Abstract
Background
Microcystic macular edema (MME) of the retinal inner nuclear layer (INL) has recently been identified in multiple sclerosis (MS) patients with optical coherence tomography (OCT). We aimed to determine if MME of the INL, and/or higher thickness of the INL, are associated with disease activity, or disability progression.
Methods
This retrospective study was performed at Johns Hopkins Hospital (between 09/2008 and 03/2012). 164 MS patients and 60 healthy-controls underwent serial OCT scans and clinical evaluation (including visual function). OCT scanning, including automated intra-retinal layer segmentation, yielded thicknesses of the retinal nerve fiber layer, ganglion cell layer (plus inner plexiform layer), INL (plus outer plexiform layer), and outer nuclear layer. MS patients also underwent annual brain MRI scans. Disability scores were compared with the Wilcoxon rank-sum test. Mixed-effects linear regression was used to compare OCT measures and letter-acuity scores. Logistic regression was used to examine the relationships of baseline OCT thicknesses with clinico-radiological parameters.
Findings
Mean follow-up (standard deviation) for MS patients and healthy-controls was 25·8-months (9·1-months) and 22·4-months (11·4-months) respectively. 10 MS patients (6·1% of the cohort) demonstrated MME during at least one study visit, but MME was not visible at baseline in 6 of these patients. MS patients with vs. without MME (151 MS patients) at any time during the study had higher baseline multiple sclerosis severity scores (p=0·032), although expanded disability status scale (EDSS) scores were not significantly different (p=0·097). MS eyes with MME (12 eyes) vs. without MME (302 eyes) had lower letter-acuity scores (100%-contrast: p=0·017; 2·5%-contrast: p=0·031; 1·25%-contrast: p=0·014), and higher INL thicknesses (p=0·003) at baseline. Higher baseline INL thickness in MS predicted the development of contrast-enhancing lesions (p=0·007), new T2 lesions (p=0·015), EDSS progression (p=0·034), and relapses (in relapsing-remitting MS; p=0·008) during the study. MME was not associated with disease activity during follow-up. Healthy-controls did not demonstrate MME.
Interpretation
Increased INL thickness on OCT, potentially representing inflammation of the unmyelinated retina, is associated with disease activity in MS. If this finding is confirmed, INL thickness may be a useful predictor of disease progression in MS.
Funding
National Multiple Sclerosis Society (TR3760-A-3, RG4212-A-4), National Eye Institute (R01-EY014993, R01-EY019473), Braxton Debbie Angela Dillon and Skip Donor Fund.
Keywords: Multiple Sclerosis, Retinalpathology, Inner nuclear layer, Microcystic macular edema
Introduction
Multiple sclerosis (MS) is regarded as an immune-mediated inflammatory demyelinating disorder of the central nervous system, in which neurodegeneration is a secondary phenomenon.1 Gray matter degeneration is however common in MS and more closely linked with disability than white matter degeneration.2,3 It remains unclear if gray matter degeneration in MS only occurs as a result of white matter injury.4 Recently, it has been proposed that neuronal loss in the retina may occur as a primary process in MS, independent of demyelination or axonal injury.5,6
The retina, although unmyelinated, is a frequent site of inflammation, blood-retinal-barrier disruption, and neuronal loss in MS. Retinal perivascular inflammation (periphlebitis), indicating blood-retinal-barrier disruption, occurs in up to 20% of MS patients.7 Active retinal periphlebitis tends to occur simultaneously with blood-brain-barrier disruption in MS,8 and may be a risk factor for relapses and gadolinium-enhancing lesions.9 Intermediate uveitis, particularly parsplanitis, also occurs in up to 16% of MS patients.10 Consistent with clinical observations, post-mortem analyses reveal retinal inflammation with activated microglia in MS eyes.11 Collectively, these findings suggest myelin may not be necessary for maintaining or propagating inflammation in MS.
Spectral-domain optical coherence tomography (OCT) renders images with outstanding resolution (<5μm),12 from which the individual retinal layers can be demarcated, qualitatively assessed, and objectively and precisely quantified.5 These layers include the retinal nerve fiber layer (RNFL), ganglion cell layer (GCL), inner nuclear layer (INL), and outer nuclear layer (ONL). The RNFL is the innermost layer of the retina and primarily consists of the axons of ganglion cell neurons, which are located below the RNFL. These axons coalesce at the optic discs to form the optic nerves, and exit the eye through the lamina cribrosa, where they acquire myelin.6
MS affects the optic nerves both clinically (from optic neuritis; ON) and subclinically, resulting in retrograde degeneration of the axons of the optic nerve, culminating in RNFL and GCL atrophy7,11,13 that may be detected and quantified in-vivo with OCT.6,14 In addition, deeper retinal layer pathology has been shown to occur in MS, although not in all studies.15 Consistent with post-mortem demonstration of INL atrophy in 40% of MS eyes,11 and the electroretinographic identification of INL and ONL dysfunction,16,17 OCT studies utilizing macular segmentation demonstrate lower INL or ONL thicknesses in MS eyes, with and without a history of ON. These have been termed mixed retinal pathology phenotype and macular thinning predominant phenotype, respectively.5,6 These findings may represent primary retinal neuronal mechanisms of pathology, since atrophy of the INL or ONL has not been demonstrated following optic nerve transection in animals.18 The presence of this deeper retinal neuronal layer pathology in MS may be associated with greater disability.5
A recent study utilizing OCT identified microcystic macular edema (MME) of the INL in approximately 5% of MS patients.19 MME was thought to represent breakdown of the blood-retinal-barrier and retinal inflammation, potentially due to subclinical uveitis or retinitis, and was associated with greater disability and visual dysfunction. However, longitudinal data was only available for 6-patients, and the INL was not quantitatively assessed with OCT-segmentation. Therefore, the prevalence of MME of the INL, and its evolution over time in MS remains unclear. Since MME of the INL may be inflammatory, we postulate that some MS patients may harbor INL inflammation in the absence of visible MME, resulting in increased thickness of the INL. Indeed, a recent cross-sectional study found that higher INL thickness (but not thicknesses of other retinal layers) correlated with higher T2-FLAIR lesion volume in MS.20 The clinical relevance of MME of the INL and/or higher INL thickness(which may both represent myelin-independent neuronal compartment inflammation)remains to be elucidated.
The primary objectives of this retrospective study were:
To confirm the occurrence of MME of the INL in MS, ascertain its prevalence and evolution of over time, and evaluate whether MME occurs in healthy controls (in order to determine whether MME in MS represents a pathological process, or a normal phenomenon).
To determine if MME in MS is associated with disease activity and disability, both at baseline and during follow-up.
To determine if higher thickness of the INL at baseline in MS is associated with disease activity, or disability progression, during follow-up.
Methods
Patients
Johns Hopkins University Institutional Review Board approval was acquired and written informed consent was obtained from all participants prior to study enrollment. Two longitudinal cohorts were recruited by unselected convenience sampling from one center (Johns Hopkins Hospital): (1) An MS cohort (without acute ON or evidence of optic disc swelling on fundoscopy within 3-months of baseline assessment; approximately 40% of this cohort have been included in other cross-sectional studies performed by our group)and (2) A healthy control (HC) cohort. Study participants were recruited between 09/2008 and 12/2010, and studied between 09/2008 and 03/2012.MS patients were enrolled from the Johns Hopkins MS Center. MS diagnosis was confirmed by the treating neurologist (PAC) based on the 2010 revised McDonald criteria.21 MS patients underwent clinical evaluation and OCT every 6-months and brain magnetic resonance imaging (MRI) annually.
