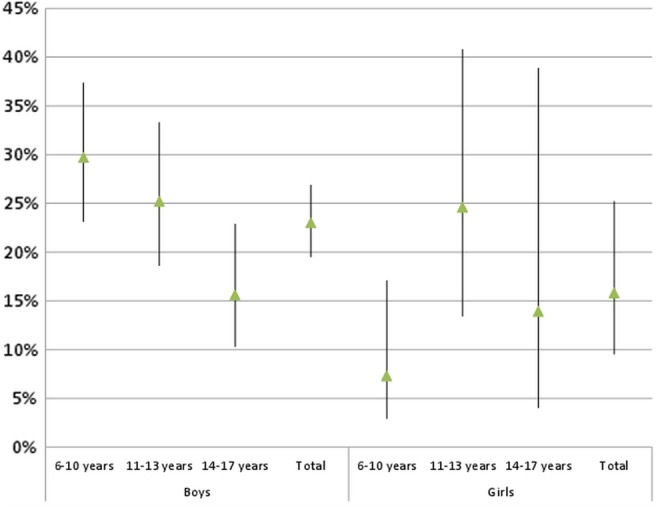
Proportion (% and 95% CI) of attention-deficit hyperactivity disorder (ADHD) medication use in children with ADHD diagnosis (n=145).
When potential confounders and risk factors were considered simultaneously, gender, age, city size, immigration status and last visit a doctor showed stable associations with stimulant and atomexetine use (table 3). In addition, a decreasing general health status was significantly associated with increased chances of ADHD medication. However, this was only true for the change from a very good to a good general status of health. As suggested by the bivariate analyses, the associations of mother's smoking tobacco and alcohol consumption during pregnancy remained statistically insignificant. In addition, separate models for boys and girls were run. For boys, the results were similar to the total model. However, the analysis did not result in a stable model solution for girls because only a small number of girls were users of ADHD medication. Further, we examined in a multivariate binary logistic regression analysis whether youth diagnosed with ADHD and ADHD medication differed from those with ADHD without medication with respect to all previously used predictors. In summary, we found that after mutual adjustment of all predictors only higher degree of urbanisation, younger age, more recent last visit a doctor uniquely contributed to the model (table 3). Excessive multicollinearity between the predictor variables was not detected. None of the correlations exceeded r=0.42.
Table 3.
Multivariate associations between ADHD medication and risk factors in youth (total sample and youth with ADHD diagnosis only) (OR and 95% CI)
| All children
N=11,142* |
Children with ADHD diagnosis N=586† |
|||
|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Gender | <0.001 | 0.171 | ||
| Boys | 5.16 (3.15 to 8.47) | 1.50 (0.84 to 2.7) | ||
| Girls | 1 (Ref.) | 1 (Ref.) | ||
| Age (years) | 0.018 | 0.036 | ||
| 6–10 | 1.60 (0.94 to 2.72) | 2.04 (1.09 to 3.81) | ||
| 11–13 | 2.24 (1.28 to 3.94) | 2.19 (1.16 to 4.11) | ||
| 14–17 | 1 (Ref.) | 1 (Ref.) | ||
| Region | 0.257 | 0.136 | ||
| East | 1 (Ref.) | 1 (Ref.) | ||
| West | 1.32 (0.83 to 2.12) | 1.41 (0.90 to 2.23) | ||
| Urbanicity | 0.014 | |||
| Rural | 1 (Ref.) | 1 (Ref.) | 0.127 | |
| Small-size urban | 1.60 (0.81 to 3.18) | 1.60 (0.84 to 3.04) | ||
| Medium-size urban | 1.78 (0.94 to 3.41) | 1.96 (1.07 to 3.60 | ||
| Metropolitan | 2.18 (1.13 to 4.22) | 2.02 (1.03 to 3.98) | ||
| Migrant background | 0.008 | 0.727 | ||
| Yes | 1 (Ref.) | 1 (Ref.) | ||
| No | 3.06 (1.34 to 6.99) | 1.22 (0.39 to 3.81) | ||
| SES | 0.500 | 0.128 | ||
| Low | 1.29 (0.75 to 2.21) | 0.52 (0.27 to 0.98) | ||
| Middle | 1.33 (0.82 to 2.14) | 0.64 (0.35 to 1.19) | ||
| High | 1 (Ref.) | 1 (Ref.) | ||
| Parent-rated subjective health status | 0.018 | 0.127 | ||
| Excellent | 1 (Ref.) | 1 (Ref.) | ||
| Good | 1.91 (1.18 to 3.08) | 1.22 (0.68 to 2.19) | ||
