Abstract
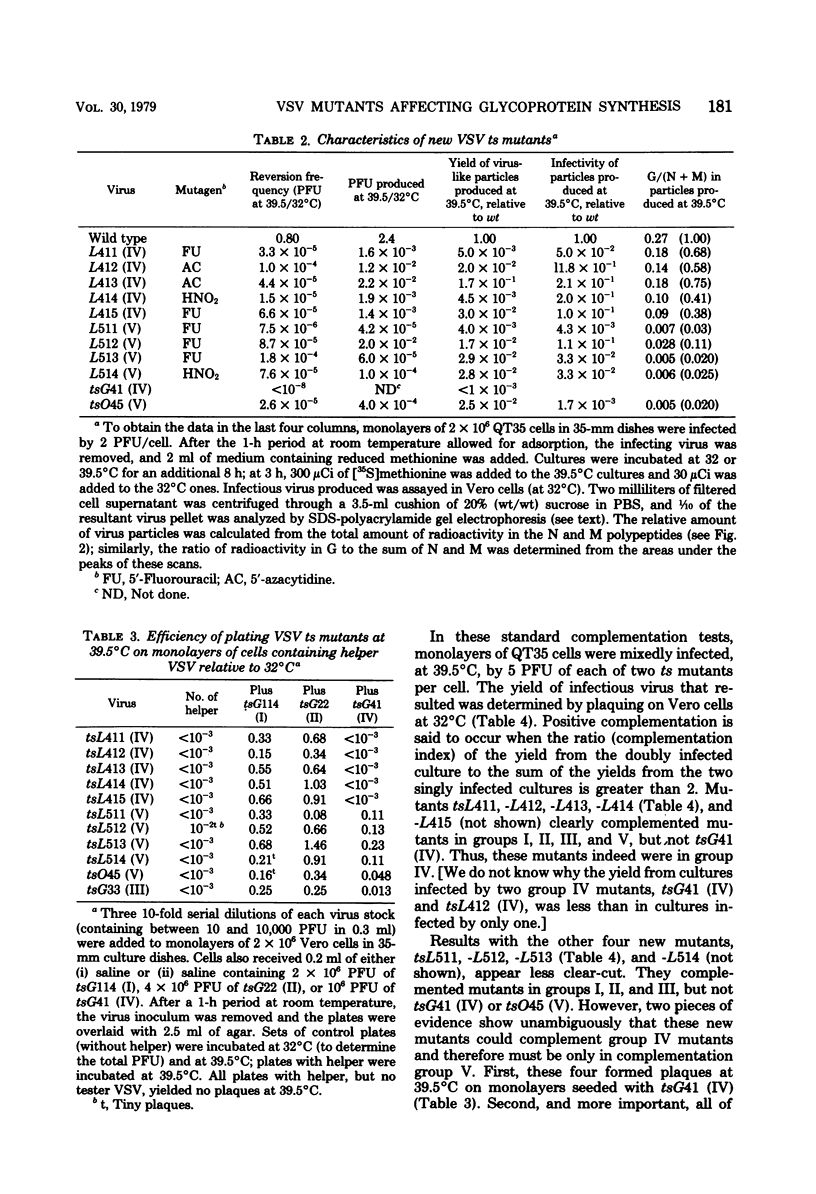
We describe a procedure that enriches for temperature-sensitive (ts) mutants of vesicular stomatitis virus (VSV), Indiana serotype, which are conditionally defective in the biosynthesis of the viral glycoprotein. The selection procedure depends on the rescue of pseudotypes of known ts VSV mutants in complementation group V (corresponding to the viral G protein) by growth at 39.5 degrees C in cells preinfected with the avian retrovirus Rous-associated virus 1 (RAV-1). Seventeen nonleaky ts mutants were isolated from mutagenized stocks of VSV. Eight induced no synthesis of VSV proteins at the nonpermissive temperature and hence were not studied further. Four mutants belonged to complementation group V and resembled other ts (V) mutations in their thermolability, production at 39.5 degrees C of noninfectious particles specifically deficient in VSV G protein, synthesis at 39.5 degrees C of normal levels of viral RNA and protein, and ability to be rescued at 39.5 degrees C by preinfection of cells by avian retroviruses. Five new ts mutants were, unexpectedly, in complementation group IV, the putative structural gene for the viral nucleocapsid (N) protein. At 39.5 degrees C these mutants also induced formation of noninfectious particles relatively deficient in G protein, and production of infectious virus at 39.5 degrees C was also enhanced by preinfection with RAV-1, although not to the same extent as in the case of the group V mutants. We believe that the primary effect of the ts mutation is a reduced synthesis of the nucleocapsid and thus an inhibition of synthesis of all viral proteins; apparently, the accumulation of G protein at the surface is not sufficient to envelope all the viral nucleocapsids, or the mutation in the nucleocapsid prevents proper assembly of G into virions. The selection procedure, based on pseudotype formation with glycoproteins encoded by an unrelated virus, has potential use for the isolation of new glycoprotein mutants of diverse groups of enveloped viruses.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Deutsch V., Brun G. Rescue at nonpermissive temperature of complementation group II temperature-sensitive mutants of vesicular stomatitis virus by uv-irradiated VSV. Virology. 1978 Jun 1;87(1):96–108. doi: 10.1016/0042-6822(78)90162-9. [DOI] [PubMed] [Google Scholar]
- Etchison J. R., Robertson J. S., Summers D. F. Partial structural analysis of the oligosaccharide moieties of the vesicular stomatitis virus glycoprotein by sequential chemical and enzymatic degradation. Virology. 1977 May 15;78(2):375–392. doi: 10.1016/0042-6822(77)90115-5. [DOI] [PubMed] [Google Scholar]