Patients who developed acute ON during study follow-up (which may confound OCT measures due to edema), and patients with diabetes, uncontrolled hypertension, glaucoma, refractive errors of +/− 6-diopters, or other ophthalmologic or neurologic disorders were excluded from the study. HCs without known ocular disease, refractive errors of +/− 6-diopters, or neurologic disease were recruited from amongst Johns Hopkins University staff, and invited for annual OCT scans. Johns Hopkins University staff members live in a similar geographic area as our patient population, and were chosen to have a similar range of ages and gender ratio as our cohort, as well as their willingness to return for repeat OCT scanning.
Optical coherence tomography
Retinal imaging was performed with spectral-domain Cirrus HD-OCT (model 4000, software version 5·0; Carl Zeiss Meditec, Dublin, California), as described in detail elsewhere.5,6 Briefly, peri-papillary and macular scans were obtained with the Optic Disc Cube 200×200 and Macular Cube 512×128 protocols respectively. Scans with signal strength less than 7/10 or with artifact were excluded from analyses.
Macular cube scans were analyzed in a blinded fashion utilizing segmentation software, as described in detail elsewhere.6 Briefly, segmentation performed in 3-D yields the thicknesses of the following macular layers – the RNFL, GCL+ inner plexiform layer (GCIP), INL (including the outer plexiform layer), and ONL (including inner and outer photoreceptor segments). This segmentation protocol has been shown to be reproducible in MS and HCs (inter-raterintra-class correlation coefficients: 0·91–0·99 for all segmentation measurements; 0.94 and 0.91 for INL (including the outer plexiform layer) thicknesses in MS and HCs respectively).5
All acquired macular cube scans were qualitatively assessed for MME or other retinal abnormalities by two reviewers blinded to clinical status (SS & ESS). MME was defined as cystic, lacunar areas of hypo-reflectivity with clear boundaries, evident on at least two contiguous B-scans, or visible in a comparable region on at least two separate acquisitions. Scans designated as fulfilling MME criteria, or demonstrating other retinal abnormalities by either reviewer were reviewed and verified by a retinal specialist, blinded to clinical status (QDN).
Clinical data
MS disease subtype was classified as relapsing-remitting (RRMS), secondary-progressive (SPMS), or primary-progressive MS (PPMS). Disease duration, co-morbidities, and history of ON, including the date and side of occurrence were recorded. Expanded Disability Status Scale (EDSS) scores were determined by a Neurostatus-certified EDSS examiner at study visits (within 30-days of OCT and/or MRI examinations). Baseline disease duration and EDSS scores were used to determine subjects’ baseline MS Severity Scale (MSSS) scores. Disability progression was defined as a ≥1-point or a ≥0·5-point increase in EDSS score from baseline to final end-of-study EDSS exam, if the baseline EDSS score was <6 or ≥6 respectively. Ocular (ON) and non-ocular relapses during study follow-up were verified and recorded.
Visual function
Standardized 100% high-contrast, 2·5% low-contrast, and 1·25% low-contrast letter acuity scores were determined at study visits with retro-illuminated eye charts, as described in detail elsewhere.6,14 High-contrast and low-contrast (2·5% and 1·25%) visual loss were defined as a decrease of ≥5-letters or ≥7-letters respectively during follow-up, in accordance with published data.14
Magnetic Resonance Imaging
Brain MRI was performed with a 3-tesla Philips Intera scanner (Philips Medical System, Best, The Netherlands). The following axial, whole-brain sequences were acquired: double-echo fast-spin-echo to obtain T2-weighted scans (acquired resolution: 1·1×1·1×2·2 mm; TE: 12 ms and 80 ms; TR: 4169 ms; SENSE factor: 2; repetitions: 1) and a T1 weighted gadolinium enhanced scan (acquired resolution: 0·9×0·9×3·0; TE: 10 ms; TR: 0·5 s). The same scanner and sequence protocols were used at each study visit. Contrast-enhancing lesions (both at baseline, as well as during follow-up) and new T2-hyperintense lesions (defined as the development of one or more new T2-hyperintense lesions during follow-up, not evident on baseline imaging) were recorded by a reviewer blinded to both clinical and OCT status.
Statistical methods
Statistical analyses were performed with Stata11 (StataCorp, College Station, TX). The Shapiro-Wilk test was used to assess normality of distributions. Comparisons between groups were performed with the Wilcoxon rank-sum test (for disease duration, EDSS, and MSSS; these variables were not normally distributed), student’s t-test (for age; normally distributed), and χ2 test (for ON history, sex, immunomodulatory treatment, clinico-radiological disease activity, and MS subtype proportions). Mixed-effects linear regression, accounting for within-subject inter-eye correlations, was used to compare OCT measures and letter-acuity scores between groups. Logistic regression with robust standard error, accounting for within-subject inter-eye correlations, was used to examine the relationships of baseline INL thicknesses with clinico-radiological parameters. Multivariate models were adjusted for age and sex in comparisons of MS patients with healthy controls, and also disease duration and ON history in analyses between MS subgroups. Levene’s variance ratio testing was used to assess differences in INL thickness variance by disease duration. Statistical significance was defined as p<0·05.
Role of the funding source
The sponsors of this study had no role in conceptualization of the study, study design, acquisition of the data, analysis of the data, interpretation of the data, drafting and revising of the manuscript, or critical revision of the manuscript for important intellectual content. The corresponding authors had full access to all of the data and had final responsibility for the decision to submit for publication.
Results
Study Population
164 MS patients (123 RRMS, 25 SPMS, 16 PPMS) and 60 HCs participated in the study (Figure 1, Table 1). Mean follow-up durations of the MS and HC cohorts were 25·8±9·1-monthsand 22·4±11·4-months respectively.
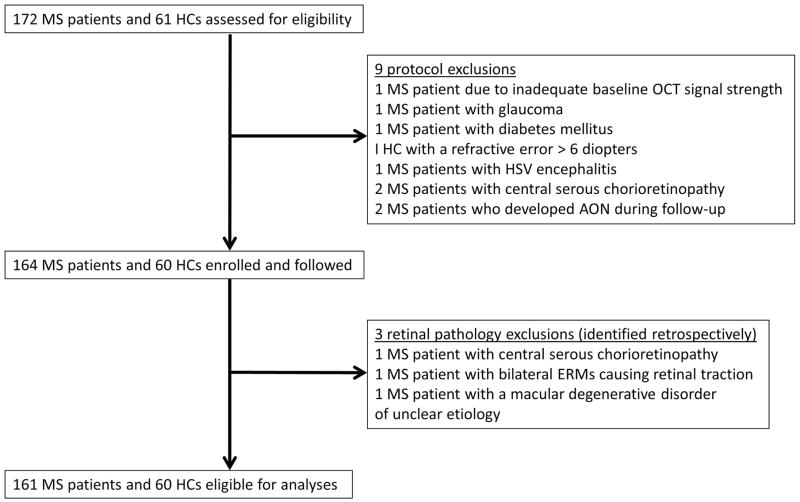
Figure 1. Study profile.
MS: multiple sclerosis, AON: acute optic neuritis, HCs: healthy controls, OCT: optical coherence tomography, HSV: herpes simplex encephalitis, ERM: epiretinal membrane
Table 1.