| Moderate/Bad/Very Bad | 1.18 ( 0.51 to 2.74) | 0.53 (0.21 to 1.33) | ||
| Tobacco smoking during pregnancy | 0.221 | |||
| Regular | 1.64 (0.85 to 3.18) | 0.46 (0.16 to 1.28) | 0.322 | |
| From time to time | 0.78 (0.41 to 1.48) | 0.71 (0.32 to 1.57) | ||
| Never | 1 (Ref.) | 1 (Ref.) | ||
| Alcohol consumption during pregnancy | 0.427 | 0.675 | ||
| Regular | 3.16 (0.27 to 37.01) | 0.82 (0.06 to 11.64) | ||
| From time to time | 0.77 (0.44 to 1.25) | 1.13 (0.09 to 14.66) | ||
| Never | 1 (Ref.) | 1 (Ref.) | ||
| Last visit to a doctor | <0.001 | 0.025 | ||
| < 4 weeks | 3.18 (1.29 to 7.85) | 2.07 (0.77 to 5.54) | ||
| 1−< 3 months | 3.59 (1.45 to 8.90) | 2.96 (1.12 to 7.77) | ||
| 3−12 months | 1.35 (0.52 to 3.52) | 1.29 (0.44 to 3.79) | ||
| 12+months | 1 (Ref.) | 1 (Ref.) | ||
| Nagelkerke pseudo-R2 | 10.6% | 11.7% |
Italicised values indicate significant results.
*6308 subjects are missing due to list-wise deletion.
†68 subjects are missing due to list-wise deletion.
ADHD, attention-deficit hyperactivity disorder; SES, socioeconomic status.
Indications and patterns of current ADHD medication use
A total of 171 preparations were mentioned by the 158 children and adolescents currently using ADHD medication. As expected, with 88.9% ADHD (ICD-10; F.90) was the most frequently mentioned indication. Methylphenidate (93.6%) was the most frequently mentioned substance. Atomoxetine and amphetamine-containing drugs were less commonly used (4.1% and 1.8%, respectively) at the time of the survey. As shown in table 4 the use of all drugs was completely based on prescription. Generally, use was mediumterm to long term. Almost one in two drugs (43.9%) had been used for at least 1 year. More than 90% of the parents associated the drug use with an improvement in symptoms, and 88% stated that the tolerability of the preparations was very good or good. Of the 171 drugs used, a total of 21 ADRs (12.3%) were reported. The corresponding percentage for the most frequently mentioned drug, methylphenidate, was 11.9%. In most cases, the reported ADRs were a reduction in appetite (13 cases, 61.9%, data not shown in table). Although the use of ADHD medication is much less common in girls than in boys, the range of indications is almost identical. Hyperkinetic disorder is the main indication for both sexes (94% of the boys and 93% of the girls). Medium-term and long-term use of the drugs outweighs the short-term application. While it is generally reported for both sexes that the symptoms were considerably improved when using the ADHD medication, the rate of ADRs was twice as high in boys as in girls (13.2 vs 7.4%, table 4). This should be noted, although the difference is not statistically significant. However, the latter is primarily due to the small cell sizes. Significant differences between those with reported ADRs and those without were not observed with respect to age, gender, immigration background, region and degree of urbanisation.
Table 4.