- Flamand A., Bishop D. H. In vivo synthesis of RNA by vesicular stomatitis virus and its mutants. J Mol Biol. 1974 Jul 25;87(1):31–53. doi: 10.1016/0022-2836(74)90558-0. [DOI] [PubMed] [Google Scholar]
- Flamand A. Etude génétique du virus de la stomatite vésiculaire: classement de mutants thermosensibles spontanés en groupes de complémentation. J Gen Virol. 1970 Sep;8(3):187–195. doi: 10.1099/0022-1317-8-3-187. [DOI] [PubMed] [Google Scholar]
- Hecht T. T., Summers D. F. Interactions of vesicular stomatitis virus with murine cell surface antigens. J Virol. 1976 Sep;19(3):833–845. doi: 10.1128/jvi.19.3.833-845.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. S., Baltimore D., Stampfer M. Ribonucleic acid synthesis of vesicular stomatitis virus. 3. Multiple complementary messenger RNA molecules. Virology. 1970 Dec;42(4):946–957. doi: 10.1016/0042-6822(70)90343-0. [DOI] [PubMed] [Google Scholar]
- Katz F. N., Rothman J. E., Knipe D. M., Lodish H. F. Membrane assembly: synthesis and intracellular processing of the vesicular stomatitis viral glycoprotein. J Supramol Struct. 1977;7(3-4):353–370. doi: 10.1002/jss.400070308. [DOI] [PubMed] [Google Scholar]
- Keller P. M., Uzgiris E. E., Cluxton D. H., Lenard J. Aggregation and thermolability of some group V (G protein) and group III (M protein) mutants of vesicular stomatitis virus. Virology. 1978 Jun 1;87(1):66–72. doi: 10.1016/0042-6822(78)90158-7. [DOI] [PubMed] [Google Scholar]
- Knipe D. M., Baltimore D., Lodish H. F. Maturation of viral proteins in cells infected with temperature-sensitive mutants of vesicular stomatitis virus. J Virol. 1977 Mar;21(3):1149–1158. doi: 10.1128/jvi.21.3.1149-1158.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipe D., Lodish H. F., Baltimore D. Analysis of the defects of temperature-sensitive mutants of vesicular stomatitis virus: intracellular degradation of specific viral proteins. J Virol. 1977 Mar;21(3):1140–1148. doi: 10.1128/jvi.21.3.1140-1148.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lafay F. Envelope proteins of vesicular stomatitis virus: effect of temperature-sensitive mutations in complementation groups III and V. J Virol. 1974 Nov;14(5):1220–1228. doi: 10.1128/jvi.14.5.1220-1228.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovici C., Moscovici M. G., Jimenez H., Lai M. M., Hayman M. J., Vogt P. K. Continuous tissue culture cell lines derived from chemically induced tumors of Japanese quail. Cell. 1977 May;11(1):95–103. doi: 10.1016/0092-8674(77)90320-8. [DOI] [PubMed] [Google Scholar]
- Murphy H. M. A new replication-defective variant of the Bryan high-titer strain Rous sarcoma virus. Virology. 1977 Apr;77(2):705–721. doi: 10.1016/0042-6822(77)90493-7. [DOI] [PubMed] [Google Scholar]