Demographics and Clinico-radiological Characteristics
| RRMS | SPMS | PPMS | HCs | |
|---|---|---|---|---|
|
| ||||
| Baseline Demographics | ||||
| n (eyes) | 123 (246) | 25 (50) | 16 (32) | 60 (120) |
| Age (SD) | 39·6 (10·7) | 55·9 (5·5) | 55·7 (6·8) | 36·8 (9·6) |
| Female, n (%) | 92 (75%) | 17 (68%) | 9 (56%) | 39 (65%) |
| Follow-up time in months (SD) | 25·2 (9·5) | 28·5 (6·9) | 26·4 (7·8) | 22·4 (11·4) |
|
| ||||
| Baseline Clinico-Radiological Characteristics | ||||
| ON eyes (%) | 83 (33·7%) | 11 (22%) | 0 (0%) | |
| EDSS, median (range) | 2·0 (0–6·5) | 6·0 (2·5–8.0) | 6·0 (2·5–6·5) | |
| Disease Duration in years (SD) | 8·2 (6·7) | 21·3 (8·1) | 11·9 (8·9) | |
| MSSS, median (range) | 3·34 (0.13–9·47) | 5·16 (1·69–9.2) | 6·74 (1·28–9·08) | |
| Baseline Gd-enhancing lesions1 (%) | 14 (18%) | 0 (0%) | 0 (0%) | |
|
| ||||
| Longitudinal Clinico-Radiological Characteristics | ||||
| Non-ocular relapse, n (%) | 35 (28%) | 0 (0%) | 0 (0%) | |
| EDSS progression2,3, n (%) | 38 (32%) | 4 (16%) | 2 (13%) | |
| New Gd-enhancing lesion4, n (%) | 24 (20%) | 1 (4%) | 2 (14%) | |
| New T2 lesion4, n (%) | 46 (38%) | 3 (13%) | 1 (7%) | |
| Relapse or New Gd-enhancing lesion3, n (%) | 47 (39%) | 1 (4%) | 2 (14%) | |
Data presented above represent means, with their corresponding standard deviation (SD), unless otherwise noted.
RRMS: relapsing remitting multiple sclerosis; SPMS: secondary progressive multiple sclerosis; PPMS: primary progressive multiple sclerosis; HCs: healthy controls; ON: optic neuritis; EDSS: expanded disability status scale; MSSS: multiple sclerosis severity score; Gd: gadolinium
Available for 76 RRMS, 19 SPMS and 13 PPMS patients
EDSS progression defined as a ≥ 1-point increase if EDSS < 6·0 and a ≥ 0·5 point increase if EDSS ≥ 6·0
Available for 120 RRMS, 25 SPMS and 15 PPMS patients
Available for 121 RRMS, 24 SPMS and 14 PPMS patients
MME occurs in a subset of MS patients
MME was identified in ten of the 164 MS patients during the study (6·1%; four of these ten patients had MME at baseline), and was bilateral in two patients (Figure 2). In one of these bilateral MME patients, bilateral epiretinal membranes (ERMs), without associated retinal traction, were also present (ERMs with retinal traction may produce appearances similar to MME on OCT images, while ERMs without retinal traction do not). ERMs, again without associated retinal traction, were identified in an additional four MS patients (five eyes) without MME, including one patient with a prior history of uveitis ipsilateral to the ERM (no other patients in the study had a history of symptomatic uveitis). Three patients were determined in retrospect to have non-microcystic retinal pathology on OCT, including bilateral ERMs causing significant retinal traction, central serous chorioretinopathy, and a macular degenerative disorder of unclear etiology, and were excluded from subsequent analyses. HC eyes did not demonstrate any qualitative OCT abnormalities, including MME, during the study.
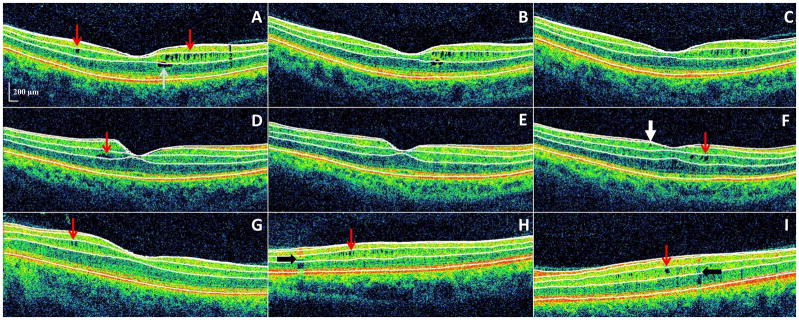
Figure 2. Microcystic macular edema (MME) of the inner nuclear layer (INL) as identified by spectral-domain optical coherence tomography, with automated segmentation lines displayed.
Panels A–C: All images were acquired from the same patient during a 3-year period of observation, and are presented in chronological order in consecutive panels. Panel A: MME of the INL (red arrows) was present at baseline, as well as a foveal cyst of the outer nuclear layer (ONL). Panels B–C: The ONL cyst progressively resolved during follow-up.
Panels D–F: All images were acquired from the same patient during a 2-year period of observation, and are presented in chronological order in consecutive panels. Panel D: A single INL cyst (red arrow) was present at baseline. Panel E: The cyst spontaneously resolved after one year. Panel F: Following fingolimod treatment (initiated after scan E) the patient developed new cystic changes of the INL (red arrow). An epiretinal membrane is noted (white arrow) that had been present on previous scans as well (not illustrated though in either panel D or E).
Panels G–I: Three different patients with MME of the INL (red arrows) are presented in each panel. Vessel artifacts (black arrows) are demonstrated for comparison.
OCT-segmentation performed in 3D identifies the inner limiting membrane, the outer boundaries of the RNFL, inner plexiform layer, and outer plexiform layer, and the inner boundary of the retinal pigment epithelium. The identification of these retinal boundaries enables the determination of the thicknesses of the following retinal layers – the macular RNFL, GCL+innerplexiform layer (GCIP; labeled 1 in panel A), INL (including the outer plexiform layer; labeled 2 in panel A), and ONL (including the inner and outer photoreceptor segments; labeled 3 in panel A).
Only one patient demonstrating MME was exposed to fingolimod (a known cause of macular edema) during the study, although in this patient (identified in retrospect) bilateral MME was already evident on OCT, prior to the exposure (Figure 2D). Fingolimod treatment was discontinued after 2·5-months due to the development of bilateral visual blurring. Extensive retinal evaluation revealed bilateral perivascular sheathing and diffuse bilateral fluoresce in leakage on fluorescein angiography. These findings continue to persist1-year following visible resolution of the MME, and despite the short exposure to fingolimod. A complete list of disease modifying therapies patients were receiving at baseline in the MS cohort (including those with and without MME) can be found in eTable 1. Detailed ophthalmologic examination (including fluorescein angiography) in two additional patients with MME was normal.
In general, the distribution of MME was patchy, and predominantly localized to the INL, although in three eyes microcysts were additionally present in the ONL. MME was dynamic over time; five eyes exhibited fluctuating MME (i.e. improving and then worsening, or vice versa), four eyes worsening MME, and three eyes improving MME (Figure 2).
Characteristics of patients and eyes demonstrating MME
Of the ten patients demonstrating MME at any time during the study, only four patients had visible MME at baseline (40%), and in five of the remaining six patients without initially visible MME at baseline, the MME did not become visible for over 1-year of follow-up (50%). Despite this, all patients that exhibited MME (regardless of the stage in the study at which the MME first became evident) had significantly higher baseline (beginning of the study) MSSS scores compared to patients not demonstrating MME at any stage in the study(p=0·032; Table 2). Similarly, eyes demonstrating MME at any time during the study (regardless of the stage in the study at which the MME first became evident) had significantly lower high and low-contrast letter-acuity scores (100%:p=0·017; 2·5%:p=0·031; 1·25%:p=0·014), lower GCIP thickness (p=0·011), and higher INL thickness (p=0·003) at baseline relative to MS eyes not demonstrating MME at any stage in the study. These findings remained significant for all measures except 2·5% letter-acuity when adjusting for age, sex, disease duration and ON history (Table 3). Moreover, those eyes in which MME developed during follow-up (n=6) i.e. was not visible at baseline, still trended towards having higher INL thicknesses compared to non-MME MS eyes at baseline (mean difference=2·5μm; 95% CI: 0·0003–4·9μm; p=0·050). Figure 1 and Figure 3 depict the retinal boundary lines as identified by the automated segmentation technique utilized in this study in the presence of MME pathology.