ADHD medication by indication (ICD-10.Rev.), substance group, origin, self-rated improvement of conditions treated, tolerance, duration of use and perceived adverse drug reactions (ADRs)
| Total |
Boys |
Girls |
|||||
|---|---|---|---|---|---|---|---|
| N* | %* | N* | %* | N* | %* | p Value | |
| 171 | 100 | 144 | 100 | 27 | 100 | ||
| Indications (ICD 10.Rev.) | 0.219 | ||||||
| Unknown | 1 | 0.6 | 1 | 0.7 | |||
| Severe depressive episode | |||||||
| without psychotic symptoms (F32.2) | 1 | 0.6 | 1 | 0.7 | |||
| Specific reading disorder F81.0 | 1 | 0.6 | 1 | 0.7 | |||
| Disturbance of activity and attention (F90.0) | 149 | 87.1 | 125 | 86.8 | 24 | 88.9 | |
| Hyperkinetic disorder, unspecified (F90.9) | 3 | 1.8 | 3 | 2.1 | |||
| Combined vocal and multiple | |||||||
| motor tic disorder (de la Tourette) (F95.2) | 1 | 0.6 | 1 | 0.7 | |||
| Other specified behavioural and | |||||||
| emotional disorders with onset usually occurring | |||||||
| in childhood and adolescence (F98.8) | 3 | 1.8 | 3 | 2.1 | |||
| Epilepsy, unspecified (G40.9) | 1 | 0.6 | 0 | 1 | 3.7 | ||
| Malaise and fatigue (R53) | 11 | 6.4 | 9 | 6.3 | 2 | 7.4 | |
| Medicine (ATC code) | 0.826 | ||||||
| Amfetamine (N06BA01) | 3 | 1.8 | 2 | 1.4 | 1 | 3.7 | |
| Dexamfetamine (N06BA02) | 1 | 0.6 | 1 | 0.7 | |||
| Methylphenidate (N06BA04) | 160 | 93.6 | 135 | 93.8 | 25 | 92.6 | |
| Atomoxetine (N06BA09) | 7 | 4.1 | 6 | 4.2 | 1 | 3.7 | |
| Origin of the medicine | |||||||
| Prescription | 171 | 100 | 144 | 100 | 27 | 100 | |
| Duration of use | 0.344 | ||||||
| <1 week | 11 | 6.4 | 9 | 6.3 | 2 | 7.4 | |
| 1−<4 weeks | 22 | 12.9 | 21 | 14.6 | 1 | 3.7 | |
| 1−<12 months | 63 | 36.8 | 50 | 34.7 | 13 | 48.1 | |
| >=1 year | 75 | 43.9 | 64 | 44.4 | 11 | 40.7 | |
| Missing | |||||||
| Self-rated improvement of conditions treated | 0.944 | ||||||
| Great | 98 | 59.4 | 83 | 57.6 | 15 | 55.6 | |
| Partial | 57 | 34.5 | 48 | 33.3 | 9 | 33.3 | |
| Little | 6 | 3.6 | 5 | 3.5 | 1 | 3.7 | |
| Missing | 10 | 8 | 2 | ||||
| Tolerance for the medicine | 0.860 | ||||||
| Very good/good | 151 | 88.8 | 126 | 87.5 | 25 | 92.6 | |
| Partial | 16 | 9.4 | 14 | 9.7 | 2 | 7.4 | |
| Not good | 1 | 0.6 | 1 | 0.7 | |||
| Poor | 2 | 1.2 | 2 | 1.4 | |||
| Missing | 1 | ||||||
| Perceived ADR | 21 | 12.3 | 19 | 13.2 | 2 | 7.4 | 0.317 |
| Amfetamine (N06BA01) | 1 | 0.6 | 1 | 0.7 | |||
| Methylphenidate (N06BA04) | 19 | 11.1 | 17 | 11.8 | 2 | 7.4 | |
| Atomoxetine (N06BA09) | 1 | 0.6 | 1 | 0.7 | |||
*N and % unweighted.
ADHD, attention-deficit hyperactivity disorder; ATC, Anatomical Therapeutic Chemical; ICD, International Classification of Diseases.
Discussion
Prevalence and determinants
In the nationwide representative KiGGS with more than 17 000 participants, the prevalence of ADHD medication (drugs from the ATC group N06BA) was 0.9%. Boys used these medications 5 times more frequently than girls did. Besides, we found use peaks among the 6-year-olds to 10-year-olds and 11-year-olds to 13-year-olds. Parents of children with no immigration background and parents of children living in large cities reported ADHD medication use significantly more frequently.