- Pringle C. R., Duncan I. B. Preliminary physiological characterization of temperature-sensitive mutants of vesicular stomatitis virus. J Virol. 1971 Jul;8(1):56–61. doi: 10.1128/jvi.8.1.56-61.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle C. R. Genetic characteristics of conditional lethal mutants of vesicular stomatitis virus induced by 5-fluorouracil, 5-azacytidine, and ethyl methane sulfonate. J Virol. 1970 May;5(5):559–567. doi: 10.1128/jvi.5.5.559-567.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Printz P., Wagner R. R. Temperature-sensitive mutants of vesicular stomatitis virus: synthesis of virus-specific proteins. J Virol. 1971 May;7(5):651–662. doi: 10.1128/jvi.7.5.651-662.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson M. A., Etchison J. R., Robertson J. S., Summers D. F., Stanley P. Specific changes in the oligosaccharide moieties of VSV grown in different lectin-resistnat CHO cells. Cell. 1978 Mar;13(3):515–526. doi: 10.1016/0092-8674(78)90325-2. [DOI] [PubMed] [Google Scholar]
- Rothman J. E., Lenard J. Membrane asymmetry. Science. 1977 Feb 25;195(4280):743–753. doi: 10.1126/science.402030. [DOI] [PubMed] [Google Scholar]
- Schlesinger S., Gottlieb C., Feil P., Gelb N., Kornfeld S. Growth of enveloped RNA viruses in a line of chinese hamster ovary cells with deficient N-acetylglucosaminyltransferase activity. J Virol. 1975 Jan;17(1):239–246. doi: 10.1128/jvi.17.1.239-246.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzer T. J., Dickson C., Weiss R. A. Morphological and biochemical characterization of viral particles produced by the tsO45 mutant of vesicular stomatitis virus at restrictive temperature. J Virol. 1979 Jan;29(1):185–195. doi: 10.1128/jvi.29.1.185-195.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger J. T., Reichmann M. E. RNA synthesis in temperature-sensitive mutants of vesicular stomatitis virus. J Virol. 1973 Sep;12(3):570–578. doi: 10.1128/jvi.12.3.570-578.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. A., Boettiger D., Murphy H. M. Pseudotypes of avian sarcoma viruses with the envelope properties of vesicular stomatitis virus. Virology. 1977 Feb;76(2):808–825. doi: 10.1016/0042-6822(77)90261-6. [DOI] [PubMed] [Google Scholar]
- Závada J. Viral pseudotypes and phenotypic mixing. Arch Virol. 1976;50(1-2):1–15. doi: 10.1007/BF01317996. [DOI] [PubMed] [Google Scholar]
- Závada J., Závodská E. Complementation and phenotypic stabilization of vesicular stomatitis virus temperature-sensitive and thermolabile mutants by avian myeloblastosis virus. Intervirology. 1974;2(1):25–32. doi: 10.1159/000149401. [DOI] [PubMed] [Google Scholar]