Table 2.
Comparison of baseline demographics and clinical characteristics between MS patients demonstrating MME and not demonstrating MME during the study
| MS Patients that demonstrated MME during the study (n=10) | MS patients that did not demonstrate MME during the study (n=151) | P-value | |
|---|---|---|---|
|
| |||
| Age (SD) | 43·4 (11·5) | 43·7 (12·0) | 0·92 |
| Female (%) | 5 (50%) | 111 (74%) | 0·11 |
| Disease Duration (SD) | 8·5 (5·0) | 10·8 (8·7) | 0·60 |
| EDSS, median (range) | 5·25 (1·0–6·5) | 2·5 (0–8·0) | 0·097 |
| MSSS, median (range) | 5.93 (2·44–8·91) | 3·81 (0·13–9·47) | 0·032 |
| MS Subtypes | |||
| RRMS, n (%) | 7 (70%) | 114 (75%) | |
| SPMS, n (%) | 1 (10%) | 24 (16%) | 0·46 |
| PPMS, n (%) | 2 (20%) | 13 (9%) | |
Data presented above represent means, with their corresponding standard deviation (SD), unless otherwise noted.
MME: microcystic macular edema; MS: multiple sclerosis; EDSS: expanded disability status scale; MSSS: multiple sclerosis severity score; RRMS: relapsing remitting multiple sclerosis; SPMS: secondary progressive multiple sclerosis; PPMS: primary progressive multiple sclerosis; EDSS: expanded disability status scale; MSSS: multiple sclerosis severity score;
Table 3.
Comparisons of baseline OCT and visual function measures
| MS eyes demonstrating MME (n=12) | MS eyes that did not demonstrate MME (n=3021) | All MS eyes (n=322) | Healthy Control eyes (n=120) | MS MME vs MS non-MME eyes
|
All MS vs HCs2,4 p-value | ||
|---|---|---|---|---|---|---|---|
| Univariate Model2 p-value | Multivariate Model2,3 p-value | ||||||
|
| |||||||
| Visual function measures | |||||||
| 100% LA (SD) | 47·7 (20·8) | 58·3 (10·7) | 58·0 (11·5) | 61·8 (6·3) | 0.017 | 0.028 | 0·037 |
| 2·5% LA (SD) | 17·0 (16·2) | 27·1 (12·4) | 26·9 (12·7) | 33·9 (8·1) | 0.031 | 0·095 | 0·0001 |
| 1·25% LA (SD) | 4·2 (5·8) | 13·4 (11·5) | 13·1 (11·5) | 20·8(9·1) | 0.014 | 0·042 | <0·0001 |
|
| |||||||
| OCT measures (μm) | |||||||
| p-RNFL (SD) | 78·6 (12·4) | 84·9 (12·4) | 84·8 (12·4) | 92·5 (10·3) | 0·11 | 0·22 | <0·0001 |
| GCIP (SD) | 62·4 (11·9) | 71·8 (9·8) | 71·5 (10·0) | 81·3 (6·5) | 0.011 | 0.032 | <0·0001 |
| INL (SD) | 69·5 (7·7) | 65·3 (4·6) | 65·4 (4·7) | 64·2 (4·3) | 0·003 | 0·005 | 0·074* |
| ONL (SD) | 120·8 (13·0) | 119·1 (7·5) | 119·2 (7·7) | 120·8 (7·1) | 0.22 | 0·43 | 0·083** |
Data presented above represent means, with their corresponding standard deviation (SD), unless otherwise noted.
MME: microcystic macular edema; LA: letter-acuity; HCs: healthy controls; p-RNFL: peripapillary retinal nerve fiber layer; GCIP: ganglion cell layer + inner plexiform layer; INL: inner nuclear layer; ONL: outer nuclear layer;
Excluding fellow eyes of MME eyes
Adjusted for within-subject inter eye correlation
Additionally adjusted for age, sex, disease duration and history of optic neuritis
Additionally adjusted for age and sex
In RRMS: p=0·038
When excluding MME eyes: p=0·054
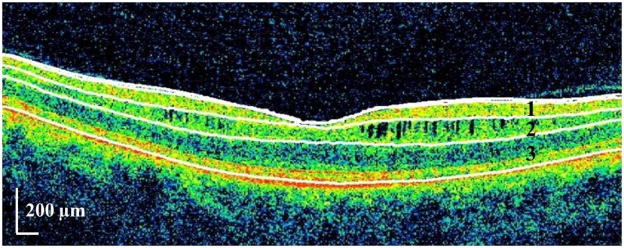
Figure 3. Illustration of the retinal layer boundaries identified by automated OCT segmentation in an eye exhibiting microcystic macular edema.
OCT-segmentation performed in 3D identifies the inner limiting membrane, the outer boundaries of the RNFL, inner plexiform layer, and outer plexiform layer, and the inner boundary of the retinal pigment epithelium. The identification of these retinal boundaries enables the determination of the thicknesses of the following retinal layers – the macular RNFL, GCL+innerplexiform layer (GCIP; labeled 1), INL (including the outer plexiform layer; labeled 2), and ONL (including the inner and outer photoreceptor segments; labeled 3).
ON history was present in a higher proportion of MS eyes demonstrating MME at any time in the study (n=6), as compared to eyes not demonstrating MME during the study (n=87) (50% vs. 28%; p=0·10). The mean time from ON to MME identification in MS was 7·6±5·7-years (range: 1·6–17·6 years). Interestingly, MS eyes with a history of ON had significantly higher INL thicknesses than eyes without ON history (mean difference=2·1μm; 95% CI: 1·3–2·9 μm;p<0·0001). After excluding eyes that developed MME, and adjusting for age, sex and disease duration, this difference remained significant. Only 1 out of the 10 MME patients had been exposed to steroid therapy in the 30-day period prior to the identification of MME.
Baseline INL thickness predictsclinico-radiological disease activity and disability progression in MS
Baseline INL thickness was significantly higher in RRMS (p=0·038), and trended towards being higher across the entire MS cohort, relative to HCs (p=0·074)(Table 3).
Higher baseline INL thickness was associated with a significantly increased risk of developing new gadolinium-enhancing lesions (p=0·007), new T2-lesions (p=0·015), and disability progression (p=0·034) during follow-up. In RRMS (since relapses only occurred in this subtype), increased INL thickness at baseline was additionally associated with a significantly increased risk of developing relapses during follow-up (p=0·008) (Figure 4, Table 4, eFigure 1, eFigure 2).
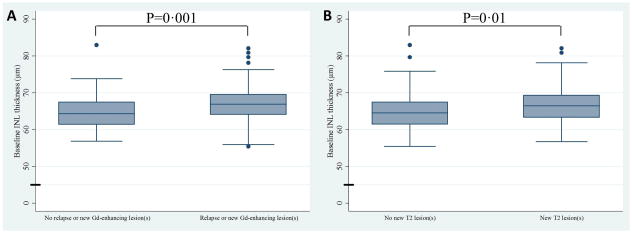
Figure 4. Box and whisker plots of baseline INL thickness.