The overall prevalence rate reported within our study is much lower than prevalence rates reported from US studies. Yet in the late 1990s and at the beginning of the 21st century, data published by the Medical Expenditure Panel Survey (MEPS) referred to prevalence rates between 2.7% (1997) and 2.9% (2002).6 After analysing the pharmaceutical data of a private insurance company in the USA, Castle et al30 stated that 4.4% of all under-19s were prescribed preparations for treating ADHD in 2005. The results of a population-based regional longitudinal study in Israel revealed a prevalence of methylphenidate prescription of 1.1% in girls and 3.8% in boys of school age.7 A similar level of stimulant prescription and use is reported by analyses of population-related pharmacy data31 and representative household surveys32 in Australia. In contrast, prevalence rates reported for Western European and Scandinavian countries are markedly lower. A national cohort study in Sweden conducted in 2005 shows a low level of ADHD medication comparable with our study.33 34 Study results from the Netherlands35 and France36 as well as an analysis of data of one of the biggest health insurances in Germany37 also indicate a lower use of stimulants in Western Europe than in the USA that is comparable with the results from our study. Significant differences in the prevalence of ADHD medication between the single years of the survey period of our study were not observed. Based on health insurance data Schubert et al37 report a doubling in prevalence of ADHD medication use from 0.54% to 1.06% between 2000 and 2007. The prevalence in our study had an average value of 0.9% from the years 2003 to 2006 within this range. Differences in study design and the date when the survey was carried out must be taken into account when comparing our data with published results. A growing trend in prescription and use can be observed over the past few decades.10 30 37 Furthermore, the prescription and use of ADHD drugs is highly dependent on gender and age. Studies looking at different populations in terms of age and gender and carried out at different times will thus arrive at different prevalence estimates for these reasons alone. Another decisive factor is the period over which the use of a drug is observed. If data collection covers a period of 7 days, as in our study, any interruption of intake can lead to prevalence rates being underestimated. On the other hand, shorter periods reduce recall bias. However, a three-country comparison carried out by Zito et al38 shows that the prevalence of stimulant prescription in children and adolescents in the USA (4.29%) is much higher than the corresponding rates in the Netherlands (1.18%) and Germany (0.71%) even when these differences in study design are largely excluded. The lower prevalence rate in western Europe (including Germany) and above all the Scandinavian countries is thought to be caused by more restrictive legislation on the prescription of stimulants11 39
Most international studies report boys to be much more likely to be treated with stimulants than girls and that there is a marked increase when children reach school age.7 30–36 This corresponds well with the prevalence rates that we found in our study and consistently, in our sample, boys were found to be four times more likely to be diagnosed with ADHD than girls.27 Results from the USA40 and from Canada41 show that although ADHD is diagnosed more frequently among children from socially deprived families, children from such families receive a corresponding drug therapy less frequently. In our study, we found no significant differences for SES concerning ADHD medication, although children diagnosed with ADHD were twice as prevalent in families with low SES in our sample, too.27 This suggests that children from families with low SES may be disadvantaged in receiving adequate drug therapy when being diagnosed with ADHD. In contrast, however, a lower educational level of a child's mother and the status of being a ‘single parent’ and a ‘social welfare recipient’ proved to be significant determinants of a greater likelihood of ADHD medication in a Swedish cohort study.33 The fact that we found youth living in large urban centres to be more frequently using ADHD medication may be due to differences in access to healthcare services in rural versus urban regions. This is supported by the fact that we did not find significant differences in the prevalence of diagnosed ADHD with respect to the degree of urbanisation.27 When comparing children with ADHD diagnosis with and without ADHD medication in an additional analysis, we found that differences in the degree of urbanisation were still present even after adjusting for potential confounders. With respect to the association of ADHD medication use and immigration background our results correspond to those of various studies.9–21 42 In a population-based study of the prescription data of the largest health insurance company in the Netherlands, Wittkampf et al42 report that ADHD medication is less likely to be prescribed to children of Turkish and Moroccan families than to Dutch boys and girls. This may either point to differential thresholds to healthcare services for immigrants or may even be due to culturally altered attitudes towards symptoms of ADHD. Olfson et al19 suggest that cultural factors, rather than economic factors, may explain the lower prevalence of ADHD treatment in racial or ethnic minorities. Also, in our sample we found that children from families with immigration background were less likely to be diagnosed with ADHD than children from non-immigrant families. Nevertheless, the prevalence of clinically relevant ADHD symptoms was far higher in immigrant children,27 which argues against a ‘healthy immigrant’ effect.
In our study, most parents of children with ADHD medication rated the general status of health of their child as good but not excellent. This is plausible as on the one hand stimulant medication is known to significantly improve ADHD symptoms which may result in a better subjective health. On the other hand, the fact that their child is dependent on a long-term medication may not encourage parents to rate general health status as excellent.43
Risky health behaviours during pregnancy including regularly smoking tobacco and regularly drinking alcohol during pregnancy are known to occur more frequently in socially deprived families.15 18 In multivariate analysis, however, we did not find any significant associations between those prenatal risk factors and ADHD medication. This corresponds to the results of Lindblad and Hjern who found that the associations of maternal tobacco consume during pregnancy and ADHD medication in the offspring were largely explained by confounding by genetic and socioeconomic factors.15
Treatment of ADHD with stimulants and atomoxetine
In our study, about one-fifth of all children and adolescents with diagnosed ADHD were treated with stimulants and atomoxetine. This rate is well below figures from the USA; more than half (50–60%) of the affected children receive appropriate medication.19 29 30 Boys with ADHD diagnosis have a higher prevalence of drug therapy as ADHD-affected girls. In our sample, the ratio of boys to girls with a parent-reported ADHD diagnosis and treatment with stimulants and atomoxetine was 1.5:1. However, these differences were statistically significant only in children of primary school age. Derks et al44 suggest that higher ADHD medication use rates in boys may be due to differential behavioural ratings by teachers in the schools. They observed that teachers reported higher problem scores of attention and aggression for boys with ADHD than for ADHD-affected girls in the school setting, whereas mothers reported similarly high levels in boys and girls with ADHD at home. The observed increase in the rate of ADHD medication use in primary school age aligns with the increase of diagnosed ADHD in our sample27 and is likely explained by the fact that in Germany the use of ADHD medication is licenced only for the age 6 years and above. A further explanation for the increase of ADHD diagnoses and ADHD medication use with the beginning of school is also due to increased demands (eg, sitting still) from the preschool to the school setting, when symptoms of ADHD impact more strongly.
Indications and patterns of current ADHD medication use
Among all preparations methylphenidate was used most frequently, that is, in almost 94% of cases. Consistent with other studies,34 35 43 45 our results indicate that methylphenidate is most frequent among the drugs used. The improvement in ADHD symptoms under medication reported in KiGGS confirms findings by Thorell and Dalström in a survey of Swedish children.43 With a proportion of 12.3%, however, parents reported for more than 1 in 10 children and adolescents with ADHD medication an ADR, in particular for methylphenidate. Against the background of a generally low overall prevalence of ADRs in our sample, these effects after ADHD medication are nevertheless among the ones most frequently mentioned.46
Strengths and limitations
KiGGS is a population-based study and due to its representative design it allows generalisations to be made on the use of ADHD medication in children and adolescents in Germany. The survey of drug use together with the collection and measurement of health-related data allows a representative description of ADHD medication in the German child and adolescent population under everyday conditions and regardless of the use of medical services. The fact that information on drug use relates to the last 7 days does have a limiting effect, however. Although, on the one hand, this reduces the likelihood of a recall bias, on the other it can lead to misclassifications as users when children and adolescents who have interrupted intake at the time of the survey reported that they are not currently taking any medication. The data on ADHD medication use are based on self-reports by study participants or their parents. Drug use that is consciously or unconsciously concealed can thus lead to an underestimation of the true use of ADHD medication. The particular focus of the KiGGS study as a population-representative cross-sectional health survey is to identify risk groups and describe any risk constellations. Cause−effect relations cannot be generated on the basis of these cross-sectional data. As the KiGGS study is recently continued as cohort study, this will be made possible for the first time in 2012 when the field work of the next wave is completed.
Conclusions
The results of the KiGGS study representative for the German child and adolescent population show a prevalence rate of stimulant use that is considerably lower than the published prevalence rates from the USA, but comparable with those of western European and Scandinavian countries. The data on higher prevalence in boys and an increase in pharmacotherapy when children reach school age are comparable in all published studies. The associations with living conditions (large urban centres, immigrant background) which were confirmed in multivariate analysis potentially point to differences in access to healthcare services and culture-specific differences in attitudes towards ADHD. Further clues on the determinacy of ADHD medication, on efficacy and long-term results will be provided by the data from the KiGGS cohort study.
Supplementary Material
Footnotes
Contributors: HK conducted the literature review, performed the statistical analysis, draughted the manuscript and assisted in the conceptualisation of the study. HH provided specific knowledge and assisted in the conceptualisation of the study. MH provided assistance in analysing the data and interpreting the results. RS assisted the data analysis, provided specific knowledge and contributed to the conceptualisation of the study as well as writing of the final manuscript. All authors read and approved the final manuscript.