Panel A illustrates that RRMS patients that developed relapses or new Gd-enhancing lesions during study follow-up (n=47 subjects [94 eyes])had higher baseline INL thicknesses, as compared to patients that did not develop relapses or new Gd-enhancing lesions (n=73 subjects [146 eyes]). Panel Billustrates that MS patients exhibiting new T2 lesions during follow-up (n=49 subjects [98 eyes]), as compared to patients that did not (n=109 subjects [218 eyes]), had higher baseline INL thicknesses. P-values were calculated utilizing mixed effects linear regression accounting for within subject inter-eye correlations.
The top and bottom of the box represent the 25th and 75th percentiles respectively. The line in the center of the box represents the median (50th percentile). The ends of the whiskers represent the largest value within the 75th percentile + 1·5 * inter-quartile range (IQR) and the smallest value within the 25th percentile – 1·5 * IQR. Values outside of the ends of the whiskers are represented with dots.
Table 4.
Prediction of clinico-radiological disease activity & disability progression with baseline INL thicknesses in MS
| Univariate Model1 | Multivariate Model1,2 | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Odds ratio per 5μm increase in INL thickness in RRMS (95%CI) | p-value | Odds ratio per 5μm increase in INL thickness in all MS (95%CI) | p-value | Odds ratio per 5μm increase in INL thickness in RRMS (95%CI) | p-value | Odds ratio per 5μm increase in INL thickness in all MS (95%CI) | p-value | |
| Non-ocular relapse | 1·76 (1·16–2·67) | 0·008 | 1·77 (1·14–2·74) | 0·01 | ||||
| EDSS progression3,4 | 1·48 (1·02–2·15) | 0.039 | 1·41 (1·03–1·95) | 0·034 | 1·49 (1·01–2·21) | 0·047 | 1·40 (1·001–1·94) | 0·049 |
| New Gd-enhancing lesion5 | 1·90 (1·24–2·90) | 0·003 | 1·70 (1·16–2·50) | 0·007 | 1·98 (1·29–3·03) | 0·002 | 1·71 (1·16–2·52) | 0·007 |
| New T2 lesion5 | 1·59 (1·08–2·34) | 0·020 | 1·51 (1·08–2·09) | 0·015 | 1·56 (1·03–2·37) | 0·035 | 1·46 (1·05–2·02) | 0·025 |
| Relapse or New Gd-enhancing lesion5 | 1·92 (1·27–2·90) | 0·002 | 1·95 (1·27–2·99) | 0·002 | ||||
INL: inner nuclear layer; RRMS: relapsing remitting multiple sclerosis; EDSS: expanded disability status scale; CI: confidence intervals;
Adjusted for within-subject inter eye correlation
Additionally adjusted for age, sex, disease duration and history of optic neuritis
EDSS progression defined as a ≥1-point increase if baseline EDSS < 6·0 and a ≥0·5 point increase if baseline EDSS ≥ 6·0
Available for 118 RRMS, 25 SPMS and 14 PPMS patients
Available for 120 RRMS, 24 SPMS and 14 PPMS patients
Adjusting for characteristics known to be associated with disease course and activity (including age, sex, disease duration and ON history), baseline INL thickness remained independently predictive of clinico-radiological disease activity and disability progression, both across the cohort and in RRMS (Table 4). Other retinal layer thicknesses and development of MME were not associated with significant inflammatory disease activity (Table 5), except GCIP thickness which was predictive of relapses in RRMS (p=0·016). Although baseline INL thickness was not predictive of clinically significant visual loss, a greater proportion of MME eyes exhibited high-contrast letter-acuity loss during the study compared to non-MME eyes (p=0·026).
Table 5.
Comparison of clinico-radiological characteristics between MS patients demonstrating MME and not demonstrating MME during the study
| MS Patients that demonstrated MME during the study (n=10) | MS patients that did not demonstrate MME during the study (n=151) | P-value | |
|---|---|---|---|
|
| |||
| Clinico-radiological characteristics | |||
| Baseline Gd-enhancing lesion1 | 2 (25%) | 12 (12%) | 0.29 |
| EDSS progression2,3, n (%) | 2 (20%) | 41 (28%) | 0.59 |
| New Gd-enhancing lesion4, n (%) | 2 (20%) | 24 (16%) | 0.76 |
| New T2 lesion4, n (%) | 4 (40%) | 45 (30%) | 0.53 |
| Non-ocular relapse5, n (%) | 3 (43%) | 32 (28%) | 0.41 |
| Relapse or New Gd-enhancing lesion5, n (%) | 4 (57%) | 45 (39%) | 0.30 |
Available for 8 MME and 100 non-MME patients
EDSS progression defined as a ≥ 1-point increase if EDSS < 6·0 and a ≥ 0·5 point increase if EDSS ≥ 6·0
Available for 10 MME patients and 147 non-MME patients
Available for 10 MME patients and 148 non-MME patients
Only patients with relapsing-remitting multiple sclerosis (7 MME patients and 114 non-MME) were included in these analyses
Scatter plots of baseline INL thicknesses in MS with disease duration and age (eFigure 3) were examined to determine whether there are differences in INL thickness later vs. early in the MS disease course, which are not related to normal aging. A relationship between age and INL thickness was not observed, but a trend for greater inter-subject variation of INL thicknesses early in the disease course was visible and Levene’s variance ratio testing revealed that INL thicknesses in MS patients with <20-years vs. ≥20-years disease duration had significantly greater variance (p=0.009).
Discussion
Results of this study confirm the occurrence of MME (predominantly of the INL) in MS, that MME is dynamicover time, and highlight MME as a pathologic process which does not seem to occur in HCs.19 Although only 40% of MS patients demonstrating MME at any stage during the study exhibited visible MME at baseline, baseline INL thickness was higher in these patients than MS patients not demonstrating MME during the study. This highlights the potential for earlier and broader identification of this INL process with OCT-segmentation, even when MME has not become visible. INL thickness in MS, particularly RRMS, was also higher than in HCs, suggesting similar INL processes occur in MS patients without visible MME. Furthermore, baseline analyses revealed greater disability and visual dysfunction in MS patients that exhibited MME (regardless of the stage in the study at which the MME became evident), compared to those that did not demonstrate MME during the study, indicating a potential phenotype effect. A similar phenotype may be present in MS patients without demonstrable MME, such as those with higher INL thickness, for the reasons outlined above. Indeed, analyses utilizing INL thickness to broaden identification of this potential retinal process revealed higher INL thickness at baseline independently predicted the development of relapses, new gadolinium-enhancing lesions, new T2 lesions, and disability progression.
Although the pathobiology underlying MME or higher INL thickness in MS remains unclear, aninflammatory etiology seems plausible, perhaps related to subclinical uveitis or retinal periphlebitis (which may be confused with optic neuropathy, particularly in the absence of comprehensive ophthalmologic evaluation). The plexiform layers surrounding the INL contain the primary networks of retinalmicroglia,23 and act as diffusion barriers, making the INL susceptible to fluid accumulation during inflammation. Consistent with an inflammatory etiology, higher INL thickness at baseline in MS predicted inflammatory disease activity. INL swelling also appeared most marked in RRMS, and earlier in the disease, during which MS tends to be more active. Conversely, INL neuronal degeneration and loss may predominate later in the disease course,5,6,11 although a longer study is likely required to demonstrate this.
Another observation that may be relevant for our findings is that approximately 50% of MS patients (and no healthy controls) have been found to harbor anti-KIR4.1 antibodies.24 KIR4.1 is expressed on Müller glia, located in the INL, and is thought to play an important role in regulation of water fluxes in the retina. Additionally, dysregulated KIR4.1 mediated potassium conductance in Müller cells is implicated in impaired water transport of these cells and formation of macular edema in several ocular disorders.25 Accordingly, a potential relationship between MME and anti-KIR4.1 antibodies in MS warrants further exploration.