Funding: The German Health Interview and Examination Survey for Children and Adolescents (KiGGS) was funded by the German Federal Ministry of Health and the Ministry of Education and Research.
Competing interests: All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author). HK, HH and RS declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous 3 years, no other relationships or activities that could appear to have influenced the submitted work. MH declares the following conflict of interest: board membership with companies producing medication for ADHD (attention-deficit hyperactivity disorder) in Germany (Medice, Lilly, Novartis, Shire); consultancy (Medice); unrestricted grant for adherence study (Medice); paid lectures (Medice, Lilly, Janssen-Cilaq, Shire).
Patient consent: Obtained.
Ethics approval: Charité/Universitätsmedizin Berlin Ethics Committee.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: The data of the German Health Interview and Examination Survey for Children and Adolescents (KiGGS) is available on demand as a public-use file. Address: Robert Koch Institute, PO Box 650261, D-13302 Berlin, Germany.
Correction notice: This article has been corrected since it was first published. The equal contributors statement has been added.
References
- 1.MTA-Cooperative-Group National Institute of Mental Health Multimodal Treatment Study of ADHD follow-up: changes in effectiveness and growth after the end of treatment. Pediatrics 2004;113:762–9 [DOI] [PubMed] [Google Scholar]
- 2.Deutsche Gesellschaft, für Kinder- und Jugendpsychiatrie, Psychotherapie. u Leitlinien zur Diagnostik und Therapie von psychischen Störungen im Säuglings-, Kindes- und Jugendalter. In 3. überarbeitete Auflage edn.: Deutscher Ärzte Verlag, 2007:239–54 [Google Scholar]
- 3.Barkley RA, Fischer M, Smallish L, et al. Does the treatment of attention-deficit/hyperactivity disorder with stimulants contribute to drug use/abuse? A 13-year prospective study. Pediatrics 2003;111:97–109 [DOI] [PubMed] [Google Scholar]
- 4.Pliszka SR. Pharmacologic treatment of attention-deficit/hyperactivity disorder: efficacy, safety and mechanisms of action. Neuropsychol Rev 2007;17:61–72 [DOI] [PubMed] [Google Scholar]
- 5.Cormier E. Attention deficit/hyperactivity disorder: a review and update. J Pediatr Nurs 2008;23:345–57 [DOI] [PubMed] [Google Scholar]
- 6.Zuvekas SH, Vitiello B, Norquist GS. Recent trends in stimulant medication use among U.S. children. Am J Psychiatry 2006;163:579–85 [DOI] [PubMed] [Google Scholar]
- 7.Vinker S, Vinker R, Elhayany A. Prevalence of methylphenidate use among Israeli children: 1998–2004. Clin Drug Invest 2006;26:161–7 [DOI] [PubMed] [Google Scholar]
- 8.Hugtenburg JG, Heerdink ER, Egberts AC. Increased psychotropic drug consumption by children in the Netherlands during 1995–2001 is caused by increased use of methylphenidate by boys. Eur J Clin Pharmacol 2004;60:377–9 [DOI] [PubMed] [Google Scholar]
- 9.Schmidt-Troschke SO, Ostermann T, Melcher D, et al. (The use of methylphenidate in children: analysis of prescription usage based in routine data of the statutory health insurance bodies concerning drug prescriptions). Gesundheitswesen 2004;66:387–92 [DOI] [PubMed] [Google Scholar]
- 10.Lohse M J, Müller-Oerlinghausen B. Psychopharmaka. In: Arzneiverordnungsreport 2009 Aktuelle Daten, Kosten Trends und Kommentare edn.. Schwabe U, Paffrath D.eds. Heidelberg: Springer Medizin Verlag, 2009:767–810 [Google Scholar]
- 11.Glaeske G, Janhsen K. GEK-Arzneimittel-Report 2007. Auswertungsergebnisse der GEK-Arzneimitteldaten aus den Jahren 2005–2006. GEK Edition Schriftenreihe zur Gesundheitsanalyse, Band 55, 2007
- 12.Bessou H, Zeeb H, Puteanus U. (Methylphenidate prescriptions in the city of Cologne: overrepresentation of privately insured patients. Results of an analysis based on prescription data). Gesundheitswesen 2007; 69:292–6 [DOI] [PubMed] [Google Scholar]
- 13.Atzori P, Usala T, Carucci S, et al. Predictive factors for persistent use and compliance of immediate-release methylphenidate: a 36-month naturalistic study. J Child Adolesc Psychopharmacol 2009;19:673–81 [DOI] [PubMed] [Google Scholar]
- 14.Hugtenburg JG, Witte I, Heerdink ER. Determinants of compliance with methylphenidate therapy in children. Acta Paediatr 2006;95:1674–6 [DOI] [PubMed] [Google Scholar]
- 15.Lindblad F, Hjern A. ADHD after fetal exposure to maternal smoking. Nicotine Tobacco Res: Off J Soc Res Nicotine Tobacco 2010;12:408–15 [DOI] [PubMed] [Google Scholar]
- 16.Milberger S, Biederman J, Faraone SV, et al. Is maternal smoking during pregnancy a risk factor for attention deficit hyperactivity disorder in children? Am J Psychiatry 1996;153:1138–42 [DOI] [PubMed] [Google Scholar]
- 17.Banerjee TD, Middleton F, Faraone SV. Environmental risk factors for attention-deficit hyperactivity disorder. Acta Paediatr 2007;96:1269–74 [DOI] [PubMed] [Google Scholar]
- 18.Knopik VS, Heath AC, Jacob T, et al. Maternal alcohol use disorder and offspring ADHD: disentangling genetic and environmental effects using a children-of-twins design. Psychol Med 2006;36:1461–71 [DOI] [PubMed] [Google Scholar]
- 19.Olfson M, Gameroff MJ, Marcus SC, et al. National trends in the treatment of attention deficit hyperactivity disorder. Am J Psychiatry 2003;160:1071–7 [DOI] [PubMed] [Google Scholar]
- 20.Visser SN, Lesesne CA, Perou R. National estimates and factors associated with medication treatment for childhood attention-deficit/hyperactivity disorder. Pediatrics 2007;119(Suppl 1):S99–106 [DOI] [PubMed] [Google Scholar]
- 21.Foster BA, Read D, Bethell C. An analysis of the association between parental acculturation and children's medication use. Pediatrics 2009;124:1152–61 [DOI] [PubMed] [Google Scholar]
- 22.Kurth BM, Kamtsiuris P, Holling H, et al. The challenge of comprehensively mapping children's health in a nation-wide health survey: design of the German KiGGS-Study. BMC Public Health 2008;8:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kamtsiuris P, Lange M, Schaffrath Rosario A. (The German Health Interview and Examination Survey for Children and Adolescents (KiGGS): sample design, response and nonresponse analysis). Bundesgesundheitsblatt, Gesundheitsforschung, Gesundheitsschutz 2007;50:547–56 [DOI] [PubMed] [Google Scholar]
- 24.Winkler J, Stolzenberg H. (Social class index in the Federal Health Survey). Gesundheitswesen 1999, 61 Spec No:S178–83 [PubMed] [Google Scholar]
- 25.Lange M, Kamtsiuris P, Lange C, et al. Sociodemographic characteristics in the German Health Interview and Examination Survey for Children and Adolescents (KiGGS)—operationalization and public health significance, taking as an example the assessment of the general status of health. Bundesgesundheitsbl Gesundheitsforsch Gesundheitsschutz 2007;50:578–99 [DOI] [PubMed] [Google Scholar]
- 26.Schenk L, Ellert U, Neuhauser H. Children and adolescents in Germany with a migration background. Methodical aspects in the German Health Interview and Examination Survey for Children and Adolescents (KiGGS). Bundesgesundheitsblatt, Gesundheitsforschung, Gesundheitsschutz 2007;50:590–9 [DOI] [PubMed] [Google Scholar]
- 27.Schlack R, Holling H, Kurth BM, et al. The prevalence of attention-deficit/hyperactivity disorder (ADHD) among children and adolescents in Germany. Initial results from the German Health Interview and Examination Survey for Children and Adolescents (KiGGS). Bundesgesundheitsblatt, Gesundheitsforschung, Gesundheitsschutz 2007;50:827–35 [DOI] [PubMed] [Google Scholar]
- 28.Kamtsiuris P, Bergmann E, Rattay P, et al. Use of medical services. Results of the German Health Interview and Examination Survey for Children and Adolescents (KiGGS). Bundesgesundheitsblatt, Gesundheitsforschung, Gesundheitsschutz 2007;50:836–50 [DOI] [PubMed] [Google Scholar]