Approximately 25% of eyes with macular edema that also demonstrate diffuse fluoresce in leakage exhibit INL microcysts on OCT,26 further supporting an inflammatory etiology. Indeed, fluorescein angiography revealed bilateral leakage in one of the patients with bilateral MME in this study, which persisted for over 1-year following visible resolution of the MME, suggesting MME may not be visible in the face of ongoing inflammation, highlighting the potential utility of measuring INL thicknesses. Additionally, this patient had bilateral venous sheathing due to retinal periphlebitis.27 Although this patient was exposed to fingolimod (which may cause macular edema),28 this was thought to be non-contributory since the exposure was short (2.5-months), and the MME developed prior to the exposure.
During acute ON, the blood-retinal-barrier may be susceptible to breakdown, as evidenced by fluorescein leakage and features of uveitis in approximately 25 % of eyes during acute ON.29 This may explain the tendency for higher INL thicknesses and MME to occur in eyes with a history of ON.19 Alternatively, INL aberrations may represent sequelae of retrograde transynaptic degeneration (instigated by demyelination in the myelinated portions of the optic nerve).30 Long observational studies may be required to demonstrate such retrograde transynaptic degeneration. Careful evaluation for MME in other optic neuropathies will be important to help further evaluate for retrograde transynaptic processes. It is also worth considering that MME/increased thickness of the INL in MS may not be related to the MS disease process, but rather may be due to concomitant pathology (such as hypertension and diabetes mellitus) or a potential medication that may be independently associated with increased MS disease activity and the development of macular edema,.31 This seems unlikely however since MS patients with known diabetes or uncontrolled hypertension were excluded, and no such medications were identified in this study. Alternatively, MME/increased thickness of the INL may be due to an MS-related auto-immune disorder targeting the retina.
This study has several limitations. Although detailed ophthalmological assessment, including fluorescein angiography, was performed in a subset of MME patients, these were not performed systematically, as many of the cases were identified retrospectively. Systematic longitudinal ophthalmological assessment is necessary in future studies to confirm an inflammatory vs. non-inflammatory etiology of identified INL aberrations in MS. Since the majority of patients had RRMS, more accurate characterization of this novel potential retinal phenotype by MS subtype is warranted, which will require the enrollment of greater numbers of RRMS, SPMS, and PPMS patients, and also HCs. Although HCs in this study had a similar range of ages as RRMS patients, the enrollment of older HCs age-matched to SPMS and PPMS, as well as RRMS patients, is necessary in future studies. Since HCs underwent OCT annually, and MS patients underwent OCT 6-monthly, this may have impeded detection of MME in HCs. In future studies HCs should undergo OCT as frequently as MS patients.
Longitudinal acute ON studies are warranted to characterize the relationship between ON and MME/higher INL thickness. Since we did not have a validation cohort, our findings need to be recapitulated in other MS cohorts, as well as using other OCT devices and OCT-segmentation techniques. The retinal segmentation technique utilized has not yet been extended to separate the INL from the neighboring thin outer plexiform layer. Therefore, the combined thickness of these two layers is used as a surrogate of INL thickness. Since the microcysts observed in this study were predominantly located in the INL, and INL pathology has been shown to occur in MS, while outer plexiform layer pathology has not, we strongly suspect that increased thicknesses of this composite measure primarily reflect increased INL thickness. Nonetheless, the possibility of outer plexiform layer pathology cannot be excluded, which if determined to be the case would represent an equally intriguing and novel finding. Future advances in automated OCT segmentation algorithms facilitating accurate assessment of the INL alone are necessary to confirm our findings. Longer studies are required to determine whether MME or higher INL thickness, potentially reflecting INL inflammation, ultimately lead to INL or ONL atrophy. Finally, there may be some bias in our MS cohort (exemplified by the high rate of disease activity in our treated RRMS patients) since our center is a major MS referral center. This may have implications for the translation of our findings to smaller clinical centers.
In summary, we have identified a novel retinal phenotype in MS characterized by dynamic aberrations of the neuronal INL, in which high-definition OCT demonstrates quantitatives welling of the INL, with or without accompanying microcysts. Our findings raise the possibility that inflammation compartmentalized to the myelin free neuronal INL of the retina may be operative in MS, suggesting myelin may not be necessary for instigating or propagating inflammation in MS, and that the pathobiology of MS may include neuronally targeted inflammation. Moreover, this retinal phenotype appears to be a harbinger of inflammatory disease activity. Higher INL thickness predicts the development of relapses, new T2 lesions, new gadolinium-enhancing lesions, and disability progression. The identification of this intriguing and clinically relevant retinal phenotype in MS may be an important step toward unraveling the elusive pathophysiology of MS, and merits further study in the future.
Supplementary Material
eFigure 1: Dotplots of baseline INL thickness.
The same data is presented as box and whiskers plots in Figure 4. Panel A shows baseline INL thicknesses of patients that developed relapses or new Gd-enhancing lesions during study follow-up (n= 47 subjects [94 eyes]) vs. patients that did not (n=73 subjects [146 eyes]). Panel B shows baseline INL thicknesses of MS patients exhibiting new T2 lesions during follow-up (n=49 subjects [98 eyes]), as compared to patients that did not (n=109 subjects [218 eyes]). The horizontal lines superimposed on the dotplots represent the corresponding mean INL thicknesses.
eFigure 2: Receiving operator characteristic (ROC) curve of baseline INL thickness for the prediction of development of a relapse or new Gd-enhancing lesion during follow-up in RRMS.
Data available for 120 RRMS patients (240 eyes). The area under the ROC curve (AUROC) is 0.67 (95% confidence intervals: 0.60–0.74).
eFigure 3: Scatterplots of baseline INL thickness with disease duration and age in MS.
Panel A illustrates increased variance of INL thickness measures earlier in the course of MS. The p-value result is from Levene’s variance ratio testing of baseline INL thickness in MS patients with disease duration <20 years vs. MS patients with disease duration ≥ 20 years. Panel B illustrates the lack of relationship between baseline INL thickness and age in MS.
Research in context.
Systematic review
We searched pubmed for original articles published in English between June 1, 1960 and June 1, 2012 with the search terms “multiple sclerosis” and “inner nuclear layer” that describe assessment of the INL in MS. Nine studies were identified, of which one was a large end-of-life ocular pathology study in MS.11 This study demonstrated qualitative atrophy of the INL in over 40% of MS eyes, as well as the presence of activated microglia in the retina of MS eyes at the end-of-life. The remaining eight studies utilized OCT for the in-vivo assessment of the INL in MS (five of these studies were published by our group). Utilizing segmentation techniques to quantitatively assess the INL, two studies demonstrated lower thicknesses of the INL in subsets of MS eyes,5,6 and one study demonstrated lower thickness of the INL across a cohort of PPMS patients, relative to HCs.32 Two studies reported that aberrations in the thickness of the INL were not the derivative of acute ON in MS.22,33 In two studies, significant differences in INL thickness between MS patients and HCs were not detected,34,35 although in one of these studies there was a suggestion that the thickness of the INL across a large cohort of MS patients may be higher than in HCs.35 Finally, a recent study identified and described for the first time MME of the INL in a subset of MS eyes,19 which was thought to represent breakdown of the blood-retinal-barrier and retinal inflammation. OCT segmentation techniques were not utilized in this study to quantitatively assess the INL. This study was performed by the same group that described ocular pathology in MS.11 Longitudinal studies assessing the clinical and radiological predictive utility of INL thickness in MS has not been previously performed.