- 29.Knopf H. Medicine use in children and adolescents. Data collection and first results of the German Health Interview and Examination Survey for Children and Adolescents (KiGGS). Bundesgesundheitsblatt, Gesundheitsforschung, Gesundheitsschutz 2007;50:863–70 [DOI] [PubMed] [Google Scholar]
- 30.Castle L, Aubert RE, Verbrugge RR, et al. Trends in medication treatment for ADHD. J Attention Disord 2007;10:335–42 [DOI] [PubMed] [Google Scholar]
- 31.Preen DB, Calver J, Sanfilippo FM, et al. Patterns of psychostimulant prescribing to children with ADHD in Western Australia: variations in age, gender, medication type and dose prescribed. Austr N Z J Public Health 2007;31:120–6 [DOI] [PubMed] [Google Scholar]
- 32.Sawyer MG, Rey JM, Graetz BW, et al. Use of medication by young people with attention-deficit/hyperactivity disorder. Med J Austr 2002;177:21–5 [DOI] [PubMed] [Google Scholar]
- 33.Hjern A, Weitoft GR, Lindblad F. Social adversity predicts ADHD-medication in school children—a national cohort study. Acta Paediatr 2010;99:920–4 [DOI] [PubMed] [Google Scholar]
- 34.Lindblad F, Weitoft GR, Hjern A. ADHD in international adoptees: a national cohort study. Eur Child Adolesc Psychiatry 2010;19:37–44 [DOI] [PubMed] [Google Scholar]
- 35.Schirm E, Tobi H, Zito JM, et al. Psychotropic medication in children: a study from the Netherlands. Pediatrics 2001;108:E25. [DOI] [PubMed] [Google Scholar]
- 36.Acquaviva E, Legleye S, Auleley GR, et al. Psychotropic medication in the French child and adolescent population: prevalence estimation from health insurance data and national self-report survey data. BMC Psychiatry 2009;9:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schubert I, Koster I, Lehmkuhl G. The changing prevalence of attention-deficit/hyperactivity disorder and methylphenidate prescriptions: a study of data from a random sample of insurees of the AOK Health Insurance Company in the German State of Hesse, 2000–2007. Deutsches Arzteblatt Int 2010;107:615–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zito JM, Safer DJ, de Jong-van den Berg LT, et al. A three-country comparison of psychotropic medication prevalence in youth. Child Adolesc Psychiatry Mental Health 2008;2:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scheffler RM, Hinshaw SP, Modrek S, et al. The global market for ADHD medications. Health Affairs 2007;26:450–7 [DOI] [PubMed] [Google Scholar]
- 40.Froehlich TE, Lanphear BP, Epstein JN, et al. Prevalence, recognition, and treatment of attention-deficit/hyperactivity disorder in a national sample of US children. Arch Pediatr Adolesc Med 2007;161:857–64 [DOI] [PubMed] [Google Scholar]
- 41.Miller AR, Lalonde CE, McGrail KM, et al. Prescription of methylphenidate to children and youth, 1990–1996. CMAJ 2001;165:1489–94 [PMC free article] [PubMed] [Google Scholar]
- 42.Wittkampf LC, Smeets HM, Knol MJ, et al. Differences in psychotropic drug prescriptions among ethnic groups in the Netherlands. Soc Psychiatry Psychiatric Epidemiol 2010;45:819–26 [DOI] [PubMed] [Google Scholar]
- 43.Thorell LB, Dahlstrom K. Children's self-reports on perceived effects on taking stimulant medication for ADHD. J Attention Disord 2009;12:460–8 [DOI] [PubMed] [Google Scholar]
- 44.Derks EM, Hudziak JJ, Boomsma DI. Why more boys than girls with ADHD receive treatment: a study of Dutch twins. Twin Res Hum Genet: Off J Int Soc Twin Stud 2007;10:765–70 [DOI] [PubMed] [Google Scholar]
- 45.Perwien A, Hall J, Swensen A, et al. Stimulant treatment patterns and compliance in children and adults with newly treated attention-deficit/hyperactivity disorder. J Managed Care Pharm: JMCP 2004;10:122–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Knopf H, Du Y. Perceived adverse drug reactions among non-institutionalized children and adolescents in Germany. Br J Clin Pharmacol 2010;70:409–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