Interpretation
Our findings implicate a novel and dynamic phenotype of the unmyelianted retina in MS that is characterized by an increase of INL thickness on OCT, with or without accompanying MME. This phenotype is a harbinger of inflammatory disease activity, with higher INL thickness in MS predicting the development of relapses, new T2 lesions, new gadolinium-enhancing lesions, and disability progression. Our findings raise the possibility that inflammation compartmentalized to the neuronal INL of the retina may be operative in MS, and that myelin may not be necessary for instigating or propagating the MS disease process. These findings have major implications for our understanding of the disease process in MS, and for guiding future research endeavors in MS.
Acknowledgments
Study Funding: National Multiple Sclerosis Society (TR 3760-A-3 to P.A.C and RG 4212-A-4 to Laura J. Balcer subcontracted to P.A.C), National Eye Institute (R01 EY 014993 and R01 EY 019473 to Laura J. Balcer subcontracted to P.A.C), and Braxton Debbie Angela Dillon and Skip (DADS) Donor Advisor Fund (to P.A.C, E.M.F, L.J.B.)
Disclosures
Dr. ShivSaidha has received consulting fees from Medical Logix for the development of continuing medical education programs in neurology, and has received educational grant support from TEVA Neurosciences.
Dr. Ciprian Crainiceanu has received consulting fees from Merck and On-X.
Dr. Jeffrey Gelfand receives grant support from an American Academy of Neurology Clinical Research Training Fellowship, and has received honoraria from the National MS Society for patient education.
Dr. John Ratchford has consulted for Bristol-Myers Squibb and Diogenix, received a speaking honorarium from Teva, and receives research support from Novartis and Biogen-Idec.
Dr. Jiwon Oh has received educational grant support from Teva Neurosciences.
Dr. Scott Newsome is a consultant for Biogen-Idec & Novartis & received speakers honoraria from Biogen-Idec and Teva Neuroscience.
Dr. Laura Balcerhas received speaking and consulting honoraria from Biogen Idec, Bayer, and Novartis.
Dr. Elliot Frohman has received speaker honoraria and consulting fees from Biogen Idec, Teva, and Athena. He has also received consulting fees from Abbott Laboratories.
Dr. Ari Green has provided consulting services for Prana Pharmaceuticals, Novartis, Biogen, Roche, and Acorda Pharmaceuticals. He has served on an end point adjudication committee for a Biogen sponsored trial and provided expert legal advice for Mylan Pharmaceuticals.
Dr. Quan Nguyen serves on the Scientific Advisory Board for Heidelberg Engineering, Inc. The Johns Hopkins University has received research support from Heidelberg to conduct studies; however, Heidelberg has had no inputs into the design, conduct, or analyses of the studies.
Dr. Peter Calabresi has provided consultation services to Novartis, Teva, Biogen Idec, Vertex, Vaccinex, Genentech; and has received grant support from EMD-Serono, Teva, Biogen Idec, Genentech, Bayer, Abbott, and Vertex.
Dr. Elias Sotirchos, Dr. Mohamed Ibrahim, Dr. Yasir Sepah and Michaela Seigohave no disclosures.
Footnotes
Statistical analysis conducted by Shiv Saidha & Elias Sotirchos, Department of Neurology, Johns Hopkins University School of Medicine, Baltimore, MD and Ciprian Crainiceanu, Department of Biostatistics, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD
Author Contributions:
Conceptualization of the study/Study concept & design: Saidha, Sotirchos, Nguyen, Calabresi
Acquisition of data: Saidha, Sotirchos, Seigo
Analysis & interpretation of the data: Saidha, Sotirchos, Crainiceanu, Nguyen, Calabresi
Drafting/Revising the manuscript: Saidha, Sotirchos, Ibrahim, Crainiceanu, Gelfand, Sepah, Ratchford, Oh, Seigo, Newsome, Balcer, Frohman, Green, Nguyen, Calabresi
Critical revision of the manuscript for important intellectual content: Saidha, Sotirchos, Ibrahim, Crainiceanu, Gelfand, Sepah, Ratchford, Oh, Seigo, Newsome, Balcer, Frohman, Green, Nguyen, Calabresi
Interpretation of the data: Saidha, Sotirchos, Crainiceanu, Nguyen, Calabresi
Study supervision: Nguyen, Calabresi
Strobe Statement:
This observational study is reported according to the STROBE statement, the checklist of which has been uploaded as supplementary material.
Conflicts of Interest
We declare that we have no conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Prineas J. Pathology of multiple sclerosis. In: Cook S, editor. Handbook of multiple sclerosis. New York: Marcel Dekker; 2001. pp. 289–324. [Google Scholar]
- 2.Calabrese M, Atzori M, Bernardi V, et al. Cortical atrophy is relevant in multiple sclerosis at clinical onset. J Neurol. 2007;254(9):1212–1220. doi: 10.1007/s00415-006-0503-6. [DOI] [PubMed] [Google Scholar]
- 3.Calabrese M, Agosta F, Rinaldi F, et al. Cortical lesions and atrophy associated with cognitive impairment in relapsing-remitting multiple sclerosis. Arch Neurol. 2009;66(9):1144–1150. doi: 10.1001/archneurol.2009.174. [DOI] [PubMed] [Google Scholar]
- 4.Moll NM, Rietsch AM, Ransohoff AJ, et al. Cortical demyelination in PML and MS: Similarities and differences. Neurology. 2008;70(5):336–343. doi: 10.1212/01.WNL.0000284601.54436.e4. [DOI] [PubMed] [Google Scholar]
- 5.Saidha S, Syc SB, Ibrahim MA, et al. Primary retinal pathology in multiple sclerosis as detected by optical coherence tomography. Brain. 2011;134(Pt 2):518–533. doi: 10.1093/brain/awq346. [DOI] [PubMed] [Google Scholar]
- 6.Saidha S, Syc SB, Durbin MK, et al. Visual dysfunction in multiple sclerosis correlates better with optical coherence tomography derived estimates of macular ganglion cell layer thickness than peripapillary retinal nerve fiber layer thickness. Mult Scler. 2011;17(12):1449–63. doi: 10.1177/1352458511418630. [DOI] [PubMed] [Google Scholar]
- 7.Kerrison JB, Flynn T, Green WR. Retinal pathologic changes in multiple sclerosis. Retina. 1994;14(5):445–451. doi: 10.1097/00006982-199414050-00010. [DOI] [PubMed] [Google Scholar]
- 8.Engell T, Hvidberg A, Uhrenholdt A. Multiple sclerosis: Periphlebitis retinalis et cerebro-spinalis. A correlation between periphlebitis retinalis and abnormal technetium brain scintigraphy. Acta Neurol Scand. 1984;69(5):293–297. doi: 10.1111/j.1600-0404.1984.tb07815.x. [DOI] [PubMed] [Google Scholar]
- 9.Sepulcre J, Murie-Fernandez M, Salinas-Alaman A, Garcia-Layana A, Bejarano B, Villoslada P. Diagnostic accuracy of retinal abnormalities in predicting disease activity in MS. Neurology. 2007;68(18):1488–1494. doi: 10.1212/01.wnl.0000260612.51849.ed. [DOI] [PubMed] [Google Scholar]
- 10.Donaldson MJ, Pulido JS, Herman DC, Diehl N, Hodge D. Pars planitis: A 20-year study of incidence, clinical features, and outcomes. Am J Ophthalmol. 2007;144(6):812–817. doi: 10.1016/j.ajo.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 11.Green AJ, McQuaid S, Hauser SL, Allen IV, Lyness R. Ocular pathology in multiple sclerosis: Retinal atrophy and inflammation irrespective of disease duration. Brain. 2010;133(6):1591–1601. doi: 10.1093/brain/awq080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saidha S, Eckstein C, Ratchford JN. Optical coherence tomography as a marker of axonal damage in multiple sclerosis. CML - Multiple Sclerosis. 2010;2(2):33–43. [Google Scholar]
- 13.Shindler KS, Ventura E, Dutt M, Rostami A. Inflammatory demyelination induces axonal injury and retinal ganglion cell apoptosis in experimental optic neuritis. Exp Eye Res. 2008;87(3):208–213. doi: 10.1016/j.exer.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Talman LS, Bisker ER, Sackel DJ, et al. Longitudinal study of vision and retinal nerve fiber layer thickness in multiple sclerosis. Ann Neurol. 2010;67(6):749–760. doi: 10.1002/ana.22005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brandt AU, Oberwahrenbrock T, Ringelstein M, et al. Primary retinal pathology in multiple sclerosis as detected by optical coherence tomography. Brain. 2011;134(Pt 11):e193. doi: 10.1093/brain/awr095. author reply e194. [DOI] [PubMed] [Google Scholar]
- 16.Forooghian F, Sproule M, Westall C, et al. Electroretinographic abnormalities in multiple sclerosis: Possible role for retinal autoantibodies. Doc Ophthalmol. 2006;113(2):123–132. doi: 10.1007/s10633-006-9022-0. [DOI] [PubMed] [Google Scholar]
- 17.Gundogan FC, Demirkaya S, Sobaci G. Is optical coherence tomography really a new biomarker candidate in multiple sclerosis?--A structural and functional evaluation. Invest Ophthalmol Vis Sci. 2007;48(12):5773–5781. doi: 10.1167/iovs.07-0834. [DOI] [PubMed] [Google Scholar]
- 18.Levkovitch-Verbin H, Quigley HA, Kerrigan-Baumrind LA, D’Anna SA, Kerrigan D, Pease ME. Optic nerve transection in monkeys may result in secondary degeneration of retinal ganglion cells. Invest Ophthalmol Vis Sci. 2001;42(5):975–982. [PubMed] [Google Scholar]
- 19.Gelfand JM, Nolan R, Schwartz DM, Graves J, Green AJ. Microcystic macular oedema in multiple sclerosis is associated with disease severity. Brain. 2012 doi: 10.1093/brain/aws098. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saidha S, Sotirchos ES, Oh S, et al. Retinal axonal and neuronal measures in multiple sclerosis reflect global CNS pathology. Archives of Neurology. doi: 10.1001/jamaneurol.2013.573. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Syc SB, Saidha S, Newsome SD, et al. Optical coherence tomography segmentation reveals ganglion cell layer pathology after optic neuritis. Brain. 2012 Feb;135(Pt 2):521–33. doi: 10.1093/brain/awr264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hume DA, Perry VH, Gordon S. Immunohistochemical localization of a macrophage-specific antigen in developing mouse retina: Phagocytosis of dying neurons and differentiation of microglial cells to form a regular array in the plexiform layers. J Cell Biol. 1983;97(1):253–257. doi: 10.1083/jcb.97.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Srivastava R, Aslam M, Kalluri SR, et al. Potassium channel KIR4.1 as an immune target in multiple sclerosis. N Engl J Med. 2012;367(2):115–123. doi: 10.1056/NEJMoa1110740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reichenbach A, Wurm A, Pannicke T, Iandiev I, Wiedemann P, Bringmann A. Müller cells as players in retinal degeneration and edema. Graefes Arch Clin Exp Ophthalmol. 2007;245(5):627–636. doi: 10.1007/s00417-006-0516-y. [DOI] [PubMed] [Google Scholar]
- 26.Brar M, Yuson R, Kozak I, et al. Correlation between morphologic features on spectral-domain optical coherence tomography and angiographic leakage patterns in macular edema. Retina. 2010;30(3):383–389. doi: 10.1097/IAE.0b013e3181cd4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rucker CW. Sheathing of the retinal veins in multiple sclerosis. review of pertinent literature. Mayo Clin Proc. 1972;47(5):335–340. [PubMed] [Google Scholar]
- 28.Cohen JA, Barkhof F, Comi G, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010;362(5):402–415. doi: 10.1056/NEJMoa0907839. [DOI] [PubMed] [Google Scholar]
- 29.Lightman S, McDonald WI, Bird AC, et al. Retinal venous sheathing in optic neuritis. its significance for the pathogenesis of multiple sclerosis. Brain. 1987;110(Pt 2):405–414. doi: 10.1093/brain/110.2.405. [DOI] [PubMed] [Google Scholar]
- 30.Gills JP, Jr, Wadsworth JA. Degeneration of the inner nuclear layer of the retina following lesions of the optic nerve. Trans Am Ophthalmol Soc. 1966;64:66–88. [PMC free article] [PubMed] [Google Scholar]
- 31.Marrie RA, Horwitz RI. Emerging effects of comorbidities on multiple sclerosis. Lancet Neurol. 2010;9(8):820–828. doi: 10.1016/S1474-4422(10)70135-6. [DOI] [PubMed] [Google Scholar]
- 32.Albrecht P, Ringelstein M, Mueller A, et al. Degeneration of retinal layers in multiple sclerosis subtypes quantified by optical coherence tomography. Mult Scler. 2012 doi: 10.1177/1352458512439237. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 33.Sriram P, Graham SL, Wang C, Yiannikas C, Garrick R, Klistorner A. Transsynaptic retinal degeneration in optic neuropathies: Optical coherence tomography study. Invest Ophthalmol Vis Sci. 2012;53(3):1271–1275. doi: 10.1167/iovs.11-8732. [DOI] [PubMed] [Google Scholar]
- 34.Seigo MA, Sotirchos ES, Newsome S, et al. In vivo assessment of retinal neuronal layers in multiple sclerosis with manual and automated optical coherence tomography segmentation techniques. J Neurol. 2012 doi: 10.1007/s00415-012-6466-x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 35.Walter SD, Ishikawa H, Galetta KM, et al. Ganglion cell loss in relation to visual disability in multiple sclerosis. Ophthalmology. 2012;119(6):1250–1257. doi: 10.1016/j.ophtha.2011.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1: Dotplots of baseline INL thickness.
The same data is presented as box and whiskers plots in Figure 4. Panel A shows baseline INL thicknesses of patients that developed relapses or new Gd-enhancing lesions during study follow-up (n= 47 subjects [94 eyes]) vs. patients that did not (n=73 subjects [146 eyes]). Panel B shows baseline INL thicknesses of MS patients exhibiting new T2 lesions during follow-up (n=49 subjects [98 eyes]), as compared to patients that did not (n=109 subjects [218 eyes]). The horizontal lines superimposed on the dotplots represent the corresponding mean INL thicknesses.
eFigure 2: Receiving operator characteristic (ROC) curve of baseline INL thickness for the prediction of development of a relapse or new Gd-enhancing lesion during follow-up in RRMS.
Data available for 120 RRMS patients (240 eyes). The area under the ROC curve (AUROC) is 0.67 (95% confidence intervals: 0.60–0.74).
eFigure 3: Scatterplots of baseline INL thickness with disease duration and age in MS.
Panel A illustrates increased variance of INL thickness measures earlier in the course of MS. The p-value result is from Levene’s variance ratio testing of baseline INL thickness in MS patients with disease duration <20 years vs. MS patients with disease duration ≥ 20 years. Panel B illustrates the lack of relationship between baseline INL thickness and age in MS.